Abstract
With growing interest in the gut microbiome, prebiotics and probiotics have received considerable attention as potential treatments for depression and anxiety. We conducted a random-effects meta-analysis of 34 controlled clinical trials evaluating the effects of prebiotics and probiotics on depression and anxiety. Prebiotics did not differ from placebo for depression (d = −.08, p = .51) or anxiety (d = .12, p = .11). Probiotics yielded small but significant effects for depression (d = −.24, p < .01) and anxiety (d = −.10, p =.03). Sample type was a moderator for probiotics and depression, with a larger effect observed for clinical/medical samples (d = −.45, p < .001) than community ones. This effect increased to medium-to-large in a preliminary analysis restricted to psychiatric samples (d = −.73, p < .001). There is general support for antidepressant and anxiolytic effects of probiotics, but the pooled effects were reduced by the paucity of trials with clinical samples. Additional randomized clinical trials with psychiatric samples are necessary fully to evaluate their therapeutic potential.
Keywords: anxiety, depression, microbiome, prebiotic, probiotic
1. Introduction
Depression and anxiety disorders are the two most common mental health conditions, with lifetime prevalence rates in the U.S. of 16.6% and 28.8%, respectively (Kessler et al., 2005). The societal and personal costs of these conditions are considerable. In terms of years living with disability in the U.S., these two disorders rank second and fifth, respectively, out of all mental and physical health conditions (US Burden of Disease Collaborators, 2013). Consistent with these findings, depression and anxiety are also the top two mental health conditions in terms of personal health care expenditures, with $71.1 billion spent annually in the U.S. to treat depression and $29.7 billion anxiety disorders (Dieleman et al., 2016). Furthermore, the burden of these disorders is increasing (Vos et al., 2016).
The development of novel therapeutic modalities is needed to reduce the burden of these conditions. Of several possibilities that have garnered substantial interest of late, prebiotics (i.e., chemical compounds that yield health benefits through their influence on the host gut microbiome) and probiotics (i.e., microorganisms that contribute to the host gut microbial flora when consumed, and thereby produce beneficial effects on health) hold particular appeal, in part, for being potentially free of cognitive side effects and the addictive properties of several currently available treatments for these disorders (Liu, 2017). Although the first study to evaluate the therapeutic efficacy of prebiotics or probiotics on depression or anxiety was conducted over a decade ago,(Marcos et al., 2004) approximately half of all existing studies were published in the last two years alone, reflecting the rapidly growing interest in this area.
Also reflective of this burgeoning interest, there have been several recent systematic reviews of probiotics in this area (Huang et al., 2016; Liu et al., 2018; Ng et al., 2018; Pirbaglou et al., 2016; Reis et al., 2018; Wallace and Milev, 2017), including two meta-analyses of depression (Huang et al., 2016; Ng et al., 2018) and anxiety (Liu et al., 2018; Reis et al., 2018), respectively. Although these prior reviews are important for providing the first syntheses of the empirical literature in this area, they are also characterized by several notable limitations. In particular, the two aforementioned meta-analyses of probiotics and depression each included a very small number of studies (ks = 4 and 9 for studies meeting the eligibility criteria of the current review), precluding any analyses of publication bias and moderating effects to account for between-study heterogeneity in effect sizes. These meta-analyses also included a study that combined anxiety and depression as a single outcome in their analyses (Mohammadi et al., 2016) which when considered within the context of the small number of studies included in each review and important etiological distinctions between these outcomes (Clark and Watson, 1991), complicates interpretations regarding the effect of probiotics specifically in relation to depression. Additionally, and perhaps in some measure a function of the number of studies included in each, these meta-analyses yielded contradictory findings, with one finding support for an ameliorative effect of probiotics (Huang et al., 2016) and the other reporting no such effect overall (Ng et al., 2018).
Interpretation of the findings of the recent meta-analyses of probiotics and anxiety is also complicated by certain methodological concerns. In particular, over half of the effects included in one of these meta-analyses (Reis et al., 2018) were based on non-independent samples. The other meta-analysis (Liu et al., 2018) included several studies of outcomes other than anxiety as typically conceptualized (e.g., visceral sensitivity; Lorenzo-Zúñiga et al., 2014), and 42% of studies did not meet the eligibility criteria of the current review. Altogether, these meta-analyses included 7 and 11 trials eligible for inclusion in this review.
Addressing these considerations, we conducted a systematic meta-analytic review of controlled clinical trials evaluating the efficacy of prebiotics and probiotics for treating depression and anxiety.. With 28 studies, including 18 with 19 unique effects for probiotics for depression and anxiety, the current review builds substantially upon the aforementioned meta-analyses. Additionally, the current review presents preliminary meta-analyses of prebiotics in relation to depression and anxiety, respectively.
2. Method
2.1. Search strategy and eligibility criteria
A systematic search of the literature was conducted in Embase, MEDLINE, and PsycINFO to identify studies relevant to the current review. The following search string was applied: (“leaky gut” OR dysbiosis OR metagenom* OR microbiome* OR microbiota OR prebiotic* OR probiotic* OR “bacterial translocation” OR “colon flora” OR “fecal flora” OR “gut flora” OR “intestinal flora” OR “enteric bacteria” OR “fecal bacteria” OR “gut bacteria” OR “intestinal bacteria” OR “fecal microflora” OR “gut microflora” OR “intestinal microflora” OR “gut microbial” OR bifidobacter* OR lactobacill*) AND (depress* OR “affective disorder” OR “affective illness” OR “mood disorder” OR anxi* OR internalizing OR “mental health” OR “mental illness” OR “psychiatric disorder” OR “psychiatric illness”). The search results were limited to: (i) English-language publications1 and (ii) peer-reviewed journals. This was supplemented with a search of the references of all prior systematic reviews of probiotics in relation to mental health as a general construct and depression and anxiety specifically (Huang et al., 2016; Liu et al., 2018; McKean et al., 2017; Ng et al., 2018; Pirbaglou et al., 2016; Reis et al., 2018; Romijn and Rucklidge, 2015; Wallace and Milev, 2017). This search strategy yielded a total of 1,911 articles, of which 1,475 were unique reports. In cases where eligibility could not be ruled out based on the title and abstract, the full text was also examined. Each search result was independently reviewed for eligibility by two of the authors, with discrepancies resolved by the first author.
The study inclusion criteria were: (i) a controlled clinical trial investigating the effects of prebiotics or probiotics on depression or anxiety (i.e., naturalistic studies assessing self-reported consumption of prebiotics or probiotics in relation to depression or anxiety were excluded); (ii) studies conducted with human participants (i.e., preclinical studies with animal models were excluded); (iii) prebiotics and/or probiotics were the only active components of the treatment condition(s); and (iv) depression and anxiety were analyzed separately from each other and other outcomes.
2.2. Study quality assessment
The quality of eligible studies was evaluated based on the risk of bias criteria detailed in the Cochrane Handbook for Systematic Reviews of Interventions (The Cochrane Collaboration, 2011): (i) condition allocation through random sequence generation (selection bias); (ii) concealment of condition allocation (allocation bias); (iii) blinding of participants and study personnel to condition (performance bias); (iv) blinding of outcome assessment (detection bias); (v) incomplete outcome data (attrition bias); and (vi) selective outcome reporting of results for depression or anxiety (reporting bias).
2.3. Data extraction
Six eligible studies (Marcos et al., 2004; Pinto-Sanchez et al., 2017; Reale et al., 2012; Roman et al., 2018; Sanchez et al., 2017; Vaghef-Mehrabany et al., 2014) presented data for both state and trait anxiety. In these cases, data for state anxiety were selected for use in our analyses, as such data would be more suitable for evaluating the immediate treatment effects of prebiotic or probiotics on anxiety.
To conduct sub-analyses and to assess potential moderators in meta-analyses, data on eight study characteristics were extracted. These included four sample characteristics: (i) sample age group (adolescent or adult); (ii) mean age of sample; (iii) sample type (community or clinical/medical); and (iv) percentage of female participants in the sample. Data for four study design characteristics were extracted: (i) form(s) of prebiotic or probiotic administered in the treatment condition; (ii) method of measuring depression and anxiety (interview/clinician ratings or self-report); (iii) type of controlled clinical trial (i.e., cross-over design or randomized clinical trial with parallel-group design [RCT]); and (iv) duration of prebiotic/probiotic administration.
2.4. Data analysis
Analyses were conducted with Comprehensive Meta-Analysis Version 3.3.070 (Biostat, 2014). Standardized mean difference (Cohen’s d) was used as the primary index of effect size, and calculated such that negative values indicating lower depression and anxiety, respectively, in the treatment condition compared to the control condition. Heterogeneity across the studies was evaluated using the I2 statistic, which indicates the percentage of the variance in an effect estimate that is a product of heterogeneity across studies rather than sampling error. Low heterogeneity is indicated by I2 values of approximately 25%, moderate heterogeneity by I2 values of 50%, and substantial heterogeneity by I2 values of 75% (Higgins et al., 2003). In cases where high heterogeneity is observed, random-effects models are more appropriate than fixed-effects models, as the former accounts for this heterogeneity by incorporating both sampling and study-level errors, with the pooled effect size representing the mean of a distribution of true effect sizes instead of a single true effect size. In contrast, fixed-effects models assume that a single true effect size exists across all studies and any variance detected is due strictly to sampling error. It thus estimates only within-study variance. For all analyses, random-effects models were generated to account for heterogeneity across studies resulting from differences in samples, measures, and design.
In cases where significant heterogeneity was observed, moderator analyses were conducted to account for potential sources of this heterogeneity. Each potential moderator was assessed separately, with an estimate of the effect size at each level of the moderator calculated.
To assess for publication bias inflating estimates of pooled effect size, the following indices were calculated: Duval and Tweedie’s trim-and-fill analysis (Duval and Tweedie, 2000), and Egger’s regression intercept (Egger et al., 1997). Duval and Tweedie’s trim-and-fill analysis provides an estimate of the number of missing studies based on asymmetry in a funnel plot of the standard error of each study in a meta-analysis against its effect size, and an effect size estimate and confidence interval, adjusting for these missing studies. This analysis assumes homogeneity of effect sizes, and thus its results need to be interpreted with caution in the presence of significant heterogeneity. Egger’s regression intercept estimates potential publication bias using a linear regression approach assessing study effect sizes relative to their standard error.
3. Results
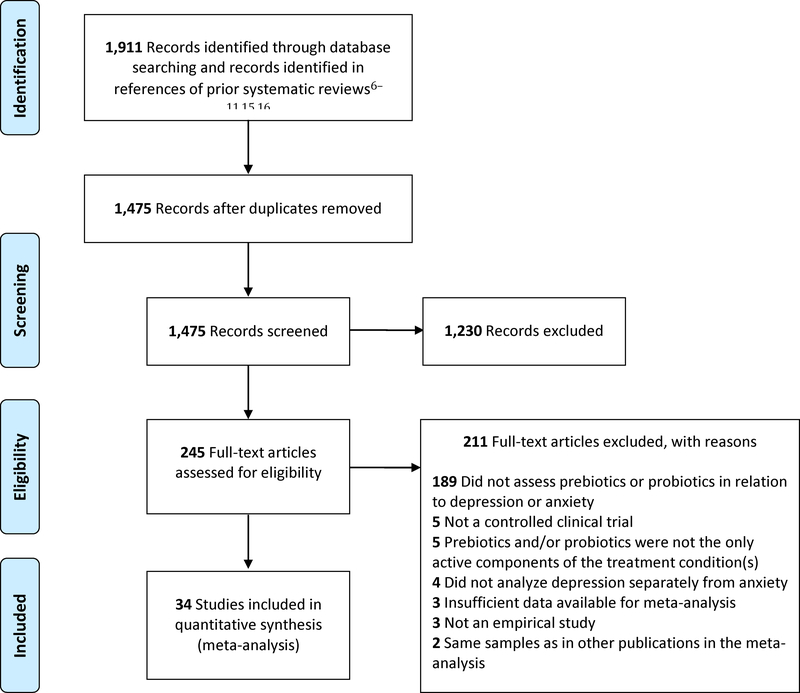
Of the 1,475 unique records identified, 1,230 reports were excluded based on their titles and abstracts. An additional 211 articles were excluded based on a detailed full-text review. Whenever it remained unclear after full-text inspection whether two studies reported on overlapping samples, the study authors were contacted to seek clarity on this issue. In one case where multiple studies featured overlapping samples, preference was given to the study that assessed the outcome of interest at the end of the clinical trial rather than prior to its completion. Eight studies (Azpiroz et al., 2017; Colica et al., 2017; Cremon et al., 2017; Kazemi et al., 2019; Messaoudi et al., 2011; Reale et al., 2012; Tillisch et al., 2013; Yang et al., 2016) did not report data required for meta-analysis, but were included after the necessary data were obtained from the study authors and prior meta-analyses (Huang et al., 2016; Reis et al., 2018). A final set of 34 publications satisfied the eligibility criteria for this review (Figure 1 and Table 1), including seven prebiotic (Azpiroz et al., 2017; Kazemi et al., 2019; Sanchez et al., 2017; Schmidt et al., 2015; Silk et al., 2009; Smith, 2005; Smith et al., 2015) and 29 probiotic trials (Akkasheh et al., 2016; Chung et al., 2014; Colica et al., 2017; Cremon et al., 2017; Ghorbani et al., 2018; Kato-Kataoka et al., 2016; Kazemi et al., 2019; Kelly et al., 2017; Kitaoka et al., 2009; Kouchaki et al., 2017; Lyra et al., 2016; Majeed et al., 2018; Marcos et al., 2004; Messaoudi et al., 2011; Nishihira et al., 2014; Östlund-Lagerström et al., 2016; Pinto-Sanchez et al., 2017; Reale et al., 2012; Roman et al., 2018; Romijn et al., 2017; Sanchez et al., 2017; Sashihara et al., 2013; Shinkai et al., 2013; Simrén et al., 2010; Slykerman et al., 2017; Steenbergen et al., 2015; Tillisch et al., 2013; Vaghef-Mehrabany et al., 2014; Yang et al., 2016). Two studies (Ghorbani et al., 2018; Sanchez et al., 2017) featured both prebiotics and a probiotic within the treatment condition, and was included only in meta-analyses of probiotics. Another study (Kazemi et al., 2019) employed a three-arm parallel design, with separate conditions for prebiotics, probiotics, and placebo, and the relevant conditions were included in separate meta-analyses of prebiotics and probiotics. With only two studies featuring interview or clinician-based measures of anxiety (Colica et al., 2017; Yang et al., 2016), method of measuring this outcome was excluded from consideration in moderator analyses. As no study featured an adolescent-only sample, age as a categorical variable was also excluded from moderator analyses.
Figure 1.
PRISMA flow chart of literature search
Table 1.
Study characteristics
| Study Authors (year) | Na | % Femalea | Mean Agea | Sample | Prebiotic Compound(s)/Probiotic Microbe(s) | Length of Treatment | Clinical Outcome(s) | Clinical Measure (s) |
|---|---|---|---|---|---|---|---|---|
| Prebiotics | ||||||||
| Azpiroz et al. (2017)b | 79 | 75.95 | 41.67 | Medical (IBS patients) | scFOS | 4 weeks | anx, dep | HADS-A, HADS-D |
| Kazemi et al. (2019)d | 72 | 70.83 | 36.68 | Clinical (MDD patients) | GOS | 8 weeks | dep | BDI-II |
| Schmidt et al. (2015) b | 45 | 51.11 | 23.69 | Community | B-GOS, FOS | 3 weeks | anx | STAI |
| Silk et al. (2009) | 44 | 63.64 | 54.00 | Medical (IBS patients) | B-GOS | 4 weeks | anx, dep | HADS-A, HADS-D |
| Smith (2005)c | 142 | 51.00 | 32.00 | Community | FOS-enriched inulin | 2 weeks | anx, dep | HADS-A, HADS-D |
| Smith et al. (2015)c | 47 | 59.57 | 23.00 | Community | FOS-enriched inulin | 4 hours | dep | SSM |
| Probiotics | ||||||||
| Akkasheh et al. (2016)d | 40 | 85.00 | 37.20 | Clinical (MDD patients) | Bifidobacterium bifidium, Lactobacillus acidophilus, Lactobacillus casei | 8 weeks | dep | BDI-I |
| Chung et al. (2014)b | 36 | 44.40 | 65.00 | Community | Lactobacillus helveticus | 12 weeks | dep | GDS-SF |
| Colica et al. (2017) | 30 | 83.30 | 45.00 | Community | Bifidobacterium lactis, Lactobacillus acidophilus, Lactobacillus bulgari, Lactococcus lactis subspecies lactis, Lactobacillus plantarum, Lactobacillus reuteri, Streptococcus thermophiles, Streptococcus thermophilus | 3 weeks | anx | HAM-A |
| Cremon et al. (2018)c | 40 | 65.00% | 40.95 | Medical (IBS patients) | Lactobacillus paracasei | 4 weeks | anx, dep | HADS-A, HADS-D |
| Ghorbani et al. (2018)d,e | 40 | 34.98 | 70 | Clinical (MDD patients) | FOS, Bifidobacterium breve, Bifidobacterium longum, Lactobacillus acidofilus, Lactobacillus bulgarigus, Lactobacillus casaei, Lactobacillus rhamnosus, Streptococus thermophilus | 6 weeks | dep | HAM-D |
| Kato-Kataoka et al. (2016)b | 47 | 44.68 | 22.85 | Community (medical students) | Lactobacillus casei Shirota | 8 weeks | anx, dep | HADS-A, HADS-D |
| Kazemi et al. (2019)d | 74 | 68.92 | 36.08 | Clinical (MDD patients) | Bifidobacterium longum, Lactobacillus helveticus | 8 weeks | dep | BDI-II |
| Kelly et al. (2017)b,c | 29 | 0 | 24.59 | Community | Lactobacillus rhamnosus | 4 weeks | anx, dep | BAI, BDI (version unspecified), STAI |
| Kitaoka et al. (2009)b | 16 | 0 | 20.69 | Community | Lactobacillus paracasei | 8 days | anx | STAI |
| Kouchaki et al. (2017) | 60 | 83.33 | 34.10 | Medical (MS patients) | Bifidobacterium bifidum, Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus fermentum | 12 weeks | dep | BDI-I |
| Lyra et al. (2016) | 391 | 74.68 | 47.90 | Medical (IBS patients) | Lactobacillus acidophilus | 12 weeks | anx, dep | HADS-A, HADS-D |
| Majeed et al. (2018)d | 40 | 85.00 | 42.12 | Clinical and Medical (patients with MDD and IBS) | Bacillus coagulans | 90 days | dep | CES-D, HAM-D, MADRS |
| Marcos et al. (2004) | 136 | 70.59 | NR | Community (college students) | Lactobacillus casei, Lactobacillus delbrueckii subspecies bulgaricus, Streptococcus salivarius subspecies thermophiles | 6 weeks | anx | STAI |
| Messaoudi et al. (2011)b | 55 | 74.55 | 42.82 | Community | Bifidobacterium longum, Lactobacillus helveticus | 30 days | anx, dep | HADS-A, HADS-D |
| Nishihira et al. (2014) | 224 | 69.20 | 53.92 | Community | Bifidobacterium longum, Lactobacillus gasseri | 12 weeks | dep | GHQ-28 depression subscale |
| Östlund-Lagerström et al. (2016) | 238 | 61.04 | 72.30 | Community (elderly) | Lactobacillus reuteri | 12 weeks | anx, dep | HADS-A, HADS-D |
| Pinto-Sanchez et al. (2017)f | 44 | 54.00 | 43.25 | Medical (IBS patients) | Bifidobacterium longum | 6 weeks | anx, dep | HADS-A, HADS–D, STAI |
| Reale et al. (2012) | 65 | 0 | 50.30 | Community (smokers) | Lactobacillus casei Shirota | 3 weeks | anx | STAI |
| Roman et al. (2018) | 31 | 90.32 | 52.71 | Medical (fibromyalgia patients) | Bifidobacterium bifidus, Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus rhamnosus | 8 weeks | anx, dep | BDI-I, STAI |
| Romijn et al. (2017)d | 79 | 78.48 | 35.45 | Clinical | Bifidobacterium longum, Lactobacillus helveticus | 8 weeks | anx, dep | DASS, MADRS, QIDS-SR16 |
| Sanchez et al. (2017)e,f | 125 | 61.60 | 36.01 | Medical (obese subjects) | FOS, inulin, Lactobacillus rhamnosus | 24 weeks | anx, dep | BDI-I, STAI |
| Sashihara et al. (2013) | 29 | 0 | 19.99 | Community Lactobacillus gasseri (college athletes) | Lactobacillus gasseri | 4 weeks | anx, dep | POMS |
| Shinkai et al. (2013) | 278 | 50.36 | 70.90 | Community | Lactobacillus pentosus | 20 weeks | anx, dep | POMS |
| Simrén et al. (2010)b | 74 | 43.00 | 70.27 | Medical (IBS patients) | Bifidobacterium lactis, Lactobacillus acidophilus, Lactobacillus bulgaricus, Lactobacillus paracasei, Streptococcus thermophilus | 8 weeks | anx, dep | HADS-A, HADS-D |
| Slykerman et al. (2017) | 381 | 100 | 33.60 | Community (pregnant women) | Lactobacillus rhamnosus | 45 weeks | anx, dep | EPDS, STAI |
| Steenbergen et al. (2015)b | 40 | 60.00 | 19.95 | Community | Bifidobacterium bifidum, Bifidobacterium lactis, Lactobacillus acidophilus, Lactobacillus brevis, Lactobacillus casei, Lactobacillus salivarius, Lactococcus lactis | 4 weeks | anx, dep | BAI, BDI-II |
| Tiilisch et al. (2013)b | 23 | 100 | 30.00 | Community | Bifidobacterium animalis, Lactobacillus bulgaricus, Lactococcus lactis, Streptococcus thermophiles | 4 weeks | anx, dep | HADS-A, HADS-D |
| Vaghef-Mehrabany et al. (2014) | 46 | 100 | 42.78 | Medical (rheumatoid arthritis patients) | Lactobacillus casei | 8 weeks | anx | STAI Form Y |
| Yang et al. (2016)b,h | 20 | 50.00 | 58.05 | Medical (laryngeal cancer patients) | Clostridium butyricum | 2 weeks | anx | HAM-A |
Note: anx = anxiety; BAI = Beck Anxiety Inventory; BDI = Beck Depression Inventory; B-GOS = Bimuno®-galactooligosaccharide; CES-D = Center for Epidemiologic Studies – Depression Scale; DASS = Depression Anxiety and Stress Scale; dep = depression; EPDS = Edinburgh Postnatal Depression Scale; FOS = fructooligosaccharide; GDS-SF = Geriatric Depression Scale – Short Form; GHQ = General Health Questionnaire; GOS = galactooligosaccharide; HADS-A = Hospital Anxiety and Depression Scale – Anxiety subscale; HADS-D = Hospital Anxiety and Depression Scale – Depression subscale; HAM-A = Hamilton Rating Scale for Anxiety; IBS = irritable bowel syndrome; MADRS = Montgomery-Åsberg Depression Rating Scale; MDD = major depressive disorder; MS = multiple sclerosis; NR = not reported; POMS = Profile of Mood States; QIDS-SR16 = Quick Inventory of Depressive Symptomatology – 16-item short-form; scFOS = short-chain fructooligosaccharide; SSM = study-specific measure; STAI = State-Trait Anxiety Inventory
In cases where data for multiple outcome measures are available in a given study, the mean of the effects for these measures was incorporated into the relevant meta-analysis.
The number, mean age, and % female of participants included in relevant analyses, rather than for the entire study sample, are presented and were incorporated in moderator analyses whenever available and applicable.
Prospective subjects with psychopathology were screened out. These studies were categorized as having non-clinical samples for the purpose of moderator analyses.
A cross-over design was employed.
Subjects were selected for elevated depression (no studies selected for elevated anxiety).
Subjects were administered a compound consisting of both prebiotics and probiotics.
Prospective subjects with minimal and severe depression and anxiety symptoms were screened out.
Separate effects were reported by sex.
Outlier excluded from meta-analysis.
3.1. Study quality assessment
Several notable patterns emerged in study quality. Specifically, 26.5% of studies had an overall low risk bias (defined as low risk for all criteria, or unclear risk for allocation bias and low risk for all other criteria), and 41.2% had overall high risk bias (defined as high risk on at least one criterion), leaving 32.4% of studies with overall unclear risk bias. Low risk bias for all six criteria was met by 23.5% of studies. Most often, high risk bias occurred due to adoption of per-protocol rather than intent-to-treat analyses (41.2% of studies), unclear risk bias occurred most often for allocation bias (47.1% of studies), and low risk reporting bias was determined for all but one study. See Supplemental Figure 1 for a summary.
3.2. Prebiotic trials for depression and anxiety
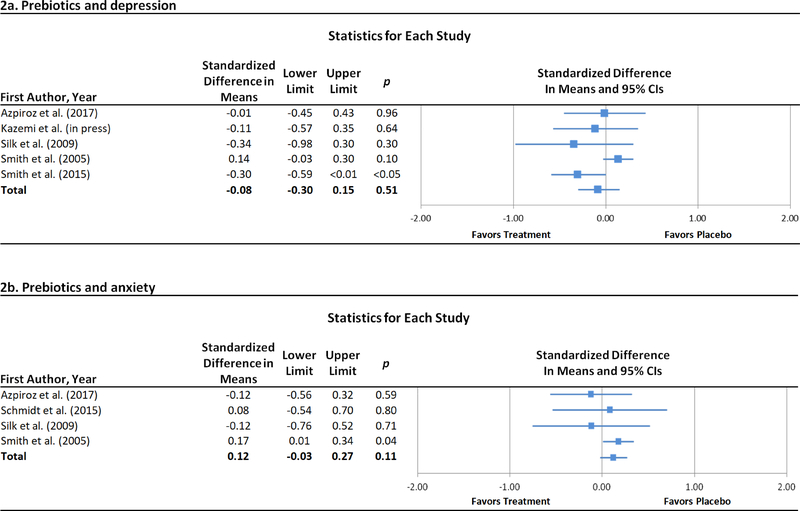
The compounds evaluated in the prebiotic trials included Bimuno®-galactooligosaccharide (B-GOS), fructooligosaccharide (FOS), GOS, and short-chain FOS (scFOS), all possessing bifidogenic properties. One three-arm study (Schmidt et al., 2015) separately evaluated B-GOS and FOS. Length of prebiotic administration across trials ranged from four hours to four weeks. Across five prebiotics trials for depression (Azpiroz et al., 2017; Kazemi et al., 2019; Silk et al., 2009; Smith, 2005; Smith et al., 2015), no difference was observed between prebiotic and control conditions (Figure 2a). Similarly, a significant effect was not observed across four prebiotic trials for anxiety (Figure 2b; Azpiroz et al., 2017; Schmidt et al., 2015; Silk et al., 2009; Smith, 2005). These results remained essentially unchanged in sensitivity analyses limited to studies of FOS and scFOS for depression and anxiety, respectively (Supplemental Table 1).
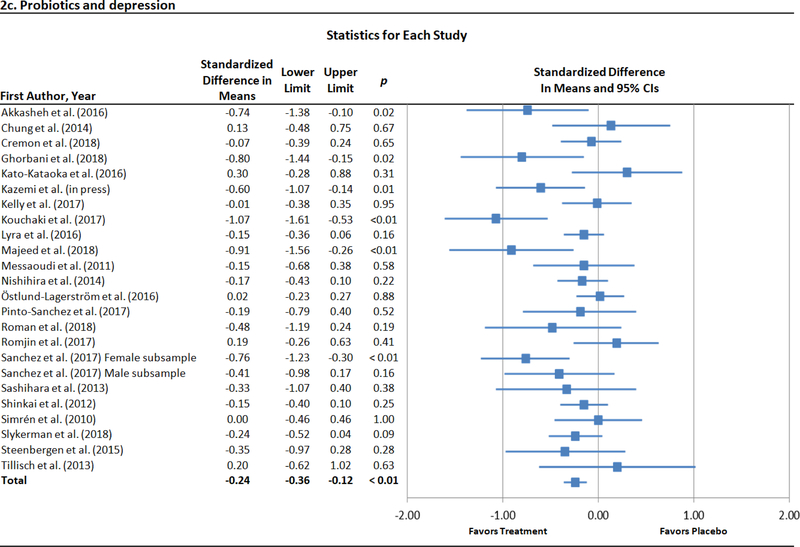
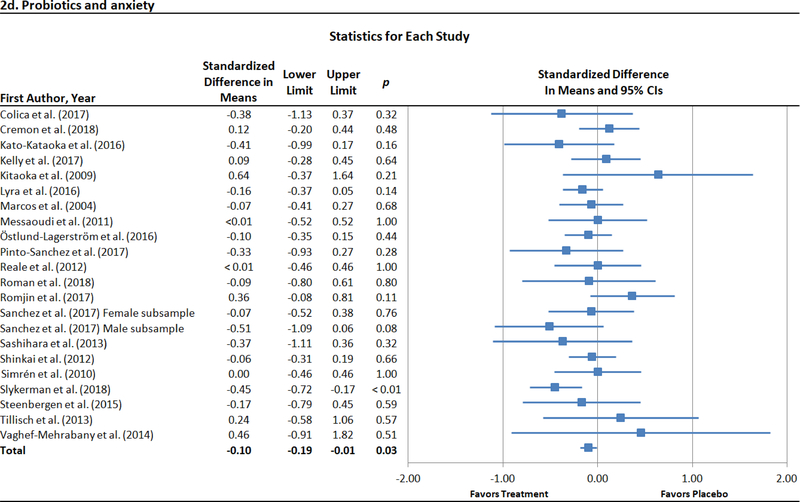
Figure 2.
Forest plots of standardized effect sizes (Cohen’s d) of prebiotic and probiotic trials for depression and anxiety.
3.3. Probiotic trials for depression and anxiety
With two exceptions focusing exclusively on Bifidobacterium longum (Pinto-Sanchez et al., 2017) and Bacillus coagulans (Majeed et al., 2018), respectively, all probiotic trials investigated lactobacilli alone or in combination with species from other genera, most often Bifidobacterium. Duration of probiotic administration across trials ranged from eight days to 45 weeks.
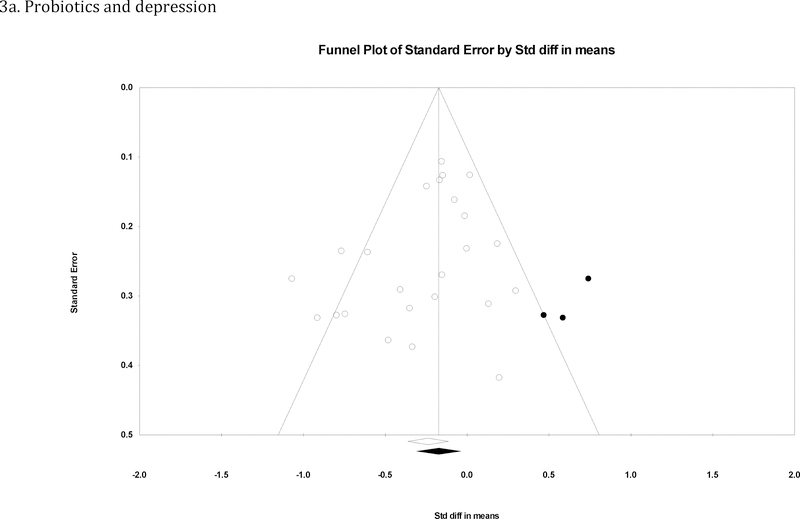
Across 23 trials with 24 unique effects for probiotics and depression (Akkasheh et al., 2016; Chung et al., 2014; Cremon et al., 2017; Ghorbani et al., 2018; Kato-Kataoka et al., 2016; Kazemi et al., 2019; Kelly et al., 2017; Kouchaki et al., 2017; Lyra et al., 2016; Majeed et al., 2018; Messaoudi et al., 2011; Nishihira et al., 2014; Östlund-Lagerström et al., 2016; Pinto-Sanchez et al., 2017; Roman et al., 2018; Romijn et al., 2017; Sanchez et al., 2017; Sashihara et al., 2013; Shinkai et al., 2013; Simrén et al., 2010; Slykerman et al., 2017; Steenbergen et al., 2015; Tillisch et al., 2013), depression was lower in probiotic than placebo conditions at the end of treatment (Figure 2c). Heterogeneity was moderately high (I2 = 48.2%, p = .01), indicting the appropriateness of moderator analyses. The strength of the observed effect did not change as a function of mean age of each sample (b < .01, p = .26), the percentage of female participants in each study (b < .01, p = .13), method of assessing depression (p = .49), or duration of probiotic administration (b <.01, p = .45). When analyses were stratified by this latter variable treated dichotomously, however, a significant treatment effect was found for trials lasting longer than a month (d = −.28 [95% CI = −.44 – −.13], p < .001), but not for trials of up to one month (d = −.10 [95% CI = −.29 – .10], p = .33). Additionally, sample type emerged as a significant moderator (p < .01), with a larger treatment effect observed for clinical or medical samples (d = −.45 [95% CI = −.68 – −.23], p < .001) than community ones (d = −.09 [95% CI = −.20 – .01], p = .09). This moderator effect held in an exploratory follow-up analysis (p < .01) directly comparing four trials with major depression (d = −.73 [95% CI = −1.02 – −.44], p < .001; Akkasheh et al., 2016; Ghorbani et al., 2018; Kazemi et al., 2019; Majeed et al., 2018) with seven trials with psychiatric disorders screened out (d < .01 [95% CI = −.20 – .20], p = .99; (Chung et al., 2014; Kato-Kataoka et al., 2016; Kelly et al., 2017; Messaoudi et al., 2011; Simrén et al., 2010; Steenbergen et al., 2015; Tillisch et al., 2013). Additional details for this analysis are presented in Supplemental Table 2. In analyses of publication bias, Egger’s regression test indicated that there was no significant publication bias (intercept = −1.26, p = .08), but the adjusted effect size produced with the trim-andfill method was smaller in absolute value terms (d = −.17 [95% CI = −.31 – −.04]) and the corresponding funnel plot of effect sizes was slightly asymmetrical (Figure 3a).
Figure 3.
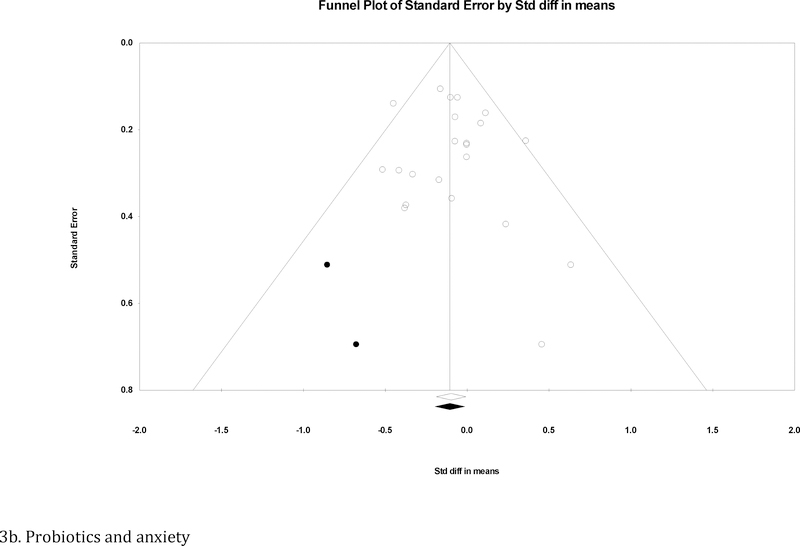
Funnel plot for effect sizes in the meta-analyses. The vertical line indicates the weighted mean effect. Open circles indicate observed effects for actual studies, and closed circles indicate imputed effects for studies believed to be missing due to publication bias. The clear diamond reflects the unadjusted weighted mean effect size, whereas the black diamond reflects the weighted mean effect size after adjusting for publication bias.
There were 22 trials with 23 unique effects for probiotics and anxiety (Colica et al., 2017; Cremon et al., 2017; Kato-Kataoka et al., 2016; Kelly et al., 2017; Kitaoka et al., 2009; Lyra et al., 2016; Marcos et al., 2004; Messaoudi et al., 2011; Östlund-Lagerström et al., 2016; Pinto-Sanchez et al., 2017; Reale et al., 2012; Roman et al., 2018; Romijn et al., 2017; Sanchez et al., 2017; Sashihara et al., 2013; Shinkai et al., 2013; Simrén et al., 2010; Slykerman et al., 2017; Steenbergen et al., 2015; Tillisch et al., 2013; Vaghef-Mehrabany et al., 2014; Yang et al., 2016). An outlier (Yang et al., 2016) was identified and excluded from all analyses. Probiotic administration was associated with lower anxiety relative to placebo at the end of treatment (Figure 2d). As significant heterogeneity was not observed (I2 = 5.0%, p = .39), moderator analyses were not conducted. Evidence of publication bias was modest. Specifically, the result of Egger’s regression test was not significant (intercept = .37, p = .49), and the pooled effect size produced with the trim-and-fill method adjusting for imputed missing significant effects remained largely unchanged (adjusted d = −.11 [95% CI = −.20 – −.01]). The funnel plot of effect sizes was essentially symmetrical, with two imputed effects favoring treatment (Figure 3b).
For both depression and anxiety, a series of sensitivity analyses was conducted (Supplemental Table 3). In analyses excluding (i) two cross-over trials (Cremon et al., 2017; Kelly et al., 2017; i.e., restricting analyses to RCTs with parallel-group design), (ii) the aforementioned trial featuring both prebiotics and a probiotic within the treatment condition (Ghorbani et al., 2018; Sanchez et al., 2017), and (iii) these cross-over trials and trials combining prebiotics and probiotics, the results remained largely unchanged. When analyses were restricted to Lactobacillus-only trials, the pooled effect was no longer significant for depression and anxiety. When the pooled effect size for Lactobacillus-only trials for depression was compared to that for the remaining probiotic trials for depression, a significant difference emerged (p < .01), the effect of probiotics on depression being larger for trials involving Lactobacillus combined with other genera or prebiotics and other genera considered alone. No difference was observed between pooled effect sizes for Lactobacillus-only trials for anxiety and other probiotic trials for this condition (p = .66).
4. Discussion
The current review provided the most comprehensive meta-analysis to date of the effects of probiotics on depression and anxiety. We also conducted the first quantitative syntheses of data on prebiotics for depression and anxiety. Although the current review did not find an ameliorative effect for prebiotics on depression or anxiety, respectively, these findings should be regarded as preliminary, given the relatively small number of eligible studies included in the analyses. We did find general support, however, for an effect of probiotics on depression and anxiety, with small pooled effects in both cases. Although Lactobacillus received the most interest among probiotic trials, when considered alone, it did not have an effect on depression, with a significant difference in effect size existing between Lactobacillus-only trials and others. Lactobacillus did not appear to have an effect on anxiety, regardless of whether considered alone or in combination with prebiotics or other probiotics.
Several considerations should be taken, however, in interpreting the observed effect sizes and the potential implications of these findings. First, and perhaps most important, is the dearth of trials featuring samples with clinical depression and anxiety. Indeed, only four trials included samples with major depression (Akkasheh et al., 2016; Ghorbani et al., 2018; Kazemi et al., 2019; Majeed et al., 2018) and none clinically significant anxiety. Moreover two prebiotic trials (Azpiroz et al., 2017; Schmidt et al., 2015) and nine probiotic trials (Chung et al., 2014; Kato-Kataoka et al., 2016; Kelly et al., 2017; Kitaoka et al., 2009; Messaoudi et al., 2011; Simrén et al., 2010; Steenbergen et al., 2015; Tillisch et al., 2013; Yang et al., 2016) specifically excluded individuals with these disorders or psychiatric conditions more generally, and most of the remaining trials featured community samples within which naturally low levels of depression and anxiety would be reasonably expected. Such trials, in which there is little room for reduction in depressive and anxiety symptoms regardless of clinical intervention, are limited in their ability to inform our understanding of the antidepressant and anxiolytic potential of prebiotics and probiotics. The high prevalence of these trials in the literature likely introduced a bias toward smaller pooled effects across analyses and such findings should therefore be interpreted with a degree of caution. For example, given that seven of the nine Lactobacillus-only trials for depression drew on healthy community samples, it may be premature to conclude that Lactobacillus has no clinically meaningful antidepressant properties based on the finding that Lactobacillus-only trials had a small, non-significant pooled effect, in contrast to the significantly larger effects for other probiotic trials for depression. Supporting this consideration regarding sample type, in moderator analyses for probiotic trials and depression, the pooled effect was essentially zero for trials with psychiatric disorders as an exclusion criterion, and in contrast, medium-to-large for trials with major depression among the inclusion criteria. Indeed, these trials with clinically depressed samples accounted for four of the six largest effects among studies of probiotics and depression. These findings are also consistent with those of an earlier meta-analysis of probiotics and depression (Ng et al., 2018), which did not test for moderator effects, but stratified studies by depressive symptom severity at enrollment and found a significant effect for studies of individuals with mild-to-moderate depressive symptoms but not for studies of healthy individuals.
It is also important to note that despite the considerable interest in the microbiome in relation to depression and anxiety, and particularly the potential for prebiotics and probiotics to treat these disorders, there is a paucity of significant findings at the level of individual studies, even in cases of significant pooled effects. In fact, just six probiotic trials for depression (Akkasheh et al., 2016; Ghorbani et al., 2018; Kazemi et al., 2019; Kouchaki et al., 2017; Majeed et al., 2018; Sanchez et al., 2017) and one for anxiety (Slykerman et al., 2017) yielded significant findings. This may in some measure be accounted for by the aforementioned predominance of trials featuring healthy community samples or samples with psychiatric disorders screened out. A complementary explanation is that the majority of studies were underpowered to detect significant effects, the median sample size of included studies being 46.5.
Several studies featured samples with chronic medical conditions, most often irritable bowel syndrome. The presence of these studies in the current review is not inconsequential, accounting for 35.3% of all trials. Although selecting for these conditions provides a greater likelihood of psychiatrically enriched samples, given meta-analytic evidence of their comorbidity with depression and anxiety (Dawes et al., 2016; Dickens et al., 2002; Fond et al., 2014; Luppino et al., 2010), it also potentially complicates interpretation of resulting findings, as these medical conditions, and treatment of them, often lead to corresponding changes in mood and anxiety (Fabricatore et al., 2011; Luppino et al., 2010), Caution should be taken in generalizing findings based on these samples, as it is unclear to what degree observed improvements in depression and anxiety are secondary to alleviation of the medical condition being treated with prebiotics or probiotics and to what degree comparable improvements in depression and anxiety would be observed in the absence of medical illness.
The current findings should also be interpreted within the context of prior meta-analyses in this area. In particular, our findings are consistent with those of an earlier meta-analysis that reported a significant probiotic effect on depression (Huang et al., 2016) but not another which failed to find a significant effect (Ng et al., 2018). One possible explanation for this pattern of findings is that the former meta-analysis and the current review featured higher proportions of clinically depressed individuals (8.3% and 8.0%, respectively) than did the meta-analysis with a non-significant effect (3.6%). A unique aspect of the current meta-analysis is its inclusion of a sufficient number of trials for moderator analyses. That a significantly larger pooled effect was found in the current review for studies featuring clinically depressed samples is congruent with this possibility that sample type may in part account for whether a significant pooled effect is found. Furthermore, that the current meta-analysis includes a substantially greater number of trials than did prior ones lends weight to our findings.
Also worth noting is that a significant pooled effect for probiotics and anxiety was found in the current review, but not in two recent ones (Liu et al., 2018; Reis et al., 2018). There are several reasons why the findings of the current meta-analysis may be accorded greater weight. In addition to addressing the previously mentioned methodological limitations pertaining to the prior meta-analyses, the current review includes a notably higher number of studies, which provides greater statistical power to detect a significant small effect, as was found in the current case. Additionally, significant heterogeneity was observed in one of the prior meta-analyses of anxiety (Liu et al., 2018), but not in the other (Reis et al., 2018), after removing an outlier, or the current review. This discrepancy between meta-analyses may, in part, be a function of the aforementioned adoption of a notably broad operationalization of anxiety (e.g., the inclusion of visceral sensitivity as an outcome) in the meta-analysis that detected significant heterogeneity.
Several limitations warrant mention. The study-level effects included in the current review were based on measures of depression and anxiety taken upon completion of prebiotic/probiotic regimens. We therefore could not evaluate to what extent potential psychotropic effects of these regimens persist after cessation of treatment. Additionally, none of the trials included adolescent samples, and thus potential differences in efficacy related to development could not be assessed. This is an important consideration, given (i) differences in microbiome composition across the lifespan (i.e., although the gut microbiome converges toward an adult-like profile in early childhood, microbial diversity increases throughout development and into adulthood [Rea et al., 2016; Yatsunenko et al., 2012] and greater inter-individual variation exists among youth than adults [Biagi et al., 2010; Claesson et al., 2012; Yatsunenko et al., 2012]), and (ii) significant age-of-onset differences in course and treatment response for depression and anxiety (Hill et al., 2004; Jaffee et al., 2002; Kaufman et al., 2001; Serretti et al., 2009; Van Ameringen et al., 2004). Addressing these limitations are important avenues for future research. Finally, the prebiotic findings are preliminary, given the relatively few studies included in the analyses, and require replication with a larger number of clinical trials.
In summary, the current evidence base for prebiotics and probiotics in the treatment of internalizing disorders appears modest. Support for the efficacy of probiotics for depression and anxiety was observed, but with generally small pooled effects. These findings are qualified, however, by the relative rarity of trials with psychiatric samples and the prevalence of non-clinical samples in the literature, which together significantly reduced the observed effects. In general, the largest effects were found for probiotics and major depression, but this should be regarded as preliminary, being limited to four trials. Future studies with clinically significant presentations are indicated and necessary adequately to evaluate the potential efficacy of prebiotics and probiotics for depression and anxiety. This is especially important given the increasing need for the development of novel psychopharmacological agents for these conditions (Hyman, 2012; Insel, 2015; Miller, 2010).
Supplementary Material
Highlights.
A meta-analysis of prebiotic and probiotic trials for depression and anxiety
Prebiotics did not differ from placebo for depression or anxiety
Probiotics yielded small but significant effects for depression and anxiety
Probiotic effects were larger for clinical than community samples for depression
More studies of clinical samples are needed fully to evaluate therapeutic potential
Acknowledgements
Preparation of this manuscript was supported in part by the National Institute of Mental Health of the National Institutes of Health under Award Number R01MH101138, R01MH115905, and R21MH112055, and the Brown Institute for Brain Science/Norman Prince Neurosciences Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
To assess the impact of restricting the search string to English-language studies, we conducted our search again with the language restriction removed. None of the new search results warranted full-text search based on their title and abstracts. Therefore, restricting the search parameters to English-language publications did not alter the current findings.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akkasheh G, Kashani-Poor Z, Tajabadi-Ebrahimi M, Jafari P, Akbari H, Taghizadeh M, Memarzadeh MR, Asemi Z, Esmaillzadeh A, 2016. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition 32, 315–320. 10.1016/j.nut.2015.09.003 [DOI] [PubMed] [Google Scholar]
- Azpiroz F, Dubray C, Bernalier-Donadille A, Cardot J-M, Accarino A, Serra J, Wagner A, Respondek F, Dapoigny M, 2017. Effects of scFOS on the composition of fecal microbiota and anxiety in patients with irritable bowel syndrome: A randomized, double blind, placebo controlled study. Neurogastroenterol. Motil 29, e12911 10.1111/nmo.12911 [DOI] [PubMed] [Google Scholar]
- Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, Nikkïla J, Monti D, Satokari R, Franceschi C, Brigidi P, De Vos W, 2010. Through ageing, and beyond: Gut microbiota and inflammatory status in seniors and centenarians. PLoS One 5, e10667 10.1371/journal.pone.0010667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biostat, 2014. Comprehensive Meta-Analysis Version 3.
- Chung YC, Jin HM, Cui Y, Kim DS, Jung JM, Park J Il, Jung ES, Choi EK, Chae SW, 2014. Fermented milk of Lactobacillus helveticus IDCC3801 improves cognitive functioning during cognitive fatigue tests in healthy older adults. J. Funct. Foods 10, 465–474. 10.1016/j.jff.2014.07.007 [DOI] [Google Scholar]
- Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, Harris HMB, Coakley M, Lakshminarayanan B, O’Sullivan O, Fitzgerald GF, Deane J, O’Connor M, Harnedy N, O’Connor K, O’Mahony D, van Sinderen D, Wallace M, Brennan L, Stanton C, Marchesi JR, Fitzgerald AP, Shanahan F, Hill C, Ross RP, O’Toole PW, 2012. Gut microbiota composition correlates with diet and health in the elderly. Nature 488, 178–184. 10.1038/nature11319 [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D, 1991. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. J. Abnorm. Psychol 100, 316–336. 10.1037/0021-843X.100.3.316 [DOI] [PubMed] [Google Scholar]
- Colica C, Avolio E, Bollero P, Costa De Miranda R, Ferraro S, Sinibaldi Salimei P, De Lorenzo A, Di Renzo L, 2017. Evidences of a new psychobiotic formulation on body composition and anxiety. Mediators Inflamm. 2017. 10.1155/2017/5650627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremon C, Guglielmetti S, Gargari G, Taverniti V, Castellazzi AM, Valsecchi C, Tagliacarne C, Fiore W, Bellini M, Bertani L, Gambaccini D, Cicala M, Germanà B, Vecchi M, Pagano I, Barbaro MR, Bellacosa L, Stanghellini V, Barbara G, 2017. Effect of Lactobacillus paracasei CNCM I-1572 on symptoms, gut microbiota, short chain fatty acids, and immune activation in patients with irritable bowel syndrome: A pilot randomized clinical trial. United Eur. Gastroenterol. J 6, 604–613. 10.1177/2050640617736478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes AJ, Maggard-Gibbons M, Maher AR, Booth MJ, Miake-Lye I, Beroes JM, Shekelle PG, 2016. Mental health conditions among patients seeking and undergoing bariatric surgery: A meta-analysis. JAMA 315, 150–63. 10.1001/jama.2015.18118 [DOI] [PubMed] [Google Scholar]
- Dickens C, McGowan L, Clark-Carter D, Creed F, 2002. Depression in rheumatoid arthritis: A systematic review of the literature with meta-analysis. Psychosom. Med 64, 52–60. https://doi.org/0033-3174/02/6401-0052 [DOI] [PubMed] [Google Scholar]
- Dieleman JL, Baral R, Birger M, Bui AL, Bulchis A, Chapin A, Hamavid H, Horst C, Johnson EK, Joseph J, Lavado R, Lomsadze L, Reynolds A, Squires E, Campbell M, DeCenso B, Dicker D, Flaxman AD, Gabert R, Highfill T, Naghavi M, Nightingale N, Templin T, Tobias MI, Vos T, Murray CJL, 2016. US spending on personal health care and public health, 1996–2013. JAMA 316, 2627–2646. 10.1001/jama.2016.16885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval S, Tweedie R, 2000. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56, 455–463. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C, 1997. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricatore AN, Wadden TA, Higginbotham AJ, Faulconbridge LF, Nguyen AM, Heymsfield SB, Faith MS, 2011. Intentional weight loss and changes in symptoms of depression: A systematic review and meta-analysis. Int. J. Obes 10.1038/ijo.2011.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fond G, Loundou A, Hamdani N, Boukouaci W, Dargel A, Oliveira J, Roger M, Tamouza R, Leboyer M, Boyer L, 2014. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): a systematic review and meta-analysis. Eur. Arch. Psychiatry Clin. Neurosci 264, 651–660. 10.1007/s00406-014-0502-z [DOI] [PubMed] [Google Scholar]
- Ghorbani Z, Nazari S, Etesam F, Nourimajd S, Ahmadpanah M, Jahromi SR, 2018. The effect of synbiotic as an adjuvant therapy to fluoxetine in moderate depression : A randomized multicenter trial. Arch. Neurosci. 5, e60507 10.5812/archneurosci.60507.Research [DOI] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG, 2003. Measuring inconsistency in meta-analyses. BMJ 327, 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J, Pickles A, Rollinson L, Davies R, Byatt M, 2004. Juvenile- versus adult-onset depression: Multiple differences imply different pathways. Psychol. Med. 34, 1483–1493. 10.1017/S0033291704002843 [DOI] [PubMed] [Google Scholar]
- Huang R, Wang K, Hu J, 2016. Effect of probiotics on depression: A systematic review and meta-analysis of randomized controlled trials. Nutrients 8 10.3390/nu8080483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, 2012. Revolution stalled. Sci. Transl. Med 4, 155cm11–155cm11. 10.1126/scitranslmed.3003142 [DOI] [PubMed] [Google Scholar]
- Insel TR, 2015. The NIMH experimental medicine initiative. World Psychiatry 14, 151–153. 10.1002/wps.20227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffee SR, Moffitt TE, Caspi A, Fombonne E, Poulton R, Martin J, 2002. Differences in early childhood risk factors for juvenile-onset and adult-onset depression. Arch. Gen. Psychiatry 59, 215–222. 10.1001/archpsyc.59.3.215 [DOI] [PubMed] [Google Scholar]
- Kato-Kataoka A, Nishida K, Takada M, Suda K, Kawai M, Shimizu K, Kushiro A, Hoshi R, Watanabe O, Igarashi T, Miyazaki K, Kuwano Y, Rokutan K, 2016. Fermented milk containing Lactobacillus casei strain Shirota prevents the onset of physical symptoms in medical students under academic examination stress. Benef. Microbes 7, 153–156. 10.3920/BM2015.0100 [DOI] [PubMed] [Google Scholar]
- Kaufman J, Martin A, King RA, Charney D, 2001. Are child-, adolescent-, and adult-onset depression one and the same disorder? Biol. Psychiatry 49, 980–1001. 10.1016/S0006-3223(01)01127-1 [DOI] [PubMed] [Google Scholar]
- Kazemi A, Noorbala AA, Azam K, Eskandari MH, Djafarian K, 2019. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: a randomized clinical trial. Clin. Nutr. 38 (2), 522–528. 10.1016/j.clnu.2018.04.010 [DOI] [PubMed] [Google Scholar]
- Kelly JR, Allen AP, Temko A, Hutch W, Kennedy PJ, Farid N, Murphy E, Boylan G, Bienenstock J, Cryan JF, Clarke G, Dinan TG, 2017. Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain. Behav. Immun 61, 50–59. 10.1016/j.bbi.2016.11.018 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE, 2005. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62, 593–602. 10.1001/archpsyc.62.6.593 [DOI] [PubMed] [Google Scholar]
- Kitaoka K, Uchida K, Okamoto N, Chikahisa S, Miyazaki T, Takeda E, Séi H, 2009. Fermented ginseng improves the first-night effect in humans. Sleep 32, 413–421. 10.1093/sleep/32.3.413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouchaki E, Tamtaji OR, Salami M, Bahmani F, Daneshvar Kakhaki R, Akbari E, Tajabadi-Ebrahimi M, Jafari P, Asemi Z, 2017. Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: A randomized, double-blind, placebo-controlled trial. Clin. Nutr 36, 1245–1249. 10.1016/j.clnu.2016.08.015 [DOI] [PubMed] [Google Scholar]
- Liu B, He Y, Wang M, Liu J, Ju Y, Zhang Y, Liu T, Li L, Li Q, 2018. Efficacy of probiotics on anxiety-A meta-analysis of randomized controlled trials. Depress. Anxiety 35, 935–945. 10.1002/da.22811 [DOI] [PubMed] [Google Scholar]
- Liu RT, 2017. The microbiome as a novel paradigm in studying stress and mental health. Am. Psychol 72, 655–667. 10.1037/amp0000058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo-Zúñiga V, Llop E, Suárez C, Álvarez B, Abreu L, Espadaler J, Serra J, 2014. I.31, a new combination of probiotics, improves irritable bowel syndrome-related quality of life. World J. Gastroenterol 20, 8709–8716. 10.3748/wjg.v20.i26.8709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppino FS, De Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BWJH, Zitman FG, 2010. Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Arch. Gen. Psychiatry. 10.1001/archgenpsychiatry.2010.2 [DOI] [PubMed] [Google Scholar]
- Lyra A, Hillilä M, Huttunen T, Männikkö S, Taalikka M, Tennilä J, Tarpila A, Lahtinen S, Ouwehand AC, Veijola L, 2016. Irritable bowel syndrome symptom severity improves equally with probiotic and placebo. World J. Gastroenterol 22, 10631–10642. 10.3748/wjg.v22.i48.10631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed M, Nagabhushanam K, Natarajan S, Sivakumar A, Ali F, Pande A, Majeed S, Karri SK, 2018. Bacillus coagulans MTCC 5856 supplementation in the management of diarrhea predominant Irritable Bowel Syndrome: A double blind randomized placebo controlled pilot clinical study. Nutr. J 62, 1218 10.1186/s12937-016-0140-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcos A, Wärnberg J, Nova E, Gómez S, Alvarez A, Alvarez R, Mateos JA, Cobo JM, 2004. The effect of milk fermented by yogurt cultures plus Lactobacillus casei DN-114001 on the immune response of subjects under academic examination stress. Eur. J. Nutr 43, 381–389. 10.1007/s00394-004-0517-8 [DOI] [PubMed] [Google Scholar]
- McKean J, Naug H, Nikbakht E, Amiet B, Colson N, 2017. Probiotics and subclinical psychological symptoms in healthy participants: A systematic review and meta-analysis. J. Altern. Complement. Med. 23, 249–258. 10.1089/acm.2016.0023 [DOI] [PubMed] [Google Scholar]
- Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, Bisson J-F, Rougeot C, Pichelin M, Cazaubiel M, Cazaubiel J-M, 2011. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr 105, 755–764. 10.1017/S0007114510004319 [DOI] [PubMed] [Google Scholar]
- Miller G, 2010. Is pharma running out of brainy ideas? Science (80-. ). 329, 502–504. 10.1126/science.329.5991.502 [DOI] [PubMed] [Google Scholar]
- Mohammadi AA, Jazayeri S, Khosravi-Darani K, Solati Z, Mohammadpour N, Asemi Z, Adab Z, Djalali M, Tehrani-Doost M, Hosseini M, Eghtesadi S, 2016. The effects of probiotics on mental health and hypothalamic-pituitaryadrenal axis: A randomized, double-blind, placebo-controlled trial in petrochemical workers. Nutr. Neurosci. 19, 387–395. 10.1179/1476830515Y.0000000023 [DOI] [PubMed] [Google Scholar]
- Ng QX, Peters C, Ho CYX, Lim DY, Yeo WS, 2018. A meta-analysis of the use of probiotics to alleviate depressive symptoms. J. Affect. Disord 228, 13–19. 10.1016/j.jad.2017.11.063 [DOI] [PubMed] [Google Scholar]
- Nishihira J, Kagami-Katsuyama H, Tanaka A, Nishimura M, Kobayashi T, Kawasaki Y, 2014. Elevation of natural killer cell activity and alleviation of mental stress by the consumption of yogurt containing Lactobacillus gasseri SBT2055 and Bifidobacterium longum SBT2928 in a double-blind, placebo-controlled clinical trial. J. Funct. Foods 11, 261–268. 10.1016/j.jff.2014.09.002 [DOI] [Google Scholar]
- Östlund-Lagerström L, Kihlgren A, Repsilber D, Björkstén B, Brummer RJ, Schoultz I, 2016. Probiotic administration among free-living older adults: A double blinded, randomized, placebo-controlled clinical trial. Nutr. J 15, 1–10. 10.1186/s12937-016-0198-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto-Sanchez MI, Hall GB, Ghajar K, Nardelli A, Bolino C, Lau JT, Martin FP, Cominetti O, Welsh C, Rieder A, Traynor J, Gregory C, De Palma G, Pigrau M, Ford AC, Macri J, Berger B, Bergonzelli G, Surette MG, Collins SM, Moayyedi P, Bercik P, 2017. Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: A pilot study in patients with irritable bowel syndrome. Gastroenterology 153, 448–459.e8. 10.1053/j.gastro.2017.05.003 [DOI] [PubMed] [Google Scholar]
- Pirbaglou M, Katz J, de Souza RJ, Stearns JC, Motamed M, Ritvo P, 2016. Probiotic supplementation can positively affect anxiety and depressive symptoms: a systematic review of randomized controlled trials. Nutr. Res 36, 889–898. 10.1016/j.nutres.2016.06.009 [DOI] [PubMed] [Google Scholar]
- Rea K, Dinan TG, Cryan JF, 2016. The microbiome: A key regulator of stress and neuroinflammation. Neurobiol. Stress 4, 23–33. 10.1016/j.ynstr.2016.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reale M, Boscolo P, Bellante V, Tarantelli C, Di Nicola M, Forcella L, Li Q, Morimoto K, Muraro R, 2012. Daily intake of Lactobacillus casei Shirota increases natural killer cell activity in smokers. Br. J. Nutr 108, 308–314. 10.1017/S0007114511005630 [DOI] [PubMed] [Google Scholar]
- Reis DJ, Ilardi SS, Punt SEW, 2018. The anxiolytic effect of probiotics: A systematic review and meta-analysis of the clinical and preclinical literature. PLoS One 13, e0199041 10.1371/journal.pone.0199041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman P, Estévez AF, Miras A, Sánchez-labraca N, Cañadas F, Vivas AB, Cardona D, 2018. A pilot randomized controlled trial to explore cognitive and emotional effects of probiotics in fibromyalgia. Sci. Rep 8, 10965 10.1038/s41598-018-29388-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romijn AR, Rucklidge JJ, 2015. Systematic review of evidence to support the theory of psychobiotics. Nutr. Rev 73, 675–693. 10.1093/nutrit/nuv025 [DOI] [PubMed] [Google Scholar]
- Romijn AR, Rucklidge JJ, Kuijer RG, Frampton C, 2017. A double-blind, randomized, placebo-controlled trial of Lactobacillus helveticus and Bifidobacterium longum for the symptoms of depression. Aust. N. Z. J. Psychiatry 51, 810–821. 10.1177/0004867416686694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez M, Darimont C, Panahi S, Drapeau V, Marette A, Taylor VH, Doré J, Tremblay A, 2017. Effects of a diet-based weight-reducing program with probiotic supplementation on satiety efficiency, eating behaviour traits, and psychosocial behaviours in obese individuals. Nutrients 9, 1–17. 10.3390/nu9030284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sashihara T, Nagata M, Mori T, Ikegami S, Gotoh M, Okubo K, Uchida M, Itoh H, 2013. Effects of Lactobacillus gasseri OLL2809 and alpha-lactalbumin on university-student athletes: a randomized, double-blind, placebo-controlled clinical trial. Appl. Physiol. Nutr. Metab 1235, 1228–1235. [DOI] [PubMed] [Google Scholar]
- Schmidt K, Cowen PJ, Harmer CJ, Tzortzis G, Errington S, Burnet PWJ, 2015. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology (Berl). 232, 1793–1801. 10.1007/s00213-014-3810-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serretti A, Chiesa A, Calati R, Perna G, Bellodi L, De Ronchi D, 2009. Common genetic, clinical, demographic and psychosocial predictors of response to pharmacotherapy in mood and anxiety disorders. Int. Clin. Psychopharmacol 24, 1–18. 10.1097/YIC.0b013e32831db2d7 [DOI] [PubMed] [Google Scholar]
- Shinkai S, Toba M, Saito T, Sato I, Tsubouchi M, Taira K, Kakumoto K, Inamatsu T, Yoshida H, Fujiwara Y, Fukaya T, Matsumoto T, Tateda K, Yamaguchi K, Kohda N, Kohno S, 2013. Immunoprotective effects of oral intake of heat-killed Lactobacillus pentosus strain b240 in elderly adults: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr 109, 1856–1865. 10.1017/S0007114512003753 [DOI] [PubMed] [Google Scholar]
- Silk DBA, Davis A, Vulevic J, Tzortzis G, Gibson GR, 2009. Clinical trial: The effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment. Pharmacol. Ther 29, 508–518. 10.1111/j.1365-2036.2008.03911.x [DOI] [PubMed] [Google Scholar]
- Simrén M, Öhman L, Olsson J, Svensson U, Ohlson K, Posserud I, Strid H, 2010. Clinical trial: The effects of a fermented milk containing three probiotic bacteria in patients with irritable bowel syndrome - A randomized, double-blind, controlled study. Aliment. Pharmacol. Ther 31, 218–227. 10.1111/j.1365-2036.2009.04183.x [DOI] [PubMed] [Google Scholar]
- Slykerman RF, Hood F, Wickens K, Thompson JMD, Barthow C, Murphy R, Kang J, Rowden J, Stone P, Crane J, Stanley T, Abels P, Purdie G, Maude R, Mitchell EA, 2017. Effect of Lactobacillus rhamnosus HN001 in Pregnancy on Postpartum Symptoms of Depression and Anxiety: A Randomised Double-blind Placebo-controlled Trial. EBioMedicine 24, 159–165. 10.1016/j.ebiom.2017.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AP, 2005. The concept of well-being: Relevance to nutrition research. Br. J. Nutr 93, 1–5. 10.1186/ar1506 [DOI] [PubMed] [Google Scholar]
- Smith AP, Sutherl D, Hewlett P, 2015. An investigation of the acute effects of oligofructose-enriched inulin on subjective wellbeing, mood and cognitive performance. Nutrients 7, 8887–8896. 10.3390/nu7115441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenbergen L, Sellaro R, van Hemert S, Bosch JA, Colzato LS, 2015. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain. Behav. Immun 48, 258–264. 10.1016/j.bbi.2015.04.003 [DOI] [PubMed] [Google Scholar]
- The Cochrane Collaboration, 2011. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons, Chichester, England. [Google Scholar]
- Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B, Guyonnet D, Legrain-Raspaud S, Trotin B, Naliboff B, Mayer EA, 2013. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 144, 1394–1401. 10.1053/j.gastro.2013.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Burden of Disease Collaborators, 2013. The state of US health, 1990–2010: Burden of diseases, injuries, and risk factors. JAMA 310, 591–608. 10.1001/jama.2013.13805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaghef-Mehrabany E, Alipour B, Homayouni-Rad A, Sharif SK, AsghariJafarabadi M, Zavvari S, 2014. Probiotic supplementation improves inflammatory status in patients with rheumatoid arthritis. Nutrition 30, 430–435. 10.1016/j.nut.2013.09.007 [DOI] [PubMed] [Google Scholar]
- Van Ameringen M, Oakman J, Mancini C, Pipe B, Chung H, 2004. Predictors of response in generalized social phobia: Effect of age of onset. J. Clin. Psychopharmacol 24, 42–48. 10.1097/01.jcp.0000104909.75206.6f [DOI] [PubMed] [Google Scholar]
- Vos T, Allen C, Arora M, Barber RM, Bhutta ZA, Brown A, Carter A, Casey DC, Charlson FJ, Chen AZ, Coggeshall M, Cornaby L, Dandona L, Dicker DJ, Dilegge T, Erskine HE, Ferrari AJ, Fitzmaurice C, Fleming T, Forouzanfar MH, Fullman N, Gething PW, Goldberg EM, Graetz N, Haagsma JA, Johnson CO, Kassebaum NJ, Kawashima T, Kemmer L, Khalil A, Kinfu Y, Kyu HH, Leung J, Liang X, Lim SS, Lopez AD, Lozano R, Marczak L, Mensah GA, Mokdad AH, Naghavi M, Nguyen G, Nsoesie E, Olsen H, Pigott DM, Pinho C, Rankin Z, Reinig N, Salomon JA, Sandar L, Smith A, Stanaway J, Steiner C, Teeple S, Thomas BA, Troeger C, Wagner A, Wang H, Wanga V, Whiteford HA, Zoeckler L, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, Abraham B, Abubakar I, Abu-Raddad LJ, Abu-Rmeileh NME, Ackerman IN, Adebiyi AO, Ademi Z, Adou AK, Afanvi KA, Agardh EE, Agarwal A, Kiadaliri AA, Ahmadieh H, Ajala ON, Akinyemi RO, Akseer N, Al-Aly Z, Alam K, Alam NKM, Aldhahri SF, Alegretti MA, Alemu ZA, Alexander LT, Alhabib S, Ali R, Alkerwi A, Alla F, Allebeck P, Al-Raddadi R, Alsharif U, Altirkawi KA, Alvis-Guzman N, Amare AT, Amberbir A, Amini H, Ammar W, Amrock SM, Andersen HH, Anderson GM, Anderson BO, Antonio CAT, Aregay AF, Ärnlöv J, Artaman A, Asayesh H, Assadi R, Atique S, Avokpaho EFGA, Awasthi A, Quintanilla BPA, Azzopardi P, Bacha U, Badawi A, Balakrishnan K, Banerjee A, Barac A, Barker-Collo SL, B??rnighausen T, Barregard L, Barrero LH, Basu A, Bazargan-Hejazi S, Bell B, Bell ML, Bennett DA, Bensenor IM, Benzian H, Berhane A, Bernab?? E, Betsu BD, Beyene AS, Bhala N, Bhatt S, Biadgilign S, Bienhoff K, Bikbov B, Biryukov S, Bisanzio D, Bjertness E, Blore J, Borschmann R, Boufous S, Brainin M, Brazinova A, Breitborde NJK, Brown J, Buchbinder R, Buckle GC, Butt ZA, Calabria B, Campos-Nonato IR, Campuzano JC, Carabin H, C??rdenas R, Carpenter DO, Carrero JJ, Casta??eda-Orjuela CA, Rivas JC, Catal??-L??pez F, Chang JC, Chiang PPC, Chibueze CE, Chisumpa VH, Choi JYJ, Chowdhury R, Christensen H, Christopher DJ, Ciobanu LG, Cirillo M, Coates MM, Colquhoun SM, Cooper C, Cortinovis M, Crump JA, Damtew SA, Dandona R, Daoud F, Dargan PI, das Neves J, Davey G, Davis AC, Leo D. De, Degenhardt L, Gobbo L.C. Del, Dellavalle RP, Deribe K, Deribew A, Derrett S, Jarlais D.C. Des, Dharmaratne SD, Dhillon PK, Diaz-Torn?? C, Ding EL, Driscoll TR, Duan L, Dubey M, Duncan BB, Ebrahimi H, Ellenbogen RG, Elyazar I, Endres M, Endries AY, Ermakov SP, Eshrati B, Estep K, Farid TA, Farinha C.S. e S., Faro A, Farvid MS, Farzadfar F, Feigin VL, Felson DT, Fereshtehnejad SM, Fernandes JG, Fernandes JC, Fischer F, Fitchett JRA, Foreman K, Fowkes FGR, Fox J, Franklin RC, Friedman J, Frostad J, Fürst T, Futran ND, Gabbe B, Ganguly P, Gankp?? FG, Gebre T, Gebrehiwot TT, Gebremedhin AT, Geleijnse JM, Gessner BD, Gibney KB, Ginawi IAM, Giref AZ, Giroud M, Gishu MD, Glaser E, Godwin WW, Gomez-Dantes H, Gona P, Goodridge A, Gopalani SV, Gotay CC, Goto A, Gouda HN, Grainger R, Greaves F, Guillemin F, Guo Y, Gupta R, Gupta R, Gupta V, Guti??rrez RA, Haile D, Hailu AD, Hailu GB, Halasa YA, Hamadeh RR, Hamidi S, Hammami M, Hancock J, Handal AJ, Hankey GJ, Hao Y, Harb HL, Harikrishnan S, Haro JM, Havmoeller R, Hay RJ, Heredia-Pi IB, Heydarpour P, Hoek HW, Horino M, Horita N, Hosgood HD, Hoy DG, Htet AS, Huang H, Huang JJ, Huynh C, Iannarone M, Iburg KM, Innos K, Inoue M, Iyer VJ, Jacobsen KH, Jahanmehr N, Jakovljevic MB, Javanbakht M, Jayatilleke AU, Jee SH, Jeemon P, Jensen PN, Jiang Y, Jibat T, JimenezCorona A, Jin Y, Jonas JB, Kabir Z, Kalkonde Y, Kamal R, Kan H, Karch A, Karema CK, Karimkhani C, Kasaeian A, Kaul A, Kawakami N, Keiyoro PN, Kemp AH, Keren A, Kesavachandran CN, Khader YS, Khan AR, Khan EA, Khang YH, Khera S, Khoja TAM, Khubchandani J, Kieling C, Kim P, Kim C. il, Kim D, Kim YJ, Kissoon N, Knibbs LD, Knudsen AK, Kokubo Y, Kolte D, Kopec JA, Kosen S, Kotsakis GA, Koul PA, Koyanagi A, Kravchenko M, Defo BK, Bicer BK, Kudom AA, Kuipers EJ, Kumar GA, Kutz M, Kwan GF, Lal A, Lalloo R, Lallukka T, Lam H, Lam JO, Langan SM, Larsson A, Lavados PM, Leasher JL, Leigh J, Leung R, Levi M, Li Y, Li Y, Liang J, Liu S, Liu Y, Lloyd BK, Lo WD, Logroscino G, Looker KJ, Lotufo PA, Lunevicius R, Lyons RA, Mackay MT, Magdy M, Razek A. El, Mahdavi M, Majdan M, Majeed A, Malekzadeh R, Marcenes W, Margolis DJ, Martinez-Raga J, Masiye F, Massano J, McGarvey ST, McGrath JJ, McKee M, McMahon BJ, Meaney PA, Mehari A, Mejia-Rodriguez F, Mekonnen AB, Melaku YA, Memiah P, Memish ZA, Mendoza W, Meretoja A, Meretoja TJ, Mhimbira FA, Miller TR, Mills EJ, Mirarefin M, Mitchell PB, Mock CN, Mohammadi A, Mohammed S, Monasta L, Hernandez JCM, Montico M, Mooney MD, Moradi-Lakeh M, Morawska L, Mueller UO, Mullany E, Mumford JE, Murdoch ME, Nachega JB, Nagel G, Naheed A, Naldi L, Nangia V, Newton JN, Ng M, Ngalesoni FN, Nguyen Q. Le, Nisar MI, Pete PMN, Nolla JM, Norheim OF, Norman RE, Norrving B, Nunes BP, Ogbo FA, Oh IH, Ohkubo T, Olivares PR, Olusanya BO, Olusanya JO, Ortiz A, Osman M, Ota E, PA M, Park EK, Parsaeian M, de Azeredo Passos VM, Caicedo AJP, Patten SB, Patton GC, Pereira DM, Perez-Padilla R, Perico N, Pesudovs K, Petzold M, Phillips MR, Piel FB, Pillay JD, Pishgar F, Plass D, Platts-Mills JA, Polinder S, Pond CD, Popova S, Poulton RG, Pourmalek F, Prabhakaran D, Prasad NM, Qorbani M, Rabiee RHS, Radfar A, Rafay A, Rahimi K, Rahimi-Movaghar V, Rahman M, Rahman MHU, Rahman SU, Rai RK, Rajsic S, Ram U, Rao P, Refaat AH, Reitsma MB, Remuzzi G, Resnikoff S, Reynolds A, Ribeiro AL, Blancas MJR, Roba HS, Rojas-Rueda D, Ronfani L, Roshandel G, Roth GA, Rothenbacher D, Roy A, Sagar R, Sahathevan R, Sanabria JR, Sanchez-Ni??o MD, Santos IS, Santos JV, Sarmiento-Suarez R, Sartorius B, Satpathy M, Savic M, Sawhney M, Schaub MP, Schmidt MI, Schneider IJC, Sch??ttker B, Schwebel DC, Scott JG, Seedat S, Sepanlou SG, Servan-Mori EE, Shackelford KA, Shaheen A, Shaikh MA, Sharma R, Sharma U, Shen J, Shepard DS, Sheth KN, Shibuya K, Shin MJ, Shiri R, Shiue I, Shrime MG, Sigfusdottir ID, Silva DAS, Silveira DGA, Singh A, Singh JA, Singh OP, Singh PK, Sivonda A, Skirbekk V, Skogen JC, Sligar A, Sliwa K, Soljak M, S??reide K, Soriano JB, Sposato LA, Sreeramareddy CT, Stathopoulou V, Steel N, Stein DJ, Steiner TJ, Steinke S, Stovner L, Stroumpoulis K, Sunguya BF, Sur P, Swaminathan S, Sykes BL, Szoeke CEI, Tabar??s-Seisdedos R, Takala JS, Tandon N, Tanne D, Tavakkoli M, Taye B, Taylor HR, Ao B.J. Te, Tedla BA, Terkawi AS, Thomson AJ, Thorne-Lyman AL, Thrift AG, Thurston GD, Tobe-Gai R, Tonelli M, Topor-Madry R, Topouzis F, Tran BX, Dimbuene ZT, Tsilimbaris M, Tura AK, Tuzcu EM, Tyrovolas S, Ukwaja KN, Undurraga EA, Uneke CJ, Uthman OA, van Gool CH, Varakin YY, Vasankari T, Venketasubramanian N, Verma RK, Violante FS, Vladimirov SK, Vlassov VV, Vollset SE, Wagner GR, Waller SG, Wang L, Watkins DA, Weichenthal S, Weiderpass E, Weintraub RG, Werdecker A, Westerman R, White RA, Williams HC, Wiysonge CS, Wolfe CDA, Won S, Woodbrook R, Wubshet M, Xavier D, Xu G, Yadav AK, Yan LL, Yano Y, Yaseri M, Ye P, Yebyo HG, Yip P, Yonemoto N, Yoon SJ, Younis MZ, Yu C, Zaidi Z, Zaki MES, Zeeb H, Zhou M, Zodpey S, Zuhlke LJ, Murray CJL, 2016. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1545–1602. 10.1016/S0140-6736(16)31678-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace CJK, Milev R, 2017. The effects of probiotics on depressive symptoms in humans: A systematic review. Ann. Gen. Psychiatry 16, 1–10. 10.1186/s12991-017-0138-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Zhao X, Tang S, Huang H, Zhao X, Ning Z, Fu X, Zhang C, 2016. Probiotics reduce psychological stress in patients before laryngeal cancer surgery. Asia. Pac. J. Clin. Oncol 12, e92–e96. 10.1111/ajco.12120 [DOI] [PubMed] [Google Scholar]
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI, 2012. Human gut microbiome viewed across age and geography. Nature 486, 222–227. 10.1038/nature11053 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.