Abstract
Voltage-gated potassium (Kv) channels play an essential role in the regulation of membrane excitability and thereby control physiological processes such as cardiac excitability, neural communication, muscle contraction, and hormone secretion. Members of the Kv1 and Kv4 families are known to associate with auxiliary intracellular Kvβ subunits, which belong to the aldo-keto reductase superfamily. Electrophysiological studies have shown that these proteins regulate the gating properties of Kv channels. Although the three gene products encoding Kvβ proteins are functional enzymes in that they catalyze the nicotinamide adenine dinucleotide phosphate (NAD[P]H)-dependent reduction of a wide range of aldehyde and ketone substrates, the physiological role for these proteins and how each subtype may perform unique roles in coupling membrane excitability with cellular metabolic processes remains unclear. Here, we discuss current knowledge of the enzymatic properties of Kvβ proteins from biochemical studies with their described and purported physiological and pathophysiological influences.
Keywords: Aldo-keto reductase, aldehyde metabolism, membrane potential, carbonyl metabolism, Kv channels
2. Introduction
The aldo-keto reductases (AKRs) comprise a group of oxidoreductase enzymes that catalyze the reduction of endogenous and xenobiotic carbonyl compounds. These enzymes are ubiquitous among eukaryotic and prokaryotic organisms and share significant structural identity in that they all possess a C-terminal active site region within a triose-phosphate isomerase (TIM) barrel (αβ8) motif with three loops at the base of the barrel that govern substrate binding [1, 2]. The utility of this structural arrangement among the AKRs allows for flexibility in binding and metabolizing a wide range of chemical substrates that includes aliphatic and aromatic aldehydes and ketones, monosaccharides, steroids, and polycyclic aromatic hydrocarbons [3–5]. All AKRs require nicotinamide adenine dinucleotides (i.e., NAD(P)H) as a cofactor for hydride transfer [6] and their function can thus be modulated by the cellular redox state of electron carriers used in many intermediary metabolic biochemical reactions.
Most human AKRs are soluble monomeric proteins that are found in the cytosolic compartment. An exception to this are members of the AKR6 subfamily, which form tetrameric complexes that are, intriguingly, associated with the pore-domains of voltage-gated potassium (Kv) channels (i.e., the Kvβ proteins) [7, 8]. The Kv channels are a large family of transmembrane K+-permeable ion channels that, via regulation of membrane potential in excitable cell types, control numerous physiological processes, including neuronal excitability, hormonal secretion, and muscle contraction [9–11]. While this assembly between a catalytically active AKR and ion channel has stimulated a number of intriguing hypotheses regarding its evolutionary conservation and potential physiological role(s) [12], there is limited information about the potential in vivo role for the Kvβ proteins in the cardiovascular, endocrine and nervous systems, and it is unclear how these proteins may uniquely regulate diverse cellular physiological processes and pathophysiological development. While the enzymatic properties and cellular functions of the AKR family have been comprehensively reviewed previously (readers are referred to [2, 13]), in this article, we discuss the enzymatic properties of the Kvβ proteins, including how these properties may relay metabolic information to the Kv channel gating apparatus, thus serving as molecular transducers that couple metabolism and membrane electrical signaling in excitable cell types. While underscoring key remaining questions that require further investigation, we discuss the potential efficacy of small molecules or peptides that selectively modulate Kvβ expression or functionality as a novel class of therapeutics that could prevent or reverse pathological changes, and therefore may be useful interventions for controlling excitability under a variety of different physiological and pathological conditions.
3. Molecular and structural biology
In the human genome, there are ~35 genes encoding Kv channel pore proteins belonging to 12 subfamilies (i.e., Kv1.x – Kv12.x) [14]. The basic Kv channel tertiary structure consists of a multi-subunit complex of pore-forming proteins with a diverse repertoire of associated auxiliary and regulatory proteins. The pore domain is formed by the tetrameric assembly of four distinct transmembrane subunits (α) that are arranged around a central axis to form a membrane-traversing ion conduction pore that is highly selective (~10,000 fold more selective for K+ than for Na+) and efficient for K+ transport (~107 K+ ions channel−1 sec−1) [15–17]. Kvα subunits are 70–100 kDa in mass and consist of six membrane-spanning α helices (S1–S6) with S1–S4 forming the voltage-sensor domain and the S5–S6 segments of each contributing to the pore lining with selectivity filter. A highly conserved series of positively charged arginine residues within the S4 region form the voltage sensor of the channel that responds to changes in membrane voltage to constrict or dilate the central pore [18]. In native channel complexes, members of a particular Kv family (e.g., Kv1) are known to promiscuously interact with other functional members of the same family, giving rise to heteromeric alpha pore complexes with variable gating properties, that could ultimately increase diversity among functional channels [19, 20]. This is thought to occur through highly conserved regions within the intracellular T1 domain, which also serves as a docking site for intracellular subunits. Association with conserved regions among accessory Kv proteins also allows for the formation of heterotetrameric auxiliary subunit complexes, which, as described below, may further add to the functional diversity of native channels.
Although the expression and assembly of four Kvα subunits is the minimum requirement to form a functional channel, association of the pore-domain with a diverse set of accessory subunits, such as Kvβ, KChAP, KChIP and MinK, imparts multimodal regulatory features to Kv channels in vivo [21]. Members of the Shaker (Kv1) and Shal (Kv4) families are known to associate with Kvβ subunits [22, 23]. The human genome contains three genes that encode Kvβ proteins (KCNAB1, KCNAB2, KCNAB3) and their transcripts are alternatively spliced to generate additional variants. Early studies suggesting the functional importance of Kvβ proteins discovered that a leg shaking phenotype in Drosophila melanogaster (i.e., ‘hyperkinetic’) was the result of a mutation in a homologue of the mammalian Kvβ peptides. Subsequent sequence analyses led to the unexpected finding that the Kvβ subunits shared significant homology (15–30% amino acid identity) with members of the AKR superfamily [12, 24]. Upon crystallization of Kv1.2-Kvβ2, it was found that Kvβ proteins possess a conserved C-terminal β-barrel structural fold with tightly bound nicotinamide cofactor and, consistent with findings from sequence alignments, the active site had all characteristic features of a catalytically active AKR, including a well-conserved cofactor binding site and a distinct substrate binding pocket [7, 25]. Indeed, in these earlier reports on the X-ray crystal structure of a Kv channel complex, and more recently in a study demonstrating the single-particle cryo-electron microscopic structure of Kv1.2-Kvβ2 expressed in lipid nanodiscs [26], electron density could be resolved from NADP+ that was bound to the β subunits.
The active site structure of the AKR6 family is unique in that the α8/β8 motif has an additional helix attached to a long loop between β9 and α7 near the cofactor binding pocket. The functional significance of this modification that is shared among AKR6 members is not presently clear. At the quaternary level, the β1 and β2, which are perpendicular to the central axis of the barrel, along with the α2-β5-α3 region, form the intersubunit interface region that participates in β tetramerization, while the α5-α6 region interacts with the T1 docking domains of the Kvα proteins [7, 8]. Thus, via the T1 domain, the active site of Kvβ can influence the conformation of the voltage sensing apparatus and thereby impact gating properties as a consequence of catalytic activity and/or pyridine nucleotide cofactor binding.
4. Enzymology and channel biophysics
A prerequisite for investigating and understanding the potential physiological or pathological roles of the Kvβ proteins is a thorough understanding of their catalytic properties and the identification of potentially relevant endogenous or xenobiotic carbonyl substrates. The Kvβ proteins have been reported to avidly bind pyridine nucleotides, with binding affinities in the low micromolar range (i.e., 0.1–4 µm). The proteins display a ~10-fold greater affinity for NADP(H) compared to NAD(H) cofactors [27]. Considering that in most metabolically active cells, the NADPH:NADP+ ratio is substantially higher than that of NADH:NAD+, while the absolute concentration of NADP(H) is much lower than that of NAD(H) [28, 29], the cofactor species predominantly used by Kvβ proteins in vivo is not presently clear and likely varies with respect to cell type. Kvβ2 catalyzes the reduction of a wide range of aldehydes and ketones, although preferential binding and reduction of aldehydes versus ketones, and higher catalytic efficiency for aromatic aldehydes was reported for this subunit [30]. For example, Kvβ2 shows higher catalytic activity with aromatic carbonyls such as phenanthrequinone than with straight chain aldehydes such as acrolein or 4-oxo-nonenal [30]. Little or no activity was observed with steroids such as cortisone. Significantly, the protein was also found to be active with products of lipid peroxidation, such as 1-palmitoyl, 2-oxovaleroyl, phosphatidyl choline (POVPC). Given that POVPC and related aldehydes are generated during the oxidation of unsaturated fatty acids in the plasma membrane and that Kvβ is tethered within close proximity to the membrane, it appears plausible that the catalytic function of Kvβ may be to detoxify lipid peroxidation products and thereby protect Kv channels from oxidative damage. Alternatively, binding to lipid peroxidation products could be a potential regulatory mechanism that could alter Kv kinetics under conditions of oxidative stress (e.g., to trigger apoptosis). Although future studies are required to distinguish between these possibilities and to identify other endogenous substrates, the catalytic reactivity of the protein with aldehydes could represent an important link that would regulate Kv channel activity as a function of Kvβ catalysis (regulation of electrical activity by metabolism) or Kvβ catalysis by Kv activity (regulation of metabolism by electrical activity). In either scenario, the link between metabolism and excitability could represent a regulatory mode with profound implications for neural, cardiac, and muscle excitability.
The catalytic activity of Kvβ2 has been found to be sensitive to both pH and ionic strength. Measurements of the enzyme activity at various pH and ionic concentrations found that enzyme activity is maximal between pH 7.2–7.4 and relatively insensitive to varied phosphate concentrations between 100 mM and 250 mM. Yet, at low phosphate concentrations (≤ 50 mM), enzymatic activity is significantly decreased [30–32] and is not impacted by the addition of NADH and or NADP+, suggesting that the enzyme functions most effectively at a specific ionic strength. As with other AKRs such as aldose reductase and aldehyde reductase, the mechanism of Kvβ catalysis was found to be consistent with an ordered bi-bi rapid equilibrium reaction in which the nucleotide cofactor is the first to bind and the last to dissociate. Consistent with this, the binding affinities for NADPH and NADP+ by Kvβ2 are significantly different, as NAD(P)H binds with 4-times greater affinity than NADP+. The sequence of cofactor and substrate binding was confirmed using variable concentrations of 4-NB and NADPH to establish the initial velocity, the starting rate of enzymatic activity. When plotting initial velocity against the different NADPH concentrations, a rapid equilibrium mechanism was predominant, indicative of NADPH binding prior to substrate [30].
The cofactor binding kinetics for Kvβ2, determined by monitoring the quenching of Kvβ2 fluorescence by addition of each respective cofactor, provided insight into the phasic behavior and rate limitation of catalysis [31]. By measuring the dependence of observed kfast and kslow of kinetic traces on NADP(H) concentration, it was suggested that the binding of NAD(P)H to Kvβ could be described as a three-step process consisting of rapid formation of a loose enzyme-cofactor association, a slow conformational change that securely seats the cofactor in the active site of the enzyme, and further stabilization of the NADPH cofactor to its binding site [30, 31]. The binding of NADP+, however, follows the 2 step model of binding affinity, suggesting that the second conformational change observed with NADPH binding that prevents nucleotide exchange is absent in the binding of oxidized nucleotide [31]. Moreover, studies performed using mutant Kvβ2 in which the catalytic site tyrosine (Y90) is replaced with phenylalanine (Kvβ2Y90F) suggest that high affinity nucleotide binding is not significantly impacted by loss of catalytic function [30]. By binding of cofactors to the Kvβ subunits, Kv channel activation and inactivation are sensitive to changes in intracellular pyridine nucleotide redox state, which is reflected in the ratio of intracellular NAD(P)H/NAD(P)+ [32]. Previous studies have suggested that modulation of Kv activity by oxidized and reduced pyridine nucleotides is determined by the identity of Kvβ subunits present [33, 34]. Unlike Kvβ1 and Kvβ3, the Kvβ2 subunit lacks an inactivating N-terminus tail-like structure [35, 36]. Despite this structural difference, Kvβ2 has a common binding affinity to the α subunit T1 domain [7]. Consistent with this, the C-terminal domain of the Kvα subunits is critical for proper association between the Kvα and β subunits [34, 37]. The underlying region of importance in the Kv1α C-terminal domain has been shown to lie precisely between Arg-543 and Val-583 of Kv1.5, a region with differential affinities for NAD(P)H-bound versus NAD(P)+-bound Kvβ. Thus, nucleotide-dependent modification in subunit binding affinity and associated conformational changes within the Kvα transmembrane region may represent a potential mechanism whereby Kvβ redox sensing could alter channel biophysical properties.
The mechanism and biochemical role of cofactor binding in Kvβ-mediated catalysis are similar to that observed with other AKRs, such as aldose reductase. As with other AKRs, NAD(P)H binding promotes a change in protein conformation that stabilizes the cofactor within the catalytic pocket [31]. The affinity of this interaction produces a large change in free energy that drives catalysis. As little energy is derived from substrate binding to achieve the transition state, the protein is capable of binding a range of carbonyl substrates. This mode of high affinity binding to pyridine coenzyme seems well-suited for ion channel regulatory functions of Kvβ, as it reduces the constraints of aldehyde binding and also renders the cofactor binding pocket an effective sensor of intracellular pyridine nucleotides. Thus, physiological changes in intracellular NAD(P)H:NAD(P)+ could readily impact Kv gating and membrane potential regulation. Under conditions of high intracellular NAD(P)H:NAD(P)+ ratio, binding of reduced cofactor generally enhances the degree and rate of channel inactivation [32, 34]. However, this effect can be effectively ‘turned off’ upon completion of a catalytic cycle resulting in substrate reduction and cofactor oxidation. Accordingly, the net effect of AKR enzymatic function on Kv channel activity likely reflects dynamic homeostatic balance between pyridine nucleotide redox potential as well as the concentrations and molecular identities of local aldehyde and ketone substrates, which collectively reflect cellular and subcellular metabolic activity.
5. Physiological roles
i. Regulation of membrane excitability by cellular metabolism.
Considering that Kv channel gating is modulated by intracellular pyridine nucleotides via the Kvβ complex as stated above, these proteins have been proposed as a link between cellular function and metabolic activity. However, a clear view of how the Kvβ proteins operate and modify channel gating behavior in their native cellular and tissue environments has not yet emerged. Complicating this issue, native Kv channels likely consist of heteromeric assemblies of multiple gene products and splice variants, which have not yet been functionally characterized. Moreover, the expression levels of particular Kvα and β proteins and how their stoichiometry within a given population of functional channels is determined may be cell-specific. It is also conceivable that the molecular identity of predominant subunits utilized by a cell may be modified as a result of changing metabolic cues. Considering the functional diversity imparted by variable N-termini of the Kvβ subtypes, the ratio of Kvβ2:Kvβ1/3 present in the Kv auxiliary complex could have a significant impact on inactivation (Fig. 1). For example, in channels with non-inactivating Kvα pore subunits (e.g., Kv1.5) and predominantly Kvβ2 subunits, channel inactivation may be substantially slower, as C-type inactivation would be the primary mode of inactivation [35]. Conversely, in channels consisting of variants of Kvβ1 or 3, channel inactivation likely occurs within a substantially faster time frame, as these subunits would contribute to rapid N-type inactivation. Interestingly, in addition to interaction and regulation of Kvα function by the Kvβ proteins, interaction between multiple types of Kvβ can influence the net function of the Kvβ complex on channel gating. For example, a previous study found that incorporation of Kvβ2 can lead to significant inhibition of N-type inactivation imposed by Kvβ1 subunits within the same channel complex [38]. Nonetheless, the extent to which these subunits impact channel activation and inactivation would also be dependent upon pyridine nucleotide redox ratios in the submembrane compartment, as discussed above. Thus, it is plausible that the cell could dynamically fine-tune the regulatory properties of membrane potential to changing metabolic conditions by altering the ratio of Kvβ subunits within the population of functional membrane-inserted channels. In the remainder of this section, we provide a brief overview of the importance of Kv channels to the cardiovascular, nervous, endocrine and immune systems, and how the functional expression of Kvβ may influence physiological processes of excitable cells types within each.
Figure 1. Balanced redox regulation of whole cell IKv by differential incorporation of Kvβ proteins in heteromultimeric channels.
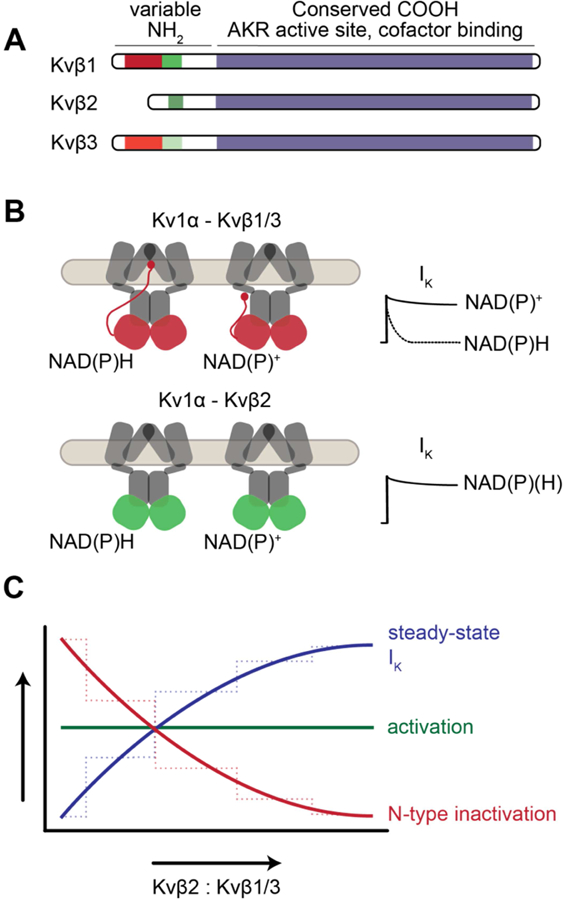
(A) Schematic of Kvβ1, Kvβ2, and Kvβ3 amino acids showing conserved COOH terminal region (blue) and variable N-termini (green/red). Several splice variants which differ in the N-terminal domain have been found for Kvβ1 (1.1–1.3_and Kvβ2 (2.1, 2.2). Ball-and-chain inactivation domain is shown in red. Adapted from [21]. (B) Differential regulation of Kv channel inactivation by Kvβ1/3 (red) and Kvβ2 (green) in the presence of oxidized and reduced pyridine nucleotides. Adapted from [54]. Kv channels expressed in membranes of excitable cells represent heterogeneous populations of structures with varying β subunit compositions. Channels that assemble with Kvβ1/Kvβ3 proteins demonstrate enhanced inactivation upon binding to reduced pyridine nucleotides. Conversely, sensitivity of channel inactivation to pyridine nucleotide redox is absent in channels that associate with Kvβ2, which does not contain the ball-and-chain. (C) Graph showing proposed regulation of Kv channel activation, inactivation, and single channel and whole-cell IKv as the ratio of expressed Kvβ2 : Kvβ1 or Kvβ3 is increased. A reduction in channel inactivation with increased Kvβ2:Kvβ1/3 in native Kv channels could produce an increase in single channel Kv activity and elevation in steady-state IK, which may lead to significant alteration in membrane excitability and responsiveness to changes in cellular metabolism. Solid lines represent predicted observed effects on whole cell Kv activation, inactivation, and IK. Dashed lines show plausible graded effects of Kvβ2:Kvβ1/3 ratio on inactivation and IK.
ii. Cardiovascular system:
In the mammalian heart, multiple types of Kv channels mediate outward K+ currents with variable activation and inactivation properties that collectively shape the cardiac action potential [39, 40]. Attesting to the importance of Kv channel function to cardiac physiology is the robust association between cardiac arrhythmias with mutations in Kv channel subunit genes [41, 42], as well as defective ventricular action potential repolarization in mice lacking Kv proteins [43–45]. The murine heart is known to express Kvβ1.1, Kvβ1.2, and Kvβ2 proteins [46]. Kvβ1 associates primarily with proteins of the Kv4 family and loss of Kvβ1 reduces the abundance of Kv4.3 in the sarcolemma, blunts transient outward K+ current, and prevents modulation of action potential duration by changes in pyridine nucleotide redox state [46, 47]. The physiological role of Kvβ2 still remains unclear. Considering that the heart expresses multiple Kvβ subtypes, it is plausible that the promiscuous association between both Kvβ1 and Kvβ2 proteins with Kv1 and Kv4 channels in the heart contributes to priming cardiac Kv channels for modulation of channel inactivation under conditions of altered nucleotide redox (e.g., altered cardiac workload stress, ischemia) to influence the duration of the early and intermediate phases of repolarization of the action potential.
The Kv channels expressed by vascular smooth muscle are a predominant regulator of vascular tone, and therefore control blood flow and organ perfusion [48]. Kv1 expression and function has been reported in a number of vascular beds, including coronary, pulmonary, mesenteric, and cerebral arteries, among others [49]. Inhibition of Kv1 channels induces vasoconstriction, suggesting that Kv1 channels are tonically active in vascular smooth muscle to oppose vascular tone development [50]. However, little is known regarding the expression and function of Kvβ subunits in the vasculature. Our laboratory recently reported that murine coronary arterial myocytes express heteromeric assemblies of Kvβ complexes in association with Kv1.5 alpha subunits and that genetic deletion of Kvβ2 reduces the membrane expression of Kv1.5 [51], similar to that reported in neurons and heterologous expression systems [52, 53]. Although we and others have speculated that these subunits may play an important regulatory role in coupling tissue oxygen demand with vasodilatory function in various vascular beds [54–56], further research is needed to increase our understanding of how the Kvβ proteins operate in the vasculature and how these may participate in functional or metabolic hyperemic responses.
Unlike most peripheral arteries and arterioles, hypoxia causes rapid and profound vasoconstriction of pulmonary arteries [57]. This phenomenon, referred to as “hypoxic pulmonary vasoconstriction” (HPV), is thought to be an important physiological response of the pulmonary circulation that shunts blood away from underventilated lung tissue [58]. However, excessive HPV can lead to pulmonary hypertension, right ventricular hypertrophy, and heart failure [59]. Kv1 channels have been shown to be an important regulator of pulmonary vascular smooth muscle membrane potential and a mediator of the HPV response [60, 61]. Previous work has shown that HPV is significantly impaired after genetic deletion of redox sensitive Kv1.5 channels, and that in vivo gene transfer of Kv1.5 normalizes HPV in a model of chronic pulmonary hypertension [62, 63]. The association of the Kv1 channels of the pulmonary vasculature with Kvβ proteins may be integral to the HPV response. In support of this, it has been shown that bovine pulmonary arteries exhibit a significant increase in Kvβ1.1 expression with further progression towards higher order pulmonary arteries and arterioles [64]. Higher expression of Kvβ1.1 may impart enhanced inactivation to Kv channels in small vessels in which HPV is apparent by allowing sensing of increases in NADH:NAD ratio upon a decrease in mitochondrial oxidative metabolism during periods of hypoxia [65]. Nonetheless, the precise role of the Kvβ1 subunits in the HPV response has not been directly tested.
iii. Nervous system:
Multiple types of Kv channels expressed in the central nervous system control membrane potential and excitability of neurons, and coordinate diverse processes such as action potential propagation and back propagation, neurotransmitter release, and apoptosis [66]. The altered activity or expression of Kv channel proteins in the nervous system has been associated with human pathological conditions such as epilepsy, multiple sclerosis, and Alzheimer’s disease [67–70]. Neurons express multiple Kv alpha subunits that form functional channels, confer A-type K+ currents, and likely associate with Kvβ proteins, including dendritic Kv4.1, Kv4.2 and Kv4.3 subunits [71–73] and presynaptic Kv1.4 subunits [71]. Variants of all three Kvβ gene products have been found in the brain, with Kvβ2 being the predominant form [74–80], suggesting that Kv1 and Kv4 channels may assemble into heteromers with considerable functional diversity that may participate in the determination of neuronal phenotype. In addition to modulation of channel activation and inactivation characteristics, Kvβ proteins may play a chaperone role and regulate the subcellular targeting of specific populations of Kv channels to distinct neuronal regions (i.e., axonal versus dendritic targeting) [52, 53]. Genetic deletion of Kvβ2 in mice increases mortality, reduces body weight and results in defects in thermoregulatory processes [81], whereas mice lacking Kvβ1.1 have reduced Kv current inactivation, frequency-dependent spike broadening, and slower afterhyperpolarization compared with wild type mice. These changes in neuronal electrical signaling are associated with impaired learning and memory in water maze and social transmission tasks [82]. Although definitive evidence is lacking, it is possible that changes in brain electrical activity could be strongly modulated by Kvβ-dependent regulation of Kv1 and Kv4 activity during periods of altered neuronal cytosolic redox potential, for example, as a result of changes in glucose metabolism.
iv. Endocrine and immune systems.
Multiple Kv channel subtypes also participate in the physiological regulation of membrane potential in a number of cell types outside of the cardiovascular and nervous systems. Prior studies have suggested that these channels, via regulation of Ca2+ influx, also control hormonal secretion in cells of the endocrine system. For example, in pancreatic beta cells, an increase in Ca2+ influx following increased cellular ATP:ADP ratio and inhibition of ATP-sensitive K+ channels (KATP) stimulates the release of insulin [70, 83]. Repolarization of the cell back to resting potential and cessation of the secretory process, is mediated, in part, by Kv-mediated outward K+ currents, which are likely mediated by a variety of Kv channel subtypes, including Kv1, Kv2, Kv4 [84]. Although the expression profile of associated Kvβ proteins in pancreatic islets is not known, modulation of Kv activity by these subunits may be essential to proper electrical signaling following a glucose-induced rise in NADPH:NADP+ ratio in beta cells [85]. In addition, Kv1 channels have also been shown to be expressed by cells of the immune system [86]. Kv1.5 and Kv1.3 are the predominant Kvα proteins in macrophages and inhibition of Kv1 channels can prevent macrophage activation and proliferation [87–89]. Previous work has shown that bone marrow-derived macrophages express all known variants of Kvβ1, and Kvβ2.1 proteins [90]. This study found that LPS- and TNF-α induced activation differentially impacted the abundance of these proteins and modified the channel inactivation, suggesting that modification of Kv1 channel pore and auxiliary subunit composition may reflect an adaptive mechanism that could alter the functional properties of cells in the immune system.
6. Therapeutic implications
Based on current knowledge of the physiological roles of Kvβ proteins, it is plausible that these proteins and their functional properties may represent an advantageous therapeutic target over conventional pharmacological ion channel blockers for a number of conditions. Classical inhibitors of AKRs show very little inhibition of Kvβ-mediated catalysis, and currently, only a few pharmacological agents are known to impact Kvβ function; these act primarily as inhibitors of catalytic activity or by disrupting the association between the Kvβ and Kvα T1 docking domain. A recent study identified the acidic dopamine metabolite 3,4-dihydroxphenylacetic acid (DOPAC) as an effective inhibitor of Kvβ2-mediated reduction of 4-nitrobenzaldehyde, inhibiting the production of 4-nitrobenzyl alcohol by ~40% [91], albeit at supraphysiological concentrations. Additional nonendogenous inhibitors such as the cardioprotective drug resveratrol and plant derived flavonoid rutin, only slightly inhibit Kvβ2 catalytic activity by ~38% each. Alternatively, corticosteroids, such as cortisone, directly interact with Kvβ to increase Kv1 channel activity through binding near the cofactor binding pocket and the intersubunit interface, resulting in dissociation of the Kvβ from the channel [92]. There are currently no known pharmacological agonists that can selectively enhance Kvβ catalytic function. Further elucidation of compounds that can selectively modulate the function of the proteins may be valuable as novel therapeutics for the treatment of multiple disorders. Although the possibility for using compounds identified by these initial studies as Kvβ modulators as therapeutics is unlikely, they provide a useful foundation for further research into more beneficial chemical analogues that may possess more specific biological actions resulting from altering Kvβ function while avoiding off target effects.
7. Summary
In summary, Kvβ proteins of the AKR6 family of aldo-keto reductases are catalytically active hydrophilic proteins that form heterotetrameric complexes at the cytosolic domain of native voltage-gated potassium channels in excitable cell types throughout the cardiovascular, nervous, endocrine and immune systems. By the non-selective binding of a wide range of carbonyl substrates, these proteins catalyze the NAD(P)H-dependent reduction of a variety of endogenous aldehydes and ketones to primary and secondary alcohols. Through differential regulation of channel activation and inactivation properties as a function of pyridine nucleotide redox status, these proteins may participate in numerous physiological processes by coupling outward K+ current and membrane excitability with intermediary metabolism. Despite nearly two decades of research on these intriguing proteins, further experimentation is necessary to fully elucidate how distinct Kvβ proteins perform unique and cell-specific roles in different organ systems during health and disease.
Supplementary Material
Highlights.
Voltage-gated potassium channels are regulated by auxiliary Kvβ proteins.
Kvβ proteins are functional aldo-keto reductases (AKR6A).
Kvβ binding of oxidized and reduced nucleotide cofactors alters Kv channel gating.
Expression of Kvβ1–3 may contribute to dynamic fine tuning of cell excitability.
Acknowledgments
Funding sources
This work was supported by the National Institutes of Health (HL142710 to MN) and American Heart Association (16SDG27260070 to MN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hoog SS, Pawlowski JE, Alzari PM, Penning TM, Lewis M, Three-dimensional structure of rat liver 3 alpha-hydroxysteroid/dihydrodiol dehydrogenase: a member of the aldo-keto reductase superfamily, Proceedings of the National Academy of Sciences of the United States of America, 91 (1994) 2517–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Barski OA, Tipparaju SM, Bhatnagar A, The aldo-keto reductase superfamily and its role in drug metabolism and detoxification, Drug Metab Rev, 40 (2008) 553–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bohren KM, Bullock B, Wermuth B, Gabbay KH, The aldo-keto reductase superfamily. cDNAs and deduced amino acid sequences of human aldehyde and aldose reductases, The Journal of biological chemistry, 264 (1989) 9547–9551. [PubMed] [Google Scholar]
- [4].Trauger JW, Jiang A, Stearns BA, LoGrasso PV, Kinetics of allopregnanolone formation catalyzed by human 3 alpha-hydroxysteroid dehydrogenase type III (AKR1C2), Biochemistry, 41 (2002) 13451–13459. [DOI] [PubMed] [Google Scholar]
- [5].Mindnich RD, Penning TM, Aldo-keto reductase (AKR) superfamily: genomics and annotation, Hum Genomics, 3 (2009) 362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Neuhauser W, Haltrich D, Kulbe KD, Nidetzky B, NAD(P)H-dependent aldose reductase from the xylose-assimilating yeast Candida tenuis. Isolation, characterization and biochemical properties of the enzyme, Biochem J, 326 ( Pt 3) (1997) 683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gulbis JM, Mann S, MacKinnon R, Structure of a voltage-dependent K+ channel beta subunit, Cell, 97 (1999) 943–952. [DOI] [PubMed] [Google Scholar]
- [8].Gulbis JM, Zhou M, Mann S, MacKinnon R, Structure of the cytoplasmic beta subunit-T1 assembly of voltage-dependent K+ channels, Science, 289 (2000) 123–127. [DOI] [PubMed] [Google Scholar]
- [9].Nerbonne JM, Molecular basis of functional voltage-gated K+ channel diversity in the mammalian myocardium, The Journal of physiology, 525 Pt 2 (2000) 285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jan LY, Jan YN, Voltage-gated potassium channels and the diversity of electrical signalling, The Journal of physiology, 590 (2012) 2591–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Philipson LH, Beta-cell ion channels: keys to endodermal excitability, Horm Metab Res, 31 (1999) 455–461. [DOI] [PubMed] [Google Scholar]
- [12].McCormack T, McCormack K, Shaker K+ channel beta subunits belong to an NAD(P)H-dependent oxidoreductase superfamily, Cell, 79 (1994) 1133–1135. [DOI] [PubMed] [Google Scholar]
- [13].Penning TM, The aldo-keto reductases (AKRs): Overview, Chem Biol Interact, 234 (2015) 236–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gonzalez C, Baez-Nieto D, Valencia I, Oyarzun I, Rojas P, Naranjo D, Latorre R, K(+) channels: function-structural overview, Compr Physiol, 2 (2012) 2087–2149. [DOI] [PubMed] [Google Scholar]
- [15].Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R, The structure of the potassium channel: molecular basis of K+ conduction and selectivity, Science, 280 (1998) 69–77. [DOI] [PubMed] [Google Scholar]
- [16].Chung SH, Allen TW, Hoyles M, Kuyucak S, Permeation of ions across the potassium channel: Brownian dynamics studies, Biophysical journal, 77 (1999) 2517–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sansom MS, Shrivastava IH, Bright JN, Tate J, Capener CE, Biggin PC, Potassium channels: structures, models, simulations, Biochim Biophys Acta, 1565 (2002) 294–307. [DOI] [PubMed] [Google Scholar]
- [18].Long SB, Campbell EB, Mackinnon R, Voltage sensor of Kv1.2: structural basis of electromechanical coupling, Science, 309 (2005) 903–908. [DOI] [PubMed] [Google Scholar]
- [19].Scott VE, Muniz ZM, Sewing S, Lichtinghagen R, Parcej DN, Pongs O, Dolly JO, Antibodies specific for distinct Kv subunits unveil a heterooligomeric basis for subtypes of alpha-dendrotoxin-sensitive K+ channels in bovine brain, Biochemistry, 33 (1994) 1617–1623. [DOI] [PubMed] [Google Scholar]
- [20].Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de Miera E, Rudy B, Molecular diversity of K+ channels, Ann N Y Acad Sci, 868 (1999) 233–285. [DOI] [PubMed] [Google Scholar]
- [21].Pongs O, Schwarz JR, Ancillary subunits associated with voltage-dependent K+ channels, Physiol Rev, 90 (2010) 755–796. [DOI] [PubMed] [Google Scholar]
- [22].McCormack K, Connor JX, Zhou L, Ho LL, Ganetzky B, Chiu SY, Messing A, Genetic analysis of the mammalian K+ channel beta subunit Kvbeta 2 (Kcnab2), The Journal of biological chemistry, 277 (2002) 13219–13228. [DOI] [PubMed] [Google Scholar]
- [23].Kilfoil PJ, Tipparaju SM, Barski OA, Bhatnagar A, Regulation of ion channels by pyridine nucleotides, Circulation research, 112 (2013) 721–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chouinard SW, Wilson GF, Schlimgen AK, Ganetzky B, A potassium channel beta subunit related to the aldo-keto reductase superfamily is encoded by the Drosophila hyperkinetic locus, Proceedings of the National Academy of Sciences of the United States of America, 92 (1995) 6763–6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Long SB, Campbell EB, Mackinnon R, Crystal structure of a mammalian voltage-dependent Shaker family K+ channel, Science, 309 (2005) 897–903. [DOI] [PubMed] [Google Scholar]
- [26].Matthies D, Bae C, Toombes GE, Fox T, Bartesaghi A, Subramaniam S, Swartz KJ, Single-particle cryo-EM structure of a voltage-activated potassium channel in lipid nanodiscs, eLife, 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Liu SQ, Jin H, Zacarias A, Srivastava S, Bhatnagar A, Binding of pyridine nucleotide coenzymes to the beta-subunit of the voltage-sensitive K+ channel, The Journal of biological chemistry, 276 (2001) 11812–11820. [DOI] [PubMed] [Google Scholar]
- [28].Williamson DH, Lund P, Krebs HA, The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver, Biochem J, 103 (1967) 514–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pollak N, Dolle C, Ziegler M, The power to reduce: pyridine nucleotides--small molecules with a multitude of functions, Biochem J, 402 (2007) 205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tipparaju SM, Barski OA, Srivastava S, Bhatnagar A, Catalytic mechanism and substrate specificity of the beta-subunit of the voltage-gated potassium channel, Biochemistry, 47 (2008) 8840–8854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Barski OA, Tipparaju SM, Bhatnagar A, Kinetics of nucleotide binding to the beta-subunit (AKR6A2) of the voltage-gated potassium (Kv) channel, Chem Biol Interact, 178 (2009) 165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Weng J, Cao Y, Moss N, Zhou M, Modulation of voltage-dependent Shaker family potassium channels by an aldo-keto reductase, The Journal of biological chemistry, 281 (2006) 15194–15200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tipparaju SM, Saxena N, Liu SQ, Kumar R, Bhatnagar A, Differential regulation of voltage-gated K+ channels by oxidized and reduced pyridine nucleotide coenzymes, American journal of physiology. Cell physiology, 288 (2005) C366–376. [DOI] [PubMed] [Google Scholar]
- [34].Tipparaju SM, Li XP, Kilfoil PJ, Xue B, Uversky VN, Bhatnagar A, Barski OA, Interactions between the C-terminus of Kv1.5 and Kvbeta regulate pyridine nucleotide-dependent changes in channel gating, Pflugers Arch, 463 (2012) 799–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Heinemann SH, Rettig J, Graack HR, Pongs O, Functional characterization of Kv channel beta-subunits from rat brain, The Journal of physiology, 493 (Pt 3) (1996) 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bahring R, Milligan CJ, Vardanyan V, Engeland B, Young BA, Dannenberg J, Waldschutz R, Edwards JP, Wray D, Pongs O, Coupling of voltage-dependent potassium channel inactivation and oxidoreductase active site of Kvbeta subunits, The Journal of biological chemistry, 276 (2001) 22923–22929. [DOI] [PubMed] [Google Scholar]
- [37].Sokolova O, Accardi A, Gutierrez D, Lau A, Rigney M, Grigorieff N, Conformational changes in the C terminus of Shaker K+ channel bound to the rat Kvbeta2-subunit, Proceedings of the National Academy of Sciences of the United States of America, 100 (2003) 12607–12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Xu J, Li M, Kvbeta2 inhibits the Kvbeta1-mediated inactivation of K+ channels in transfected mammalian cells, The Journal of biological chemistry, 272 (1997) 11728–11735. [DOI] [PubMed] [Google Scholar]
- [39].Nerbonne JM, Molecular Basis of Functional Myocardial Potassium Channel Diversity, Card Electrophysiol Clin, 8 (2016) 257–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Grant AO, Cardiac ion channels, Circ Arrhythm Electrophysiol, 2 (2009) 185–194. [DOI] [PubMed] [Google Scholar]
- [41].Olson TM, Alekseev AE, Liu XK, Park S, Zingman LV, Bienengraeber M, Sattiraju S, Ballew JD, Jahangir A, Terzic A, Kv1.5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation, Hum Mol Genet, 15 (2006) 2185–2191. [DOI] [PubMed] [Google Scholar]
- [42].Christophersen IE, Olesen MS, Liang B, Andersen MN, Larsen AP, Nielsen JB, Haunso S, Olesen SP, Tveit A, Svendsen JH, Schmitt N, Genetic variation in KCNA5: impact on the atrial-specific potassium current IKur in patients with lone atrial fibrillation, Eur Heart J, 34 (2013) 1517–1525. [DOI] [PubMed] [Google Scholar]
- [43].London B, Jeron A, Zhou J, Buckett P, Han X, Mitchell GF, Koren G, Long QT and ventricular arrhythmias in transgenic mice expressing the N terminus and first transmembrane segment of a voltage-gated potassium channel, Proceedings of the National Academy of Sciences of the United States of America, 95 (1998) 2926–2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Barry DM, Xu H, Schuessler RB, Nerbonne JM, Functional knockout of the transient outward current, long-QT syndrome, and cardiac remodeling in mice expressing a dominant-negative Kv4 alpha subunit, Circulation research, 83 (1998) 560–567. [DOI] [PubMed] [Google Scholar]
- [45].Guo W, Li H, London B, Nerbonne JM, Functional consequences of elimination of i(to,f) and i(to,s): early afterdepolarizations, atrioventricular block, and ventricular arrhythmias in mice lacking Kv1.4 and expressing a dominant-negative Kv4 alpha subunit, Circulation research, 87 (2000) 73–79. [DOI] [PubMed] [Google Scholar]
- [46].Aimond F, Kwak SP, Rhodes KJ, Nerbonne JM, Accessory Kvbeta1 subunits differentially modulate the functional expression of voltage-gated K+ channels in mouse ventricular myocytes, Circulation research, 96 (2005) 451–458. [DOI] [PubMed] [Google Scholar]
- [47].Tur J, Chapalamadugu KC, Katnik C, Cuevas J, Bhatnagar A, Tipparaju SM, Kvbeta1.1 (AKR6A8) senses pyridine nucleotide changes in the mouse heart and modulates cardiac electrical activity, American journal of physiology. Heart and circulatory physiology, 312 (2017) H571–H583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Jackson WF, KV channels and the regulation of vascular smooth muscle tone, Microcirculation, 25 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Cox RH, Molecular determinants of voltage-gated potassium currents in vascular smooth muscle, Cell Biochem Biophys, 42 (2005) 167–195. [DOI] [PubMed] [Google Scholar]
- [50].Knot HJ, Nelson MT, Regulation of membrane potential and diameter by voltage-dependent K+ channels in rabbit myogenic cerebral arteries, The American journal of physiology, 269 (1995) H348–355. [DOI] [PubMed] [Google Scholar]
- [51].Nystoriak MA, Zhang D, Jagatheesan G, Bhatnagar A, Heteromeric complexes of aldo-keto reductase auxiliary KVbeta subunits (AKR6A) regulate sarcolemmal localization of KV1.5 in coronary arterial myocytes, Chem Biol Interact, (2017). [DOI] [PMC free article] [PubMed]
- [52].Campomanes CR, Carroll KI, Manganas LN, Hershberger ME, Gong B, Antonucci DE, Rhodes KJ, Trimmer JS, Kv beta subunit oxidoreductase activity and Kv1 potassium channel trafficking, The Journal of biological chemistry, 277 (2002) 8298–8305. [DOI] [PubMed] [Google Scholar]
- [53].Shi G, Nakahira K, Hammond S, Rhodes KJ, Schechter LE, Trimmer JS, Beta subunits promote K+ channel surface expression through effects early in biosynthesis, Neuron, 16 (1996) 843–852. [DOI] [PubMed] [Google Scholar]
- [54].Dwenger MM, Ohanyan V, Navedo MF, Nystoriak MA, Coronary microvascular Kv1 channels as regulatory sensors of intracellular pyridine nucleotide redox potential, Microcirculation, 25 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Patel AJ, Honore E, Molecular physiology of oxygen-sensitive potassium channels, Eur Respir J, 18 (2001) 221–227. [DOI] [PubMed] [Google Scholar]
- [56].Thorneloe KS, Chen TT, Kerr PM, Grier EF, Horowitz B, Cole WC, Walsh MP, Molecular composition of 4-aminopyridine-sensitive voltage-gated K(+) channels of vascular smooth muscle, Circulation research, 89 (2001) 1030–1037. [DOI] [PubMed] [Google Scholar]
- [57].Yuan XJ, Tod ML, Rubin LJ, Blaustein MP, Contrasting effects of hypoxia on tension in rat pulmonary and mesenteric arteries, The American journal of physiology, 259 (1990) H281–289. [DOI] [PubMed] [Google Scholar]
- [58].Theissen IL, Meissner A, [Hypoxic pulmonary vasoconstriction], Anaesthesist, 45 (1996) 643–652. [DOI] [PubMed] [Google Scholar]
- [59].Durmowicz AG, Stenmark KR, Mechanisms of structural remodeling in chronic pulmonary hypertension, Pediatr Rev, 20 (1999) e91–e102. [PubMed] [Google Scholar]
- [60].Sweeney M, Yuan JX, Hypoxic pulmonary vasoconstriction: role of voltage-gated potassium channels, Respiratory research, 1 (2000) 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Yuan XJ, Voltage-gated K+ currents regulate resting membrane potential and [Ca2+]i in pulmonary arterial myocytes, Circulation research, 77 (1995) 370–378. [DOI] [PubMed] [Google Scholar]
- [62].Pozeg ZI, Michelakis ED, McMurtry MS, Thebaud B, Wu XC, Dyck JR, Hashimoto K, Wang S, Moudgil R, Harry G, Sultanian R, Koshal A, Archer SL, In vivo gene transfer of the O2-sensitive potassium channel Kv1.5 reduces pulmonary hypertension and restores hypoxic pulmonary vasoconstriction in chronically hypoxic rats, Circulation, 107 (2003) 2037–2044. [DOI] [PubMed] [Google Scholar]
- [63].Archer SL, London B, Hampl V, Wu X, Nsair A, Puttagunta L, Hashimoto K, Waite RE, Michelakis ED, Impairment of hypoxic pulmonary vasoconstriction in mice lacking the voltage-gated potassium channel Kv1.5, FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 15 (2001) 1801–1803. [DOI] [PubMed] [Google Scholar]
- [64].Coppock EA, Tamkun MM, Differential expression of K(V) channel alpha- and beta-subunits in the bovine pulmonary arterial circulation, Am J Physiol Lung Cell Mol Physiol, 281 (2001) L1350–1360. [DOI] [PubMed] [Google Scholar]
- [65].Wolin MS, Ahmad M, Gupte SA, Oxidant and redox signaling in vascular oxygen sensing mechanisms: basic concepts, current controversies, and potential importance of cytosolic NADPH, Am J Physiol Lung Cell Mol Physiol, 289 (2005) L159–173. [DOI] [PubMed] [Google Scholar]
- [66].Norris AJ, Foeger NC, Nerbonne JM, Neuronal voltage-gated K+ (Kv) channels function in macromolecular complexes, Neurosci Lett, 486 (2010) 73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Rangaraju S, Gearing M, Jin LW, Levey A, Potassium channel Kv1.3 is highly expressed by microglia in human Alzheimer’s disease, J Alzheimers Dis, 44 (2015) 797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Pan Y, Xu X, Tong X, Wang X, Messenger RNA and protein expression analysis of voltage-gated potassium channels in the brain of Abeta(25–35)-treated rats, J Neurosci Res, 77 (2004) 94–99. [DOI] [PubMed] [Google Scholar]
- [69].Villa C, Combi R, Potassium Channels and Human Epileptic Phenotypes: An Updated Overview, Front Cell Neurosci, 10 (2016) 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Judge SI, Lee JM, Bever CT Jr., Hoffman PM, Voltage-gated potassium channels in multiple sclerosis: Overview and new implications for treatment of central nervous system inflammation and degeneration, J Rehabil Res Dev, 43 (2006) 111–122. [DOI] [PubMed] [Google Scholar]
- [71].Sheng M, Tsaur ML, Jan YN, Jan LY, Subcellular segregation of two A-type K+ channel proteins in rat central neurons, Neuron, 9 (1992) 271–284. [DOI] [PubMed] [Google Scholar]
- [72].Song WJ, Tkatch T, Baranauskas G, Ichinohe N, Kitai ST, Surmeier DJ, Somatodendritic depolarization-activated potassium currents in rat neostriatal cholinergic interneurons are predominantly of the A type and attributable to coexpression of Kv4.2 and Kv4.1 subunits, The Journal of neuroscience : the official journal of the Society for Neuroscience, 18 (1998) 3124–3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kim J, Wei DS, Hoffman DA, Kv4 potassium channel subunits control action potential repolarization and frequency-dependent broadening in rat hippocampal CA1 pyramidal neurones, The Journal of physiology, 569 (2005) 41–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Leicher T, Bahring R, Isbrandt D, Pongs O, Coexpression of the KCNA3B gene product with Kv1.5 leads to a novel A-type potassium channel, The Journal of biological chemistry, 273 (1998) 35095–35101. [DOI] [PubMed] [Google Scholar]
- [75].Rettig J, Heinemann SH, Wunder F, Lorra C, Parcej DN, Dolly JO, Pongs O, Inactivation properties of voltage-gated K+ channels altered by presence of beta-subunit, Nature, 369 (1994) 289–294. [DOI] [PubMed] [Google Scholar]
- [76].Scott VE, Rettig J, Parcej DN, Keen JN, Findlay JB, Pongs O, Dolly JO, Primary structure of a beta subunit of alpha-dendrotoxin-sensitive K+ channels from bovine brain, Proceedings of the National Academy of Sciences of the United States of America, 91 (1994) 1637–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Coleman SK, Newcombe J, Pryke J, Dolly JO, Subunit composition of Kv1 channels in human CNS, J Neurochem, 73 (1999) 849–858. [DOI] [PubMed] [Google Scholar]
- [78].Rhodes KJ, Strassle BW, Monaghan MM, Bekele-Arcuri Z, Matos MF, Trimmer JS, Association and colocalization of the Kvbeta1 and Kvbeta2 beta-subunits with Kv1 alpha-subunits in mammalian brain K+ channel complexes, The Journal of neuroscience : the official journal of the Society for Neuroscience, 17 (1997) 8246–8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Rhodes KJ, Monaghan MM, Barrezueta NX, Nawoschik S, Bekele-Arcuri Z, Matos MF, Nakahira K, Schechter LE, Trimmer JS, Voltage-gated K+ channel beta subunits: expression and distribution of Kv beta 1 and Kv beta 2 in adult rat brain, The Journal of neuroscience : the official journal of the Society for Neuroscience, 16 (1996) 4846–4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Sandberg R, Yasuda R, Pankratz DG, Carter TA, Del Rio JA, Wodicka L, Mayford M, Lockhart DJ, Barlow C, Regional and strain-specific gene expression mapping in the adult mouse brain, Proceedings of the National Academy of Sciences of the United States of America, 97 (2000) 11038–11043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Connor JX, McCormack K, Pletsch A, Gaeta S, Ganetzky B, Chiu SY, Messing A, Genetic modifiers of the Kv beta2-null phenotype in mice, Genes Brain Behav, 4 (2005) 77–88. [DOI] [PubMed] [Google Scholar]
- [82].Giese KP, Storm JF, Reuter D, Fedorov NB, Shao LR, Leicher T, Pongs O, Silva AJ, Reduced K+ channel inactivation, spike broadening, and after-hyperpolarization in Kvbeta1.1-deficient mice with impaired learning, Learn Mem, 5 (1998) 257–273. [PMC free article] [PubMed] [Google Scholar]
- [83].Newgard CB, McGarry JD, Metabolic coupling factors in pancreatic beta-cell signal transduction, Annu Rev Biochem, 64 (1995) 689–719. [DOI] [PubMed] [Google Scholar]
- [84].Yan L, Figueroa DJ, Austin CP, Liu Y, Bugianesi RM, Slaughter RS, Kaczorowski GJ, Kohler MG, Expression of voltage-gated potassium channels in human and rhesus pancreatic islets, Diabetes, 53 (2004) 597–607. [DOI] [PubMed] [Google Scholar]
- [85].Gray JP, Alavian KN, Jonas EA, Heart EA, NAD kinase regulates the size of the NADPH pool and insulin secretion in pancreatic beta-cells, Am J Physiol Endocrinol Metab, 303 (2012) E191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Feske S, Wulff H, Skolnik EY, Ion channels in innate and adaptive immunity, Annual review of immunology, 33 (2015) 291–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Vicente R, Escalada A, Villalonga N, Texido L, Roura-Ferrer M, Martin-Satue M, Lopez-Iglesias C, Soler C, Solsona C, Tamkun MM, Felipe A, Association of Kv1.5 and Kv1.3 contributes to the major voltage-dependent K+ channel in macrophages, The Journal of biological chemistry, 281 (2006) 37675–37685. [DOI] [PubMed] [Google Scholar]
- [88].Villalonga N, David M, Bielanska J, Vicente R, Comes N, Valenzuela C, Felipe A, Immunomodulation of voltage-dependent K+ channels in macrophages: molecular and biophysical consequences, The Journal of general physiology, 135 (2010) 135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Vicente R, Escalada A, Coma M, Fuster G, Sanchez-Tillo E, Lopez-Iglesias C, Soler C, Solsona C, Celada A, Felipe A, Differential voltage-dependent K+ channel responses during proliferation and activation in macrophages, The Journal of biological chemistry, 278 (2003) 46307–46320. [DOI] [PubMed] [Google Scholar]
- [90].Vicente R, Escalada A, Soler C, Grande M, Celada A, Tamkun MM, Solsona C, Felipe A, Pattern of Kv beta subunit expression in macrophages depends upon proliferation and the mode of activation, J Immunol, 174 (2005) 4736–4744. [DOI] [PubMed] [Google Scholar]
- [91].Alka K, Dolly JO, Ryan BJ, Henehan GT, New inhibitors of the Kvbeta2 subunit from mammalian Kv1 potassium channels, Int J Biochem Cell Biol, 55 (2014) 35–39. [DOI] [PubMed] [Google Scholar]
- [92].Pan Y, Weng J, Kabaleeswaran V, Li H, Cao Y, Bhosle RC, Zhou M, Cortisone dissociates the Shaker family K+ channels from their beta subunits, Nature chemical biology, 4 (2008) 708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


