Abstract
A20, also known as TNFAIP3, is a potent regulator of ubiquitin (Ub) dependent signals. A20 prevents multiple human diseases, indicating that the critical functions of this protein are clinically as well as biologically impactful. As revealed by mouse models, cell specific functions of A20 are linked to its ability to regulate diverse signaling pathways. Aberrant expression or functions of A20 in specific cell types underlie divergent disease outcomes. Discernment of A20’s biochemical functions and their phenotypic outcomes will contribute to our understanding of how ubiquitination is regulated, how Ub mediated functions can prevent disease, and will pave the way for future therapeutic interventions.
Keywords: A20, TNFAIP3, ubiquitin, autoimmunity, inflammation, innate immunity
A20 and the Ub mediated regulation of cellular processes.
Protein ubiquitination is a post-translational modification that orchestrates diverse cellular functions. The prevalence of intracellular ubiquitination events is expansive, and proteomic analyses reveal a large fraction of the mammalian genome is dedicated to encoding Ub associated and Ub interacting proteins ([1][2][3]). Substrate proteins may be modified by the addition of a single Ub (monoubiquitination) by an enzyme complex that includes an E1 Ub activating enzyme, an E2 Ub conjugating enzyme, and an E3 Ub ligase. These enzyme complexes can further catalyze ubiquitination of substrate-conjugated Ub, resulting in substrate polyubiquitination.
Interubiquitin linkages can conjugate in different topologies depending on which of Ub’s seven lysines (e.g., K48, K63 polyubuiquitin) or N-terminal Ub methionine (M1) is conjugated to the preceeding Ub molecule ([4][5][6]). This biochemical “Ub code” provides the cell with profound post-translational plasticity. For example, while modification of proteins with K48-linked polyubiquitin chains targets proteins for proteolytic degradation, modification with K63-linked or M1-linked Ub chains stimulates a wide array of protein-protein interactions, including the recruitment of signaling proteins during signal transduction ([7]). The generation of distinct types of poly-Ub chains requires the collaboration of specific combinations of E2 and E3 Ub ligase enzymes. Meanwhile, the recognition of distinct types of poly-Ub chains requires Ub sensor proteins that directly bind to specific types of Ub oligomers ([8]Sims et al 2012).Further complexity in the Ub code has been introduced by recent evidence suggesting K63 linked Ub chains can also be used as a substrate for building M1 linked Ub chains. The resulting “hybrid” Ub chains may be integral for coordinating recruitment of diverse combinations of Ub binding proteins ([9][10][11][12][13]). Such heterotypic Ub chains — conjugates with mixed or branched chains—modulate signaling pathway output both spatially and temporally ([14]). In summary, the distinct and diverse types of Ub modifications mediate a broad spectrum of protein-protein interactions that provide a cell’s ability to dynamically regulate cellular processes, including receptor recycling, signal transduction, cell cycle, transcription, and subcellular localization. Correspondingly, the regulators of ubiquitination are critical for preserving cellular and organismal homeostasis.
Given the diverse protein functions that Ub modification coordinate, it is no surprise that misregulation of this process can lead to numerous and disparate human diseases. The multitude of human diseases that can be impacted by aberrant ubiquitination is prominently exemplified by A20, also known as TNFAIP3. A20 is a unique Ub modifying protein — exhibiting deubiquitinating, Ub E3 ligase, and Ub binding activities — that has been linked to manifold human diseases. This review focuses on how A20 expression and biochemical functions impact disease outcomes, with a concentration on immune mediated diseases.
A20’s links to human disease
A20, or TNFAIP3, is a remarkably potent regulator of Ub dependent signals and of immune homeostasis. Among ubiquitination regulators, A20 has garnered increasing attention because of its multiple links to human disease ([15][16]). Initially, genome wide association studies (GWAS) linked single nucleotide polymorphisms (SNPs) in the A20 gene locus with the incidence of rheumatoid arthritis, systemic lupus erythematosus (SLE), psoriasis, rheumatoid arthritis, celiac disease, inflammatory bowel disease (IBD), multiple sclerosis, scleroderma, and asthma. Among disease associated susceptibility SNPs, putative causal SNPs were shown to cause reduced A20 expression ([17][18][19]). More recently, it was shown that transgenic mice bearing human A20 genes with susceptibility SNPs develop autoimmunity and arthritis ([20]). In addition, reductions in A20 expression have been observed independently of germline polymorphisms in both involved and uninvolved tissues from psoriasis and asthma patients ([21] [22][23][24]). Reduced A20 expression has also been correlated with disease and anti-TNF responsiveness in inflammatory bowel disease patients ([25][26][27]).
A20’s ability to protect against human disease has been further amplified by recent descriptions of patients that carry heterozygous loss-of-function mutations of A20 that present with Behcet-like disorders, defining a new autoinflammatory syndrome termed A20 haploinsufficiency (HA20) ([28][29][30][31]). Over 45 cases of HA20 from diverse geographic regions have been reported since the first report in 2016 ([28][30]). Symptoms of HA20 patients commonly occur in early childhood, and include recurrent fever, oral, genital and/or gastrointestinal ulcers, arthralgia, arthritis, autoimmune hepatitis, and cutaneous lesions. Germline inactivating A20 mutations have also been recently found in patients presenting with early onset inflammatory bowel disease ([32]). HA20 patients have been treated with immunosuppressive therapies, including corticosteroids and biologic cytokine inhibitors (anti-TNF, anti-IL-1, anti-IL-6) with variable effectiveness ([31]). Interestingly, promising results were observed using therapeutic approaches directed by functional cytokine testing, suggesting that a more detailed mechanistic understanding of HA20 disease will allow more effective diagnosis and treatment of these patients.
Loss of A20 expression also leads to malignancies, especially B cell lymphomas. Somatic biallelic somatic mutations of A20 are found in up to 30% of human B lymphomas ([33][34][35][36]). In addition, germline and somatic genetic variations of A20 are observed in 77% of patients with mucosa-associated lymphoma associated with primary Sjogren’s syndrome ([36] [37]). Furthermore, aberrant A20 expression has been suggested to contribute to both the incidence and/or prognosis of a number of non-hematopoietic malignancies [38][39][40][41][42][43]).
Overall, these links between A20 and human disease provide compelling evidence that understanding A20 function is not only biologically important, but also clinically impactful. The mechanisms by which A20 may prevent human diseases are likely complex. Early studies in fibroblasts linked A20 with the regulation of NF-κB signaling and with the regulation of ubiquitination ([44][45][46]). Both A20 and NF-κB can be expressed in virtually all cell types. Additionally, Ub modifications regulate signaling proteins beyond the NF-κB signaling cascades. Thus, A20 regulates immune and non-immune functions via a wide array of NF-κB-dependent and -independent mechanisms. As discussed below, these functions have been most assiduously explored using mouse models bearing targeted mutations of A20 alleles.
A20 and adaptive immunity
Most cell types express baseline levels of A20 protein that is further induced by stimulation with a variety of ligands such as TNF that trigger NF-κB signaling. The induction of A20 expression by pro-inflammatory signals suggests A20 is a negative feedback regulator of inflammation. Spontaneous activation of multiple immune cell types was first observed in globally A20 deficient (A20−/−) mice, leading to multiorgan inflammation and perinatal death ([46]Lee et al). A20’s broad expression presented several potential non-cell-autonomous as well as cell-autonomous mechanisms by which global A20 deficiency could perturb immune homeostasis. The generation of mice lacking A20 in specific cell lineages has helped clarify these mechanisms.
Mice lacking A20 in CD19+ B cells (LoxP-flanked A20 (A20FL) bred to CD19-Cre mice) exhibited spontaneous B cell activation, expansion of germinal center (GC) B cells, and production of autoantibodies ([47][48][49]). The accumulation of GC B cells was correlated with increased NF-κB responses to CD40, increased Bcl-XL expression, and resistance to Fas mediated cell death. The resistance of activated A20 deficient GC B cells to undergo cell death may abrogate normal negative selection in GCs, allowing survival of autoreactive B cells and secretion of autoantibodies. No gross defects in affinity maturation or class switching have been reported to date. Whether A20 expression in B cells regulates these functions remains an open question. The presence of autoantibodies and GC B cell expansion in heterozygous A20FL/+ CD19-Cre mice showed that hypomorphic A20 expression in B cells confers spontaneous autoimmunity and dose dependent NF-κB signaling responses ([47]). The physiological impact of hypomorphic A20 expression in mice provides experimental support for the importance of reduced A20 expression in human tissues. Considered together with studies indicating that A20 expression is tightly regulated via multiple transcriptional, post-transcriptional, and post-translational mechanisms (to be discussed later in this review), these observations revealed the importance of finely tuned A20 expression in cells. Finally, A20 deficient B cells elaborate more cytokines such as IL-6, and cause myeloid activation. These studies reveal cell autonomous mechanisms by which A20 deficiency in B cells compromises B cell tolerance and causes autoimmunity.
Increased NF-κB signaling and survival of A20 deficient GC B cells also provide critical insights into A20’s tumor suppressor function in human B cell lymphomas. While A20’s ability to protect fibroblasts from TNF induced cell death demonstrate its pro-survival function ([44][46]), this pro-survival function aligns poorly with its tumor suppressor function in B cells. The discovery that loss of A20 from B cells increases their survival provides a mechanism explaining A20’s tumor suppressor function. The apparent diametric affects of A20 in regulating fibroblast versus B cell survival highlight the importance of studying pleiotropically expressed proteins in cell specific fashion. They also reinforce the notion that A20 restricts NF-κB responses via distinct mechanisms from restricting cell death. Moreover, cellular integration of activation and survival signals is known to vary at different stages within lymphocyte development and activation, i.e., A20 may regulate survival of resting B cells distinctly from activated B cells. Finally, although B cell lymphomas have not been reported to date in A20FL/FL CD19-Cre mice, histological studies of older mice revealed marked perturbations of splenic architecture that resemble lymphomatous states (Taveres and Ma, unpublished observations). Studies using mice expressing collaborating B cell oncogenes might replicate multistep carcinogenesis and further clarify the mechanisms by which A20 suppresses B cell tumorigenesis.
As in B cells, the functions of A20 in T cells include regulating NF-κB responses as well as cellular survival. A20 deficient CD8+ T cells elaborate increased NF-κB responses and production of cytokines, leading to enhanced tumor immunity ([50]). Exaggerated NF-κB signaling of these cells leads to increased IFNγ and TNFα expression as well as reduced PD-1 expression. Increased NF-κB responses are also observed in A20 deficient CD4+ T cells, resulting in enhanced TCR-induced production of IL-2 ([51]).
In contrast to activated B cells, activated A20-deficient T cells exhibit increased sensitivity to cell death. These findings again highlight the diverse outcomes associated with integration of Ub dependent NF-κB and survival signals in distinct cell types. Recently activated T cells require A20 to prevent RIP3 dependent necroptotic death during experimental allergic encephalomyelitis (EAE) ([51]). A20 also promotes CD4+ T cell survival via restriction of mTOR activity and increased autophagy [52]). As RIP3 is not known to regulate NF-κB signaling, A20’s regulation of RIP3 dependent death distinguishes A20’s capacity to regulate NF-κB signals from its ability to regulate cell death.
T cell specific A20 expression supports secondary, but not primary, T cell responses against Listeria monocytogenes ([53]). These findings reinforce the notion that A20 dependent functions may be differentially regulated at different stages of T cell differentiation. A20 also restrains intrathymic differentiation of regulatory T cells (Tregs) and NKT differentiation ([54][55]). The degrees to which these phenotypes are due to A20’s regulation of NF-κB signaling or cell survival—or potentially other cell biological functions—remain open questions. Overall, A20 exerts diverse influences on physiological T cell survival, differentiation, and function. These T cell intrinsic functions regulate host autoimmunity, anti-pathogen immunity, and anti- tumor immune responses.
Studies of A20 in T cells also unveiled an interesting post-translational mode of regulating A20 stability. This regulation implicated MALT1, a protein that was identified from its frequent translocation in mucosal associated lymphomas (MALT lymphomas), and whose overexpression drives T cell activation and proliferation. MALT1 was found to support T cell receptor (TCR) mediated activation of T cells by functioning as a paracaspase to cleave A20 protein ([56]). In addition, MALT1 undergoes ubiquitination with K63-linked polyubiquitin chains, and A20 removes these chains. Hence, MALT1 and A20 exhibit an interesting post-translational cross-talk. Combined with NF-κB dependent transcriptional induction of A20 expression, post-translational proteolytic mechanisms provide dynamic negative feedback of TCR signaling.
A20 functions in innate immunity
Spontaneous inflammation, perinatal lethality, and enhanced NF-κB signaling are the most prominent phenotypes in A20−/− mice. These inflammatory phenotypes persist in A20−/− RAG-1−/− compound mutant mice, indicating that A20 performs critical physiological functions independently of adaptive lymphocytes ([46]). T and B cell independent regulatory functions of A20 may broadly be partitioned into regulation of innate immune cells or non-hematopoietic cells.
Potent inhibition of innate immune functions by A20 was initially demonstrated by exaggerated LPS induced NF-κB and cytokine responses in A20−/− bone marrow derived macrophages (BMDMs) ([57]). A20 directly restricts both MyD88 dependent and TRIF dependent TLR signals ([58]) as well as signals triggered by the intracellular microbial sensor NOD2 ([59]). The physiological importance of these activities is seen in A20 mediated control of LPS induced circulatory “shock” in chimeric mice bearing A20 deficient hematopoietic cells ([57]). It is also evident in the amelioration of spontaneous inflammation in A20−/− mice by MyD88−/− deficiency ([58]).
Innate immune functions of A20 are evident in mice lacking A20 specifically in CD11c+ dendritic cells (DCs) ([60][61][62]). A20 deficient DCs are spontaneously activated in A20FL/FL CD11c-Cre mice, leading to production of high levels of pro-inflammatory cytokines and activation of B, T, and myeloid cells. Somewhat divergent phenotypes were observed in independently generated A20FL/FL CD11c-Cre mouse strains. In our lab, mice developed colitis and sero-negative ankylosing arthritis—a symptom complex resembling human IBD and IBD-associated spondyloarthritis [60]. Kool et al observed lymphosplenomegaly, plasma cell expansion, and autoantibody production—features resembling SLE [61]. These divergent phenotypes could be due to microbiome differences and/or to distinct targeting strategies. Overall, these marked phenotypes indicate critical roles for DC A20 expression to preserve basal immune homeostasis as well as to prevent autoimmune and autoinflammatory disease. A20 deficiency in CD11c+ DCs elaborate exaggerated amounts of cytokines such as IL-6, IL-12, TNF, and BAFF that drive inflammation and abrogate T and B cell tolerance.
Mice bearing A20 deficient LysM-Cre+ myeloid cells (macrophages and neutrophils) spontaneously develop arthritis that occurs independently of TNF, functional T and B cells, but requires MyD88 signaling ([63]). NLRP3 inflammasomes cause arthritis in these mice as deletion of NLRP3, caspase-1 or the interleukin-1 receptor ameliorate disease pathology ([64]). Similarly, A20 in CX3CR1-Cre+ myeloid cells inhibits inflammasome dependent neuroinflammation ([65]). Hence, A20 deficient macrophages drive arthritis via inflammasome products such as IL-1β, IL-18, and/or pyroptotic death. Together with studies using A20FL/FL CD11c-Cre mice, these studies highlight the critical importance of A20 expression in myeloid cells. A20 likely also regulates human myeloid cells, as HA20 patients express less A20 protein in peripheral monocytes and respond to treatments with inhibitors of TNF and IL-1β. Hence, A20 dependent functions in innate immune cells appear to be central mediators of human disease ([28][30][31]).
Mice lacking A20 expression in other innate immune cell types have revealed additional functions for this pleiotropic protein. In each cell type, the pathophysiological potential of these cells is highlighted by releasing signals normally restricted by A20. Deletion of A20 from brain-resident CX3CR1+ microglia leads to increased feeding behavior due to microglial cross-talk with hypothalamic neurons ([66]). Deletion of A20 from IL-5 expressing intestinal cells (i.e., ILC2s) resulted in spontaneous expansion of intestinal goblet cells and intestinal lengthening ([67]). While deletion of A20 from mast cells does not affect mast cell degranulation, it leads to amplified pro-inflammatory responses and aggravated models of experimentally induced arthritis and asthma, indicating that hyperactive mast cells may exacerbate inflammatory disorders ([68]). The molecular mechanisms by which A20 functions in these cell types remain unclear. Nevertheless, A20 expression in innate immune cells clearly preserves immune homeostasis and prevents inflammatory and autoimmune pathology.
3.3. Non-hematopoietic functions of A20
A20 expression is induced in non-hematopoietic cells as well as immune cells. The genetic association of A20 with a variety of distinct autoimmune diseases—including diseases afflicting specific tissues—prompted investigations of A20’s physiological functions in tissue restricted cell types such as intestinal, lung, and skin epithelial cells.
A20 deficiency in villin-expressing intestinal epithelial cells (IECs) renders mice susceptible to dextran sulfate sodium (DSS) ([69]). This defect also confers sensitivity of the intestinal tissue to non-inflammatory neoplastic transformation, a finding that correlates with reduced A20 expression in human adenomatous and cancerous intestinal tissues ([38]).
While mice bearing A20 deficient IECs exhibit minimal spontaneous disease, IEC-specific defects in A20 function can collaborate with immune cell specific A20 defects to perturb tissue homeostasis. A20FL/FL villin-Cre, LysM-Cre compound mutant mice bearing A20 deficient IECs and A20 deficient macrophages exhibit increased susceptibility to intestinal damage and cancer ([70]).
Marked synergy is observed in mice lacking A20 in combination with partial or complete deficiency of A20 binding inhibitor of NF- κB-1, or ABIN-1, selectively in IECs. Tamoxifen induced deletion of A20 and ABIN-1 triggers spontaneous IEC death that causes uniform mouse death within 72 hrs ([71]). Tamoxifen induced deletion of only one copy of ABIN-1 in conjunction with A20 deletion also leads to spontaneous IEC and mouse death, and this epistatic interaction occurs despite the fact that ABIN-1 protein levels are higher in A20FL/FL ABIN-1FL/+ than in wild type cells. Hence, elevated levels of ABIN-1 are critical for the survival of A20 deficient IECs, and ABIN-1 appears to partially compensate for A20 in protecting IECs from cell death. Therefore, studies of isolated A20 deficiency likely underestimate the importance of this protein’s physiological functions.
A20 deficiency in lung epithelial cells confers sensitivity to house dust mite induced disease, and A20 expression is reduced in primary lung epithelial cultures from asthma patients ([22]). The clinical relationship between A20 expression and asthma may be related to early life exposure to farm environs, suggesting that environmental ligands might induce mucosal tolerance via A20 expression ([22]). Decreased A20 expression is also correlated with increased asthma incidence in two distinct populations of inbred farming communities ([72]). A20 deficiency in K14+ skin epithelial cells causes keratinocyte hyperplasia and ectodermal abnormalities ([73]). These mice also exhibit sensitivity to imiquimod induced psoriasis ([24]). Reduced A20 expression is also seen in the non-involved skin of psoriasis patients, further validating the clinical relevance of these mouse models. Broadly, these studies suggest that A20 functions in tissue epithelial cells may collaborate with A20 functions in immune cells to protect against disease.
4. A20 beyond NF-κB signaling
Lineage specific studies of A20 have not only highlighted diverse cell type specific functions for this protein, but have also expanded A20’s cell autonomous functions. While A20’s capacity to prevent inflammation has largely been ascribed to its ability to restrict NF-κB signaling, A20 also appears to regulate additional Ub dependent signals.
While cells with deficient NF-κB signals are more susceptible to programmed cell death, A20 deficient fibroblasts and T cells are more susceptible to cell death while exhibiting robust or exaggerated NF-κB signaling. In addition, distinct cell populations exhibit variably increased or decreased susceptibility to cell death, despite consistently increased NF-κB responses. Hence, A20’s cell survival functions are unlikely to be due entirely to exaggerated NF-κB signaling. While the cell biological mechanisms by which A20 restricts cell death remain incompletely understood, some clues are provided by the observations that several death signaling proteins undergo physiological ubiquitination, and ubiquitinated death signaling complexes are dependent upon A20. RIP1 kinase undergoes ubiquitination after TNFR ligation, and RIP1 ubiquitination is enhanced after TNF stimulation of A20 deficient fibroblasts ([74]). However, RIP1 ubiquitination is thought to support NF-κB signaling and to inhibit death promoting RIP1 kinase activity ([75]). Hence, increased cell death in these cells is probably not due to disproportionate RIP1 ubiquitination versus RIP1 kinase activity. It is possible that A20 restricts both RIP1 ubiquitination and RIP1 kinase activity. In addition, as multiple types of polyubiqutin chains can be attached to the same signaling molecules, it is also possible that distinct types of polyubiqutin chains may be built on distinct RIP1 lysines to alter cell death signaling.
Understanding how A20 regulates cell death has been augmented by mass spectrometry (MS) studies in primary cells. For example, anti-Gly-Gly antibody assisted MS studies of ubiquitinated peptides of A20 deficient cells revealed that ubiquitination of RIP3 on the lysine at position 5 occurs during caspase independent necroptosis. This ubiquitination event supports the formation of RIP1-RIP3 complexes and necroptotic cell death ([51]). Quantitative stable isotope labeling (SILAC) MS experiments indicated that this specific ubiquitination event is highly A20 dependent. As necroptosis is an inflammatory form of cell death, exaggerated necroptosis in A20 deficient tissues could contribute to their susceptibility to inflammation and disease.
A20 regulates additional ubiquitination signaling events in myeloid cells. Activation of NLRP3 inflammasomes normally requires NF-κB dependent transcriptional induction of pro-IL-1β and NLRP3, followed by ATP triggered assembly of these inflammasome components along with ASC and caspase 1. However, A20 deficient macrophages process and secrete mature IL-1β without extracellular ATP, a phenomonen described as “spontaneous” inflammasome activation ([76]). Notably, NF-κB induction of pro-IL-1β is disconnected from IL-1 β cleavage and secretion. Hence, spontaneous inflammasome activation in A20−/− macrophages is not due to exaggerated NF-κB signaling. Inflammasome activation in these cells requires engagement of TRIF dependent TLRs, and involves both caspase 1 and caspase 8 . TRIF engages RIP1; RIP1 binds RIP3; and spontaneous inflammasome activity in A20−/− cells is entirely RIP3 dependent. Considered together with A20’s role in regulating RIP1-RIP3 complexes during necroptosis, as well as the ability of RIP3 deficiency to ameliorate perinatal lethality of A20−/− mice, it is likely that A20 plays a critical role in regulating RIP1 and RIP3 complexes. Studies of NLRP3 inflammasome induction in A20−/− cells also revealed that pro-IL-1β undergoes ubiquitination on lysine 133, an event that supports pro-IL-1 β cleavage ([76]). The physiological importance of A20’s restriction of NLRP3 inflammasome activation is evidenced by abrogation of arthritis in A20FL/FL LysM-Cre mice by NLRP3, ASC, and caspase1/11 deficiency ([64]). As inflammasomes are implicated in a number of experimental and clinical disease models, restricting inflammasome activation is another mechanism by which A20 can prevent disease.
Wnt ligands trigger β-catenin signals that support survival and proliferation of stem cells. These ligands inhibit Skip-Cullen-F box (SCF) dependent ubiqutination and degradation of β-catenin, thereby allowing β-catenin translocation to the nucleus. A20 restricts Wnt induced β-catenin signaling in IECs, and suppresses APC dependent neoplastic transformation in vivo ([38]). While the mechanism by which A20 restricts Wnt signaling remains to be determined, this finding amplifies A20’s tumor suppressor potential, and dovetails with the clinical observation that A20 expression is reduced in adenomatous intestinal tissues, and further reduced in intestinal carcinomas. Hence, A20 restricts an array of Ub dependent signals that preserve immune and tissue homeostasis.
5. Biochemical mechanisms by which A20’s mediates its functions
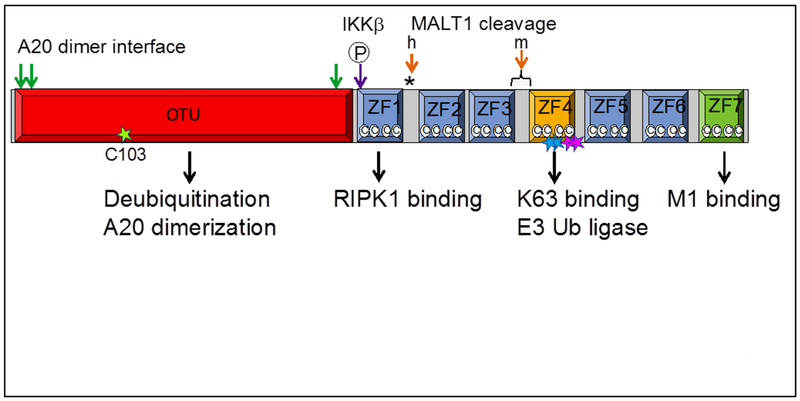
While A20 clearly regulates ubiquitination of multiple signaling complexes, the precise biochemical mechanisms by which A20 performs these functions remain surprisingly enigmatic and complex. The A20 protein is a cysteine protease with deubiquitinating (DUB) activity ([45][57]). A20 also possesses seven zinc finger motifs that mediate binding to at least two types of polyubiqutin chains, RIP1, IKKγ, TRAF2, and a number of additional Ub dependent proteins (e.g., ABIN-1, RNF11, TAX1BP1). Hence, A20 and its binding partners are extraordinarily well equipped to regulate ubiquitinated signaling complexes (Figure 1).
Figure 1.
Schematic representation of A20 protein. A20 can be divided into an N-terminal OTU (ovarian tumor) domain and seven C-terminal Cys2-Cys2 (©©) zinc finger (ZF) domains. The catalytic cysteine C103 in the OTU domain mediates A20’s deubiquitinating activity ([45][57][74][86]). A20’s DUB activity is regulated by phosphorylation by IKKβ (purple arrow) ([87]) that increases A20’s catalytic specificity for K63 polyubiqutin chains ([78]). A20’s ZF4 domain (yellow square) and ZF7 domain (green square) mediate binding to K63- and M1-polyubiquitin chains, respectively. A20’s ZF4 may also directly (or indirectly) mediate E3 Ub ligase activity to build K48 polyubiquitin chains on RIPK1 ([74][78]). Knock-in mice bear inactivating mutations of A20’s hydrolase activity (C103, green star) or ZF4-mediated polyubiquitin binding and/or E3 Ub ligase activities (Cys609Cys612, pink stars; Tyr599Phe600, blue stars). A20 forms a dimer with critical dimerization residues in the OTU domain (green arrows) ([77][88]). A20 is cleaved by MALT1 ([56]). MALT1 cleavage sites (orange arrows) in human (h) and mouse (m) are distinct.
A20’s DUB motif cleaves unanchored K48-linked ([45][57]) and K63-linked polyubiquitin chains that are anchored to TRAF6 or RIP1 ([57][74]). This activity is thought to remove “activating” K63 Ub chains that support recruitment of downstream signaling molecules, broadly parallel to the manner in which phosphatases reverse activating phosphorylation events. Knock-in mice bearing a point mutation of A20’s catalytic cysteine are grossly normal but exhibit increased sensitivity to DSS ([77]). MEFs from these A20C103A mice exhibit increased RIP1 ubiquitination and modestly enhanced NF-κB responses after TNF stimulation ([77][78]). These findings confirm A20’s DUB activity upon RIP1. However, A20C103A mice do not recapitulate the spontaneous perinatal lethality observed in A20−/− mice ([46] [77][78][79]). Hence, A20 performs critical physiological functions independently of its DUB activity.
A20’s fourth zinc finger, ZF4, binds K63-linked Ub chains and supports E3 ligase activity. Mice bearing knock-in mutations in this motif, A20ZF4 mice, are grossly healthy for several months of age, and exhibit sensitivity to DSS ([77]). Like A20C103A cells, A20ZF4 cells exhibit increased RIP1 ubiquitination and TNF induced NF-κB responses ([77][78]). These findings could be explained either by ZF4’s ability to build degradative K48 Ub chains on RIP1 or by its ability to bind Ub chains and recruit A20 or other DUBs to RIP1.
The unusual ability of A20 to exhibit both DUB and Ub E3 ligase activity raised the possibility that A20 could function as an Ub editing enzyme that exchanged one type of poly-Ub chain (e.g., K63 linked) with another (e.g., K48 linked). However, A20’s C103 based DUB activity can be physically separated from its ZF4 based activity in cell free assays, and more importantly, A20C103A proteins can complement A20ZF4 proteins in compound mutant A20C103A/ZF4 cells ([77]). This complementation occurs despite the fact that A20 proteins form dimers and potentially higher order oligomers ([77]). Thus, A20’s biochemical activities are separable. A20’s C103 and ZF4 based motifs are also integral to A20’s ability to inhibit E2-E3 complex formation and limit ubiquitination ([80]). Inhibiting E2-E3 complexes provides an alternative mechanism by which A20 may restrict ubiquitination of signaling complexes. Finally, the relatively mild phenotypes of both A20C103A mice and A20ZF4 mice compared with A20−/− mice suggests that A20 utilizes additional biochemical activities to prevent inflammatory disease. Such activities could be housed in other motifs, or could require combinations of A20’s biochemical activities.
6. Outstanding questions/future areas for discovery
If A20 performs critical non-catalytic functions, then A20 may partly function as an adaptor and/or scaffold protein. This function could involve some of A20’s binding partners, several of which display overlapping signaling functions with A20. For example, ABIN-1 and TAX1BP1 share A20’s ability to restrict TNF and TLR induced NF-κB responses as well as TNF induced cell death ([81][82][83][84][85]). A20 would be an unusual adaptor protein, as it is dynamically regulated at transcriptional and post-transcriptional levels and as it harbors enzymatic activities. In addition, the fact that ABIN-1 and TAX1BP1 lack known enzymatic functions begs the question of how such A20 bearing complexes regulate ubiquitinated signaling complexes. Overall, better definition of the physiological functions of A20’s biochemical activities should lead to better understanding of how Ub dependent signals are regulated and how specific motifs might be targeted with small molecules to achieve therapeutic benefits.
Highlights.
A20 is a potent anti-inflammatory protein that is strongly linked to multiple human diseases.
A20 is expressed in virtually all cell types, and A20 deficiency in distinct cell types confers susceptibility to different disease phenotypes.
A20 restricts NF- κB signaling as well as other ubiquitin dependent signals
A20 possesses a number of ubiquitin dependent biochemical motifs, and collaborates with other ubiquitin dependent proteins in complex and incompletely understood mechanisms to regulate signals
Acknowledgements
We apologize to contributors in this field that we have not had room to reference.
Work from the authors’ laboratory was supported by NIH R01 grants AI117908, DK095693, and AI35198.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Kim W, Bennett EJ, Huttlin EL, Guo A, Li J, Possemato A, Sowa ME, Rad R, Rush J, Comb MJ, Harper JW, Gygi SP, Systematic and Quantitative Assessment of the Ubiquitin-Modified Proteome, Molecular Cell. 44 (2011) 325–340. doi: 10.1016/j.molcel.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ordureau A, Münch C, Harper JW, Quantifying Ubiquitin Signaling, Molecular Cell. 58 (2015) 660–676. doi: 10.1016/j.molcel.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Beaudette P, Popp O, Dittmar G, Proteomic techniques to probe the ubiquitin landscape, PROTEOMICS. 16 (2016) 273–287. doi: 10.1002/pmic.201500290. [DOI] [PubMed] [Google Scholar]
- [4].Pickart CM, Fushman D, Polyubiquitin chains: polymeric protein signals, Current Opinion in Chemical Biology. 8 (2004) 610–616. doi: 10.1016/j.cbpa.2004.09.009. [DOI] [PubMed] [Google Scholar]
- [5].Heride C, Urbé S, Clague MJ, Ubiquitin code assembly and disassembly, Current Biology. 24 (2014) R215–R220. doi: 10.1016/j.cub.2014.02.002. [DOI] [PubMed] [Google Scholar]
- [6].Yau R, Rape M, The increasing complexity of the ubiquitin code, Nature Cell Biology. 18 (2016) 579–586. doi: 10.1038/ncb3358. [DOI] [PubMed] [Google Scholar]
- [7].Oh E, Akopian D, Rape M, Principles of Ubiquitin-Dependent Signaling, Annual Review of Cell and Developmental Biology. 34 (2018) null. doi: 10.1146/annurev-cellbio-100617-062802. [DOI] [PubMed] [Google Scholar]
- [8].Sims JJ, Scavone F, Cooper EM, Kane LA, Youle RJ, Boeke JD, Cohen RE, Polyubiquitin-sensor proteins reveal localization and linkage-type dependence of cellular ubiquitin signaling, Nature Methods. 9 (2012) 303–309. doi: 10.1038/nmeth.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Skaug B, Chen J, Du F, He J, Ma A, Chen ZJ, Direct, Noncatalytic Mechanism of IKK Inhibition by A20, Molecular Cell. 44 (2011) 559–571. doi: 10.1016/j.molcel.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chen J, Chen ZJ, Regulation of NF-κB by ubiquitination, Current Opinion in Immunology. 25 (2013) 4–12. doi: 10.1016/j.coi.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nakasone MA, Livnat-Levanon N, Glickman MH, Cohen RE, Fushman D, Mixed-Linkage Ubiquitin Chains Send Mixed Messages, Structure. 21 (2013) 727–740. doi: 10.1016/j.str.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Emmerich CH, Bakshi S, Kelsall IR, Ortiz-Guerrero J, Shpiro N, Cohen P, Lys63/Met1-hybrid ubiquitin chains are commonly formed during the activation of innate immune signalling, Biochemical and Biophysical Research Communications. 474 (2016) 452–461. doi: 10.1016/j.bbrc.2016.04.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cohen P, Strickson S, The role of hybrid ubiquitin chains in the MyD88 and other innate immune signalling pathways, Cell Death and Differentiation. 24 (2017) 1153–1159. doi: 10.1038/cdd.2017.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Stolz A, Dikic I, Heterotypic Ubiquitin Chains: Seeing is Believing, Trends in Cell Biology. 28 (2018) 1–3. doi: 10.1016/j.tcb.2017.11.005. [DOI] [PubMed] [Google Scholar]
- [15].Malynn BA, Ma A, Ubiquitin makes its mark on immune regulation, Immunity. 33 (2010) 843–852. doi: 10.1016/j.immuni.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Catrysse L, Vereecke L, Beyaert R, van Loo G, A20 in inflammation and autoimmunity, Trends in Immunology. 35 (2014) 22–31. doi: 10.1016/j.it.2013.10.005. [DOI] [PubMed] [Google Scholar]
- [17].Wang S, Wen F, Wiley GB, Kinter MT, Gaffney PM, An Enhancer Element Harboring Variants Associated with Systemic Lupus Erythematosus Engages the TNFAIP3 Promoter to Influence A20 Expression, PLOS Genetics. 9 (2013) e1003750. doi: 10.1371/journal.pgen.1003750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Adrianto I, Wen F, Templeton A, Wiley G, King JB, Lessard CJ, Bates JS, Hu Y, Kelly JA, Kaufman KM, Guthridge JM, Alarcón-Riquelme ME, Anaya J-M, Bae S-C, Bang S-Y, Boackle SA, Brown EE, Petri MA, Gallant C, Ramsey-Goldman R, Reveille JD, Vila LM, Criswell LA, Edberg JC, Freedman BI, Gregersen PK, Gilkeson GS, Jacob CO, James JA, Kamen DL, Kimberly RP, Martin J, Merrill JT, Niewold TB, Park S-Y, Pons-Estel BA, Scofield RH, Stevens AM, Tsao BP, Vyse TJ, Langefeld CD, Harley JB, Moser KL, Webb CF, Humphrey MB, Montgomery CG, Gaffney PM, Association of a functional variant downstream of TNFAIP3 with systemic lupus erythematosus, Nature Genetics. 43 (2011) 253–258. doi: 10.1038/ng.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang S, Wen F, Tessneer KL, Gaffney PM, TALEN-mediated enhancer knockout influences TNFAIP3 gene expression and mimics a molecular phenotype associated with systemic lupus erythematosus, Genes and Immunity. 17 (2016) 165–170. doi: 10.1038/gene.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sokhi UK, Liber MP, Frye L, Park S, Kang K, Pannellini T, Zhao B, Norinsky R, Ivashkiv LB, Gong S, Dissection and function of autoimmunity-associated TNFAIP3 (A20) gene enhancers in humanized mouse models, Nature Communications. 9 (2018) 658. doi: 10.1038/s41467-018-03081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jiang X, Tian H, Fan Y, Chen J, Song Y, Wang S, Zhu F, Guo C, Zhang L, Shi Y, Expression of Tumor Necrosis Factor Alpha-Induced Protein 3 mRNA in Peripheral Blood Mononuclear Cells Negatively Correlates with Disease Severity in Psoriasis Vulgaris, Clin. Vaccine Immunol. 19 (2012) 1938–1942. doi: 10.1128/CVI.00500-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Schuijs MJ, Willart MA, Vergote K, Gras D, Deswarte K, Ege MJ, Madeira FB, Beyaert R, van Loo G, Bracher F, von Mutius E, Chanez P, Lambrecht BN, Hammad H, Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells, Science. 349 (2015) 1106–1110. doi: 10.1126/science.aac6623. [DOI] [PubMed] [Google Scholar]
- [23].Aki A, Nagasaki M, Malynn BA, Ma A, Kagari T, Hypomorphic A20 expression confers susceptibility to psoriasis, PLOS ONE. 12 (2017) e0180481. doi: 10.1371/journal.pone.0180481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Devos M, Mogilenko DA, Fleury S, Gilbert B, Becquart C, Quemener S, Dehondt H, Tougaard P, Staels B, Bachert C, Vandenabeele P, Van Loo G, Staumont-Salle D, Declercq W, Dombrowicz D, Keratinocyte expression of A20/TNFAIP3 controls skin inflammation associated with atopic dermatitis and psoriasis, Journal of Investigative Dermatology. (2018). doi: 10.1016/j.jid.2018.06.191. [DOI] [PubMed] [Google Scholar]
- [25].Arsenescu R, Bruno MEC, Rogier EW, Stefka AT, McMahan AE, Wright TB, Nasser MS, de Villiers WJS, Kaetzel CS, Signature biomarkers in Crohn’s disease: toward a molecular classification, Mucosal Immunology. 1 (2008) 399–411. doi: 10.1038/mi.2008.32. [DOI] [PubMed] [Google Scholar]
- [26].Bruno MEC, Rogier EW, Arsenescu RI, Flomenhoft DR, Kurkjian CJ, Ellis GI, Kaetzel CS, Correlation of Biomarker Expression in Colonic Mucosa with Disease Phenotype in Crohn’s Disease and Ulcerative Colitis, Dig Dis Sci. 60 (2015) 2976–2984. doi: 10.1007/s10620-015-3700-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zaidi D, Huynh HQ, Carroll MW, Baksh S, Wine E, Tumor necrosis factor α-induced protein 3 (A20) is dysregulated in pediatric Crohn disease, Clin Exp Gastroenterol. 11 (2018) 217–231. doi: 10.2147/CEG.S148217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhou Q, Wang H, Schwartz DM, Stoffels M, Park YH, Zhang Y, Yang D, Demirkaya E, Takeuchi M, Tsai WL, Lyons JJ, Yu X, Ouyang C, Chen C, Chin DT, Zaal K, Chandrasekharappa SC, Hanson EP, Yu Z, Mullikin JC, Hasni SA, Wertz IE, Ombrello AK, Stone DL, Hoffmann P, Jones A, Barham BK, Leavis HL, van Royen-Kerkof A, Sibley C, Batu ED, Gül A, Siegel RM, Boehm M, Milner JD, Ozen S, Gadina M, Chae J, Laxer RM, Kastner DL, Aksentijevich I, Loss-of-function mutations in TNFAIP3 leading to A20 haploinsufficiency cause an early-onset autoinflammatory disease, Nature Genetics. 48 (2016) 67–73. doi: 10.1038/ng.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Franco-Jarava C, Wang H, Martin-Nalda A, de la SD Alvarez, M. García-Prat, D. Bodet, V. García-Patos, A. Plaja, F. Rudilla, V. Rodriguez-Sureda, L. García-Latorre, I. Aksentijevich, R. Colobran, P. Soler-Palacín, TNFAIP3 haploinsufficiency is the cause of autoinflammatory manifestations in a patient with a deletion of 13Mb on chromosome 6, Clinical Immunology. 191 (2018) 44–51. doi: 10.1016/j.clim.2018.03.009. [DOI] [PubMed] [Google Scholar]
- [30].Berteau F, Rouviere B, Delluc A, Nau A, Le Berre R, Sarrabay G, Touitou I, de Moreuil C, Autosomic dominant familial Behçet disease and haploinsufficiency A20: A review of the literature, Autoimmunity Reviews. 17 (2018) 809–815. doi: 10.1016/j.autrev.2018.02.012. [DOI] [PubMed] [Google Scholar]
- [31].Aeschlimann FA, Batu ED, Canna SW, Go E, Gül A, Hoffmann P, Leavis HL, Ozen S, Schwartz DM, Stone DL, van Royen-Kerkof A, Kastner DL, Aksentijevich I, Laxer RM, A20 haploinsufficiency (HA20): clinical phenotypes and disease course of patients with a newly recognised NF-kB-mediated autoinflammatory disease, Annals of the Rheumatic Diseases. 77 (2018) 728–735. doi: 10.1136/annrheumdis-2017-212403. [DOI] [PubMed] [Google Scholar]
- [32].Zheng C, Huang Y, Ye Z, Wang Y, Tang Z, Lu J, Wu J, Zhou Y, Wang L, Huang Z, Yang H, Xue A, Infantile Onset Intractable Inflammatory Bowel Disease Due to Novel Heterozygous Mutations in TNFAIP3 (A20), Inflammatory Bowel Diseases. 24 (2018) 2613–2620. doi: 10.1093/ibd/izy165. [DOI] [PubMed] [Google Scholar]
- [33].Novak U, Rinaldi A, Kwee I, Nandula SV, Rancoita PMV, Compagno M, Cerri M, Rossi D, Murty VV, Zucca E, Gaidano G, Dalla-Favera R, Pasqualucci L, Bhagat G, Bertoni F, The NF-κB negative regulator TNFAIP3 (A20) is inactivated by somatic mutations and genomic deletions in marginal zone lymphomas, Blood. 113 (2009) 4918–4921. doi: 10.1182/blood-2008-08-174110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kato M, Sanada M, Kato I, Sato Y, Takita J, Takeuchi K, Niwa A, Chen Y, Nakazaki K, Nomoto J, Asakura Y, Muto S, Tamura A, Iio M, Akatsuka Y, Hayashi Y, Mori H, Igarashi T, Kurokawa M, Chiba S, Mori S, Ishikawa Y, Okamoto K, Tobinai K, Nakagama H, Nakahata T, Yoshino T, Kobayashi Y, Ogawa S, Frequent inactivation of A20 in B-cell lymphomas, Nature. 459 (2009) 712–716. doi: 10.1038/nature07969. [DOI] [PubMed] [Google Scholar]
- [35].Compagno M, Lim WK, Grunn A, Nandula SV, Brahmachary M, Shen Q, Bertoni F, Ponzoni M, Scandurra M, Califano A, Bhagat G, Chadburn A, Dalla-Favera R, Pasqualucci L, Mutations of multiple genes cause deregulation of NF-κB in diffuse large B-cell lymphoma, Nature. 459 (2009) 717–721. doi: 10.1038/nature07968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nocturne G, Boudaoud S, Miceli-Richard C, Viengchareun S, Lazure T, Nititham J, Taylor KE, Ma A, Busato F, Melki J, Lessard CJ, Sivils KL, Dubost J-J, Hachulla E, Gottenberg JE, Lombès M, Tost J, Criswell LA, Mariette X, Germline and somatic genetic variations of TNFAIP3 in lymphoma complicating primary Sjögren’s syndrome, Blood. 122 (2013) 4068–4076. doi: 10.1182/blood-2013-05-503383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Johnsen SJ, Gudlaugsson E, Skaland I, Janssen E. a. M., Jonsson MV, Helgeland L, Berget E, Jonsson R, Omdal R, Low Protein A20 in Minor Salivary Glands is Associated with Lymphoma in Primary Sjögren’s Syndrome, Scandinavian Journal of Immunology. 83 (2016) 181–187. doi: 10.1111/sji.12405. [DOI] [PubMed] [Google Scholar]
- [38].Shao L, Oshima S, Duong B, Advincula R, Barrera J, Malynn BA, Ma A, A20 Restricts Wnt Signaling in Intestinal Epithelial Cells and Suppresses Colon Carcinogenesis, PLOS ONE. 8 (2013) e62223. doi: 10.1371/journal.pone.0062223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chen H, Hu L, Luo Z, Zhang J, Zhang C, Qiu B, Dong L, Tan Y, Ding J, Tang S, Shen F, Li Z, Wang H, A20 suppresses hepatocellular carcinoma proliferation and metastasis through inhibition of Twist1 expression, Molecular Cancer. 14 (2015). doi: 10.1186/s12943-015-0454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ghadban T, Schmidt-Yang M, Uzunoglu FG, Perez DR, El Gammal AT, Miro JT, Wellner U, Pantel K, Izbicki JR, Vashist YK, Evaluation of the germline single nucleotide polymorphism rs583522 in the TNFAIP3 gene as a prognostic marker in esophageal cancer, Cancer Genetics. 208 (2015) 595–601. doi: 10.1016/j.cancergen.2015.09.008. [DOI] [PubMed] [Google Scholar]
- [41].Pitt SC, Hernandez RA, Nehs MA, Gawande AA, Moore FD, Ruan DT, Cho NL, Identification of Novel Oncogenic Mutations in Thyroid Cancer, Journal of the American College of Surgeons. 222 (2016) 1036–1043.e2. doi: 10.1016/j.jamcollsurg.2015.12.047. [DOI] [PubMed] [Google Scholar]
- [42].Zheng H, Dai W, Cheung AKL, Ko JMY, Kan R, Wong BWY, Leong MML, Deng M, Kwok TCT, Chan JY-W, Kwong DL-W, Lee AW-M, Ng WT, Ngan RKC, Yau CC, Tung S, Lee VH, Lam K-O, Kwan CK, Li WS, Yau S, Chan K-W, Lung ML, Whole-exome sequencing identifies multiple loss-of-function mutations of NF-κB pathway regulators in nasopharyngeal carcinoma, Proceedings of the National Academy of Sciences. 113 (2016) 11283–11288. doi: 10.1073/pnas.1607606113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lee E, Ouzounova M, Piranlioglu R, Ma MT, Guzel M, Marasco D, Chadli A, Gestwicki JE, Cowell JK, Wicha MS, Hassan KA, Korkaya H, The pleiotropic effects of TNFα in breast cancer subtypes is regulated by TNFAIP3/A20, Oncogene. (2018). doi: 10.1038/s41388-018-0472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Opipari AW, Hu HM, Yabkowitz R, Dixit VM, The A20 zinc finger protein protects cells from tumor necrosis factor cytotoxicity., J. Biol. Chem 267 (1992) 12424–12427. [PubMed] [Google Scholar]
- [45].Komander D, Barford D, Structure of the A20 OTU domain and mechanistic insights into deubiquitination, Biochemical Journal. 409 (2008) 77–85. doi: 10.1042/BJ20071399. [DOI] [PubMed] [Google Scholar]
- [46].Lee EG, Boone DL, Chai S, Libby SL, Chien M, Lodolce JP, Ma A, Failure to Regulate TNF-Induced NF-κB and Cell Death Responses in A20-Deficient Mice, Science. 289 (2000) 2350. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Tavares RM, Turer EE, Liu CL, Advincula R, Scapini P, Rhee L, Barrera J, Lowell CA, Utz PJ, Malynn BA, Ma A, The ubiquitin modifying enzyme A20 restricts B cell survival and prevents autoimmunity, Immunity. 33 (2010) 181–191. doi: 10.1016/j.immuni.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hövelmeyer N, Reissig S, Xuan NT, Adams‐Quack P, Lukas D, Nikolaev A, Schlüter D, Waisman A, A20 deficiency in B cells enhances B-cell proliferation and results in the development of autoantibodies, European Journal of Immunology. 41 (2011) 595–601. doi: 10.1002/eji.201041313. [DOI] [PubMed] [Google Scholar]
- [49].Chu Y, Vahl JC, Kumar D, Heger K, Bertossi A, Wójtowicz E, Soberon V, Schenten D, Mack B, Reutelshöfer M, Beyaert R, Amann K, van Loo G, Schmidt-Supprian M, B cells lacking the tumor suppressor TNFAIP3/A20 display impaired differentiation and hyperactivation and cause inflammation and autoimmunity in aged mice, Blood. 117 (2011) 2227–2236. doi: 10.1182/blood-2010-09-306019. [DOI] [PubMed] [Google Scholar]
- [50].Giordano M, Roncagalli R, Bourdely P, Chasson L, Buferne M, Yamasaki S, Beyaert R, van Loo G, Auphan-Anezin N, Schmitt-Verhulst A-M, Verdeil G, The tumor necrosis factor alpha-induced protein 3 (TNFAIP3, A20) imposes a brake on antitumor activity of CD8 T cells, PNAS. 111 (2014) 11115–11120. doi: 10.1073/pnas.1406259111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Onizawa M, Oshima S, Schulze-Topphoff U, Oses-Prieto JA, Lu T, Tavares R, Prodhomme T, Duong B, Whang MI, Advincula R, Agelidis A, Barrera J, Wu H, Burlingame A, Malynn BA, Zamvil SS, Ma A, The ubiquitin-modifying enzyme A20 restricts ubiquitination of the kinase RIPK3 and protects cells from necroptosis, Nature Immunology. 16 (2015) 618–627. doi: 10.1038/ni.3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Matsuzawa Y, Oshima S, Takahara M, Maeyashiki C, Nemoto Y, Kobayashi M, Nibe Y, Nozaki K, Nagaishi T, Okamoto R, Tsuchiya K, Nakamura T, Ma A, Watanabe M, TNFAIP3 promotes survival of CD4 T cells by restricting MTOR and promoting autophagy, Autophagy. 11 (2015) 1052–1062. doi: 10.1080/15548627.2015.1055439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Just S, Nishanth G, Buchbinder JH, Wang X, Naumann M, Lavrik I, Schlüter D, A20 Curtails Primary but Augments Secondary CD8+ T Cell Responses in Intracellular Bacterial Infection, Sci Rep. 6 (2016). doi: 10.1038/srep39796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Fischer JC, Otten V, Kober M, Drees C, Rosenbaum M, Schmickl M, Heidegger S, Beyaert R, van Loo G, Li XC, Peschel C, Schmidt-Supprian M, Haas T, Spoerl S, Poeck H, A20 Restrains Thymic Regulatory T Cell Development, The Journal of Immunology. 199 (2017) 2356–2365. doi: 10.4049/jimmunol.1602102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Drennan MB, Govindarajan S, Verheugen E, Coquet JM, Staal J, McGuire C, Taghon T, Leclercq G, Beyaert R, van Loo G, Lambrecht BN, Elewaut D, NKT sublineage specification and survival requires the ubiquitin-modifying enzyme TNFAIP3/A20, Journal of Experimental Medicine. 213 (2016) 1973–1981. doi: 10.1084/jem.20151065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Coornaert B, Baens M, Heyninck K, Bekaert T, Haegman M, Staal J, Sun L, Chen ZJ, Marynen P, Beyaert R, T cell antigen receptor stimulation induces MALT1 paracaspase–mediated cleavage of the NF-κB inhibitor A20, Nature Immunology. 9 (2008) 263–271. doi: 10.1038/ni1561. [DOI] [PubMed] [Google Scholar]
- [57].Boone DL, Turer EE, Lee EG, Ahmad R-C, Wheeler MT, Tsui C, Hurley P, Chien M, Chai S, Hitotsumatsu O, McNally E, Pickart C, Ma A, The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses, Nature Immunology. 5 (2004) 1052–1060. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- [58].Turer EE, Tavares RM, Mortier E, Hitotsumatsu O, Advincula R, Lee B, Shifrin N, Malynn BA, Ma A, Homeostatic MyD88-dependent signals cause lethal inflamMation in the absence of A20, Journal of Experimental Medicine. 205 (2008) 451–464. doi: 10.1084/jem.20071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hitotsumatsu O, Ahmad R-C, Tavares R, Wang M, Philpott D, Turer EE, Lee BL, Shiffin N, Advincula R, Malynn BA, Werts C, Ma A, The Ubiquitin-Editing Enzyme A20 Restricts Nucleotide-Binding Oligomerization Domain Containing 2-Triggered Signals, Immunity. 28 (2008) 381–390. doi: 10.1016/j.immuni.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hammer GE, Turer EE, Taylor KE, Fang CJ, Advincula R, Oshima S, Barrera J, Huang EJ, Hou B, Malynn BA, Reizis B, DeFranco A, Criswell LA, Nakamura MC, Ma A, Expression of A20 by dendritic cells preserves immune homeostasis and prevents colitis and spondyloarthritis, Nature Immunology. 12 (2011) 1184–1193. doi: 10.1038/ni.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kool M, van Loo G, Waelput W, De Prijck S, Muskens F, Sze M, van Praet J, Branco-Madeira F, Janssens S, Reizis B, Elewaut D, Beyaert R, Hammad H, Lambrecht BN, The Ubiquitin-Editing Protein A20 Prevents Dendritic Cell Activation, Recognition of Apoptotic Cells, and Systemic Autoimmunity, Immunity. 35 (2011) 82–96. doi: 10.1016/j.immuni.2011.05.013. [DOI] [PubMed] [Google Scholar]
- [62].Hong B, Song X-T, Rollins L, Berry L, Huang XF, Chen S-Y, Mucosal and systemic anti-HIV immunity controlled by A20 in mouse dendritic cells, J Clin Invest. 121 (2011) 739–751. doi: 10.1172/JCI42656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Matmati M, Jacques P, Maelfait J, Verheugen E, Kool M, Sze M, Geboes L, Louagie E, Guire CM, Vereecke L, Chu Y, Boon L, Staelens S, Matthys P, Lambrecht BN, Schmidt-Supprian M, Pasparakis M, Elewaut D, Beyaert R, van Loo G, A20 (TNFAIP3) deficiency in myeloid cells triggers erosive polyarthritis resembling rheumatoid arthritis, Nature Genetics. 43 (2011) 908–912. doi: 10.1038/ng.874. [DOI] [PubMed] [Google Scholar]
- [64].Walle LV, Van Opdenbosch N, Jacques P, Fossoul A, Verheugen E, Vogel P, Beyaert R, Elewaut D, Kanneganti T-D, van Loo G, Lamkanfi M, Negative regulation of the NLRP3 inflammasome by A20 protects against arthritis, Nature. 512 (2014) 69–73. doi: 10.1038/nature13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Voet S, Mc Guire C, Hagemeyer N, Martens A, Schroeder A, Wieghofer P, Daems C, Staszewski O, Vande Walle L, Jordao MJC, Sze M, Vikkula H-K, Demeestere D, Van Imschoot G, Scott CL, Hoste E, Gonçalves A, Guilliams M, Lippens S, Libert C, Vandenbroucke RE, Kim K-W, Jung S, Callaerts-Vegh Z, Callaerts P, de Wit J, Lamkanfi M, Prinz M, van Loo G, A20 critically controls microglia activation and inhibits inflammasome-dependent neuroinflammation, Nature Communications. 9 (2018). doi: 10.1038/s41467-018-04376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Valdearcos M, Douglass JD, Robblee MM, Dorfman MD, Stifler DR, Bennett ML, Gerritse I, Fasnacht R, Barres BA, Thaler JP, Koliwad SK, Microglial Inflammatory Signaling Orchestrates the Hypothalamic Immune Response to Dietary Excess and Mediates Obesity Susceptibility, Cell Metabolism. 26 (2017) 185–197.e3. doi: 10.1016/j.cmet.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Schneider C, O’Leary CE, von Moltke J, Liang H-E, Ang QY, Turnbaugh PJ, Radhakrishnan S, Pellizzon M, Ma A, Locksley RM, A Metabolite-Triggered Tuft Cell-ILC2 Circuit Drives Small Intestinal Remodeling, Cell. 174 (2018) 271–284.e14. doi: 10.1016/j.cell.2018.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Heger K, Fierens K, Vahl JC, Aszodi A, Peschke K, Schenten D, Hammad H, Beyaert R, Saur D, van Loo G, Roers A, Lambrecht BN, Kool M, Schmidt-Supprian M, A20-Deficient Mast Cells Exacerbate Inflammatory Responses In Vivo, PLOS Biology. 12 (2014) e1001762. doi: 10.1371/journal.pbio.1001762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Vereecke L, Sze M, Guire CM, Rogiers B, Chu Y, Schmidt-Supprian M, Pasparakis M, Beyaert R, van Loo G, Enterocyte-specific A20 deficiency sensitizes to tumor necrosis factor–induced toxicity and experimental colitis, Journal of Experimental Medicine. 207 (2010) 1513–1523. doi: 10.1084/jem.20092474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Vereecke L, Vieira-Silva S, Billiet T, van Es JH, Guire CM, Slowicka K, Sze M, van den Born M, Hertogh GD, Clevers H, Raes J, Rutgeerts P, Vermeire S, Beyaert R, van Loo G, A20 controls intestinal homeostasis through cell-specific activities, Nature Communications. 5 (2014) 5103. doi: 10.1038/ncomms6103. [DOI] [PubMed] [Google Scholar]
- [71].Kattah MG, Shao L, Rosli YY, Shimizu H, Whang MI, Advincula R, Achacoso P, Shah S, Duong BH, Onizawa M, Tanbun P, Malynn BA, Ma A, A20 and ABIN-1 synergistically preserve intestinal epithelial cell survival, J Exp Med. 215 (2018) 1839. doi: 10.1084/jem.20180198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Stein MM, Hrusch CL, Gozdz J, Igartua C, Pivniouk V, Murray SE, Ledford JG, Marques dos Santos M, Anderson RL, Metwali N, Neilson JW, Maier RM, Gilbert JA, Holbreich M, Thorne PS, Martinez FD, von Mutius E, Vercelli D, Ober C, Sperling AI, Innate Immunity and Asthma Risk in Amish and Hutterite Farm Children, New England Journal of Medicine. 375 (2016) 411–421. doi: 10.1056/NEJMoa1508749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Lippens S, Lefebvre S, Gilbert B, Sze M, Devos M, Verhelst K, Vereecke L, Guire CM, Guérin C, Vandenabeele P, Pasparakis M, Mikkola ML, Beyaert R, Declercq W, van Loo G, Keratinocyte-specific ablation of the NF-κB regulatory protein A20 (TNFAIP3) reveals a role in the control of epidermal homeostasis, Cell Death and Differentiation. 18 (2011) 1845–1853. doi: 10.1038/cdd.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Wertz IE, O’Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S, Wu P, Wiesmann C, Baker R, Boone DL, Ma A, Koonin EV, Dixit VM, De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-κB signalling, Nature. 430 (2004) 694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- [75].O’Donnell MA, Legarda-Addison D, Skountzos P, Yeh WC, Ting AT, Ubiquitination of RIP1 Regulates an NF-κB-Independent Cell-Death Switch in TNF Signaling, Current Biology. 17 (2007) 418–424. doi: 10.1016/j.cub.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Duong BH, Onizawa M, Oses-Prieto JA, Advincula R, Burlingame A, Malynn BA, Ma A, A20 Restricts Ubiquitination of Pro-Interleukin-1β Protein Complexes and Suppresses NLRP3 Inflammasome Activity, Immunity. 42 (2015) 55–67. doi: 10.1016/j.immuni.2014.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Lu TT, Onizawa M, Hammer GE, Turer EE, Yin Q, Damko E, Agelidis A, Shifrin N, Advincula R, Barrera J, Malynn BA, Wu H, Ma A, Dimerization and Ubiquitin Mediated Recruitment of A20, a Complex Deubiquitinating Enzyme, Immunity. 38 (2013) 896–905. doi: 10.1016/j.immuni.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Wertz IE, Newton K, Seshasayee D, Kusam S, Lam C, Zhang J, Popovych N, Helgason E, Schoeffler A, Jeet S, Ramamoorthi N, Kategaya L, Newman RJ, Horikawa K, Dugger D, Sandoval W, Mukund S, Zindal A, Martin F, Quan C, Tom J, Fairbrother WJ, Townsend M, Warming S, DeVoss J, Liu J, Dueber E, Caplazi P, Lee WP, Goodnow CC, Balazs M, Yu K, Kolumam G, Dixit VM, Phosphorylation and linear ubiquitin direct A20 inhibition of inflammation, Nature. 528 (2015) 370–375. doi: 10.1038/nature16165. [DOI] [PubMed] [Google Scholar]
- [79].De A, Dainichi T, Rathinam CV, Ghosh S, The deubiquitinase activity of A20 is dispensable for NF- B signaling, EMBO Reports. 15 (2014) 775–783. doi: 10.15252/embr.201338305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Shembade N, Ma A, Harhaj EW, Inhibition of NF-kB Signaling by A20 Through Disruption of Ubiquitin Enzyme Complexes, 327 (2010) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Kattah MG, Malynn BA, Ma A, Ubiquitin-Modifying Enzymes and Regulation of the Inflammasome, Journal of Molecular Biology. 429 (2017) 3471–3485. doi: 10.1016/j.jmb.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Heyninck K, Kreike MM, Beyaert R, Structure–function analysis of the A20-binding inhibitor of NF-κB activation, ABIN-1, FEBS Letters. 536 (2003) 135–140. doi: 10.1016/S0014-5793(03)00041-3. [DOI] [PubMed] [Google Scholar]
- [83].Shembade N, Harhaj NS, Liebl DJ, Harhaj EW, Essential role for TAX1BP1 in the termination of TNF-α-, IL-1- and LPS-mediated NF-κB and JNK signaling, The EMBO Journal. 26 (2007) 3910–3922. doi: 10.1038/sj.emboj.7601823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Iha H, Peloponese J-M, Verstrepen L, Zapart G, Ikeda F, Smith CD, Starost MF, Yedavalli V, Heyninck K, Dikic I, Beyaert R, Jeang K-T, Inflammatory cardiac valvulitis in TAX1BP1-deficient mice through selective NF-κB activation, The EMBO Journal. 27 (2008) 629–641. doi: 10.1038/emboj.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Oshima S, Turer EE, Callahan JA, Chai S, Advincula R, Barrera J, Shifrin N, Lee B, Yen B, Woo T, Malynn BA, Ma A, ABIN-1 is a ubiquitin sensor that restricts cell death and sustains embryonic development, Nature. 457 (2009) 906–909. doi: 10.1038/nature07575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Evans PC, Ovaa H, Hamon M, Kilshaw PJ, Hamm S, Bauer S, Ploegh HL, Smith TS, Zinc-finger protein A20, a regulator of inflammation and cell survival, has de-ubiquitinating activity., Biochem J. 378 (2004) 727–734. doi: 10.1042/BJ20031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Hutti JE, Turk BE, Asara JM, Ma A, Cantley LC, Abbott DW, I B Kinase Phosphorylates the K63 Deubiquitinase A20 To Cause Feedback Inhibition of the NF- B Pathway, Molecular and Cellular Biology. 27 (2007) 7451–7461. doi: 10.1128/MCB.01101-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Garcia-Carbonell R, Wong J, Kim JY, Close LA, Boland BS, Wong TL, Harris PA, Ho SB, Das S, Ernst PB, Sasik R, Sandborn WJ, Bertin J, Gough PJ, Chang JT, Kelliher M, Boone D, Guma M, Karin M, Elevated A20 promotes TNF-induced and RIPK1-dependent intestinal epithelial cell death, MEDICAL SCIENCES. (n.d.) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]