Abstract
Background/objectives:
Inadequate sleep increases obesity and environmental noise contributes to poor sleep. However, women may be more vulnerable to noise and hence more susceptible to sleep disruption-induced weight gain than men. In male rats, exposure to environmental (i.e. ambient) noise disrupts sleep and increases feeding and weight gain. However, the effects of environmental noise on sleep and weight gain in female rats are unknown. Thus, this study was designed to determine whether noise exposure would disturb sleep, increase feeding and weight gain and alter the length of the estrous cycle in female rats.
Subjects/methods:
Female rats (12-weeks old) were exposed to noise for 17d (8h/d during the light period) to determine the effects of noise on weight gain and food intake. In a separate set of females, estrous cycle phase and length, EEG, EMG, spontaneous physical activity and energy expenditure were recorded continuously for 27d during baseline (control, 9d), noise exposure (8h/d, 9d) and recovery (9d) from sleep disruption.
Results:
Noise exposure significantly increased weight gain and food intake compared to females that slept undisturbed. Noise also significantly increased wakefulness, reduced sleep and resulted in rebound sleep during the recovery period. Total energy expenditure was significantly lower during both noise exposure and recovery due to lower energy expenditure during spontaneous physical activity and sleep. Notably, noise did not alter the estrous cycle length.
Conclusions:
As previously observed in male rats, noise exposure disrupted sleep and increased weight gain in females but did not alter the length of the estrous cycle. This is the first demonstration of weight gain in female rats during sleep disruption. We conclude that the sleep disruption caused by exposure to environmental noise is a significant tool for determining how sleep loss contributes to obesity in females.
Keywords: arousal, brain, obesity, sleep, estrous
INTRODUCTION
The prevalence of inadequate sleep has increased (1), which increases the risk for weight gain (2). Noise is an environmental factor that disrupts sleep and metabolism (3–5). In a population sensitive to noise, obesity risk is greater among women (4) and it is women (but not men) who report lower sleep quality (6). Women with short sleep also display a greater likelihood of weight gain (7). Thus, women exhibiting sleep disruption (SD) have a predisposition for obesity and noise exposure can mediate this relationship.
Experimental sleep restriction (SR) promotes weight gain in women (8–10) though the underlying mechanisms are unknown. In women, SR increased calorie intake (8–11) but its effects on energy expenditure (EE) are contradictory since total EE (TEE) increased (8, 12) or remained unchanged (10). Moreover, although SR reduced resting metabolic rate (11) other studies report no change to this component of TEE (13), sleeping metabolic rate (12) or physical activity (10).
The discrepancies between these studies underscore the importance of developing an animal model for weight gain due to SD in females. Studies in non-ovariectomized female rodents report weight loss after sleep deprivation (14–16), and thus disagree with the studies of SR in women (8–10). Furthermore, despite reports in males (17) and ovariectomized females (18), the effect of weight-promoting methods of SD on energy intake or EE have yet to be evaluated in non-ovariectomized females throughout the estrous cycle. The estrous cycle adds complexity to any rodent study in females but is critical for studies focusing on sleep and weight gain because sleep (20, 23, 24) and TEE (25, 26) vary across the estrous cycle. A previous study showed that sleep deprivation by the multiple platform method disrupted estrous cycle length (19), but whether this method disrupts sleep across all phases of the estrous cycle remains unknown. In female rodents, propensity to sleep and sleep time increase during a recovery period after sleep deprivation by the gentle handling or multiple platform methods (20, 21). Yet sleep during the deprivation period was either not reported or tested in all phases of the estrous cycle (20, 21). Exposure to the sweeping bar method of SD reduced sleep during proestrus and diestrus only without altering estrous cycle length (22). Since previously tested methods of sleep deprivation elicit weight loss in female rodents, they do not mirror the human condition. Thus the lack of a pre-clinical model for weight gain due to SD in women hinders efforts to determine the mechanisms that alter sleep and energy balance to promote weight gain.
We established a rodent model of SD-induced weight gain in male rats whereby environmental noise exposure resulted in SD, hyperphagia, increased weight gain and reduced spontaneous physical activity (SPA), TEE and its components (27–29). Here, we sought to validate this methodology for SD in non-ovariectomized female rats. We hypothesized that environmental noise exposure in female rats would result in SD, as indicated by increased time spent awake, sleep fragmentation and sleep propensity and also would stimulate weight gain due to hyperphagia and reduced TEE and its components.
METHODS
Animals:
Three-month old female Sprague-Dawley rats (N = 26, Charles River Laboratories, Kingston, NY USA) were housed individually in plexiglass cages with a perforated plexiglass floor (21-22 °C, 12h light/12h dark periods, lights-on 0600h). Rodent chow (Harlan Teklad 8604) and water were allowed ad libitum. Procedures were approved by the University of Arizona Institutional Animal Care and Use Committee.
Surgery:
Rats were surgically implanted with a radiotelemetric transmitter connected to EEG and EMG leads (F40-EET, Data Sciences International, Saint Paul, MN USA) for determination of vigilance states (30). Experiments began 10d post-surgery.
Determining vigilance states:
Wake, non-rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep were manually scored from 15-sec. epochs of EEG and EMG (30). Time spent in vigilance states (WAKETIME, SLEEPTIME, NREMTIME and REMTIME) and indicators of sleep fragmentation including the number and duration of episodes, transitions between vigilance states, and NREM delta power, an indicator of sleep propensity (31) were calculated as described (30, 32).
Concurrent EE, SPA, and vigilance states:
EEG and EMG signals were captured by radiotelemetry (30). EE was determined with a pull-mode open circuit indirect calorimeter that measured O2, CO2, and water vapor continuously every second from each chamber and spontaneous physical activity (SPA), indicated by distance traveled, was determined from infrared beam break sensors (Promethion-C, Expedata v1.9.13, Sable Systems Inc. Las Vegas, NV USA). Thus, EEG, EMG, SPA and EE were measured concurrently (33, 34). TEE and components of TEE (EE during SPA, rest, sleep (NREM+REM), NREM, and REM) were calculated from time-stamped EEG/EMG, SPA, and TEE (27, 33). For each component, total calories (kcal, SPAEE, RestEE, SleepEE, NREMEE and REMEE) and the metabolic rate (kcal/h, SPAEE-MR, RestEE-MR, SleepEE-MR, NREMEE-MR, REMEE-MR) were calculated since a change in the EE of a specific component can be due to a change in the rate of energy utilization (i.e. metabolic rate) with or without a change in the amount of time spent in that component (27).
Sleep disruption by noise exposure:
Rats were exposed to pre-recorded noise (8h/d, beginning at 0700) during the light period by placing two speakers in front of the cages; this has been validated to disrupt sleep in male rats (27, 28). A 15 min. recording of noises (random street noises, vehicle horn, ambulance siren, hammering, bell, etc.) was repeated for 8h. To prevent habituation, the noise events, duration of these events, frequency range of sound (800-20000 Hz), amplitude (65-100 dB, with average intensity of 85 db) and inter-noise interval (1-26 sec.) were randomly distributed. The recording also contained periods of silence followed by a sharp attack rate (85-100 dB) with noises randomly distributed in the sound sequence. The frequency range of the recording (800-20000 Hz) was matched to the rat audiogram (Audacity https://www.audacityteam.org/). The noises were selected from the Best Service Studio Box DVD3-Technical sample library (Best Service GmbH, Munchen, Germany).
Experimental Design:
Female rats were randomized by body weight to sleep undisturbed (control) or be exposed to noise (8h/d, light period) for 17d (n=10/group). Body weight and food intake, corrected for uneaten food, were measured manually every 48h. A separate group of females (N=6) were implanted with EEG/EMG leads. Vaginal smears were collected for 12d following the post-surgical recovery period to validate normal estrous cycles (35). Subsequently, rats were acclimated to indirect calorimetry chambers for 3d prior to a 27d period (9d baseline-control (i.e. undisturbed sleep-wake), 9d of noise exposure (8h/d, light period) and 9d of recovery (i.e. undisturbed sleep-wake) (27, 28). SPA, EE, and EEG/EMG were measured continuously for the 27d. Vaginal smears were performed daily (0600–0700h) to determine estrous cycle phase (proestrus, estrus, diestrus-1 and −2) and length (35) (Figure 1). Experiments were conducted once.
Figure 1. Female rats maintained 4-5d estrous cycles during and after noise-induced SD.
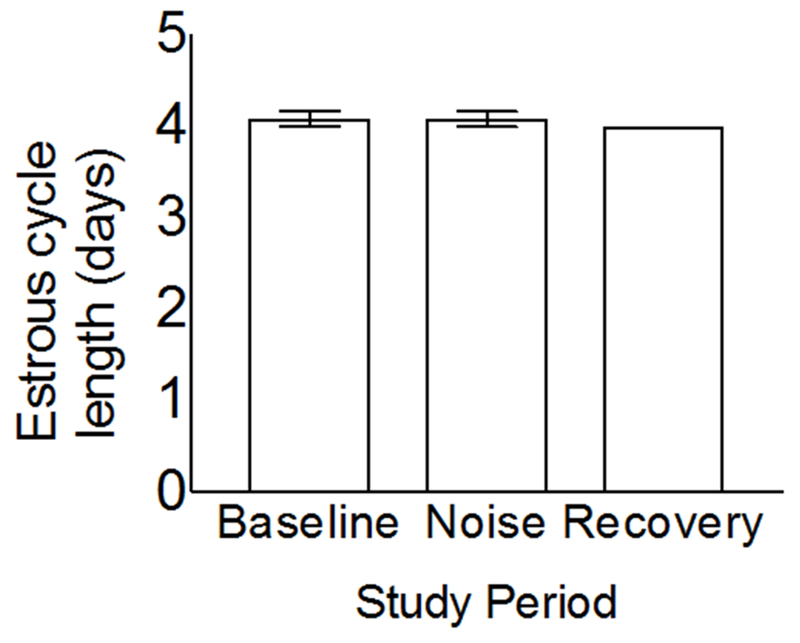
Vaginal smears were performed daily (0600-0700h) to determine estrous cycle phase (i.e. proestrus, estrus, diestrus 1 and −2) and the length of the estrous cycle during 9d of undisturbed sleep (i.e. control), 9d of noise exposure (8h/d during the light period), and 9d of recovery. The result for recovery lacks an error bar because all rats had 4 day cycles. Significant differences (P < 0.05) were determined by ANOVA. Data expressed as mean ± SEM; N = 6.
Statistics:
Unblinded data were analyzed with Prism 7.0d (GraphPad, San Diego, CA USA) and are expressed as mean±SEM. Alpha was 0.05 for statistical tests, normality was verified with the Shapiro-Wilk test and all t-tests were two-sided. Sample size was based on our prior report (28). Separate analyses were completed for each time period (light period, dark period and 24h) based on our previous data which showed that SD reduced sleep during both the 8h period of noise exposure, the 12h light cycle and the 24h period (28). Despite the fact that rats were only exposed to noise during 8h of the 12h light period, compensatory rebound sleep occurs immediately after SD, so noise exposed rats sleep more relative to baseline for the 4h in the light cycle following noise exposure. A more conservative analysis is therefore to test for significance over the 12h light cycle rather than over the 8h period of noise exposure. The effects of noise exposure on weight gain and feeding were determined with two-way ANOVA followed by multiple comparisons with false discovery rate (FDR) correction (36). The effect of estrous cycle phase on endpoints before SD was determined with repeated measures ANOVA followed by multiple comparisons with FDR correction (36). To determine the effect of noise exposure and recovery on vigilance states and EE while controlling for the effect of estrous cycle phase, the change from control (during both noise exposure and recovery) was calculated by subtracting daily values within the same phase of the estrous cycle. Cumulative change was then calculated as the sum of the change from control for each rat over the 9d period of noise exposure or the 9d or 3d periods of recovery. Subsequently, endpoints were analyzed with one sample t-tests for the null hypothesis of no change relative to control. Vigilance state data from one rat were excluded due to transmitter malfunction.
RESULTS
Noise exposure stimulates weight gain and hyperphagia in female rats:
We first tested whether noise exposure increased weight gain and feeding. Compared to females that slept undisturbed, noise exposure significantly increased weight gain (Figure 2A, treatment: F(1,18) = 9.2, P<0.007; time: F(8,144) = 36.3, P<0.0001; time x treatment: F(8,144) = 2.7, P<0.008) and feeding (Figure 2B, treatment: F(1,18) = 11.0, P<0.004; time F(8,144) = 3793, P<0.0001; time x treatment: F(8,144) = 6.1, P<0.0001). Hence, noise exposure stimulated weight gain and hyperphagia.
Figure 2. Noise exposure increases weight gain and food intake in female rats.
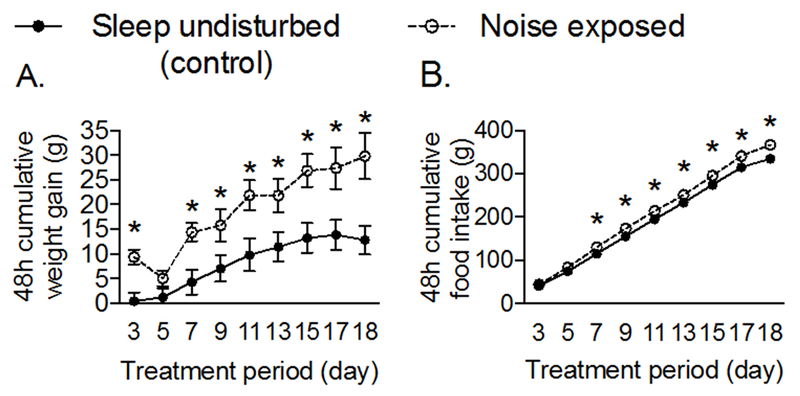
Female Sprague-Dawley rats (12-weeks old) were allowed to sleep undisturbed or exposed to pre-recorded noise (8h/d for 17d during the light period). (A) Weight gain and (B) food intake were determined every 48h and are presented as cumulative changes from day 1 of treatment. Significant differences (P < 0.05) were determined by two factor ANOVA followed by multiple comparison tests corrected for the FDR. *P < 0.05 compared to undisturbed controls for a specific treatment day. Data represent mean ± SEM. n = 10/group.
Vigilance states, SPA and EE vary across phases of the estrous cycle during undisturbed sleep-wake:
For the SD validation study, we first tested whether vigilance states, SPA, TEE and its components varied across phases of the estrous cycle before SD (20, 23–26, 37). The duration of vigilance states and several indicators of sleep fragmentation differed across phases of the estrous cycle during all time periods with proestrus exhibiting the greatest difference compared to the other phases of the estrous cycle (Figure S1A–S1I). SPA, TEE, and its components also differed significantly across the phases before SD. Over the dark and 24h periods, SPA and TEE were significantly higher during proestrus than other phases with the exception of TEE over the light and 24h periods (Figure S1J–S1K, P<0.05). During the dark period, higher TEE in proestrus was due to higher SPAEE, as RESTEE was similar across phases while NREMEE and REMEE were significantly lower in proestrus compared to the other phases (Figure S1L–S1N, P<0.05). During the dark period, SPAEE-MR was also significantly higher in proestrus compared to diestrus-1 (Figure S1O, P=0.01), while RESTEE-MR was significantly higher in proestrus than diestrus-1 and diestrus-2 (Figure S1P, P=0.02 for both). In contrast, NREMEE-MR and REMEE-MR were significantly higher in proestrus than other phases during the dark period (Figure S1R-S, P<0.05). Collectively, these data demonstrate that parameters which regulate weight gain and are affected by noise-induced SD in males (27, 28), differ across all phases of the estrous cycle.
Noise exposure increases time spent awake and sleep fragmentation:
We next tested whether noise exposure increased WAKETIME and sleep fragmentation. During the light period, noise significantly increased WAKETIME and decreased SLEEPTIME due to reductions in NREMTIME and REMTIME (Figure 3A, P<0.05). In contrast, during the dark period, rebound sleep occurred, as indicated by significantly reduced WAKETIME and increased SLEEPTIME due to prolonged REMTIME (Figure 3B, P<0.05). Yet, over 24h, WAKETIME was still significantly increased (Figure 3C, P=0.01). In conclusion, noise increased wake duration over the 24h period.
Figure 3. Noise exposure increases time spent awake and sleep fragmentation.
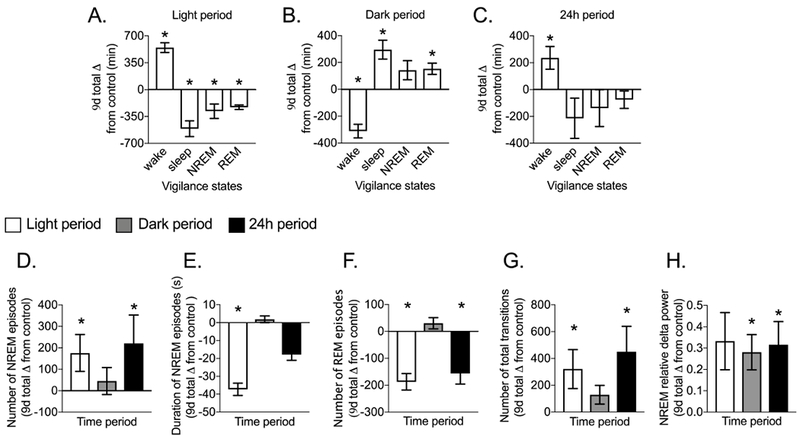
Female Sprague-Dawley rats (12-weeks old) were implanted with EEG/EMG leads. EEG and EMG signals were measured continuously for 9d before and 9d during noise exposure (8h/d during the light period). (A-C) Cumulative change from control for time spent in wake, sleep (NREM + REM), NREM and REM sleep and measures of sleep fragmentation including the (D-F) number and mean duration of NREM and REM episodes, (G) total transitions between vigilance states, and (H) sleep propensity indicated by NREM delta power during the light, dark, and 24h periods. During the first 24h of noise exposure, time spent in wake (P < 0.0001) was significantly higher while sleep (P < 0.0001) and NREM sleep (P < 0.0001) but not REM sleep (P = 0.78) were significantly lower compared to control. For each rat, change from control during noise exposure was calculated by subtracting values within the same phase of the estrous cycle. Significant differences (P < 0.05) were determined with one sample t-tests. Data expressed as mean ± SEM; N = 5. *P < 0.05.
Noise also increased sleep fragmentation during the light and 24h periods. This was due to significantly more and shorter episodes of NREM during the light period, fewer episodes of REM during both the light and 24h periods, and significantly more transitions between vigilance states during the light and 24h periods (Figure 3D-G, P<0.05). Noise also significantly increased NREM delta power over 24h, which was due to higher NREM delta power during the dark (Figure 3H, P<0.05) and light periods (P=0.06). Therefore, SD caused by noise was due to reduced sleep duration and increased sleep fragmentation.
Noise-induced SD reduces TEE:
We then tested whether SD due to noise exposure reduced SPA, TEE and its components. Noise-induced SD significantly decreased TEE during the dark (P=0.009) and 24h periods (Figure 4A, P=0.03). The reduction in TEE was due to several components (Figure 4B-D) including lower NREMEE (P=0.001) and REMEE (P=0.003) during the light period, SPAEE during the dark period (P=0.03), and NREMEE (P=0.003) and SPAEE-MR (−0.09±0.03, P=0.03) during the 24h period. Despite lower SPAEE and SPAEE-MR, SPA itself was not significantly different from control during all time periods (P>0.05, data not shown). Thus, noise-induced SD reduced TEE during the dark and 24h periods, due to lower EE during SPA and NREM sleep.
Figure 4. Noise-induced SD reduces total and individual components of total EE.
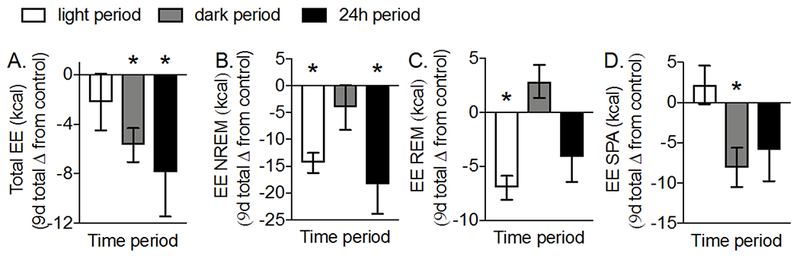
(A) TEE and components of TEE including the EE during (B) NREM, and (C) REM sleep, and (D) SPA were measured continuously for 9d before and 9d during noise exposure (8h/d) in female Sprague-Dawley rats (12-weeks old). During the first 24h of noise exposure, TEE and EE during NREM sleep were significantly lower (P = 0.04 and P = 0.01, respectively) while EE during REM sleep (P = 0.05) and SPA (P = 0.41) were similar compared to control levels. For each rat, the cumulative change from control during noise-induced SD was calculated by subtracting values within the same phase of the estrous cycle. Significant differences (P < 0.05) were determined with one sample t-tests. Data represent mean ± SEM; N = 5–6. *P < 0.05
Noise-induced SD causes rebound sleep:
Since noise resulted in SD, we tested whether SD elicited a compensatory increase in sleep during the recovery period. As expected, SLEEPTIME during recovery was significantly greater during the dark (P=0.001) and 24h periods (Figure 5A-C, P=0.02). This was due to significantly prolonged NREMTIME in the dark period (P=0.04) and significantly less WAKETIME during the dark (P=0.01) and 24h (P=0.02) periods. Noise-induced sleep fragmentation normalized during recovery. This was indicated by significantly fewer episodes of wake during the light period and fewer NREM episodes during the light and 24h periods, in addition to significantly longer episodes of NREM and REM during all time periods (Figure 5D-G, P<0.05). Furthermore, we note significantly fewer transitions between vigilance states and significantly higher NREM delta power during the light and 24h periods (Figure 5H-I, P<0.05). Thus rebound sleep during the recovery period was less fragmented and had higher NREM delta power compared to the control period before SD.
Figure 5. Female rats show rebound sleep during recovery after noise-induced SD.
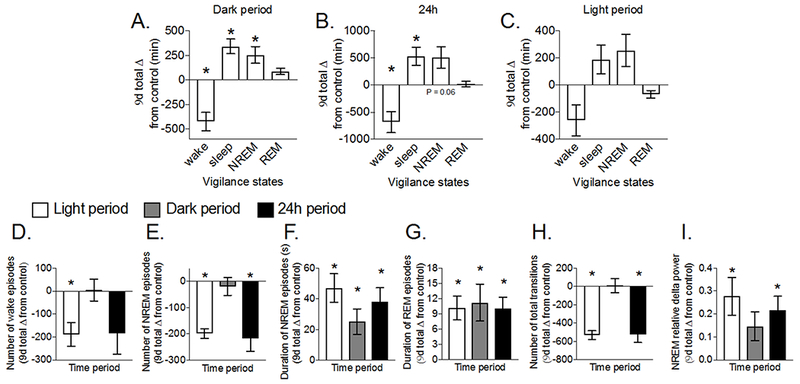
Female Sprague-Dawley rats (12-weeks old) were implanted with EEG/EMG leads. EEG/EMG signals were measured continuously for 9d before noise-induced SD and 9d during a recovery period after exposure to noise (8h/d for 9d during the light period). (A-C) cumulative time spent in wake, sleep (NREM + REM), NREM, and REM sleep and measures of sleep fragmentation including the (D) cumulative number of wake episodes, (E-F) number and mean duration of NREM episodes, (G) mean duration of REM episodes, (H) total transitions between vigilance states, and (I) sleep propensity indicated by NREM delta power during the light, dark, and 24h periods. During the first 24h of recovery, time spent in wake (P < 0.0001) was significantly lower while sleep (P < 0.0001) and NREM sleep (P < 0.0001) but not REM sleep (P = 0.73) were significantly higher than control. For each rat, cumulative or mean change from control during recovery was calculated by subtracting values within the same phase of the estrous cycle. Significant differences (P < 0.05) were determined with one sample t-tests. Data represent mean ± SEM; N = 5. *P < 0.05.
Noise-induced SD causes rebound sleep for 9d during the dark period:
We next evaluated the time course of rebound sleep during the recovery period by binning the 9d vigilance state data into 3d bins. WAKETIME was significantly lower and SLEEPTIME (data not shown) significantly higher during the dark period throughout the 9d recovery period (Figure 6A, P<0.05 for all comparisons). For the first third of recovery, WAKETIME was significantly lower and SLEEPTIME significantly higher due to increased NREMTIME during all time periods and increased REMTIME during the dark period (Figure 6A-C, P<0.05). Starting on the second third of recovery, NREMTIME and REMTIME were not significantly different from control across the light, dark or 24h periods (Figure 6B-C). Thus, vigilance states during the 24h period normalized after 3d of recovery, whereas rebound sleep during the dark period continued for 9d.
Figure 6. Female rats show prolonged rebound sleep and reduced total EE after noise-induced SD.
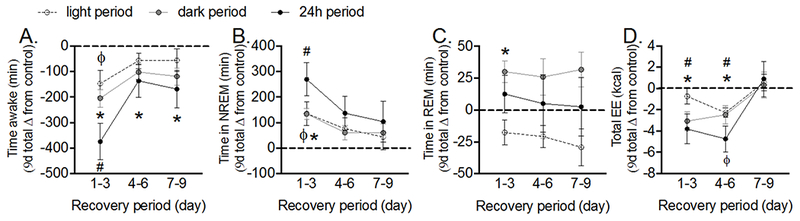
Female Sprague-Dawley rats (12-weeks old) were implanted with EEG/EMG leads. EEG, EMG and total EE were measured continuously for 9d before noise-induced SD and a 9d recovery period after exposure to noise (8h/d for 9d during the light period). Time course of cumulative time spent in (A) wake, (B) NREM, and (C) REM sleep and (D) TEE in the light, dark, and 24h periods during recovery. During the first 24h of recovery, TEE (P = 0.03) was significantly lower than control. For each rat, cumulative change from control during recovery was calculated by subtracting values within the same phase of the estrous cycle. Significant differences (P < 0.05) were determined with one sample t-tests. Data represent mean ± SEM; N = 5–6. ϕ P < 0.05, *P < 0.05 and # P < 0.05 for each tertile of the recovery period and for the light, dark and 24h periods, respectively.
Noise-induced SD reduced EE during rebound sleep:
Based on the persistent sleep rebound during recovery, we next tested whether SPA, TEE and its components were reduced during recovery. Dark and 24h period TEE were significantly lower for the first 6d of recovery (Figure 6D, P<0.05). This was due to significant differences in TEE components since SPAEE, SPAEE-MR, RESTEE, SLEEPEE-MR and NREMEE-MR were significantly lower during the dark and/or 24h periods (Figure S2A-E, P<0.05). Similar to results during noise exposure, SPA itself was not different from control during all time periods throughout recovery (P>0.05, data not shown). Thus, reductions in TEE observed during SD continued for the first 6d of recovery and this was due to lower EE during SPA and rest combined with reductions in the metabolic rates for both sleep and NREM sleep.
DISCUSSION
Evidence is accumulating that environmental noise decreases sleep quality and duration (3, 4) and increases the risk of obesity, particularly among women (4, 6, 7). We previously reported that noise exposure disrupted sleep, increased weight gain due to hyperphagia, and reduced TEE and its components in male rats (27, 28). Here, we extend those results to female rats. We demonstrate that noise exposure increases time spent awake, sleep fragmentation, sleep propensity, weight gain, and feeding while reducing TEE due to lower SPAEE and NREMEE. These changes occurred without disruption of the estrous cycle. Subsequently, during the recovery period, TEE remained reduced for 6d while rebound sleep continued for 9d. Overall, these results are consistent with population and clinical studies reporting that SD increases obesity risk and promotes weight gain in women by altering energy balance (4, 7–10). Hence we propose that noise exposure is an effective method to investigate the mechanisms underlying the effects of SD on weight gain and metabolism in females.
Indeed, this is the first report of weight gain and hyperphagia in non-ovariectomized female rats during SD, and the result is concordant with reports of weight gain in pre-menopausal women undergoing SR (8–11). But our results contrast with others who have reported weight loss in non-ovariectomized female rats using methods of sleep deprivation instead of SD (14–16), and also reports of similar food intake between ovariectomized sleep deprived females and undisturbed controls (18). These differences are likely the result of experimental differences (e.g. the duration of sleep deprivation, methods that use sleep deprivation and not SD, use of ovariectomized females, and failure to correct for uneaten food (14–16, 18)). Although a comparison of weight gain in males (28) and these results from females suggests that sex does not modulate weight gain under these circumstances, a direct comparison between males and females will be required to determine the influence of sex on SD-induced weight gain. Overall, the significance of these data is underscored by our use of a method of SD in females that parallels the phenotype of SR in women (8–10) as well as the elevated obesity risk in women exposed to noise (4).
Before investigating whether noise disrupted sleep in female rats, we determined whether vigilance states and EE, both of which influence weight gain, differed across phases of the estrous cycle. Our results for TEE components in all phases of the estrous cycle are novel but our findings of greater WAKETIME, SPA, and TEE during proestrus agree with some (20, 24) but contrast with other results (23, 25, 26, 38). These discrepancies are probably due to differences in measurement frequency, duration and timing, diets, assessment methods for EE and physical activity or the timing of vaginal lavage (25, 26), since the latter influences estrous cycle staging (35). Higher TEE during proestrus in the dark period was due to higher SPAEE since other TEE components were lower or similar in proestrus compared to other phases. This parallels higher SPA and reduced sleep during proestrus. These effects of estrous cycle on SPA and EE could be associated with higher estradiol levels (39), prepro-orexin, or orexin receptor 1 and 2 mRNA (40) during proestrus. Exogenous estradiol reduces sleep (41) and increases physical activity in ovariectomized females (42) and orexins reduce sleep (43) and increase SPA and SPAEE (34, 43–45). Nevertheless the role of estradiol and orexins on SPA and EE rhythm during the estrous cycle remains inconclusive.
We also show here that the estrous cycle has distinct effects on total calories and metabolic rates. This is exemplified by comparing the calories and metabolic rates for rest, NREM and REM. During the dark period, RESTEE was similar and NREMEE and REMEE were lower in proestrus, even though the metabolic rates for these components were higher in proestrus compared to other phases of the estrous cycle. These data highlight the importance of measuring endpoints throughout the estrous cycle in studies addressing weight gain in females since vigilance states, TEE, and each component of TEE (calories and metabolic rates) are phase dependent.
Our protocol for SD relies on noise exposure during the light period and we noted that sleep rebound during each subsequent dark period and throughout the recovery period here parallel rebound sleep in noise exposed males (27) and non-ovariectomized females after exposure to other sleep deprivation methods (20, 21). In particular, the absence of REM sleep rebound during the recovery period may be due to endogenous estrogen since exogenous estrogen suppresses REMTIME in ovariectomized females after sleep deprivation (47). Collectively, our data demonstrate that noise exposure fragmented and disrupted sleep and also caused prolonged rebound sleep.
The persistent reduction in TEE due to total calories and metabolic rates of TEE components during the noise exposure and recovery periods is significant and parallels the effect of noise on EE in male rats (27). Here, we controlled for the effect of the estrous cycle on endpoints by calculating the change from control within the same phase of estrous during the noise exposure and recovery periods. We found that TEE in females was lower in the dark period and over 24h during noise-induced SD and for the first 6d of recovery. These reductions in TEE were due to lower SPAEE and SPAEE-MR (despite unchanged SPA) during SD and recovery, NREMEE during SD, and RESTEE, SLEEPEE-MR, and NREMEE-MR during recovery. Interestingly, the rates of energy utilization (i.e. metabolic rate) for SPA, sleep, and NREM were lower during both SD and recovery, which implies that energy conservation may be one adaptation to noise-induced SD in females. These results agree with our prior report in sleep disrupted male rats (27) and the finding that resting metabolic rate is lower in women after chronic SR (11). Significantly therefore, these data suggest that clinical interventions targeting SPA and sleep may mitigate dampened TEE and weight gain due to noise-induced SD.
We found that the length of the estrous cycle was consistent throughout noise exposure, indicating that these results cannot be attributed to disruption of the estrous cycle. This is a critical finding since other methods of sleep deprivation (19), stress application (50) or corticosterone administration (51) disrupt estrous cycle length to induce constant diestrus (i.e. anestrus). Furthermore, stress disrupts ovarian hormone secretion (52). Nonetheless, we cannot exclude the possibility that noise may influence other indicators of stress since noise exposure has mixed effects on urinary or salivary cortisol and on the cortisol awakening response (53). Although further studies are now required to determine whether noise increases stress biomarkers, the maintenance of estrous cycle length throughout this study indicates that stress is unlikely to be a causative factor with this SD protocol.
Although the mechanisms underlying these results are unknown (29, 42, 54–60), alterations in hormones and neuropeptides that regulate metabolism are likely contributing factors. For example, leptin, ghrelin, neuropeptide Y, and proopiomelanocortin could contribute to noise-induced hyperphagia since they act centrally to regulate satiety and are modified by SR in humans (54) and by sleep deprivation in rodents (55, 56). Another possibility is that lower TEE and greater weight gain in response to noise-induced SD may be related to reduced orexin function. This follows from our data showing that noise-induced SD antagonizes orexin stimulated increases in SPA and SPAEE in male rats (29). Alterations in peripheral or central estrogen may also have contributed to weight gain, hyperphagia and reduction in TEE, since estradiol has been shown to increase physical activity (42) and EE (57) in ovariectomized rats. Furthermore, estradiol reverses weight gain and hyperphagia caused by estrogen depletion (58), and the estrogen rhythm is dampened by REM sleep deprivation (19). Lastly, mitochondrial bioenergetics may contribute to the reduction in TEE and its components, including the rate of energy utilization during, and after noise-induced SD since other methods of sleep deprivation have been linked to mitochondrial dysfunction (59). In view of our results in this study, experiments can now be designed to address the mechanisms mediating the effects of noise on SD, sleep fragmentation and metabolism.
In conclusion, we have shown that environmental noise exposure disturbs sleep, energy metabolism and feeding, leading to increased weight gain in female rats. These data both mirror our previous findings in male rats and indicate that the response to noise in female rats could be a model, with both internal and face validity, for the weight gain and hyperphagia observed in sleep restricted women. The present results thus provide a foundation for mechanistic studies aimed at elucidating the metabolic consequences of SD.
Supplementary Material
ACKNOWLEDGEMENTS
Funding for the publication was supported by the National Institutes of Health NS099468-01A1 (JAT), a CONICYT grant Fondecyt Regular 1150274 (CEPL), and the USDA ARZT-1372540-R23-131 (JAT).
Footnotes
Conflict of Interest Statement: The authors declare that no conflict of interest exists.
Supplementary information can be found online at the IJO homepage.
REFERENCES
- 1.Ford ES, Cunningham TJ, Croft JB. Trends in Self-Reported Sleep Duration among US Adults from 1985 to 2012. Sleep. 2015;38(5):829–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Itani O, Jike M, Watanabe N, Kaneita Y. Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression. Sleep Med. 2017;32:246–56. [DOI] [PubMed] [Google Scholar]
- 3.Basner M, McGuire S. WHO Environmental Noise Guidelines for the European Region: A Systematic Review on Environmental Noise and Effects on Sleep. Int J Environ Res Public Health. 2018;15(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oftedal B, Krog NH, Pyko A, Eriksson C, Graff-Iversen S, Haugen M, et al. Road traffic noise and markers of obesity - a population-based study. Environ Res. 2015;138:144–53. [DOI] [PubMed] [Google Scholar]
- 5.Herzog N, Jauch-Chara K, Hyzy F, Richter A, Friedrich A, Benedict C, et al. Selective slow wave sleep but not rapid eye movement sleep suppression impairs morning glucose tolerance in healthy men. Psychoneuroendocrinology. 2013;38(10):2075–82. [DOI] [PubMed] [Google Scholar]
- 6.Roosli M, Mohler E, Frei P, Vienneau D. Noise-related sleep disturbances: does gender matter? Noise Health. 2014;16(71):197–204. [DOI] [PubMed] [Google Scholar]
- 7.Lyytikainen P, Rahkonen O, Lahelma E, Lallukka T. Association of sleep duration with weight and weight gain: a prospective follow-up study. J Sleep Res. 2011;20(2):298–302. [DOI] [PubMed] [Google Scholar]
- 8.Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci U S A. 2013;110(14):5695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spaeth AM, Dinges DF, Goel N. Effects of Experimental Sleep Restriction on Weight Gain, Caloric Intake, and Meal Timing in Healthy Adults. Sleep. 2013;36(7):981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bosy-Westphal A, Hinrichs S, Jauch-Chara K, Hitze B, Later W, Wilms B, et al. Influence of partial sleep deprivation on energy balance and insulin sensitivity in healthy women. Obes Facts. 2008;1(5):266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spaeth AM, Dinges DF, Goel N. Resting metabolic rate varies by race and by sleep duration. Obesity (Silver Spring). 2015;23(12):2349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shechter A, Rising R, Albu JB, St-Onge MP. Experimental sleep curtailment causes wake-dependent increases in 24-h energy expenditure as measured by whole-room indirect calorimetry. Am J Clin Nutr. 2013;98(6):1433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shechter A, Rising R, Wolfe S, Albu JB, St-Onge MP. Postprandial thermogenesis and substrate oxidation are unaffected by sleep restriction. Int J Obes (Lond). 2014;38(9):1153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersen ML, Ribeiro DA, Alvarenga TA, Silva A, Araujo P, Zager A, et al. Are endogenous sex hormones related to DNA damage in paradoxically sleep-deprived female rats? Horm Behav. 2010;57(2):216–21. [DOI] [PubMed] [Google Scholar]
- 15.de Oliveira RA, Cunha GM, Borges KD, de Bruin GS, dos Santos-Filho EA, Viana GS, et al. The effect of venlafaxine on behaviour, body weight and striatal monoamine levels on sleep-deprived female rats. Pharmacol Biochem Behav. 2004;79(3):499–506. [DOI] [PubMed] [Google Scholar]
- 16.Hajali V, Sheibani V, Esmaeili-Mahani S, Shabani M. Female rats are more susceptible to the deleterious effects of paradoxical sleep deprivation on cognitive performance. Behav Brain Res. 2012;228(2):311–8. [DOI] [PubMed] [Google Scholar]
- 17.Everson CA, Bergmann BM, Rechtschaffen A. Sleep deprivation in the rat: III. Total sleep deprivation. Sleep. 1989;12(1):13–21. [DOI] [PubMed] [Google Scholar]
- 18.Longuski PA, Cudillo CA, Stern JJ. Brief communication effects of estradiol on feeding and locomotion in REM deprived rats. Physiol Behav. 1976;16(1):97–9. [DOI] [PubMed] [Google Scholar]
- 19.Antunes IB, Andersen ML, Baracat EC, Tufik S. The effects of paradoxical sleep deprivation on estrous cycles of the female rats. Horm Behav. 2006;49(4):433–40. [DOI] [PubMed] [Google Scholar]
- 20.Schwierin B, Borbely AA, Tobler I. Sleep homeostasis in the female rat during the estrous cycle. Brain Res. 1998;811(1–2):96–104. [DOI] [PubMed] [Google Scholar]
- 21.Andersen ML, Antunes IB, Silva A, Alvarenga TA, Baracat EC, Tufik S. Effects of sleep loss on sleep architecture in Wistar rats: gender-specific rebound sleep. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(4):975–83. [DOI] [PubMed] [Google Scholar]
- 22.Cordeira J, Kolluru SS, Rosenblatt H, Kry J, Strecker RE, McCarley RW. Learning and memory are impaired in the object recognition task during metestrus/diestrus and after sleep deprivation. Behav Brain Res. 2018;339:124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang J, Fishbein W. Sex differences in paradoxical sleep: influences of estrus cycle and ovariectomy. Brain Res. 1996;734(1–2):275–85. [PubMed] [Google Scholar]
- 24.Colvin GB, Whitmoyer DI, Lisk RD, Walter DO, Sawyer CH. Changes in sleep-wakefulness in female rats during circadian and estrous cycles. Brain Res. 1968;7(2):173–81. [DOI] [PubMed] [Google Scholar]
- 25.Giles ED, Jackman MR, Johnson GC, Schedin PJ, Houser JL, MacLean PS. Effect of the estrous cycle and surgical ovariectomy on energy balance, fuel utilization, and physical activity in lean and obese female rats. Am J Physiol Regul Integr Comp Physiol. 2010;299(6):R1634–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anantharaman-Barr HG, Decombaz J. The effect of wheel running and the estrous cycle on energy expenditure in female rats. Physiol Behav. 1989;46(2):259–63. [DOI] [PubMed] [Google Scholar]
- 27.Parrish JB, Teske JA. Acute partial sleep deprivation due to environmental noise increases weight gain by reducing energy expenditure in rodents. Obesity (Silver Spring). 2017;25(1):141–6. [DOI] [PubMed] [Google Scholar]
- 28.Mavanji V, Teske JA, Billington CJ, Kotz CM. Partial sleep deprivation by environmental noise increases food intake and body weight in obesity-resistant rats. Obesity (Silver Spring). 2013;21(7):1396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DePorter DP, Coborn JE, Teske JA. Partial Sleep Deprivation Reduces the Efficacy of Orexin-A to Stimulate Physical Activity and Energy Expenditure. Obesity (Silver Spring). 2017;25(10):1716–22. [DOI] [PubMed] [Google Scholar]
- 30.Mavanji V, Teske JA, Billington CJ, Kotz CM. Elevated sleep quality and orexin receptor mRNA in obesity-resistant rats. Int J Obes (Lond). 2010;34(11):1576–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borbely AA, Tobler I, Hanagasioglu M. Effect of sleep deprivation on sleep and EEG power spectra in the rat. Behav Brain Res. 1984;14(3):171–82. [DOI] [PubMed] [Google Scholar]
- 32.Sinton CM, Kovakkattu D, Friese RS. Validation of a novel method to interrupt sleep in the mouse. J Neurosci Methods. 2009;184(1):71–8. [DOI] [PubMed] [Google Scholar]
- 33.Coborn JE, DePorter DP, Mavanji V, Sinton CM, Kotz CM, Billington CJ, et al. Role of orexin-A in the ventrolateral preoptic area on components of total energy expenditure. Int J Obes (Lond). 2017;41(8):1256–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mavanji V, Perez-Leighton CE, Kotz CM, Billington CJ, Parthasarathy S, Sinton CM, et al. Promotion of Wakefulness and Energy Expenditure by Orexin A in the Ventrolateral Preoptic Area. Sleep. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anita M M The Phases of the Oestrous Cycle in the Adult White Rat. J Exp Biol. 1951;28:576–84. [Google Scholar]
- 36.Benjamini Y HY. Controlling the false discovery rate: a practical and powerful approach to multiple hypothesis testing. Journal of the Royal Statistical Society Series B 1995. 57 289–300. [Google Scholar]
- 37.Parker GC, McKee ME, Bishop C, Coscina DV. Whole-body metabolism varies across the estrous cycle in Sprague-Dawley rats. Physiol Behav. 2001;74(3):399–403. [DOI] [PubMed] [Google Scholar]
- 38.Brobeck JR, Wheatland M, Strominger JL. Variations in regulation of energy exchange associated with estrus, diestrus and pseudopregnancy in rats. Endocrinology. 1947;40(2):65–72. [DOI] [PubMed] [Google Scholar]
- 39.Oshima I, Morishita H, Omura K, Saito S. Changes in hypothalamic LH-RH content and blood levels of LH-RH, gonadotropin and estradiol during the preovulatory stage of rat estrous cycle. Endocrinol Jpn. 1978;25(6):607–11. [DOI] [PubMed] [Google Scholar]
- 40.Silveyra P, Catalano PN, Lux-Lantos V, Libertun C. Impact of proestrous milieu on expression of orexin receptors and prepro-orexin in rat hypothalamus and hypophysis: actions of Cetrorelix and Nembutal. Am J Physiol Endocrinol Metab. 2007;292(3):E820–8. [DOI] [PubMed] [Google Scholar]
- 41.Colvin GB, Whitmoyer DI, Sawyer CH. Circadian sleep-wakefulness patterns in rats after ovariectomy and treatment with estrogen. Exp Neurol. 1969;25(4):616–25. [DOI] [PubMed] [Google Scholar]
- 42.Kawashima S, Shinoda A. Spontaneous activity of neonatally estrogenized female rats. Endocrinol Jpn. 1968;15(3):305–12. [DOI] [PubMed] [Google Scholar]
- 43.Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, et al. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A. 1999;96(19):10911–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kiwaki K, Kotz CM, Wang C, Lanningham-Foster L, Levine JA. Orexin A (hypocretin 1) injected into hypothalamic paraventricular nucleus and spontaneous physical activity in rats. Am J Physiol Endocrinol Metab. 2004;286(4):E551–9. [DOI] [PubMed] [Google Scholar]
- 45.Nichols J, Gardner RL. Effect of damage to the zona pellucida on development of preimplantation embryos in the mouse. Hum Reprod. 1989;4(2):180–7. [DOI] [PubMed] [Google Scholar]
- 46.Baratta AM, Buck SA, Buchla AD, Fabian CB, Chen S, Mong JA, et al. Sex Differences in Hippocampal Memory and Kynurenic Acid Formation Following Acute Sleep Deprivation in Rats. Sci Rep. 2018;8(1):6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwartz MD, Mong JA. Estradiol suppresses recovery of REM sleep following sleep deprivation in ovariectomized female rats. Physiol Behav. 2011;104(5):962–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shechter A, Varin F, Boivin DB. Circadian variation of sleep during the follicular and luteal phases of the menstrual cycle. Sleep. 2010;33(5):647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Webb P 24-hour energy expenditure and the menstrual cycle. Am J Clin Nutr. 1986;44(5):614–9. [DOI] [PubMed] [Google Scholar]
- 50.Williams NI, Berga SL, Cameron JL. Synergism between psychosocial and metabolic stressors: impact on reproductive function in cynomolgus monkeys. Am J Physiol Endocrinol Metab. 2007;293(1):E270–6. [DOI] [PubMed] [Google Scholar]
- 51.Luo E, Stephens SB, Chaing S, Munaganuru N, Kauffman AS, Breen KM. Corticosterone Blocks Ovarian Cyclicity and the LH Surge via Decreased Kisspeptin Neuron Activation in Female Mice. Endocrinology. 2016;157(3):1187–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wagenmaker ER, Moenter SM. Exposure to Acute Psychosocial Stress Disrupts the Luteinizing Hormone Surge Independent of Estrous Cycle Alterations in Female Mice. Endocrinology. 2017;158(8):2593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Basner M, Samel A, Isermann U. Aircraft noise effects on sleep: application of the results of a large polysomnographic field study. J Acoust Soc Am. 2006;119(5 Pt 1): 2772–84. [DOI] [PubMed] [Google Scholar]
- 54.Reutrakul S, Van Cauter E. Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. Metabolism. 2018;84:56–66. [DOI] [PubMed] [Google Scholar]
- 55.Koban M, Swinson KL. Chronic REM-sleep deprivation of rats elevates metabolic rate and increases UCP1 gene expression in brown adipose tissue. Am J Physiol Endocrinol Metab. 2005;289(1):E68–74. [DOI] [PubMed] [Google Scholar]
- 56.Martins PJ, Marques MS, Tufik S, D’Almeida V. Orexin activation precedes increased NPY expression, hyperphagia, and metabolic changes in response to sleep deprivation. Am J Physiol Endocrinol Metab. 2010;298(3):E726–34. [DOI] [PubMed] [Google Scholar]
- 57.Richard D Effects of ovarian hormones on energy balance and brown adipose tissue thermogenesis. Am J Physiol. 1986;250(2 Pt 2):R245–9. [DOI] [PubMed] [Google Scholar]
- 58.Wade GN, Zucker I. Modulation of food intake and locomotor activity in female rats by diencephalic hormone implants. J Comp Physiol Psychol. 1970;72(2):328–36. [DOI] [PubMed] [Google Scholar]
- 59.Rodrigues NR, Macedo GE, Martins IK, Gomes KK, de Carvalho NR, Posser T, et al. Short-term sleep deprivation with exposure to nocturnal light alters mitochondrial bioenergetics in Drosophila. Free Radic Biol Med. 2018;120:395–406. [DOI] [PubMed] [Google Scholar]
- 60.Rangtell FH, Schmidt F, Wurfel J, Karamchedu S, Andersson P, Vogel H, et al. Morning Enzymatic Activity of DPP-4 Is Differentially Altered by Sleep Loss in Women and Men. Diabetes Care. 2018;41(2):e10–e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


