Abstract
SCOPE:
Palmitoleic acid (palmitoleate; C16:1 n-7), an omega-7 monounsaturated fatty acid (MUFA) found in plants and marine sources, has been shown to favorably modulate lipid and glucose metabolism. Its impact, however, on atherosclerosis has not been examined in detail.
METHODS AND RESULTS:
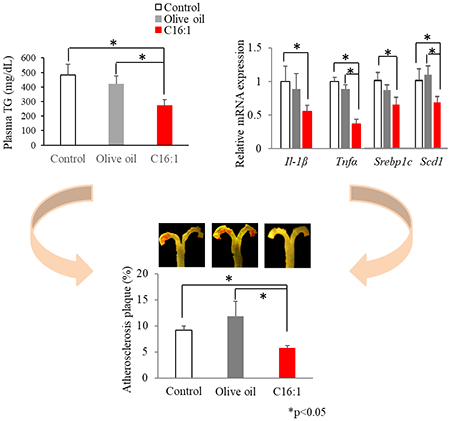
LDL receptor knock-out (LDLR-KO) mice were fed a Western diet supplemented with 5% (w/w) palmitoleate concentrate, oleic-rich olive oil, or none (control) for 12 weeks. Dietary palmitoleate increased hepatic C16:1 levels, improved plasma and hepatic lipid/lipoprotein profiles (~40% decrease in triglycerides), and reduced the atherosclerotic plaque area by ~45% compared with control or olive oil group (p<0.05). These favorable changes were accompanied by the down-regulation of key genes, such as Srebp1c, Scd1, Il-1β, and Tnfα. ApoB-depleted plasma from mice fed palmitoleate had increased cholesterol efflux capacity by 20% from ABCA1-expressing cells (p<0.05). We further observed a beneficial effect of palmitoleate on glucose metabolism (54% decreased in HOMA-IR, p<0.05).
CONCLUSIONS:
Dietary supplemented palmitoleate reduces atherosclerosis development in LDLR-KO mice, and was associated with improvement of lipid and glucose metabolism and favorable changes in regulatory genes involved in lipogenesis and inflammation. These findings imply the potential role of dietary palmitoleate in the prevention of cardiovascular disease and diet-induced metabolic disorders.
Keywords: Palmitoleate, atherosclerosis, hyperlipidemia, insulin resistance, inflammation
Graphical Abstract
Dietary supplemented palmitoleate (C16:1 n-7) reduces atherosclerosis development in LDLR-KO mice, which was associated with improvement of lipid and glucose metabolism and favorable changes in regulatory genes involved in lipogenesis and inflammation.
1. Introduction
Atherosclerosis is a key underlying pathological process that leads to cardiovascular disease (CVD), with dyslipidemia and inflammation as the two major drivers [1]. Dietary nutrients have a major effect in both promoting and combating atherosclerotic processes, by modulating lipid metabolism and inflammation. Dietary fat is an essential macronutrient, and there is growing evidence on the importance of the quality of fat in atherogenesis development. Compared with pro-atherogenic saturated fatty acids, numerous studies have demonstrated the beneficial effect of monounsaturated fatty acid (MUFA) consumption on lipid metabolism, inflammation, and cell membrane function [2]. The most abundant MUFA in the diet is oleic acid (oleate; C18:1 n-9), and to date it has been the most common type of MUFA studied in various nutritional studies on CVD. As a consequence, it is largely unknown whether MUFA with different chain length have beneficial or perhaps even adverse effects on metabolism and CVD risk factors.
Palmitoleic acid (palmitoleate; C16:1 n-7) is an omega-7 MUFA that is produced by de novo lipogenesis pathway and is found in naturally occurring sources. The natural sources of palmitoleate include fatty fish, fish oil and some nuts and seeds and their oils such as macadamia and sea buckthorn [3,4]. Palmitoleate has been recently proposed as a novel adipose tissue-derived lipokine that regulates lipogenesis, insulin action, and coordinates hemostasis [5]. In further support of this hypothesis, several studies have shown a beneficial effect of palmitoleate on CVD risk factors. For example, in vitro studies have shown that palmitoleate suppresses inflammation in macrophages and human endothelial cells through inhibiting inflammasome pathways, and prevents the beta-cell apoptosis induced by glucose or saturated fatty acids [6,7]. In animals, our previous study showed that orally administered palmitoleate improved insulin sensitivity and suppressed hepatosteatosis and inflammation in type II diabetic mice, partly through regulating lipogenic and inflammatory genes [8]. The positive effect of palmitoleate in restoring metabolic function was also reported in various other animal models [9,10]. Furthermore, data from a recent clinical study demonstrated that purified palmitoleate supplementation for 30 days had beneficial effect in improving serum lipoprotein profile and systemic inflammation in adults with dyslipidemia [11]. Other nutritional intervention trials, using macadamia nut, also showed a more favorable lipid profile among the subjects with high-palmitoleate diet compared with high-saturated fatty acid diet or typical American diet [12,13].
In the present study, we examined the effect of dietary palmitoleate purified from fish oil on atherosclerosis development in LDL receptor knock-out (LDLR-KO) mice fed with high-fat Western diet. Compared to olive oil enriched in oleate, which was used as a MUFA control, we observed several potentially beneficial changes in lipid and glucose metabolism from palmitoleate supplementation and an overall reduction of atherosclerosis in our mouse model.
2. Materials and methods
2.1. Ethical Statement
Animal handling and experiments were performed in accordance with guidelines provided by the NIH Guide for the Care and Use of Laboratory Animals. All procedures were approved by the Animal Care and Use Committee in the National Heart, Lung and Blood Institute (protocol # H0050).
2.2. Animals and experimental design
Eight-week old LDLR-KO female mice on a C57Bl/6J background were purchased from Jackson Lab (Bar Harbor, ME). Mice were housed in a room with controlled temperature (24°C ± 1°C) and a 12:12-hour light-dark cycle. Oleate-rich olive oil and palmitoleate concentrate oil (AlaskOmega® Omega7-700) were provided by Organic Technologies (Coshocton, OH), with respective fatty acid composition shown in Table 1. After consuming standard mouse chow for 1 week, retro-orbital bleeds were performed on mice 5-hour after fasting and baseline plasma total cholesterol (TC) was measured. Forty-five mice were divided into 3 groups (n = 15/group) with similar mean plasma TC concentrations and body weight, and were fed with one of the following diets for 12 weeks: (I) control diet: Western diet TD.88137 Adjusted Calories Diet (Harlan Teklad/Envigo, Madison, WI), (II) control diet supplemented with 5% (wt/wt) palmitoleate concentrate or (III) control diet supplemented with 5% (wt/wt) olive oil. The experimental diet compositions are summarized in Table 2. During the experiment, fasting blood samples collection and body weight record were performed at 3-week intervals. Glucose tolerance test and body composition analysis was performed at Week 10, and the study of mouse metabolism (n=4/group) were performed at Week 11 using a Comprehensive Lab Animal Monitor System (CLAMS; Columbus Instruments, Columbus, OH). At week 12, mice were terminated and aortas were isolated for atherosclerosis analysis, and a portion of liver and white adipose tissues were kept in tissue preparation medium (RNAlater, Ambion, Austin, TX) for RNA isolation or stored at −80°C for lipid analysis. In two additional separate experiments, eight-week female C57Bl/6J (n=7) and LDLR-KO mice (n=10) on a C57Bl/6J background (Jackson Lab) were fed a chow diet (NIH-31 Open Formula Mouse/Rat Sterilizable Diet; Harlan Teklad/Envigo) for 12 weeks. The plasma lipid levels at baseline and week 12 were measured as described above. The aortas of LDLR-KO mice on chow diet were isolated at week 12 for atherosclerosis analysis.
Table 1.
Major fatty acid composition of dietary oils
| FA (%) | Olive oil | Palmitoleate concentrate |
|---|---|---|
| C14:0 | 0.1 | ND |
| C16:0 | 12 | 8.2 |
| C16:1(n-7) | 0.8 | 71.2 |
| C16:2(n-6) | ND | 8.4 |
| C16:3(n-3) | ND | 1.2 |
| C18:0 | 3.6 | ND |
| C18:1(n-9) | 74 | ND |
| C18:2(n-6) | 7.5 | ND |
| C18:3(n-3) | 0.7 | ND |
| Total SFA | 15.7 | 8.2 |
| Total MUFA | 74.8 | 71.2 |
| Total n-3 PUFA | 0.8 | 1.2 |
| Total n-6 PUFA | 7.5 | 8.4 |
FA: fatty acid; MUFA: monounsaturated fatty acids; ND: not detected; PUFA: polyunsaturated fatty acid; SFA: saturated fatty acid.
Table 2.
Diet compositions for LDLR-KO mice fed Western diet-based diet
| Ingredient (g/kg) | Western diet (control) | Olive oil-supplemented Western diet (Olive oil group) | Palmitoleate-supplemented Western diet (C16:1 group) |
|---|---|---|---|
| Casein | 195 | 195 | 195 |
| DL-methionine | 3 | 3 | 3 |
| Sucrose | 341 | 341 | 341 |
| Corn starch | 150 | 150 | 150 |
| Cholesterol | 1.5 | 1.5 | 1.5 |
| Cellulose | 50 | 50 | 50 |
| Mineral mix | 35 | 35 | 35 |
| Calcium carbonate | 4 | 4 | 4 |
| Vitamin mix | 10 | 10 | 10 |
| Ethoxyquin | 0.04 | 0.04 | 0.04 |
| Milk fat | 210 | 160 | 160 |
| Olive oil | 0 | 50 | 0 |
| Palmitoleate concentrate | 0 | 0 | 50 |
| Nutritional values | |||
| Energy content, Kcal/g | 4.5 | 4.5 | 4.5 |
| Protein, % of energy | 15.2 | 15.4 | 15.3 |
| Carbohydrate, % of energy | 42.7 | 42.9 | 42.8 |
| Fat, % of energy | 42 | 42.1 | 41.9 |
2.3. Dosage Information
Currently, there is no recommended human daily intake of palmitoleate. Five percent (w/w) of palmitoleate concentrate oil (70% of purity) was supplemented in diet, which is equivalent to approximately 6 g/kg BW/day. Overall, the diet contained 7% total energy as palmitoleate, and this dose is comparable with previous human study using palmitoleate-supplemented food [14]. Furthermore, the majority of health organizations suggest between 10–20% of our total energy intake should be derived from MUFAs [15]. Palmitoleate supplementation was calculated according to a metabolic body weight formula [16] to produce a human (70 kg) equivalent dose of 13 g/day of palmitoleate. From the NHANES study, the average daily consumption of the most common MUFA in natural source, oleic acid, in the US is nearly 30 grams per day[17]; therefore, the dose is achievable in a daily diet, although it is relatively high.
2.4. Plasma metabolites and lipoprotein analysis
Fasting plasma were analyzed for triglycerides (TG), TC, free cholesterol (FC), and phospholipids (PL), using commercial enzymatic methods (Wako Chemicals USA, Inc., Richmond, VA). Plasma glucose concentration were measured using a commercially available kit (Crystal Chem, Inc., IL), and plasma insulin concentration were analyzed using ELISA method, with Ultra Sensitive Mouse Insulin ELISA Kits (Crystal Chem. Inc.). Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as glucose concentration (mmol/L) × insulin concentration (mU/L)/22.5 [18]. Plasma lipoprotein profiles from pooled plasma (n= 3/group) were analyzed after separation by fast protein liquid chromatography (FPLC).
2.5. Atherosclerotic lesion analysis
At the end of the 12-week feeding period, mouse aortas were prepared and analyzed en face for atherosclerosis [19]. Briefly, aortas were dissected free from adipose tissue, cut open along the longitudinal axis. Lipids were stained with Sudan IV solution, following fixation with 10% neutral buffered formalin and wash with phosphate-buffered saline (PBS). After de-staining with 80% ethanol solution, aortas were mounted on glass slides and images were recorded with an Olympus digital camera mounted on an Olympus dissecting microscope. Image analysis was performed with Image-Pro software (Media Cybernetics, Silver Spring, MD). The extent of atherosclerosis was expressed as the percentage of lesion area to the total aortic area.
2.6. Hepatic lipid and fatty acid composition analysis
Total lipids from frozen liver tissue samples (100 mg) were extracted overnight using chloroform/methanol (2:1, v/v) with Folch method. The separated organic layers were collected, and the solvent was evaporated at room temperature under a nitrogen stream. A portion of the extract was used to determine fatty acid profiles by gas chromatography (GC). In brief, the dry residue was redissolved in 1 mL of 0.5 mol/L KOH-methanol in a sealed vial in a 60°C bath under a nitrogen stream for 10 min. Next, 1.5 mL of 13% methanolic BF3 was added, and the mixture was incubated at 60°C for 30 min. Fatty acid methyl esters were extracted with hexane and the fatty acid composition was analyzed on a Shimadzu GC2010 system using a capillary GC column. Another portion of the lipid extract was redissolved in hexane/2-propaonol (3:2 vol/vol) to measure TC, FC, PL and TG using enzymatic Wako assay kits.
2.7. In vitro cholesterol efflux assay
To estimate HDL function, pooled apoB-depleted plasma from each diet group (n = 5/group) were used to measure cholesterol efflux capacity at 37°C in BHK cells stably transfected with ABCA1, ABCG1, or not (MOCK) as described previously [19]. In brief, cells in 24-well plates were incubated with DMEM containing 1 μCi of [3H] cholesterol per ml and for 24 h, followed by efflux (4 h), using 1% (vol/vol) apoB-depleted plasma prepared in DMEM containing 0.1% BSA as the cholesterol receptor. The cholesterol efflux rate was expressed as the % of [3H]-cholesterol transferred from cells to medium over time, and values were normalized to untreated control cells.
2.8. Glucose Tolerance Test
Mice were fasted for 6hr and injected i.p. with a 1.5 mg/g lean body mass glucose solution. Blood glucose was then measured at 0, 15, 30, 60, 90 and 120 min post injection, using whole blood drawn from the tail and analyzed using an AlphaTrak glucometer (Zoetis, Parsippany, New Jersey). Dose was determined by lean body mass that was measured by nuclear magnetic resonance (NMR) body composition analysis.
2.9. Body Composition Analysis
Whole-body composition of LDLR-KO mice on different diets for 10 weeks was analyzed by NMR. The EchoMRI™ (EchoMRI LLC, Houston, TX) was used to scan un-anesthetized mice, to estimate the mass of lean and fat tissue. Two scans were performed per mouse and the results were averaged. The fat and lean mass percentages were calculated based on fat and lean mass and body weight.
2.10. Indirect Calorimetry
Changes in whole-body energy expenditure and ambulatory activity of LDLR-KO mice in response to the different diets were assessed by indirect calorimetry. The food intake, activity and metabolic parameters were measured within the CLAMS container. The mice (n=4/group) were placed in individual chambers with 12hr light/dark phase cycles and standard animal room temperature was maintained throughout the 5-day study and water and food were provided ad libitum.
2.11. RT-qPCR analysis
Total RNA was extracted from liver and white adipose tissue, using Trizol reagent (Qiagen Inc., CA, USA). First strand cDNA was synthesized from total RNA (100 ng), using the PrimeScript II 1st strand cDNA Synthesis kit (TaKaRa Bio, Otsu, Japan). The products of reverse transcription were used for reverse transcription quantitative polymerase chain reaction (RT-qPCR) to evaluate gene expression. TaqMan® Gene Expression Assays were used on a 7900HT Fast Real Time PCR system (Applied Biosystems). Probes and primer sets of the following genes were purchased from Applied Biosystems (Foster City, CA): Sterol regulatory element-binding protein 1c (Srebp1c), Stearoyl-CoA desaturase-1 (Scd1), Fatty acid synthase (Fasn), Sterol regulatory element-binding protein 2 (Srebp2), Cholesterol 7α-Hydroxylase (Cyp7a1), Interleukin 1-β (Il-1β), Tumor necrosis factor alpha (Tnfα), Peroxisome proliferator-activated receptor alpha (Pparα), and Peroxisome proliferator-activated receptor gamma (Pparγ). mRNA quantification was assayed, using the ddCT method, and gene expression was normalized to the expression of the beta-actin gene.
2.12. Statistical analysis
GraphPad Prism statistical software (GraphPad Software Inc. version 6.00, La Jolla, CA) was used for all statistical analyses. Statistical analysis was completed using one-way analysis of variance (ANOVA) followed by Bonferroni post hoc test where appropriate for multiple comparisons. Differences were considered significant at p ≤ 0.05. Data are presented in text, figures, and tables as means ± standard error of the mean (SEM).
3. Results
3.1. Effect of dietary palmitoleate on body weight and food intake
All of the diets were well tolerated. No significant differences were observed in food intake or body weight (Supplemental Fig. 1A,B) among three dietary groups of mice throughout the 12-week feeding period.
3.2. Effect of dietary palmitoleate on hepatic fatty acid composition
As shown in Table 3, olive oil- and palmitoleate-rich diets significantly (p<0.05) increased hepatic total MUFA composition by greater than 35% compared with control, but the compositions of total saturated fatty acids (SFA), n-6 and n-3 polyunsaturated fatty acids (PUFA) were similar among the three diet groups. Palmitoleate-rich diet resulted in a significant (p<0.05) increase in hepatic palmitoleate by 4.8- and 10.6-fold compared with control and olive oil group, respectively. Similarly, olive oil-rich diet significant (p<0.05) increased oleic acid (C18:1 n-9) compared with control and palmitoleate group.
Table 3.
Hepatic major fatty acid composition (%) of mice from different diet groups after 12 weeks of feeding
| FA (%) | Control | Olive oil | Palmitoleate concentrate |
|---|---|---|---|
| C14:0 | 0.4 ± 0.06 | 0.3 ± 0.06 | 0.5 ± 0.02 |
| C16:0 | 20 ± 0.7 | 18.8 ± 0.6 | 20.4 ± 0.4 |
| C16:1n-7 | 0.6 ± 0.09a | 0.3 ± 0.1b | 3.5 ± 0.01c |
| C18:0 | 17.6 ± 0.7a | 15.9 ± 0.7b | 15.8 ± 0.3b |
| C18:1n-9 | 6.3 ± 0.4a | 9.4 ± 0.3b | 5.9 ± 0.8a |
| C18:1n-7 | 1.7 ± 0.07 | 1.6 ± 0.1 | 1.7 ± 0.04 |
| C18:2n-6 | 19.6 ± 0.80 | 20.5 ± 0.4 | 19.1 ± 1.5 |
| C18:3n-6 | 0.3 ± 0.01 | 0.2 ± 0.01 | 0.3 ± 0.01 |
| C18:3n-3 | 0.5 ± 0.04 | 0.6 ± 0.03 | 0.6 ± 0.1 |
| C20:0 | ND | 0.2 ± 0.04 | 0.1 ± 0.01 |
| C20:1n-9 | 0.1 ± 0.06 | 0.2 ± 0.05 | 0.1 ± 0.02 |
| C20:2n-6 | 0.2 ± 0.06 | 0.4 ± 0.01 | 0.2 ± 0.05 |
| C20:3n-6 | 0.6 ± 0.2 | 0.6 ± 0.4 | 0.6 ± 0.2 |
| C20:4n-6 | 20.9 ± 1.3 | 20.5 ± 1.8 | 21.3 ± 1.8 |
| C20:5n-3 | 1 ± 0.3 | 0.9 ± 0.1 | 0.6 ± 0.5 |
| C22:0 | 0.4 ± 0.03 | 0.4 ± 0.01 | 0.4 ± 0.05 |
| C22:4n-6 | 0.4 ± 0.03 | 0.4 ± 0.1 | 0.4 ± 0.01 |
| C22:5n-3 | 0.6 ± 0.03 | 0.5 ± 0.04 | 0.6 ± 0.02 |
| C24:0 | 1.2 ± 0.1 | 1.1 ± 0.01 | 1 ± 0.05 |
| C22:6n-3 | 4.9 ± 0.3 | 4.5 ± 0.03 | 4.3 ± 0.2 |
| Total SFA | 39.6 ± 0.7 | 38.2 ± 1.1 | 36.6 ± 1.2 |
| Total MUFA | 7 ± 1.8a | 9.5 ± 2.1b | 9.9 ± 1.5b |
| Total n-6 PUFA | 42 ± 0.5 | 41.9 ± 0.6 | 42.5 ± 0.4 |
| Total n-3 PUFA | 7.1 ± 0.3 | 6.1 ± 1.1 | 6.4 ± 0.6 |
Data are expressed as mean ± SEM (n = 12). Labled means in a row without a common superscript letter differ, p< 0.05. FA: fatty acid; MUFA: monounsaturated fatty acid; ND: not detected; PUFA: polyunsaturated fatty acid; SFA: saturated fatty acid.
3.3. Effect of dietary palmitoleate on lipid and lipoprotein profiles
LDLR-KO mice developed hyperlipidemia after 12 weeks on the Western diet, with plasma lipid levels (TC, FC, PL and TG) significantly (p<0.05) increased by ~ 3-fold and ~9-fold compared with chow diet-fed LDLR-KO mice and wild-type mice, respectively (Supplemental Fig. 2). In the diet-induced hyperlipidemic LDLR-KO mice, palmitoleate-rich diet lowered by less than 10% plasma levels of cholesterol and phospholipid, and had a more profound effect (>30%) in lowering TG compared with control and olive oil group at most of the time points during the 12-week feeding study (Fig. 1A–E). The further analysis of Area Under the Curve (AUC) revealed that the plasma lipid AUC results were in agreement with the results of the overall lipid lowering-effects of palmitoleate. Compared with control or olive oil group, the AUC for FC, PL and TG in palmitoleate group was significantly (p<0.05) lowered by approximately 10%, 9%, and 33%, respectively. Dietary palmitoleate also lowered hepatic lipid levels. Compared with control or olive oil-rich diet, palmitoleate-rich diet significantly (p<0.05) decreased hepatic PL and TG by approximately 20% and 45%, respectively (Fig. 1F). Analysis of lipoproteins by FPLC analysis showed that diet enriched in palmitoleate also favorably altered the plasma lipoprotein profile, particularly VLDL and HDL fractions (Fig. 2). TC, PL and TG in VLDL fraction were significantly (p<0.05) decreased in palmitoleate group compared with control and olive oil group. In contrast, dietary palmitoleate, but not olive oil, increased (p<0.05) cholesterol and PL in HDL fraction compared with control (Fig. 2D–F).
Figure 1.

Effects of dietary palmitoleate on plasma and hepatic lipid profiles in LDLR-KO mice. Mice (n=15/group) were fed a Western diet supplemented with 5% olive oil, palmitoleate concentrate (C16:1), or none (control) for 12 weeks. Time course of plasma concentrations of (A) TC, (B) FC, (C) PL, and (D) TG. (E) Area under the curve (AUC) for plasma TC, FC, PL, and TG. (F) Hepatic contents of TC, PL, and TG. Values represent the mean ± SEM. Labled means without a common letter differ (p<0.05).
Figure 2.

Effects of dietary palmitoleate on FPLC elution profile in LDLR-KO mice. Mice (n=15/group) were fed a Western diet supplemented with 5% olive oil, palmitoleate concentrate (C16:1), or none (control) for 12 weeks. FPLC analysis of plasma (A) TC, (B) PL, and (C) TG. Area under the curve (AUC) for plasma (D) TC, (E) PL, and (F) TG in VLDL, LDL, and HDL fractions. Values represent the mean ± SEM. Labled means without a common letter differ (p<0.05).
3.4. Effect of dietary palmitoleate on atherosclerosis lesion development
En face Sudan IV staining confirmed that the Western diet induced atherosclerosis in LDLR-KO mice. Atherosclerotic plaque area in LDLR-KO mice fed a Western diet was 22-fold higher (p<0.001) than that in LDLR-KO mice on the chow diet (Supplemental Fig.3). This result is in line with previous studies that have demonstrated that chow diet-fed LDLR-KO mice show moderate lipid levels and develop no or minor atherosclerosis, in contrast to dramatically elevated lipid levels and prominent aorta lesions on the high-fat, high-cholate diet [20]. When the mice were fed a Western diet supplemented with palmitoleate, the total atherosclerotic lesions were suppressed significantly (p<0.05) by 37 % and 51% compared to the control and olive oil groups, respectively (Fig. 3). There was no difference in plaque areas between the control and olive oil group. Most of the atherosclerotic lesions were restricted to the arch of the aorta for all 3 diet groups.
Figure 3.
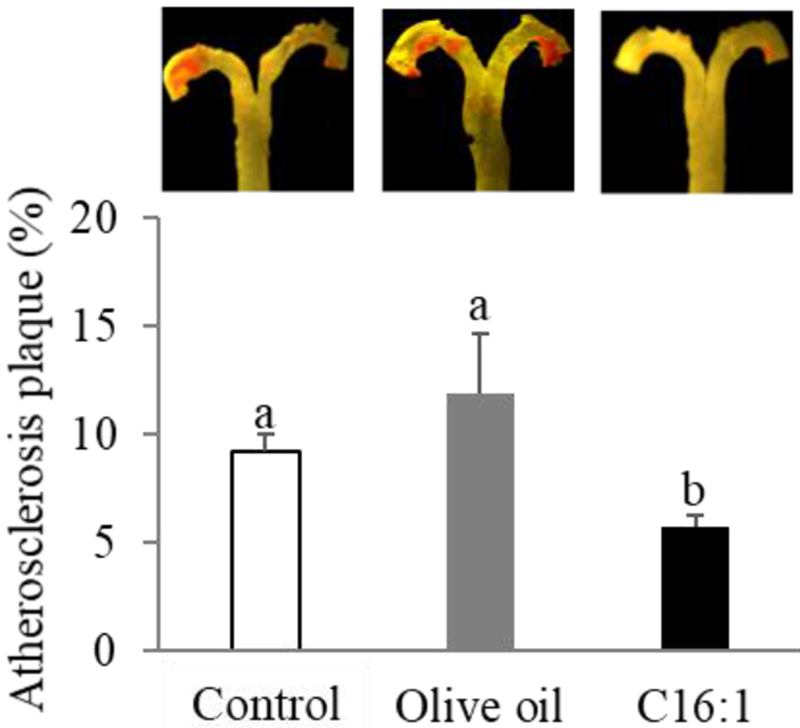
Effects of dietary palmitoleate on progression of atherosclerosis in LDLR-KO mice. Mice (n=15/group) were fed a Western diet supplemented with 5% olive oil, palmitoleate concentrate (C16:1), or none (control) for 12 weeks. Representative en face Sudan IV staining of aorta (upper panel), and quantitative analysis of Sudan IV-positive area of aorta (lower panel). Values represent the mean ± SEM. Labled means without a common letter differ (p<0.05).
3.5. Effect of dietary palmitoleate on cholesterol efflux capability
As a measure of HDL function, the effect of palmitoleate-rich diet on plasma cholesterol efflux capacity from BHK cells stably transfected with either ABCA1 (BHK-ABCA1), ABCG1 (BHK-ABCG1) or none (BHK-MOCK) was examined. As shown in Fig. 4A, apoB-depleted plasma from palmitoleate group significantly (p<0.05) increased [3]-H cholesterol release by 20% in BHK-ABCA1 cells compared with those from control or olive oil group. There was no difference in cholesterol efflux capacity in BHK-ABCA1 cells between the control and olive oil group. No differences were detected in cholesterol efflux capacity among the three groups when BHK-ABCG1 cells (Fig. 4B) and BHK-MOCK cells (Fig. 4C) were used as the donor cells.
Figure 4.

Effects of dietary palmitoleate on cholesterol efflux capacity in LDLR-KO mice. Mice (n=15/group) were fed a Western diet supplemented with 5% olive oil, palmitoleate concentrate (C16:1), or not (control) for 12 weeks. Cholesterol efflux rate from (A) BHK-ABCA1 cells, (B) BHK-ABCG1 cells, and (C) BHK-MOCK cells. Values represent the mean ± SEM. Labled means without a common letter differ (p<0.05).
3.6. Effect of dietary palmitoleate on glucose metabolism
Glucose tolerance test showed that glucose levels were lower in palmitoleate group than in control during the time post glucose load, and they decreased significantly (p<0.05) by 17% at 30 min after glucose administration, although there was no difference between the control and olive oil group (Fig. 5A). The mice fed palmitoleate-rich diet, but not olive oil-rich diet, had significantly lower (p<0.05) plasma glucose levels by 14% at 12 weeks compared with mice fed control diet (Fig. 5B). Furthermore, palmitoleate-rich diet significantly (p<0.05) lowered plasma insulin levels by 47% compared with control, and no difference was detected between the control and olive oil group (Fig. 5C). When the HOMA index was calculated the mice fed palmitoleate-rich diet had 54% lower score than control diet (p<0.05), consistent with improved glucose tolerance on this diet (Fig. 5D). Olive oil-rich diet also decreased HOMA-IR by 36% compared with control (p<0.05).
Figure 5.
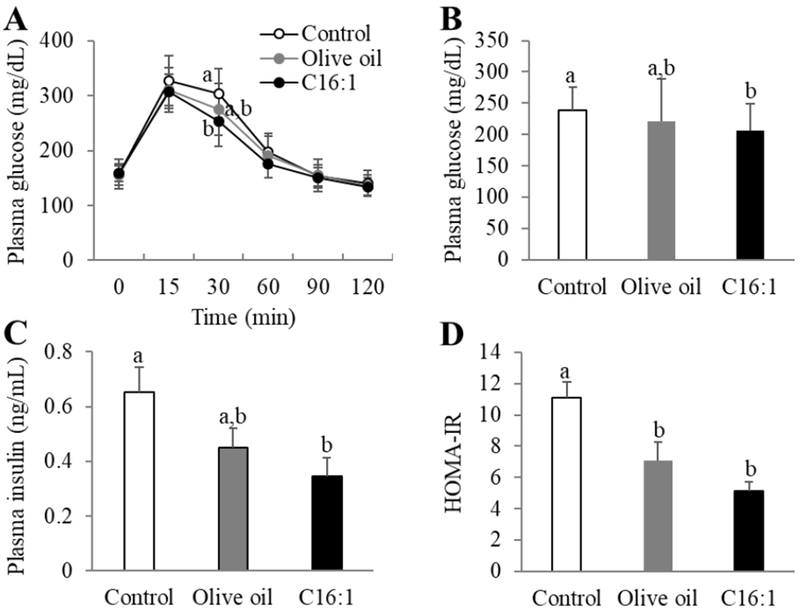
Effects of dietary palmitoleate on glucose metabolism in LDLR-KO mice. Mice (n=15/group) were fed a Western diet supplemented with 5% olive oil, palmitoleate concentrate (C16:1), or none (control) for 12 weeks. (A) Time course of plasma glucose concentrations in glucose tolerance test in mice. (B) Plasma glucose levels. (C) Plasma insulin levels. (D) Calculated HOMA-IR score. Values represent the mean ± SEM. Labled means without a common letter differ (p<0.05).
3.7. Effect of dietary palmitoleate on body composition and metabolism
We next investigated whether body composition and whole-body energy expenditure and ambulatory activity were affected by dietary palmitoleate. There were no differences in fat and lean mass percentages among the three diet groups (Supplemental Fig. 1C, D). In the course of the dark-phase, palmitoleate-rich diet significantly (p<0.05) increased both oxygen consumption (VO2) by approximately 17% (Supplemental Fig. 4A, C) and carbon dioxide produced (VCO2) (Supplemental Fig. 4B, D) by approximately 15% compared with control and olive oil group, and there were no differences in VO2 and VCO2 among the three diet groups during the light phase. Nevertheless, no differences were detected in respiratory Exchange Ratio (RER) (Supplemental Fig. 4E) and heat generation (Supplemental Fig. 4F) among the three diet groups, although palmitoleate diet tended to increase overall motor activity compared with control or olive oil diet in dark phases (p=0.054) (Supplemental Fig. 4G, H).
3.8. RT-qPCR analysis
To explore the potential molecular mechanisms underlying the beneficial metabolic effects of palmitoleate on diet-induced atherosclerosis in LDLR-KO mice, mRNA levels of candidate genes in livers and white adipose tissue from the control, olive oil and palmitoleate groups were measured by RT-qPCR. Gene expressions relevant to lipid/cholesterol metabolism and inflammation were measured. SREBP1c, FASN and SCD1 play major roles in de novo synthesis of fatty acids, and liver mRNA expression levels of Srebp1c and Scd1 were found to be significantly (p<0.05) decreased in the palmitoleate group compared to the control and olive oil group (Fig 6A). There were no differences in the expression of Pparα and Fasn among the three diet groups. For genes involved in cholesterol metabolism, palmitoleate- and olive oil-rich diet upregulated (p<0.05) liver mRNA of the key bile acid synthase gene Cyp7a1 compared with control, although there was no difference in Pparα and Srebp2 mRNA expression (Fig.6A). Furthermore, dietary palmitoleate, but not olive oil, downregulated (p<0.05) mRNA expression of the pro-inflammatory Il-1β and Tnfα genes compared with control in both liver (Fig. 6B) and adipose tissues (Fig. 6C). No difference was detected in Pparγ gene expression among the three groups in adipose tissue (Fig. 6C).
Figure 6.

Effects of dietary palmitoleate on gene expression in LDLR-KO mice. Mice (n=15/group) were fed a Western diet supplemented with 5% olive oil, palmitoleate concentrate (C16:1), or none (control) for 12 weeks. (A) Liver mRNA expression of genes involved in lipogenesis and cholesterol metabolism. (B) mRNA expression of genes related to inflammation in liver (left panel) and white adipose tissue (right panel). Values represent the mean ± SEM. Labled means without a common letter differ (p<0.05).
4. Discussion
Numerous studies have demonstrated that foods containing unsaturated fatty acids reduce the risk of developing CVD, and thus current dietary guidelines recommend replacing dietary saturated fatty acids with unsaturated fatty acids including MUFAs such as oleic acid. Recent discoveries related to omega-7 MUFA palmitoleate as acting as a lipokine suggest that other types of MUFAs may also have health benefits [5]. The main finding of this study is that palmitoleate may have beneficial effects in reducing cardiometabolic risk factors and in reducing atherosclerosis.
In the present study, we first confirmed that the Western diet induced hyperlipidemia and atherosclerosis in LDLR-KO mice, which agrees well with previous studies [20]. We then supplemented the Western diet with two types of MUFA (i.e., oleate and palmitoleate), and investigated the effect of palmitoleate on atherosclerosis development and related CVD risk factors. Despite the equal energy intake and whole-body composition among the three diet groups, our data showed that palmitoleate-rich diet reduced atherosclerotic plaque areas and hyperlipidemia in LDLR-KO mice, although such effect was not observed in mice fed oleate-rich diet. Mice fed palmitoleate-rich diet also had increased oxygen consumption and carbon dioxide production compared with those fed control or oleic-rich diet, which was consistent with overall increased motor activity tendency. A recent study from Çimen et al., also reported the anti-atherogenic effect of oral gavaged palmitoleate in ApoE-KO mice, through suppressing inflammasome activation and endoplasmic reticulum stress [21]. Compared with ApoE-KO mice, LDL-KO mice used in the present study exhibits less elevated plasma lipid levels, and may be a better model for human dyslipidemia, particularly for patients with Familial Hypercholesterolemia with a defective LDL receptor [20]. The dose of palmitoleate used in the current study (~6 g/kg BW/day) is comparable with that used in atherosclerotic ApoE-KO mice by Çimen et al (1.4 g/kg BW/day) [16]. It is higher than that used in our previous study in type II diabetic mice orally gavaged with 0.3 g/kg/day of palmitoleate [8], due to the differences in mouse type and absorption efficacy rate from different routes of administration.
In terms of the major plasma lipids, palmitoleate supplementation had its greatest effect on TG. Growing evidence suggests that an elevated TG level is an independent risk factor for coronary heart disease, and that TG play and important role in the pathogenesis of atherosclerosis [22]. The TG-reducing effect of palmitoleate observed in LDLR-KO mice was consistent with previous studies in diabetic mice and obese sheep [8,10]. Several species of TG-rich lipoproteins, such as VLDL, VLDL remnants, and chylomicron remnants, appear to promote atherogenesis independently of LDL [23]. VLDL stimulates multiple processes that are involved in atherosclerosis, including activation of monocytes to produce inflammatory mediators, such as TNFα and IL-1β, and activation of insulin resistance pathways in endothelial cells reducing their production of nitric oxide causing vascular dysfunction [24]. Our data show that dietary palmitoleate, but not olive oil, decreased hepatic contents of lipids, particularly TG, and down-regulated expression of Srebp1c and Scd1. These data are in agreement of our previous findings from animal studies [8]. Western diet used in the current study is enriched in fat and carbohydrate, and is known to stimulate de novo lipogenesis, a process that occurs via the transcriptional activation of lipogenic transcription factors, such as SREBP and carbohydrate response-element binding protein [25]. The upregulation of SREBP-1c is known to induce SCD1, a rate-limiting enzyme that converts saturated fatty acids 16:0 and 18:0 into the corresponding MUFA palmitoleate and oleate, respectively [26]. SCD1-deficient mice are known to be protected from insulin resistance, hypertriglyceridemia, hepatic steatosis, and diet-induced obesity [27]. Our data, therefore, suggested that a decrease in steatosis of liver by down-regulating lipogenic genes could potentially explain the decrease in the plasma levels of TG and VLDL observed in mice fed palmitoleate-rich diet.
In terms of cholesterol metabolism, dietary palmitoleate did not decrease plasma and hepatic total cholesterol, as well as cholesterol in LDL, although cholesterol in VLDL fraction is lower than that in control, suggesting a minor role of palmitoleate in modulating LDL cholesterol levels. Although palmitoleate did not alter hepatic expression of Srebp2, a major regulator for cholesterol biosynthesis, it markedly upregulated Cyp7a1, which encodes the rate-limiting enzyme in the classic pathway of bile acid biosynthesis and has been shown to reduce atherosclerosis when overexpressed in mice [28]. Future studies are needed for estimating fecal bile acid excretion to confirm the effect of palmitoleate on bile acid metabolism. Another potential mechanism for reduced atherosclerosis in the palmitoleate supplemented mice may be related to the changes observed in HDL. At least in animals, numerous studies have demonstrated that HDL exerts a protective effect on the development of atherosclerosis through a wide range of mechanisms, such as reverse cholesterol transport (RCT) and anti-inflammatory properties [29]. Our in vitro cholesterol efflux assay revealed that dietary palmitoleate, but not olive oil, improved cholesterol efflux capacity by ABCA1, an ATP-binding cassette transporter that promotes cellular phospholipid and cholesterol efflux [30]. Furthermore, the favorable impact of dietary palmitoleate on HDL observed in the present study is in agreement with previous studies in rodents and human subjects [31,11]
Besides alterations in lipoprotein metabolism, inflammation also contributes to the pathogenesis of atherosclerosis. In particular, infiltrating monocytes and macrophages into plaques play a major role in the production of pro-inflammatory cytokines, such as IL-1β and TNFα [32]. Proinflammatory cytokines were previously shown to be upregulated in LDLR-KO mice fed high-fat diet [33], and our RT-PCR results showed that dietary palmitoleate down-regulated Il-1β and Tnfα expressions. Increased energy intake promotes adipocyte hypertrophy in adipose tissue, an endocrine organ that secretes various proinflammatory adipokines. In vitro studies in human adipocytes showed that palmitoleate treatment led to increased activity of PPARγ that promotes the differentiation of fat cells [34]. However, palmitoleate diet in the present study did not change fat mass or expression of PPAR such as Pparα and Pparγ, suggesting a minor effect of palmitoleate on PPAR in the present mouse model.
A strong association between atherosclerosis and insulin resistance commonly linked to proatherogenic lipid phenotype has been demonstrated [35]. Our results showed that dietary palmitoleate improved glucose metabolism and insulin sensitivity in a mouse model of atherosclerosis. Prior in vitro studies have revealed that palmitoleate can influence pancreatic β-cell survival, insulin secretion, and skeletal muscle insulin response [7,36] and improve glucose control in animal studies [8,10]. Collectively, these data suggest that another beneficial effect of palmitoleate supplementation may relate to glucose homeostasis and warrants further investigation. Limitations of the present study include that only one gender of mice, females, and only a single dose of palmitoleate were used in the present study. More studies in the future are needed in both male and female LDLR-KO mice to explore whether there is any sexual dimorphism in response to the diet and to establish the minimum effective dose of palmitoleic acid needed to induce the beneficial changes observed in the present study. Furthermore, the mechanism underlying triglyceride-lowering effect of palmitoleate is not fully understood, and further studies are needed to address this issue.
In summary, we have shown for the first time that aortic atherosclerosis plaques, as well as impaired lipid and glucose metabolism, were improved in LDLR-KO mice fed Western diet supplemented with palmitoleate compared to those fed Western diet or oleate-rich olive oil-supplemented diet. These positive findings have led us to initiate a clinical trial comparing oleic acid to palmitoleic acid supplementation on various cardiometabolic parameters (ClinicalTrials.gov Identifier: NCT03372733).
Supplementary Material
Acknowledgements
Conceived and designed the experiments: Z.-H.Y., M. A., and A.R. Performed the experiments: Z.-H.Y., M.P., A.N., B.J., M.S., M.P., K.D. Analyzed the data: Z.-H.Y., A.N., Wrote the manuscript: Z.-H.Y. and A.R. This research was supported by the Intramural Research Program of the National Heart, Lung and Blood Institute (NHLBI) at National Institutes of Health. The authors declare no conflict of interest.
Abbreviations
- CVD
cardiovascular disease
- KO
knockout
- FC
free cholesterol
- MUFA
monounsaturated fatty acids
- PL
phospholipid
- TC
total cholesterol
- TG
triglyceride
- VLDL
very-low-density lipoprotein
- LDL
low-density lipoprotein
- HDL
high-density lipoprotein
References
- [1].Rafieian-Kopaei M, Setorki M, Doudi M, Baradaran A, Nasri H, Atherosclerosis: process, indicators, risk factors and new hopes. Int. J. Prev. Med 2014, 5, 927–946. [PMC free article] [PubMed] [Google Scholar]
- [2].Gillingham LG, Harris-Janz S, Jones PJ, Dietary monounsaturated fatty acids are protective against metabolic syndrome and cardiovascular disease risk factors. Lipids 2011, 46, 209–228. [DOI] [PubMed] [Google Scholar]
- [3].Fatima T, Snyder CL, Schroeder WR, Cram D, Datla R, Wishart D, Weselake RJ, Krishna P, Fatty acid composition of developing sea buckthorn (Hippophae rhamnoides L.) berry and the transcriptome of the mature seed. PLoS One 2012, 7, e34099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Maguire LS, O’Sullivan SM, Galvin K, O’Connor TP, O’Brien NM, Fatty acid profile, tocopherol, squalene and phytosterol content of walnuts, almonds, peanuts, hazelnuts and the macadamia nut. Int. J. Food Sci. Nutr 2004, 55, 171–178. [DOI] [PubMed] [Google Scholar]
- [5].Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS, Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 2008, 134, 933–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].de Souza CO, Valenzuela CA, Baker EJ, Miles EA, Rosa Neto JC, Calder PC, Palmitoleic Acid has Stronger Anti-Inflammatory Potential in Human Endothelial Cells Compared to Oleic and Palmitic Acids. Mol. Nutr. Food Res 2018, 62, e1800322. [DOI] [PubMed] [Google Scholar]
- [7].Maedler K, Oberholzer J, Bucher P, Spinas GA, Donath MY, Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic beta-cell turnover and function. Diabetes 2003, 52, 726–733. [DOI] [PubMed] [Google Scholar]
- [8].Yang ZH, Miyahara H, Hatanaka A, Chronic administration of palmitoleic acid reduces insulin resistance and hepatic lipid accumulation in KK-Ay Mice with genetic type 2 diabetes. Lipids Health Dis. 2011, 10, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].de Souza CO., Teixeira AAS, Biondo LA, Lima Junior EA, Batatinha HAP, Rosa Neto JC, Palmitoleic Acid Improves Metabolic Functions in Fatty Liver by PPARα-Dependent AMPK Activation. J. Cell Physiol 2017, 232, 2168–2177. [DOI] [PubMed] [Google Scholar]
- [10].Duckett SK, Volpi-Lagreca G, Alende M, Long NM, Palmitoleic acid reduces intramuscular lipid and restores insulin sensitivity in obese sheep. Diabetes Metab. Syndr. Obes 2014, 7, 553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bernstein AM, Roizen MF, Martinez L, Purified palmitoleic acid for the reduction of high-sensitivity C-reactive protein and serum lipids: a double-blinded, randomized, placebo controlled study. J. Clin. Lipidol 2014, 8, 612–617. [DOI] [PubMed] [Google Scholar]
- [12].Curb JD, Wergowske G, Dobbs JC, Abbott RD, Huang B, Serum lipid effects of a high-monounsaturated fat diet based on macadamia nuts. Arch. Intern. Med 2000, 160, 1154–1158. [DOI] [PubMed] [Google Scholar]
- [13].Griel AE, Cao Y, Bagshaw DD, Cifelli AM, Holub B, Kris-Etherton PM, A macadamia nut-rich diet reduces total and LDL-cholesterol in mildly hypercholesterolemic men and women. J. Nutr 2008, 138, 761–767. [DOI] [PubMed] [Google Scholar]
- [14].Nestel P, Clifton P, Noakes M, Effects of increasing dietary palmitoleic acid compared with palmitic and oleic acids on plasma lipids of hypercholesterolemic men. J. Lipid Res 1994, 35, 656–662. [PubMed] [Google Scholar]
- [15].Schwingshackl L, Hoffmann G, Monounsaturated fatty acids and risk of cardiovascular disease: synopsis of the evidence available from systematic reviews and meta-analyses. Nutrients 2012, 4, 1989–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hawk CT, Leary SL, Morris TH, American College of Laboratory Animal Medicine., European College of Laboratory Animal Medicine Formulary for laboratory animals. 3rd ed. Ames, Iowa: Blackwell Pub., 2005. [Google Scholar]
- [17].Ervin RB, Wright JD, Wang CY, Kennedy-Stephenson J, Dietary intake of fats and fatty acids for the United States population: 1999-2000. Adv. Data 2004, 8, 1–6. [PubMed] [Google Scholar]
- [18].Bernal-Mizrachi C, Weng S, Feng C, Finck BN, Knutsen RH, Leone TC, Coleman T, Mecham RP, Kelly DP, Semenkovich CF, Examethasone induction of hypertension and diabetes is PPAR-alpha dependent in LDL receptor-null mice. Nat Med. 2003, 9, 1069–1075. [DOI] [PubMed] [Google Scholar]
- [19].Vaisman BL, Klein HG, Rouis M, Bérard AM, Kindt MR, Talley GD, Meyn SM, Hoyt RF Jr., Marcovina SM, Albers JJ, Overexpression of human lecithin cholesterol acyltransferase leads to hyperalphalipoproteinemia in transgenic mice. J. Biol. Chem 1995, 270, 12269–12275. [DOI] [PubMed] [Google Scholar]
- [20].Ishibashi S, Goldstein JL, Brown MS, Herz J, Burns DK, Massive xanthomatosis and atherosclerosis in cholesterol-fed low density lipoprotein receptor-negative mice. J Clin Invest. 1994, 93, 1885–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Çimen I, Kocatürk B, Koyuncu S, Tufanlı Ö, Onat UI, Yıldırım AD, Apaydın O, Demirsoy Ş, Aykut ZG, Nguyen UT, Watkins SM, Hotamışlıgil GS, Erbay E, Prevention of atherosclerosis by bioactive palmitoleate through suppression of organelle stress and inflammasome activation. Sci. Transl. Med 2016, 8, 358ra126. [DOI] [PubMed] [Google Scholar]
- [22].Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, Boekholdt SM, Khaw KT, Gudnason V, Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation 2007, 115, 450–458. [DOI] [PubMed] [Google Scholar]
- [23].Nordestgaard BG, Triglyceride-Rich Lipoproteins and Atherosclerotic Cardiovascular Disease: New Insights From Epidemiology, Genetics, and Biology. Circ. Res 2016, 118, 547–563. [DOI] [PubMed] [Google Scholar]
- [24].Kawakami A, Osaka M, Tani M, Azuma H, Sacks FM, Shimokado K, Yoshida M, Apolipoprotein CIII links hyperlipidemia with vascular endothelial cell dysfunction. Circulation 2008, 118, 731–742. [DOI] [PubMed] [Google Scholar]
- [25].Shimano H, Yahagi N, Amemiya-Kudo M, Hasty AH, Osuga J, Tamura Y, Shionoiri F, Iizuka Y, Ohashi K, Harada K, Gotoda T, Ishibashi S, Yamada N, Sterol regulatory element-binding protein-1 as a key transcription factor for nutritional induction of lipogenic enzyme genes. J. Biol. Chem 1999, 274, 35832–35839. [DOI] [PubMed] [Google Scholar]
- [26].Flowers MT, Ntambi JM, Stearoyl-CoA desaturase and its relation to high-carbohydrate diets and obesity. Biochim. Biophys. Acta 2009, 1791, 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ntambi JM, Miyazaki M, Regulation of stearoyl-CoA desaturases and role in metabolism. Prog. Lipid Res 2004, 43, 91–104. [DOI] [PubMed] [Google Scholar]
- [28].Miyake JH, Duong-Polk XT, Taylor JM, Du EZ, Castellani LW, Lusis AJ, Davis RA, Transgenic expression of cholesterol-7-alpha-hydroxylase prevents atherosclerosis in C57BL/6J mice. Arterioscler. Thromb. Vasc. Biol 2002, 22, 121–126. [DOI] [PubMed] [Google Scholar]
- [29].Remaley AT, Norata GD, Catapano AL, Novel concepts in HDL pharmacology. Cardiovasc Res. 2014, 103, 423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yvan-Charvet L, Wang N, Tall AR, The role of HDL, ABCA1 and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler. Thromb. Vasc. Biol 2010, 30, 139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Matthan NR, Dillard A, Lecker JL, Ip B, Lichtenstein AH, Effects of dietary palmitoleic acid on plasma lipoprotein profile and aortic cholesterol accumulation are similar to those of other unsaturated fatty acids in the F1B golden Syrian hamster. J. Nutr 2009, 139, 215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tedgui A, Mallat Z, Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol. Rev 2006, 86, 515–581. [DOI] [PubMed] [Google Scholar]
- [33].Bieghs V, Van Gorp PJ, Wouters K, Hendrikx T, Gijbels MJ, van Bilsen M, Bakker J, Binder CJ, Lütjohann D, Staels B, Hofker MH, Shiri-Sverdlov R, LDL receptor knock-out mice are a physiological model particularly vulnerable to study the onset of inflammation in non-alcoholic fatty liver disease. PLoS One 2012, 7, e30668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sauma L, Stenkula KG, Kjølhede P, Strålfors P, Söderström M, Nystrom FH, PPAR-gamma response element activity in intact primary human adipocytes: effects of fatty acids. Nutrition 2006, 22, 60–68. [DOI] [PubMed] [Google Scholar]
- [35].Semenkovich CF, Insulin resistance and atherosclerosis. J. Clin. Invest 2006, 116, 1813–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Talbot NA, Wheeler-Jones CP, Cleasby ME, Palmitoleic acid prevents palmitic acid-induced macrophage activation and consequent p38 MAPK-mediated skeletal muscle insulin resistance. Mol. Cell Endocrinol 2014, 393, 129–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


