Abstract
Numerous cross-sectional and longitudinal studies have implicated saturated fat-enriched diets in the etio-pathogenesis of Alzheimer’s disease (AD). Emerging evidence shows that saturated fat-enriched diets, such as palmitate-enriched diets, increase amyloid-beta (Aβ) production, the histopathological hallmark of AD. However, the molecular mechanisms that underlie the deleterious effects of palmitate-enriched diets in the augmentation of Aβ genesis are yet to be characterized. Sterol Response Element binding protein 1 (SREBP1) is a transcription factor that is modulated by saturated fatty acids, such as palmitate, and consequently regulates the expression of genes that code for proteins involved in almost all facets of lipid metabolism. Herein, we determined the role of changes in SREBP1 expression and transcriptional activity in the palmitate-induced effects on Aβ genesis and BACE1 expression, the enzyme that catalyzes the rate limiting step in Aβ biosynthesis. We demonstrate that palmitate-induced SREBP1 activation, directly regulates BACE1 expression at the transcriptional level in the mouse hippocampus and mouse Neuro-2a (N2a) neuroblastoma cells. Chromatin Immunoprecipitation (ChIP) studies show that palmitate increases the binding of SREBP1 to the Bace1 promoter region in the mouse hippocampus and mouse N2a neuroblastoma cells. Ectopic expression of the dominant negative SREBP1 mutant and knocking -down SREBP1 expression, significantly reduced the palmitate-induced increase in BACE1 expression and subsequent Aβ genesis in mouse N2a neuroblastoma cells. Our study unveils SREBP1 activation as a novel molecular player in the palmitate-induced up-regulation of BACE1 expression and subsequent Aβ genesis.
Keywords: Aβ, Alzheimer’s disease, BACE 1, palmitate, saturated free fatty acids, SREBP1
Introduction
The membrane-bound aspartyl protease β-site AβPP cleaving enzyme 1 (BACE1) catalyzes the rate-limiting step in Amyloid-β (Aβ) genesis from the transmembrane protein Amyloid-β precursor protein (AβPP) [1]. The accumulation and aggregation of the Aβ peptide is considered as the central biochemical event that triggers a cascade of deleterious pathophysiological events involved in the neurodegenerative changes that characterize Alzheimer’s disease (AD) [2]. The protein levels and the enzymatic activity of BACE1 are markedly up-regulated in the AD brain [3,4]. The etiological factors that underlie the AD pathogenesis and disease progression are poorly characterized and egregiously comprehended. More evidence continues to accumulate implicating dietary factors in increasing the risk as well as abetting the biochemical and signaling mechanisms that are involved in the pathogenesis of AD. High fat diets significantly increase the risk of AD and evoke cognitive impairment in a multitude of rodent models [5-8]. There is a direct correlation between the degree of saturated fatty acids and saturated fat content of the diet to the degree of risk for developing AD [9,10]. Palmitic acid (palmitate) is the most abundant long-chain sFFA in the brain [11] and the diet [12]. Increased levels of palmitate in the plasma, due to direct consumption of palmitate-enriched diet or due to the de novo lipogenesis in the liver, contributes to the burden of the palmitate pool and the total saturated fatty acid pool in the brain [13,14]. The transcription factors, Sterol Response Element Binding Proteins (SREBP) are the master regulators of lipid [15] and cholesterol metabolism [16]. There are two genes in mammals (SREBF1 and SREBF2) that code for three different isoforms – SREBP1a, SREBP1c, and SREBP2 [15]. The SREBP are basic helix-loop-helix leucine zipper transcription factors that regulate distinct target genes [15]. SREBP1c and SREBP2 are master regulators of genes involved in de novo lipogenesis and cholesterol biosynthesis respectively, while SREBP1a regulates genes involved in both, DNL and cholesterol biosynthesis [15]. Palmitate-enriched diets and saturated fat-enriched diets cause SREBP1c activation in the β-cells of the islets of pancreas [17,18] and regulate both SREBP1c and SREBP2 expression and activity in hepatocytes [19]. A recent study found that SREBP2 activity mediates the cholesterol-induced up-regulation in BACE1 expression [20]. We have shown that palmitate-enriched diets and exogenous palmitate-treatment of cells results in increased BACE1 expression and activity leading to increased Aβ genesis [21,22]. However, the extent to which palmitate modulates the expression and activities of SREBP in the brain is not known. Furthermore, the role of palmitate-induced dysregulation in the expression and activities of SREBP that underlie the palmitate-induced up-regulation in BACE1 expression and activity leading to increased Aβ genesis is not known. In this study, we determined the impact of palmitate-enriched diet and exogenous palmitate treatment in the mouse hippocampus and in mouse neuroblastoma Neuro-2a (N2a) cells respectively on the expression and transcriptional activities of SREBP. We also determined the role of SREBP as well as delineated the associated molecular mechanisms that underlie the palmitate-induced up-regulation in BACE1 expression, BACE1 enzymatic activity, and Aβ genesis.
Materials and Methods
Materials.
Mouse Neuro-2a (N2a) neuroblastoma cells stably expressing the AβPP695 bearing the double Swedish (KM595/596NL) and the Indiana (V642F) mutation (N2a-APPSwe/In) were cultured in DMEM : Ham’s F12 with Glutamax (1:1; v/v), 10% fetal bovine serum, and 1% antibiotic/antimycotic mix. Cells were maintained at 37°C in a saturated humidity atmosphere containing 95% air and 5% CO2. All cell culture reagents, with the exception of fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA) and antibiotic/antimycotic mix (Sigma Aldrich, Saint Louis, MO) were purchased from Invitrogen (Carlsbad, CA). Palmitic acid was purchased from Sigma Aldrich (St. Louis, MO. The expression plasmids for ectopically expressing the dominant negative SREBP1 mutant (dnSREBP1) (CMV500 A-SREBP1, Addgene plasmid # 33357) and the dominant negative SREBP2 mutant (dnSREBP2) (CMV500 A-SREBP2, Addgene plasmid # 33358) were a gift from Dr. Charles Vinson [23]. The mouse Srebp1 (Srebf1) and Srebp2 (Srebf2) double-stranded siRNA (Silencer® Select Pre-Designed & Validated siRNA) and their respective scrambled non-silencing control siRNA were purchased from Thermo Fisher Scientific (Waltham, MA). The list of siRNA and their respective targets are enumerated in Table 1. The mouse Srebp1 (Srebf1) and Srebp2 (Srebf2) shRNA encoded in pLKO.1 lentiviral vector were purchased from Open Biosystems (GE Dharmacon, Lafayette, CO) and their respective target sequences are enumerated in Table 2.
Table 1.
List of siRNA used for RNA interference
| Species | Gene ID | mRNA target | RNA interference | RefSeq GenBank |
siRNA location |
|---|---|---|---|---|---|
| Mouse | 20787 | Srebf1 | siRNA | NM_011480.3 | 374 |
| Mouse | 20787 | Srebf1 | siRNA | AB017337.1 | 190 |
| Mouse | 20787 | Srebf1 | siRNA | AF374266.1 | 153 |
| Mouse | 20787 | Srebf1 | siRNA | AK052628.1 | 375 |
| Mouse | 20787 | Srebf1 | siRNA | AK150052.1 | 363 |
| Mouse | 20787 | Srebf1 | siRNA | AK154424.1 | 357 |
| Mouse | 20787 | Srebf1 | siRNA | AK169607.1 | 375 |
| Mouse | 20787 | Srebf1 | siRNA | BC056922.1 | 356 |
| Mouse | 20788 | Srebf2 | siRNA | NM_033218.1 | 608 |
| Mouse | 20788 | Srebf2 | siRNA | AF374267.1 | 461 |
| Mouse | 20788 | Srebf2 | siRNA | AK032847.1 | 632 |
| Mouse | 20788 | Srebf2 | siRNA | AK155857.1 | 556 |
Table 2.
List of shRNA and mature antisense sequences used for RNA interference
| Species | Gene ID | mRNA target | RNA interference |
Sequence |
|---|---|---|---|---|
| Mouse | 20787 | Srebf1 | shRNA | AAACGTGTCAAGAAGTGCAGG |
| Mouse | 20787 | Srebf1 | shRNA | AATACCCTCCTCATAGCAGGC |
| Mouse | 20787 | Srebf1 | shRNA | AAGAAGCGGATGTAGTCGATG |
| Mouse | 20787 | Srebf1 | shRNA | AAAGGCCAGT ACACACAGGGC |
| Mouse | 20787 | Srebf1 | shRNA | ATGAGGTTCCAAAGCAGACTG |
| Mouse | 20788 | Srebf2 | shRNA | TTTAAGAAGTAGCTAGCCAAG |
| Mouse | 20788 | Srebf2 | shRNA | AATGAACAAGGCTTAGTCAGG |
| Mouse | 20788 | Srebf2 | shRNA | TTCTGGTATATCAAAGGCTGC |
| Mouse | 20788 | Srebf2 | shRNA | ATGATATTGTGTGTTGTCCGC |
| Mouse | 20788 | Srebf2 | shRNA | AACAACAAAGAGAACCAGAGG |
Cell Culture and Treatments.
N2a-APPSwe/In cells were transfected with the designated vectors as previously described [24]. Transfected N2a-APPSwe/In cells were treated with BSA (bovine serum albumin)-conjugated palmitate as shown previously [21,22]. Briefly, palmitate stock solution of 250mM was prepared in 100% ethanol. BSA (5mM) stock solution was prepared in MilliQ water (18MΩ). Both, the palmitate and BSA stock solution were sterile filtered using a 0.2μm filter. The requisite amounts of palmitate and BSA were added to sterile serum-free medium to yield the designated terminal palmitic acid concentrations with the ratio of palmitate and BSA being 6:1. The respective media were incubated for 1.5 hours to conjugate the palmitic acid to the BSA. The cells were treated with the designated concentration of palmitic acid conjugated to BSA for 24 hours. The BSA concentration (6.0μM) corresponding to the palmitate concentration (100μM of palmitate) was used as the experimental control. At concentrations above 100μM, palmitate will likely form micelles, likely making the data interpretation difficult.
Mouse experiments.
The wild-type C57BL/6J mice were procured from The Jackson Laboratory (Stock # 000664). The mice were housed in individually ventilated cages at an ambient room temperature (23-25°C) and ambient relative humidity ranging between 50-70%. The mice were maintained on 12:12 hour light:dark cycle and allowed access to food and water ad libitum. The wild-type C57BL/6J mice (all males, nine months of age) counterparts were segregated into two groups, one fed a palmitate-enriched diet (TD 110616, Harlan Teklad, 2.2% w/w palmitic acid; n=6) and the other fed the corresponding control diet (TD 85172, Harlan Teklad, 0.8% w/w palmitic acid; n=6) for three months. The diets were isocaloric in relation to each other and the respective composition of the diets is shown in Table 3. Food-intake was monitored for the span of 24 hours, once every two weeks. Body weights were measured every two weeks. No significant changes in body weight and food intake were observed among the different cohorts of mice. Necropsy was performed at twelve (12) months of age. Mice were anesthetized with sodium pentobarbital and perfused transcardially with Dulbecco’s phosphate-buffered saline. Brains were promptly removed and hippocampi immediately dissected and homogenized. All animal procedures were carried out in accordance with the U.S. Public Health Service Policy on the Humane Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of North Dakota.
Table 3.
Composition of the control chow diet and palmitate-enriched diet
| Control chow diet NIH07 open formula rodent diet – original - 0.8 % palmitic acid |
palmitate-enriched diet NIH07 open formula rodent diet – palmitate enriched – 2.2 % palmitic acid |
|
|---|---|---|
| Protein | 23.60 % w/w | 23.60 % w/w |
| Carbohydrates | 65.80 % w/w | 65.80 % w/w |
| Total Fat | 5.60 % w/w | 5.60 % w/w |
| Total Energy | 4.08 Kcal/gram | 4.08 Kcal/gram |
| Myristic acid (14:0) | 0.10 % w/w | 0.10 % w/w |
| Palmitic acid (16:0) | 0.80 % w/w | 2.20 % w/w |
| Stearic acid (18:0) | 0.20 % w/w | 0.20 % w/w |
| Palmitoleic acid (16:1) | trace | trace |
| Oleic acid (18:1) | 1.20 % w/w | 1.20 % w/w |
| Gadoleic acid (20:1) | trace | trace |
| Linoleic acid (18:2 n6) | 2.20 % w/w | 0.80 % w/w |
| Linolenic acid (18:3 n3) | 0.20 % w/w | 0.20 % w/w |
| Arachidonic acid (20:4 n6) | trace | trace |
| EPA (20:5 n3) | 0.10 % w/w | 0.10 % w/w |
| DHA (22:6 n3) | 0.30 % w/w | 0.30 % w/w |
Western Blot analysis.
Whole cell, cytosolic and nuclear homogenates from N2a-APPSwe/In cells as well as the mouse cortices and hippocampi were prepared as previously described [25,26]. In brief, homogenates were washed with PBS, trypsinized, and centrifuged at 5000 g. The pellets were washed again with PBS and homogenized in mammalian protein extraction reagent (MPER, Thermo Scientific, Rockford, IL) supplemented with protease and phosphatase inhibitors for whole cell homogenates or homogenized in NE-PER tissue protein extraction reagent (Thermo Scientific, Rockford, IL) supplemented with protease and phosphatase inhibitors for cytosolic and nuclear homogenates. Proteins (10-40μg) were resolved on SDS-PAGE gels followed by transfer to a polyvinylidene difluoride membrane (BioRad, Hercules, CA) and incubation with the monoclonal antibodies listed in Table 4. The origin, source, the dilutions of the respective antibodies used for this study is compiled in Table 4. β-actin was used as a gel loading control for whole cell and cytosolic homogenates, whereas Histone H3 was used as a gel loading control for nuclear homogenates. The blots were developed with enhanced chemiluminescence (Clarity™ Western ECL blotting substrate, Bio-Rad, Hercules, CA) and imaged using a LiCOR Odyssey Fc imaging system.
Table 4.
List of monoclonal and polyclonal antibodies used in the study
| Antibody | Application | Dilution | Amount | Host | Manufacturer | Catalog # |
|---|---|---|---|---|---|---|
| β-Actin | WB | 1:2500 | 2μg | mouse | Santa Cruz BioTechnology | sc-47778 (C4) |
| BACE1 | WB | 1:1000 | 5 μg | rabbit | EMD Millipore | AB5832 |
| Histone H3 | WB | 1:1000 | 5 μg | rabbit | Santa Cruz BioTechnology | sc-8654 (C16) |
| SREBP1 | WB | 1:500 | 10 μg | mouse | Abcam | ab3259 |
| SREBP1 | WB | 1:500 | 10 μg | mouse | Active Motif | 39939 |
| SREBP1 | ChIP | — | 5 μg | mouse | Active Motif | 39939 |
| SREBP2 | WB | 1:500 | 10 μg | mouse | R & D systems | MAB7119 |
| SREBP2 | WB | 1:500 | 10 μg | rabbit | Abcam | ab30682 |
| SREBP2 | ChIP | — | 5 μg | goat | R & D systems | AF7119 |
Enzyme-linked immunosorbent assay (ELISA).
Aβ1-42 levels in N2a-APPSwe/In cells were quantified in the conditioned media (secreted) and cellular homogenates (intracellular) using an amyloid β mouse ELISA kit (Invitrogen, Carlsbad, CA) following the manufacturer’s protocol and as described earlier [27]. Intracellular Aβ1-42 levels in the cellular homogenates were normalized to total protein content in the samples (pg/mg protein). Treatments were performed in quadruplet, (n=4, four biological replicates with three technical replicates within each biological replicate). The secreted Aβ1-42 levels measured in the culture medium are expressed in pg/mL of media.
Quantitative real time RT-PCR analysis.
Total RNA was isolated and extracted from treated N2a-APPSwe/In cells using the 5 prime “PerfectPure RNA tissue kit” (5 Prime, Inc., Gaithersburg, MD) following manufacturer’s instructions and as described previously [28,29]. cDNA was obtained by reverse transcribing 1μg of extracted RNA using an iScript cDNA synthesis kit” (BioRad, Hercules, CA). cDNA was obtained by reverse transcribing 1μg of extracted RNA using an iScript cDNA synthesis kit" (BioRad, Hercules, CA).The quantitative Real-time RT-PCR analysis for BACE1 was performed using TaqMan chemistry using “Assays-on-Demand” probes (ABI, Foster City, CA) for mouse Bace1 (Bace1 gene) (Mm00478664_m1). The expression of specific transcripts amplified was normalized to the expression of 18s rRNA. Real-time qRT-PCR analysis to quantify mouse Srebp1A, Srebp1C, and Srebp2 mRNA transcripts was performed using amplification kit from ThermoFisher Scientific (Power SYBR® Green RNA-to-CT™ 1-Step Kit, Catalog# 4389986) and transcript-specific primers following manufacturer’s instructions. The exon-specific primers are enumerated in Table 5. The amplification was performed using the “StepOnePlus” PCR System (ABI, Foster City, CA). The expression of specific Srebp1a, Srebp1c, and Srebp2 transcripts amplified was normalized to the expression of β-Actin (Actb). The data were quantified and expressed as fold-change compared to the control by using the ΔΔCT method.
Table 5.
List of primers used for qRT-PCR and ChIP analysis
| Species | Gene | mRNA | ChIP target |
Gene ID |
Application | Reference Sequence |
Primers |
|---|---|---|---|---|---|---|---|
| Mouse | Srebf1 | Srebp1a | — | 20787 | qRT-PCR | NM_011480.4 | 5’-tagtccgaagccgggtgggcgccggcgccat-3’ [73] 5’-gatgtcgttcaaaaccgctgtgtgtccagttc-3’ [73] |
| Mouse | Srebf1 | Srebp1c | — | 20787 | qRT-PCR | NM_001358314.1 | 5’-atcggcgcggaagctgtcggggtagcgtc-3’ [73] 5’-actgtcttggttgttgatgagctggagcat-3’ [73] |
| Mouse | Srebf1 | Srebp2 | — | 20788 | qRT-PCR | NM_033218.1 | 5’-cacaatatcattgaaaagcgctaccggtcc-3’ [73] 5’-tttttctgattggccagcttcagcaccatg-3’ [73] |
| Mouse | Actb | β-Actin | — | 11461 | qRT-PCR | NM_007393.5 | 5’-ggtcgtaccacaggcattgtgatg-3’ [73] 5’-ggagagcatagccctcgtagatgg-3’ [73] |
| Mouse | Bacel | — | Srebp1 | 23821 | ChIP | NC_000075.6 | 5’-gagctgggagctggattatg-3’ 5’-ggacccagctacatctggac-3’ |
| Mouse | Bacel | — | Srebp2 | 23821 | ChIP | NC_000075.6 | 5’-gagctgggagctggattatg- 3’ 5’-ggacccagctacatctggac- 3’ |
FRET-based BACE1 activity assay.
BACE1 activity in N2a-APPSwe/In cellular and brain tissue homogenates was determined using a FRET-based kit from Sigma-Aldrich (St. Louis, MO, Catalog # CS0010) following manufacturer’s protocol and as described previously [30]. The raw data expressing the BACE1 activity in terms of percentage of substrate cleaved in respective samples was further normalized and expressed as fold-change compared to control.
SREBP1 and SREBP2 transcriptional activity assays.
The transcriptional activities of SREBP1 and SREBP2 were determined by the ELISA method using kits from Abcam (ab133125 for SREBP1 and ab133111 for SREBP2) following manufacturer’s protocol. Briefly, nuclear homogenate equivalent to 30μg of the protein content was added to each of the wells of the 96-well plate containing the double stranded DNA sequence harboring the consensus SREBP-binding sequence (sterol regulatory element, SRE) coated onto the wells. The nuclear extract was allowed to hybridize with the coated double stranded DNA sequence harboring the consensus SRE (sterol regulatory element) in the plate overnight at 4°C. The activated SREBP transcription factor complex was detected by addition of a specific primary antibody directed against either SREBP1 or SREBP2 and a secondary antibody conjugated to HRP added to provide a sensitive colorimetric readout at 450 nm.
Chromatin Immunoprecipitation (ChIP) Analysis.
ChIP analysis was performed to evaluate the extent of SREBP1 and SREBP2 binding to the SRE in the mouse Bace1 promoter region using “SimpleChIP™ Enzymatic Chromatic IP kit” from Cell Signaling (Boston, MA) following manufacturer’s instructions and as described earlier [31]. The relative abundance of the SREBP1- and SREBP2-antibody precipitated chromatin containing the SRE binding site in the mouse Bace1 promoter region was determined by qPCR using sequence specific primers (Qiagen Inc. Valencia, CA) (Table 5). The amplification was performed using the “StepOnePlus” PCR System (ABI, Foster City, CA). The fold enrichment of the bound - SREBP1 and SREBP2 in the mouse Bace1 promoter region was calculated using the ΔΔCT method which normalizes ChIP CT values of each sample to the % input and background. The data was further normalized and expressed as fold-change compared to control.
Statistical analysis.
The significance of differences among the samples was assessed by non-parametric Kruskal-Wallis One Way Analysis of Variance followed by Dunn’s post-hoc test. Statistical analysis was performed with GraphPad Prism 6. Quantitative data for all the assays are presented as mean values ± S.D (mean values ± standard deviation) with unit value assigned to control and the magnitude of differences among the samples being expressed relative to the unit value of control as fold-change. Quantitative data for ELISA analysis are presented as mean values ± S.D with absolute concentrations of Aβ42 reported.
Results
Palmitate increases the expression and transcriptional activities of SREBP1 and SREBP2 in the mouse hippocampus and the N2a-APPSwe/In mouse neuroblastoma cells
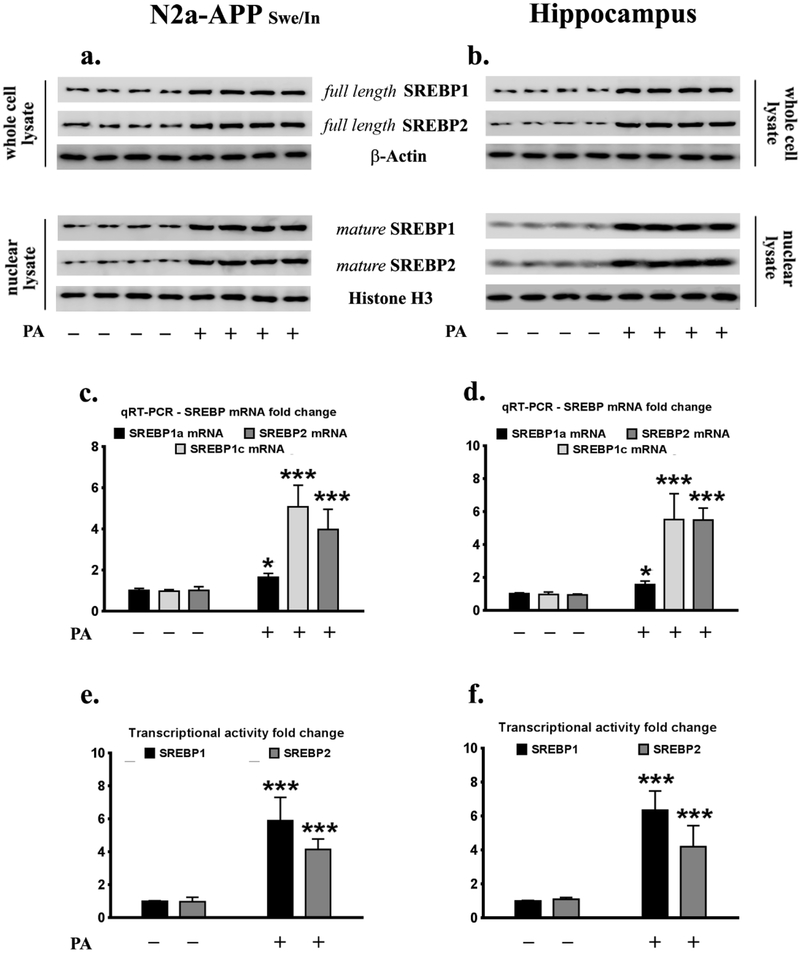
We first determined the effects of palmitate treatment on the expression levels and the transcriptional activities of SREBP1 and SREBP2 in cultured N2a-APPSwe/In mouse neuroblastoma cells that stably express the Swedish (KM595/596NL) and the Indiana (V642F) double mutant AβPP695. Our previous studies have demonstrated that palmitate treatment, for 24 hours at 100μM concentration, increases BACE1 expression in cultured SH-SY5Y-APPSwe cells [21,22]. In this study, we used the same validated treatment paradigm, palmitate conjugated to BSA (molar ratio 6:1) at a concentration of 100μM, to treat N2a-APPSwe/In mouse neuroblastoma cells for 24 hours. We also determined the effects of feeding a palmitate-enriched diet (2.2% w/w palmitic acid vs 0.8% w/w palmitic acid in the control chow) for three months on the expression levels and the transcriptional activities of SREBP1 and SREBP2 in the hippocampi of C57BL/6J mice. Exogenous palmitate treatment of N2a-APPSwe/In cells and feeding C57BL/6J mice the palmitate-enriched diet for three months increased the protein levels of the precursor or full length SREBP1 (flSREBP1) (the antibody does not distinguish between isoform 1a and 1c) and precursor or full length SREBP2 (flSREBP2) in the whole cell lysates from N2a-APPSwe/In cells (Fig. 1a) and the whole cell homogenates from the hippocampi of mice (Fig. 1b). Exogenous palmitate treatment and the palmitate-enriched diet, more profoundly increased the levels of the processed mature SREBP1 (mSREBP1) (the antibody does not distinguish between isoform 1a and 1c) and mature SREBP2 (mSREBP2) in the nuclear lysates from N2a-APPSwe/In cells (Fig. 1a) and the nuclear fraction of the homogenates from the hippocampi of mice (Fig. 1b). There was a greater increase in mSREBP1 and mSREBP2 in the nuclear lysates, both in the palmitate-treated N2a-APPSwe/In cells (Fig. 1a) and the hippocampi of mice fed a palmitate-enriched diet (Fig. 1b), indicative of a pronounced increase in the processing and subsequent nuclear translocation of the cleaved mature forms of SREBP1 and SREBP2. Exogenous palmitate treatment also increased the Srebp1a, srebp1c, and Srebp2 mRNA expression by 1.6-fold, 5.1-fold, and 3.9-fold respectively in N2a-APPSwe/In cells (Fig. 1c), while feeding C57BL/6J mice a palmitate-enriched diet resulted in a 1.5-fold, 5.6-fold and 5.5-fold augmentation in the Srebp1a, Srebp1c, and Srebp2 mRNA expression in the hippocampus, respectively (Fig. 1d). Transcriptional activity assays validated the functional relevance of this increase in mRNA expression and protein levels of SREBP1 and SREBP2, as exogenous palmitate treatment of N2a-APPSwe/In cells increased the transcriptional activities of SREBP1 and SREBP2 by 5.9-fold and 4.1-fold respectively (Fig. 1e). The transcriptional activities of SREBP1 and SREBP2 were also profoundly increased, by 6.4-fold and 4.2-fold respectively in the nuclear lysates from the hippocampi of C57BL/6J mice fed a palmitate-enriched diet (Fig. 1f).
Fig.1. Palmitate increases SREBP1 and SREBP2 expression in N2a-APPSwe/In cells and the mouse hippocampus.
Representative western blots show that treatment with palmitate (100μM for 24 hours) significantly increases the protein levels of full length SREBP1 (flSREBP1) and flSREBP2, in the whole cell lysates, as well as mature SREBP1 (mSREBP1) and mSREBP2 in the nuclear fractions from N2a-APPSwe/In cells (a). Palmitate-enriched diet for three months, also results increased the protein levels of full length SREBP1 (flSREBP1) and flSREBP2, in the whole cell homogenates from the hippocampus, as well as mature SREBP1 (mSREBP1) and mSREBP2 in the nuclear homogenates from the hippocampus of C57BL/6J wild-type mice (b). Real-time qRT-PCR demonstrates that palmitate treatment (100μM for 24 hours) and a palmitate enriched diet significantly increase the mRNA expression of Srebp1a, Srebp1c, and Srebp2 isoforms in N2a-APPSwe/In cells (c) and the mouse hippocampus (d), respectively. Palmitate treatment (100μM for 24 hours) and a palmitate-enriched diet significantly increase the transcriptional activities of SREBP1 and SREBP2 in N2a-APPSwe/In cells (e) and the mouse hippocampus (f), respectively. Data is expressed as Mean ± S.D and includes determination made in four (n=4) separate cell culture experiments as well as in six (n=6) different animals from each group. *p < 0.05, ***p < 0.001 versus BSA-treated cells or C57BL/6J wild-type mice fed a control chow diet. PA = palmitic acid
Palmitate-induced BACE1 expression and the subsequent Aβ genesis is contingent on SREBP1 expression and activation.
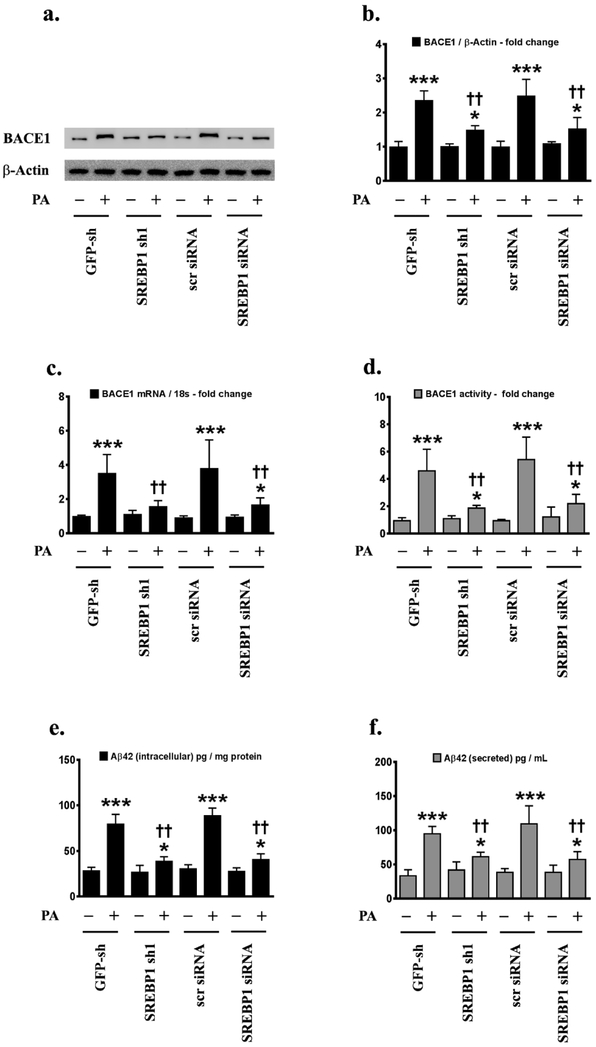
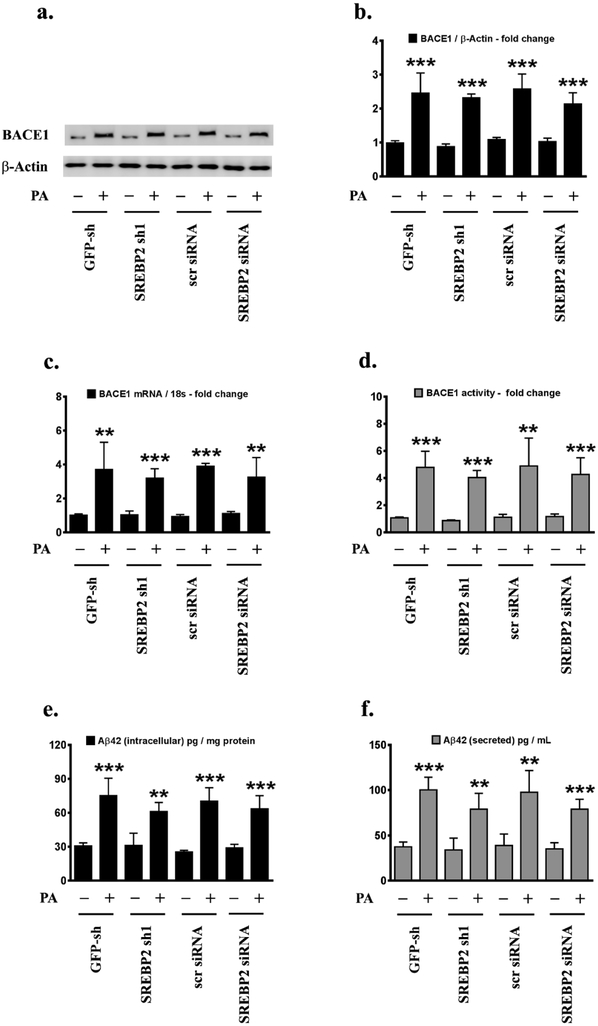
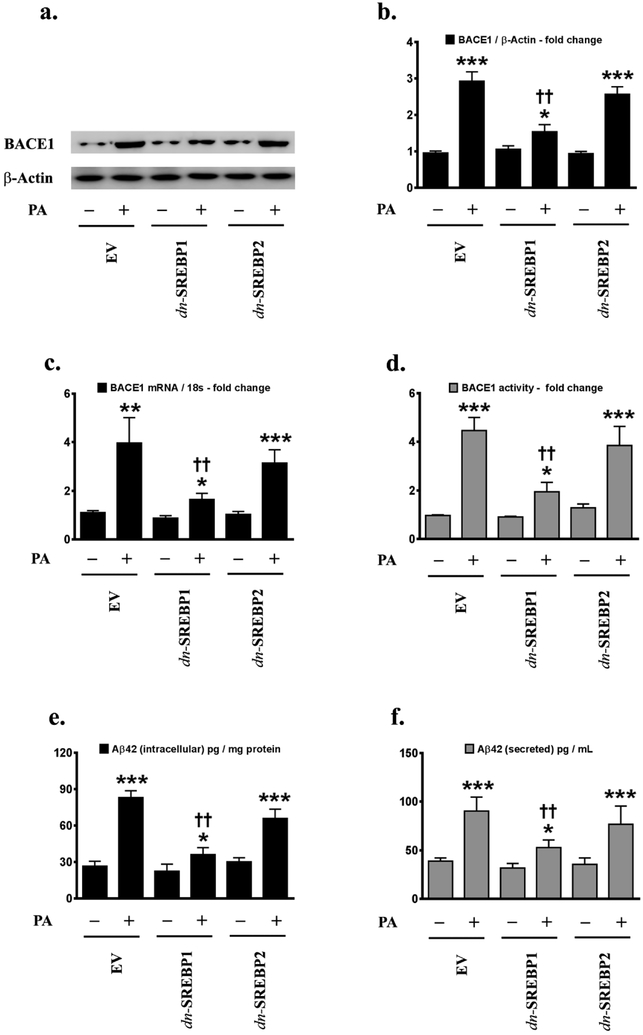
Our previous study has demonstrated that palmitate induces BACE1 expression and Aβ production in treated SH-SY5Y-APPSwe and the hippocampi of C57BL/6J mice fed a palmitate-enriched diet [22]. We next determined the role and contribution of palmitate-induced activation of SREBPs in the palmitate-induced increase in BACE1 expression and Aβ genesis in N2a-APPSwe/In cells. To this end, we knocked-down either SREBP1 or SREBP2 expression using an RNA-interference approach and determined BACE1 expression, BACE1 enzymatic activity, and Aβ genesis in palmitate-treated N2a-APPSwe/In cells. Palmitate-induced increase in BACE1 protein levels (Fig.2a,2b), BACE1 mRNA expression (Fig.2c) and BACE1 enzymatic activity (Fig.2d), as well as an increase in intracellular Aβ42 (Fig.2e) and secreted Aβ42 levels (Fig.2f), were significantly mitigated in SREBP1 knocked-down N2a-APPSwe/In cells compared to palmitate-treated N2a-APPSwe/In cells transfected with GFP-shRNA control or scrambled siRNA control (Fig.2a-2f). However, knocking-down SREBP2 expression did not attenuate the palmitate-induced increase in BACE1 protein levels (Fig.3a,3b), BACE1 mRNA expression (Fig.3c) and BACE1 enzymatic activity (Fig.3d), as well as the increase in intracellular Aβ42 (Fig.3e) and secreted Aβ42 levels (Fig.3f) in N2a-APPSwe/In cells (Fig.3a-3f). The aforementioned findings demonstrated that SREBP1 expression, but not SREBP2 expression, is necessary for the full spectrum effects of palmitate on increased BACE1 expression, augmnented BACE1 enzymatic activity, and enhanced Aβ genesis. To further unequivocally implicate enhanced SREBP1 transcriptional activity in the palmitate-induced increase in BACE1 expression and Aβ production, we determined the effects of exogenous palmitate in N2a-APPSwe/In cells ectopically expressing either the transcriptionally dead dominant negative SREBP1 (dnSREBP1) or the transcriptionally dead dominant negative SREBP2 (dnSREBP2). N2a-APPSwe/In cells ectopically expressing dnSREBP1 did not exhibit the commensurate degree of increase in palmitate-induced BACE1 protein levels (Fig.4a,4b), BACE1 mRNA expression (Fig.4c) and BACE1 enzymatic activity (Fig.4d), as well as the commensurate degree of increase in the palmitate-induced augmentation of intracellular Aβ42 (Fig.4e) and secreted Aβ42 levels (Fig.4f) compared to the palmitate-treated empty vector transfected native N2a-APPSwe/In cells (Fig.4a-4f). However, ectopically expressing the transcriptionally dead dominant negative SREBP2 (dnSREBP2) did not affect the palmitate-induced increase in BACE1 protein levels (Fig. 4a,4b), BACE1 mRNA expression (Fig.4c) and BACE1 enzymatic activity (Fig.4d), as well as palmitate-elicited increase in intracellular Aβ42 (Fig.4e) and secreted Aβ42 levels (Fig.4f), compared to the palmitate-treated empty vector transfected native N2a-APPSwe/In cells (Fig.4a-4f).
Fig.2. SREBP1 mediates the palmitate-induced increase in BACE1 expression and subsequent Aβ genesis.
Representative western blots (a) and densitometric analysis (b) show that knocking-down SREBP1 expression using an RNAi approach significantly attenuates the palmitate-induced increase in BACE1 protein levels in the whole cell homogenates from N2a-APPSwe/In cells. Knocking-down SREBP1 expression attenuates the palmitate-induced increase in BACE1 mRNA expression (c), BACE1 enzymatic activity (d), and intracellular Aβ42 species in the whole cell lysates (e) and secreted Aβ42 species in the conditioned media (f) of N2a-APPSwe/In cells. Data is expressed as Mean ± S.D and includes determination made in four (n=4) separate cell culture experiments. *p < 0.05, ***p < 0.001 versus BSA-treated GFP knock-down cells or BSA-treated scrambled siRNA transfected cells; ††p < 0.01, versus palmitate-treated GFP knock-down cells or palmitate-treated scrambled siRNA transfected cells. PA = palmitic acid
Fig.3. Palmitate-induced increase in BACE1 expression and Aβ genesis is not contingent on SREBP2.
Representative western blots (a) and densitometric analysis (b) show that knocking-down SREBP2 expression using an RNAi approach does not significantly affect the palmitate-induced increase in BACE1 protein levels, BACE1 mRNA expression (c), or BACE1 enzymatic activity (d) in N2a-APPSwe/In cells. ELISA immunoassays show that knocking-down SREBP2 expression does not significantly mitigate the exogenous palmitate treatment-induced increase in the levels of the intracellular Aβ42 species in the whole cell lysates (e) and secreted Aβ42 species in the conditioned media (f), from N2a-APPSwe/In cells. Data is expressed as Mean ± S.D and includes determination made in four (n=4) separate cell culture experiments. **p < 0.01, ***p < 0.001 versus BSA-treated GFP knock-down cells or BSA-treated scrambled siRNA transfected cells. PA = palmitic acid
Fig.4. Ectopically expressed dominant negative SREBP1, but not dominant negative SREBP2, abrogates the palmitate-induced BACE1 expression.
Representative western blots (a) and densitometric analysis (b) show that the ectopic expression of the dominant negative SREBP1 (dnSREBP1), but not the dominant negative SREBP2 (dnSREBP2), abrogates the palmitate-induced increase in BACE1 protein levels, BACE1 mRNA expression (c) and BACE1 enzymatic activity (d) in mouse N2a-APPSwe/In cells. ELISA immunoassays show that the ectopic overexpression of the dominant negative SREBP1 (dnSREBP1), but not the dominant negative SREBP2 (dnSREBP2), abrogates the palmitate-induced increase in the levels of the intracellular Aβ42 species in the whole cell lysates (e) and secreted Aβ42 species in the conditioned media (f), from N2a-APPSwe/In cells. Data is expressed as Mean ± S.D and includes determination made in three (n=4) separate cell culture experiments. *p < 0.05, **p < 0.01, ***p < 0.001 versus BSA-treated empty vector (EV)-transfected cells; ††p <0.01, versus palmitate-treated empty vector (EV)-transfected cells. PA = palmitic acid
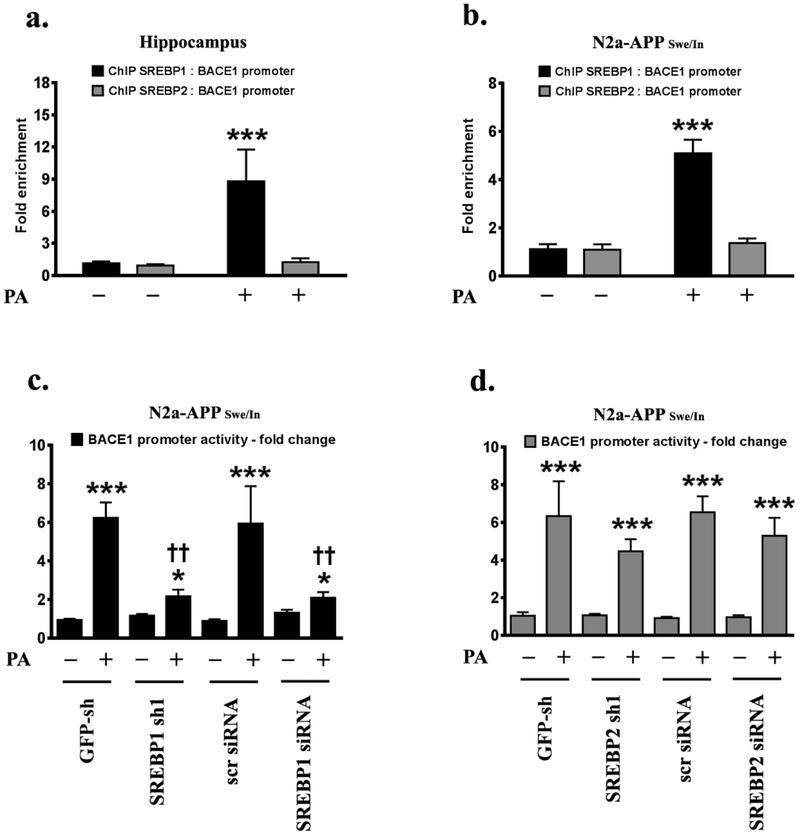
Palmitate increases the binding of SREBP1, but not SREBP2, in the proximal Bace1 promoter resulting in the transactivation of the Bace1 promoter
We further characterized and delineated the mechanism that underlies the palmitate-induced SREBP1-mediated increase in BACE1 expression. ChIP analysis revelaed a significant 8.5-fold increase in SREBP1 binding to the SRE in the Bace1 promoter region in the hippocampi of C57BL/6J mice fed a palmitate-enriched diet (Fig.5a). This increase in SREBP1-binding to the SRE site in the Bace1 promoter, was recapitulated in the exogenous palmitate-treated N2a-APPSwe/In cells with a significant 5.1-fold increase in enrichment of SREBP1 at the SRE site in the Bace1 promoter (Fig.5b). However, no changes in SREBP2 binding to the SRE site in the Bace1 promoter region were observed in the hippocampi of C57BL/6J mice fed a palmitate-enriched diet (Fig.5a) as well as in the exogenous palmitate-treated N2a-APPSwe/In cells (Fig. 5b). Exogenous palmitate treatment increased the SRE-constituting Bace1 promoter-driven luciferase reporter activity by 6.3-fold in treated N2a-APPSwe/In cells (Fig.5c, Fig.5d). Knocking-down SREBP1 significantly attenuated the palmitate-evoked increase in SRE-constituting Bace1 promoter-driven luciferase reporter activity (Fig.5c). However, knocking-down SREBP2 did not elicit any effect on the palmitate-induced augmentation of SRE-constituting Bace1 promoter-driven luciferase reporter activity (Fig.5d), further reinforcing the mediatory role of SREBP1, and not SREBP2, in the palmitate-induced transactivation of the Bace1 promoter.
Fig.5. Palmitate-induced increase in Bace1 promoter transactivation is mediated by SREBP1.
ChIP analysis demonstrates that a palmitate-enriched diet increases the binding of SREBP1, but not SREBP2, to the canonical SRE site in the Bace1 promoter in the hippocampi of C57BL/6J mice (a) and in the mouse N2a-APPSwe/In cells (b). Luciferase reporter assays to determine the SRE-driven Bace1 promoter activity show that knocking-down SREBP1 (c), but not SREBP2 (d), mitigates the exogenous palmitate treatment (100μM for 24 hours)-induced increase in Bace1 promoter activity. Data is expressed as Mean ± S.D and includes determination made in four (n=4) separate cell culture experiments as well as in six (n=6) different animals from each group. *p < 0.05, ***p < 0.001 versus BSA-treated GFP shRNA transfected cells or BSA-treated scrambled siRNA transfected cells or C57BL/6J wild-type mice fed a control chow diet; ††p <0.01, versus palmitate-treated GFP shRNA transfected cells or palmitate-treated scrambled siRNA transfected cells PA = palmitic acid
Discussion
Our current study highlights the critical role of SREBP1 transcriptional activity in mediating the palmitate-induced increase in BACE1 expression and subsequent Aβ genesis. Our study is the first todate to demonstrate that SREBP1 directly regulates BACE1 expression by transcriptional regulation and the subsequent Aβ genesis in response to a palmitate-enriched diet or exogenous palmitate treatment. Our unique study implicates enhanced expression of SREBP1 and augmented SREBP1 transcriptional activity in the palmitate-induced increase in BACE1 expression and Aβ genesis. The role of SREBP1 in Alzheimer’s disease is not well characterized at the molecular level. On the other hand, overexpression of SREBP2 in APP/PS1 and 3xTg-AD mouse models of Alzheimer’s disease results in increased accumulation of Aβ burden and enhancement of tau tangle pathology as a consequence of increased SREBP2-mediated cholesterol biosynthesis and enhanced mitochondrial cholesterol loading [32]. Furthermore, SREBP2 has been shown to directly up-regulate BACE1 expression in male Wistar rats fed a high cholesterol diet [20]. However, until our current study, no molecular studies todate have yet implicated the involvement of SREBP1 dysregulation in the regulation of mechanisms inherent to AD. The effects of palmitate-enriched diets and exogenous palmitate on the regulatory mechanisms involved in BACE1 expression and Aβ genesis have also not been exhaustively elucidated, with very few studies having determined the role of palmitate per se, and not high saturated fat diet, in the regulation of BACE1 expression and Aβ genesis. Our previous study has demonstrated that a palmitate-enriched diet, as well as treatment of cultured cells with exogenous palmitate, increases phosphorylation of tau, enhances BACE1 expression and the subsequent Aβ genesis, by evoking endoplasmic reticulum (ER) stress and C/EBP Homologous Protein (CHOP) activation [21,22,33]. Other studies have shown the effects of exogenous palmitate on BACE1 expression in primary cortical neuronal cultures and primary astrocytic cultures in vitro [34-37]. Our study is the first to demonstrate the effects of a palmitate-enriched diet in eliciting an increase in SREBP1 expression and transcriptional activity in the hippocampus which consequently transactivates the Bace1 promoter culminating in augmented BACE1 expression, BACE1 enzymatic activity, and subsequent Aβ genesis.
In the context of high fat diet (HFD) feeding studies that entailed feeding AD-transgenic mice diets that lacked caloric parity, a plethora of studies found an increase in BACE1 activity and a commensurate augmentation of Aβ plaque burden [38-43]. However, the aforementioned studies utilized a dietary regimen that had other confounding variables such as high sucrose content [38] or high cholesterol content [41,42] coupled with high fat content. It is imperative to appreciate that high sucrose diets as well as high cholesterol diets alone, increase Aβ plaque burden in rodents [44-47] and are associated with the pathophysiology of other diseases [48]. Our study is the first to address the effects of a palmitate-enriched diet that is isocaloric with parity in total calories and caloric density. While we show that palmitate increased SREBP1 and BACE1 expression and activity, and subsequent Aβ genesis in N2a-APPSwe/In cells, however, the effects of palmitate-enriched diet on SREBP1, BACE1, and Aβ in mice may also be due in part to the reduction in linoleate in the diet. When formulating the palmitate-enriched diet, we have offset the increase in palmitic acid by decreasing linoleic acid to ensure that both the palmitate-enriched and the control diet are isocaloric. In line with the suggestion that the reduction in linoleic acid is a possible contributor in the increase in SREBP1, BACE1, and Aβ levels are data showing that linoleic acid decreased the nuclear content of SREBP-1 in cultured human embryonic kidney (HEK)-293 cells [49]. It is therefore prudent to suggest that although the in vitro data are mirrored by the in vivo data, the increase in palmitic acid and the reduction in linoleic acid may both contributed to the increase in SREBP1, BACE1, and Aβ levels in mice brains. Linoleic acid, the parent fatty acid of the omega-6 family of fatty acids, is considered essential fatty acids because it cannot be synthesized by humans and is mostly obtained from the diet. It is suggested that a diet rich in omega-6s but low in omega-3s increases inflammation [50]. In addition to the laboratory studies involving rodents, there is also compelling epidemiological data implicating high saturated fat diets in precipitating cognitive impairment and the increased risk for developing neurodegenerative disorders, including Alzheimer’s disease (AD) in humans [51-55]. Emerging data from three separate and independent prospective cohort studies - i) The Rotterdam study in the Netherlands which included 5395 participants, aged 55 years and older [52]; ii) The Washington Heights - Inwood Columbia Aging Project (WHICAP) in the United States that included 980 New Yorkers aged ≥65 years [54]; and iii) The Chicago Health and Aging Project (CHAP) in the United States that included 895 Chicagoans aged between 65-94 [5]; have all posited a positive correlation between a high saturated fat intake and the risk for AD [49]. Laboratory studies have also cogently demonstrated the deleterious effects of diets enriched in saturated fat in precipitating cognitive impairment as well as deficits in learning and memory in a multitude of rodent models [7,8,56,57].
Conclusions and future perspectives
In light of the data emanating from this study, it is important to determine the mechanisms and endogenous factors that are involved in the palmitate-induced SREBP1 activation. However, the role of linoleic acid per se on SREBP1 actication in vitro and in vivo should also be determined. Our previous study demonstrated that palmitate-enriched diet evokes ER stress and CHOP activation that results in enhanced BACE1 expression and activity leading to augmented Aβ burden in the hippocampus [21,22]. Interestingly, ER stress has been linked to SREBP1 activation in the β-cells of the pancreas [58-60]. In this study we demonstrated that palmitate-enriched diet induces SREBP1 expression and transcriptional activity that results in augmented BACE1 expression and activity. It is therefore imperative to determine the role of palmitate-induced ER stress and CHOP activation in the increased expression and activation of SREBP1, and the role this ER stress / CHOP / SREBP1 axis plays in the regulation of BACE1 expression and Aβ genesis. Also, our preliminary studies have shown that palmitate-enriched diet attenuates the expression of SIRT1 [61], the histone deacetylase known to deacetylate and inhibit SREBP1 transcriptional activity [62]. Furthermore, our studies have shown that palmitate-enriched diets cause an attenuation the expression of leptin [24,63], a key neurotrophic factor in the brain that regulates BACE1 activity, Aβ burden and accumulation [64-67]. Interestingly, leptin has been shown to reduce SREBP1 activation in the white adipose tissue [68]. It is therefore required to determine the role of palmitate-induced mitigation of leptin expression in the SREBP1 activation and to determine the efficacy of exogenous leptin to abrogate the palmitate-induced SREBP1 activation. Saturated fatty acids, such as palmitate, are known to bind to and modulate the transcriptional activity of Liver X receptor alpha (LXRα) [69,70], which is a master regulator of lipogenic gene expression [71] as it regulates SREBP1 expression [72,73]. Furthermore, our studies have shown that palmitate-induced activation of the transcription factor, NF-κB (Nuclear factor of kappa-light-polypeptide gene enhancer of activated B cells) underlies the palmitate-induced increase in BACE1 expression and Aβ genesis [21,74]. Our current on-going work is also exploring the role of palmitate-induced NF-κB activation in the increased expression and activity of SREBP1 to determine the extent of NF-κB - SREBP1 signaling crosstalk, if any, in the regulation of BACE1 expression. Future studies should also examine the effects of supplementation with or deficiency in the unsaturated fatty acid linoleate on ER stress, CHOP and NF-κB activation and subsequent effects on SREBP1, BACE1 and Aβ status.
Acknowledgements:
This work was supported by a Grant from the National Institute of Health (R01AG045264) to Dr. Othman Ghribi.
List of Abbreviations
- Aβ
Amyloid beta
- AβPP
Amyloid beta precursor protein
- AD
Alzheimer’s disease
- BACE1
β-site AβPP cleaving enzyme 1
- CHOP
C/EBP Homologous Protein
- ChIP
Chromatin Immunoprecipitation
- ER
Endoplasmic Reticulum
- LXRα
Liver X Receptor alpha
- N2a
Neuro-2a mouse neuroblastoma cells
- NCoA
nuclear receptor coactivator
- NFT
Neurofibrillary tangles
- PA
palmitic acid
- sFFA
saturated free fatty acids
- SRC
steroid receptor coactivator
- SRE
sterol response element
- SREBP
Sterol Response Element Binding Protein
- WB
Western Blotting
Footnotes
Ethical Approval of animal studies
All animal procedures and studies carried out were approved by the Institutional Animal Care and Use Committee at the University of North Dakota. All animal procedures and studies were carried out in accordance with the U.S Public Health Service's "Policy on Humane Care and Use of Laboratory Animals" and "Guide for the Care and Use of Laboratory Animals". All animal procedures and studies carried out are in compliance with the U.S National Research Council's "Guide for the Care and Use of Laboratory Animals".
Conflict of Interest
The authors declare that they have no conflict of interest.
Contributor Information
Gurdeep Marwarha, Email: gurdeep.marwarha@ndus.edu.
Jonah Lund, Email: jonah.c.lund@ndus.edu.
Othman Ghribi, Email: othman.ghribi@ndus.edu.
References
- 1.Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis J-C, Collins F, Treanor J, Rogers G, Citron M (1999) β-Secretase Cleavage of Alzheimer's Amyloid Precursor Protein by the Transmembrane Aspartic Protease BACE. Science 286 (5440):735–741. doi: 10.1126/science.286.5440.735 [DOI] [PubMed] [Google Scholar]
- 2.Hardy J, Higgins G (1992) Alzheimer's disease: the amyloid cascade hypothesis. Science 256 (5054):184–185. doi: 10.1126/science.1566067 [DOI] [PubMed] [Google Scholar]
- 3.Fukumoto H, Cheung BS, Hyman BT, Irizarry MC (2002) B-secretase protein and activity are increased in the neocortex in alzheimer disease. Archives of Neurology 59 (9): 1381–1389. doi: 10.1001/archneur.59.9.1381 [DOI] [PubMed] [Google Scholar]
- 4.Holsinger RM, McLean CA, Beyreuther K, Masters CL, Evin G (2002) Increased expression of the amyloid precursor beta-secretase in Alzheimer's disease. Ann Neurol 51 (6):783–786. doi: 10.1002/ana.10208 [DOI] [PubMed] [Google Scholar]
- 5.Busquets O, Ettcheto M, Pallàs M, Beas-Zarate C, Verdaguer E, Auladell C, Folch J, Camins A. (2017) Long-term exposition to a high fat diet favors the appearance of β-amyloid depositions in the brain of C57BL/6J mice. A potential model of sporadic Alzheimer's disease. Mech Ageing Dev (162):38–45. doi: 10.1016/j.mad.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Thériault P, ElAli A, Rivest S (2016) High fat diet exacerbates Alzheimer’s disease-related pathology in APPswe/PS1 mice. Oncotarget (42):67808–67827. doi: 10.18632/oncotarget.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kothari V, Luo Y, Tornabene T, O'Neill AM, Greene MW, Geetha T, Babu JR (2017) High fat diet induces brain insulin resistance and cognitive impairment in mice. Biochim Biophys Acta Mol Basis Dis 1863(2):499–508. doi: 10.1016/j.bbadis.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Janssen CI, Jansen D, Mutsaers MP, Dederen PJ, Geenen B, Mulder MT, Kiliaan AJ (2016) The Effect of a High-Fat Diet on Brain Plasticity, Inflammation and Cognition in Female ApoE4-Knockin and ApoE-Knockout Mice. PLoS One 11(5):e0155307. doi: 10.1371/journal.pone.0155307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grant WB (1999) Dietary links to Alzheimer’s disease: 1999 update. J Alzheimers Dis 1 (4-5):197–201. doi: 10.3233/JAD-1999-14-501 [DOI] [PubMed] [Google Scholar]
- 10.Solfrizzi V, D'Introno A, Colacicco AM, Capurso C, Del Parigi A, Capurso S, Gadaleta A, Capurso A, Panza F (2005) Dietary fatty acids intake: possible role in cognitive decline and dementia. Exp Gerontol 40 (4):257–270. doi: 10.1016/j.exger.2005.01.001 [DOI] [PubMed] [Google Scholar]
- 11.Ulmann L, Mimouni V, Roux S, Porsolt R, Poisson JP (2001) Brain and hippocampus fatty acid composition in phospholipid classes of aged-relative cognitive deficit rats. Prostaglandins Leukot Essent Fatty Acids 64 (3): 189–195. doi: 10.1054/plef.2001.0260 [DOI] [PubMed] [Google Scholar]
- 12.Lovejoy JC, Smith SR, Champagne CM, Most MM, Lefevre M, DeLany JP, Denkins YM, Rood JC, Veldhuis J, Bray GA (2002) Effects of Diets Enriched in Saturated (Palmitic), Monounsaturated (Oleic), or trans (Elaidic) Fatty Acids on Insulin Sensitivity and Substrate Oxidation in Healthy Adults. Diabetes Care 25 (8): 1283–1288. doi: 10.2337/diacare.25.8.1283 [DOI] [PubMed] [Google Scholar]
- 13.Hamilton JA, Brunaldi K (2007) A model for fatty acid transport into the brain. J Mol Neurosci 33 (1): 12–17. doi: 10.1007/s12031-007-0050-3 [DOI] [PubMed] [Google Scholar]
- 14.Rapoport SI (2001) In vivo fatty acid incorporation into brain phosholipids in relation to plasma availability, signal transduction and membrane remodeling. J Mol Neurosci 16 (2-3):243–261; discussion 279-284. doi: 10.1385/JMN:16:2-3:243 [DOI] [PubMed] [Google Scholar]
- 15.Horton JD, Goldstein JL, Brown MS (2002) SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest 109 (9): 1125–1131. doi: 10.1172/JCI15593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown MS, Goldstein JL (1997) The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 89 (3):331–340. doi: 10.1016/S0092-8674(00)80213-5 [DOI] [PubMed] [Google Scholar]
- 17.Kato T, Shimano H, Yamamoto T, Ishikawa M, Kumadaki S, Matsuzaka T, Nakagawa Y, Yahagi N, Nakakuki M, Hasty AH, Takeuchi Y, Kobayashi K, Takahashi A, Yatoh S, Suzuki H, Sone H, Yamada N (2008) Palmitate impairs and eicosapentaenoate restores insulin secretion through regulation of SREBP-1c in pancreatic islets. Diabetes 57 (9):2382–2392. doi: 10.2337/db06-1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Natalicchio A, Biondi G, Marrano N, Labarbuta R, Tortosa F, Spagnuolo R, D'Oria R, Carchia E, Leonardini A, Cignarelli A, Perrini S, Laviola L, Giorgino F (2016) Long-Term Exposure of Pancreatic beta-Cells to Palmitate Results in SREBP-1C-Dependent Decreases in GLP-1 Receptor Signaling via CREB and AKT and Insulin Secretory Response. Endocrinology 157 (6):2243–2258. doi: 10.1210/en.2015-2003 [DOI] [PubMed] [Google Scholar]
- 19.Vallim T, Salter AM (2010) Regulation of hepatic gene expression by saturated fatty acids. Prostaglandins Leukot Essent Fatty Acids 82 (4-6):211–218. doi: 10.1016/j.plefa.2010.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mastrocola R, Guglielmotto M, Medana C, Catalano MG, Cutrupi S, Borghi R, Tamagno E, Boccuzzi G, Aragno M (2011) Dysregulation of SREBP2 induces BACE1 expression. Neurobiol Dis 44 (1): 116–124. doi: 10.1016/j.nbd.2011.06.010 [DOI] [PubMed] [Google Scholar]
- 21.Marwarha G, Schommer J, Lund J, Schommer T, Ghribi O (2018) Palmitate-induced C/EBP homologous protein activation leads to NF-κB-mediated increase in BACE1 activity and amyloid beta genesis. Journal of Neurochemistry 144 (6):761–779. doi:doi: 10.1111/jnc.14292 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Marwarha G, Rostad S, Lilek J, Kleinjan M, Schommer J, Ghribi O (2017) Palmitate Increases beta-site AbetaPP-Cleavage Enzyme 1 Activity and Amyloid-beta Genesis by Evoking Endoplasmic Reticulum Stress and Subsequent C/EBP Homologous Protein Activation. J Alzheimers Dis 57 (3):907–925. doi: 10.3233/JAD-161130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rishi V, Gal J, Krylov D, Fridriksson J, Boysen MS, Mandrup S, Vinson C (2004) SREBP-1 dimerization specificity maps to both the helix-loop-helix and leucine zipper domains: use of a dominant negative. J Biol Chem 279 (12): 11863–11874. doi: 10.1074/jbc.M308000200 [DOI] [PubMed] [Google Scholar]
- 24.Marwarha G, Claycombe K, Schommer J, Collins D, Ghribi O (2016) Palmitate-induced Endoplasmic Reticulum stress and subsequent C/EBPalpha Homologous Protein activation attenuates leptin and Insulin-like growth factor 1 expression in the brain. Cell Signal 28 (11): 1789–1805. doi: 10.1016/j.cellsig.2016.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marwarha G, Dasari B, Prasanthi JR, Schommer J, Ghribi O (2010) Leptin reduces the accumulation of Abeta and phosphorylated tau induced by 27-hydroxycholesterol in rabbit organotypic slices. J Alzheimers Dis 19 (3): 1007–1019. doi: 10.3233/JAD-2010-1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marwarha G, Dasari B, Prabhakara JP, Schommer J, Ghribi O (2010) beta-Amyloid regulates leptin expression and tau phosphorylation through the mTORC1 signaling pathway. J Neurochem 115 (2):373–384. doi: 10.1111/j.1471-4159.2010.06929.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marwarha G, Raza S, Prasanthi JR, Ghribi O (2013) Gadd153 and NF-kappaB crosstalk regulates 27-hydroxycholesterol-induced increase in BACE1 and beta-amyloid production in human neuroblastoma SH-SY5Y cells. PLoS One 8 (8):e70773.doi: 10.1371/journal.pone.0070773 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Marwarha G, Prasanthi JR, Schommer J, Dasari B, Ghribi O (2011) Molecular interplay between leptin, insulin-like growth factor-1, and beta-amyloid in organotypic slices from rabbit hippocampus. Mol Neurodegener 6 (1):41. doi: 10.1186/1750-1326-6-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marwarha G, Dasari B, Ghribi O (2012) Endoplasmic reticulum stress-induced CHOP activation mediates the down-regulation of leptin in human neuroblastoma SH-SY5Y cells treated with the oxysterol 27-hydroxycholesterol. Cell Signal 24 (2):484–492. doi: 10.1016/j.cellsig.2011.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marwarha G, Raza S, Meiers C, Ghribi O (2014) Leptin attenuates BACE1 expression and amyloid-beta genesis via the activation of SIRT1 signaling pathway. Biochim Biophys Acta 1842 (9):1587–1595. doi: 10.1016/j.bbadis.2014.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Marwarha G, Claycombe-Larson K, Schommer J, Ghribi O (2017) Maternal low-protein diet decreases brain-derived neurotrophic factor expression in the brains of the neonatal rat offspring. J Nutr Biochem 45:54–66. doi: 10.1016/j.jnutbio.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barbero-Camps E, Fernandez A, Martinez L, Fernandez-Checa JC, Colell A (2013) APP/PS1 mice overexpressing SREBP-2 exhibit combined Abeta accumulation and tau pathology underlying Alzheimer's disease. Hum Mol Genet 22 (17):3460–3476. doi: 10.1093/hmg/ddt201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marwarha G, Ghribi O (2017) Palmitate-enriched diet-induced ER stress and CHOP activation causes tau hyperphosphorylation in the cultured human neuroblatoma cells and the mouse brain Alzheimer's & Dementia: The Journal of the Alzheimer's Association 13 (7):P326. doi: 10.1016/j.jalz.2017.06.036 [DOI] [Google Scholar]
- 34.Liu L, Martin R, Chan C (2013) Palmitate-activated astrocytes via serine palmitoyltransferase increase BACE1 in primary neurons by sphingomyelinases. Neurobiol Aging 34 (2):540–550. doi: 10.1016/j.neurobiolaging.2012.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu L, Martin R, Kohler G, Chan C (2013) Palmitate induces transcriptional regulation of BACE1 and presenilin by STAT3 in neurons mediated by astrocytes. Exp Neurol 248:482–490. doi: 10.1016/j.expneurol.2013.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patil S, Melrose J, Chan C (2007) Involvement of astroglial ceramide in palmitic acid-induced Alzheimer-like changes in primary neurons. Eur J Neurosci 26 (8):2131–2141. doi: 10.1111/j.1460-9568.2007.05797.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patil S, Sheng L, Masserang A, Chan C (2006) Palmitic acid-treated astrocytes induce BACE1 upregulation and accumulation of C-terminal fragment of APP in primary cortical neurons. Neurosci Lett 406 (1-2):55–59. doi: 10.1016/j.neulet.2006.07.015 [DOI] [PubMed] [Google Scholar]
- 38.Cao D, Lu H, Lewis TL, Li L (2007) Intake of sucrose-sweetened water induces insulin resistance and exacerbates memory deficits and amyloidosis in a transgenic mouse model of Alzheimer disease. J Biol Chem 282 (50):36275–36282. doi: 10.1074/jbc.M703561200 [DOI] [PubMed] [Google Scholar]
- 39.Julien C, Tremblay C, Phivilay A, Berthiaume L, Emond V, Julien P, Calon F (2010) High-fat diet aggravates amyloid-beta and tau pathologies in the 3xTg-AD mouse model. Neurobiol Aging 31 (9):1516–1531. doi: 10.1016/j.neurobiolaging.2008.08.022 [DOI] [PubMed] [Google Scholar]
- 40.Maesako M, Uemura K, Kubota M, Kuzuya A, Sasaki K, Asada M, Watanabe K, Hayashida N, Ihara M, Ito H, Shimohama S, Kihara T, Kinoshita A (2012) Environmental enrichment ameliorated high-fat diet-induced Abeta deposition and memory deficit in APP transgenic mice. Neurobiol Aging 33 (5): 1011 e1011–1023. doi: 10.1016/j.neurobiolaging.2011.10.028 [DOI] [PubMed] [Google Scholar]
- 41.Refolo LM, Malester B, LaFrancois J, Bryant-Thomas T, Wang R, Tint GS, Sambamurti K, Duff K, Pappolla MA (2000) Hypercholesterolemia accelerates the Alzheimer's amyloid pathology in a transgenic mouse model. Neurobiol Dis 7 (4):321–331. doi: 10.1006/nbdi.2000.0304 [DOI] [PubMed] [Google Scholar]
- 42.Thirumangalakudi L, Prakasam A, Zhang R, Bimonte-Nelson H, Sambamurti K, Kindy MS, Bhat NR (2008) High cholesterol-induced neuroinflammation and amyloid precursor protein processing correlate with loss of working memory in mice. J Neurochem 106 (1):475–485. doi: 10.1111/j.1471-4159.2008.05415.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vandal M, White PJ, Tremblay C, St-Amour I, Chevrier G, Emond V, Lefrancois D, Virgili J, Planel E, Giguere Y, Marette A, Calon F (2014) Insulin reverses the high-fat diet-induced increase in brain Abeta and improves memory in an animal model of Alzheimer disease.Diabetes 63 (12):4291–4301. doi: 10.2337/db14-0375 [DOI] [PubMed] [Google Scholar]
- 44.Ghribi O (2008) Potential mechanisms linking cholesterol to Alzheimer's disease-like pathology in rabbit brain, hippocampal organotypic slices, and skeletal muscle. J Alzheimers Dis 15 (4):673–684. doi: 10.3233/JAD-2008-15412 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Marwarha G, Ghribi O (2015) Does the oxysterol 27-hydroxycholesterol underlie Alzheimer's disease-Parkinson's disease overlap? Exp Gerontol 68:13–18. doi: 10.1016/j.exger.2014.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Selvi Y, Gergerlioglu HS, Akbaba N, Oz M, Kandeger A, Demir EA, Yerlikaya FH, Nurullahoglu-Atalik KE (2016) Impact of enriched environment on production of tau, amyloid precursor protein and, amyloid-beta peptide in high-fat and high-sucrose-fed rats. Acta Neuropsychiatr:1–8. doi: 10.1017/neu.2016.63 [DOI] [PubMed] [Google Scholar]
- 47.Ghribi O, Marwarha G (2010) Cholesterol causes Alzheimer pathology through Akt/mTOR inhibition. Alzheimer's & Dementia: The Journal of the Alzheimer's Association 6 (4):S402–S403. doi: 10.1016/j.jalz.2010.05.1355 [DOI] [Google Scholar]
- 48.Marwarha G, Raza S, Hammer K, Ghribi O (2017) 27-hydroxycholesterol: A novel player in molecular carcinogenesis of breast and prostate cancer. Chem Phys Lipids. doi: 10.1016/j.chemphyslip.2017.05.012 [DOI] [PubMed] [Google Scholar]
- 49.Hannah VC, Ou J, Luong A, Goldstein JL, Brown MS. Unsaturated fatty acids down-regulate srebp isoforms 1a and 1c by two mechanisms in HEK-293 cells. J Biol Chem. 2001. February 9;276(6):4365–72) [DOI] [PubMed] [Google Scholar]
- 50.Russo GL (2009) Dietary n-6 and n-3 polyunsaturated fatty acids: from biochemistry to clinical implications in cardiovascular prevention. Biochem Pharmacol 77(6):937–46 [DOI] [PubMed] [Google Scholar]
- 51.Barnard ND, Bunner AE, Agarwal U (2014) Saturated and trans fats and dementia: a systematic review. Neurobiol Aging 35 Suppl 2:S65–73. doi: 10.1016/j.neurobiolaging.2014.02.030 [DOI] [PubMed] [Google Scholar]
- 52.Kalmijn S, Launer LJ, Ott A, Witteman JC, Hofman A, Breteler MM (1997) Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol 42 (5):776–782. doi: 10.1002/ana.410420514 [DOI] [PubMed] [Google Scholar]
- 53.Laitinen MH, Ngandu T, Rovio S, Helkala EL, Uusitalo U, Viitanen M, Nissinen A, Tuomilehto J, Soininen H, Kivipelto M (2006) Fat intake at midlife and risk of dementia and Alzheimer's disease: a population-based study. Dement Geriatr Cogn Disord 22 (1):99–107. doi: 10.1159/000093478 [DOI] [PubMed] [Google Scholar]
- 54.Luchsinger JA, Tang MX, Shea S, Mayeux R (2002) Caloric intake and the risk of Alzheimer disease. Arch Neurol 59 (8):1258–1263. doi: 10.1001/archneur.59.8.1258 [DOI] [PubMed] [Google Scholar]
- 55.Parrott MD, Greenwood CE (2007) Dietary influences on cognitive function with aging: from high-fat diets to healthful eating. Ann N Y Acad Sci 1114:389–397. doi: 10.1196/annals.1396.028 [DOI] [PubMed] [Google Scholar]
- 56.Winocur G, Greenwood CE (1999) The effects of high fat diets and environmental influences on cognitive performance in rats. Behav Brain Res 101 (2): 153–161. doi: 10.1016/S0166-4328(98)00147-8 [DOI] [PubMed] [Google Scholar]
- 57.Winocur G, Greenwood CE (2005) Studies of the effects of high fat diets on cognitive function in a rat model. Neurobiol Aging 26 Suppl 1:46–49.doi: 10.1016/j.neurobiolaging.2005.09.003 [DOI] [PubMed] [Google Scholar]
- 58.Wang H, Kouri G, Wollheim CB (2005) ER stress and SREBP-1 activation are implicated in beta-cell glucolipotoxicity. J Cell Sci 118 (Pt 17):3905–3915. doi: 10.1242/jcs.02513 [DOI] [PubMed] [Google Scholar]
- 59.Basseri S, Austin RC (2012) Endoplasmic reticulum stress and lipid metabolism: mechanisms and therapeutic potential. Biochem Res Int 2012:841362. doi: 10.1155/2012/841362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, Ferre P, Foufelle F (2009) GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest 119 (5): 1201–1215. doi: 10.1172/JCI37007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marwarha G, Ghribi O (2018) Saturated fat-enriched diet decreases SIRT1 expression in the mouse hippocampus - The SIRTain effects of saturated fat in the brain. The FASEB Journal 32 (1_supplement):1b7–1b7. doi: 10.1096/fasebj.2018.32.1_supplement.lb729295886 [DOI] [Google Scholar]
- 62.Ponugoti B, Kim D-H, Xiao Z, Smith Z, Miao J, Zang M, Wu S-Y, Chiang C-M, Veenstra TD, Kemper JK (2010) SIRT1 Deacetylates and Inhibits SREBP-1C Activity in Regulation of Hepatic Lipid Metabolism. Journal of Biological Chemistry 285 (44):33959–33970. doi: 10.1074/jbc.M10.122978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marwarha G, Ghribi O (2018) Leptin alleviates the saturated fatty acid-induced increase in BACE1 expression and Amyloid-β production - Relevance to Alzheimer’s disease pathogenesis. The FASEB Journal 32 (1_supplement):659.652–659.652. doi: 10.1096/fasebj.2018.32.1_supplement.659.2 [DOI] [Google Scholar]
- 64.Marwarha G, Ghribi O (2012) Leptin signaling and Alzheimer's disease. Am J Neurodegener Dis 1 (3):245–265 [PMC free article] [PubMed] [Google Scholar]
- 65.Marwarha G, Ghribi O (2012) Cellular model of Alzheimer's disease--relevance to therapeutic testing. Exp Neurol 233 (2):733–739. doi: 10.1016/j.expneurol.2011.11.011 [DOI] [PubMed] [Google Scholar]
- 66.Marwarha GSA (2011) Leptin expression and signaling at the confluence of neurodegenerative mechanisms in Alzheimer disease. The University of North Dakota ProQuest Dissertations Publishing; 3515504 [Google Scholar]
- 67.Marwarha G (2011) Mutual upregulation of IGF-1 and leptin expression prevents their β- amyloid-induced down regulation. Alzheimer's & Dementia: The Journal of the Alzheimer's Association 7 (4): S586. doi: 10.1016/j.jalz.2011.05.1659 [DOI] [Google Scholar]
- 68.Nogalska A, Sucajtys-Szulc E, Swierczynski J (2005) Leptin decreases lipogenic enzyme gene expression through modification of SREBP-1c gene expression in white adipose tissue of aging rats. Metabolism 54 (8):1041–1047. doi: 10.1016/j.metabol.2005.03.007 [DOI] [PubMed] [Google Scholar]
- 69.Bedi S, Hines GV, Lozada-Fernandez VV, de Jesus Piva C, Kaliappan A, Rider SD Jr., Hostetler HA (2017) Fatty acid binding profile of the liver X receptor alpha. J Lipid Res 58 (2):393–402. doi: 10.1194/jlr.M072447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tobin KA, Steineger HH, Alberti S, Spydevold O, Auwerx J, Gustafsson JA, Nebb HI (2000) Cross-talk between fatty acid and cholesterol metabolism mediated by liver X receptor-alpha. Mol Endocrinol 14 (5):741–752. doi: 10.1210/mend.14.5.0459 [DOI] [PubMed] [Google Scholar]
- 71.Hong C, Tontonoz P (2014) Liver X receptors in lipid metabolism: opportunities for drug discovery. Nat Rev Drug Discov 13 (6):433–444. doi: 10.1038/nrd4280 [DOI] [PubMed] [Google Scholar]
- 72.Calkin AC, Tontonoz P (2012) Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat Rev Mol Cell Biol 13 (4):213–224. doi: 10.1038/nrm3312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JM, Shimomura I, Shan B, Brown MS, Goldstein JL, Mangelsdorf DJ (2000) Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev 14 (22):2819–2830. doi: 10.1101/gad.844900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marwarha G, Ghribi O (2017) Nuclear Factor Kappa-light-chain-enhancer of Activated B Cells (NF-κB) – a Friend, a Foe, or a Bystander - in the Neurodegenerative Cascade and Pathogenesis of Alzheimer’s Disease. CNS & Neurological Disorders - Drug Targets 16 (10):1050–1065. doi: 10.2174/1871527316666170725114652 [DOI] [PubMed] [Google Scholar]