Abstract
Objective
This randomized, double‐blind, placebo‐controlled study assessed the necessity of early intervention, safety and efficacy of intravenous zoledronic acid 5 mg/year in East China women with newly diagnosed osteoporosis at high risk of fracture during a 24‐month treatment period.
Methods
Subjects (57 [52–62] years old) were randomized 3:2 to zoledronic acid versus placebo (randomized at baseline, zoledronic acid [175 cases], placebo‐zoledronic acid [110 cases]). The bone mineral density of the lumbar spine and total hip was measured every 6 months with the use of dual‐energy X‐ray absorptiometry. Serum procollagen I N‐terminal pro‐peptide (PINP) and serum C‐telopeptide of type I collagen (CTX) levels were measured every 6 months. The primary end point was the rate of change in the bone mineral density at the posteroanterior spine.
Results
For subjects with measurements at 24 months, zoledronic acid significantly increased bone mineral density (BMD) at the lumbar spine (mean percent change ± SD, zoledronic acid 5.390% ± 0.854% versus placebo‐zoledronic acid −1.038% ± 0.599%), the total hip (zoledronic acid 1.900% ± 0.262% versus placebo–zoledronic acid −1.631% ± 0.649%). Serum procollagen I N‐terminal pro‐peptide (PINP) and CTX decreased rapidly with zoledronic acid 5 mg treatment (P < 0.001 versus placebo at 6 month and 24 months) and changed from baseline in the zoledronic acid 5 mg and placebo–zoledronic acid 5 mg at 6 months by a mean of −66.348% and −75.375%, respectively (P < 0.001), and at 24 months by −49.950% and −52.325%, respectively (P < 0.001). No cases of serious adverse events were observed in two groups. Headache, pyrexia and myalgia occurred more commonly within the first 3 days after infusion with zoledronic acid 5 mg than with placebo (13.7% versus 2.1%, P = 0.0018; 28.0% versus 3.2%, P < 0.001; 21.7% versus 4.2%, P < 0.001, respectively).
Conclusions
These data show that early application of zoledronic acid 5 mg/year was well stimulated and tolerated for bone mass in newly diagnosed east china subjects with osteoporosis in a 24‐month treatment.
Keywords: Bone mineral density, Bone turnover markers, Early intervention, Placebo‐controlled study, Zoledronic acid
Introduction
Osteoporosis is a skeletal system disease characterized by degradation of bone strength and a consequent increase in fracture risk1. Currently, China has the largest number of osteoporosis patients worldwide. According to one survey in China, the incidence of osteoporosis was two times higher in women than in men, with approximately 112 million female patients2. With its rapidly increasing aging population, osteoporosis and osteoporotic fractures are a serious threat to public health in China, and are associated with large medical costs and severe burden for society and families3, 4. Therefore, osteoporosis and osteoporotic fractures have become a serious public health concern, which require further attention in China5. Osteoporotic fractures, the most serious complications of osteoporosis, are responsible for more days of disability than most types of cancer6.
Recent studies on osteoporosis medications have let to big improvements in treatment options. Currently, strategies for treating osteoporosis are based on pharmaceutical intervention. The drugs preventing and curing this extremely complex and challenging disease mostly include bone absorption‐inhibitor drugs and bone formation‐acceleration drugs. Many studies report that bone formation‐acceleration drugs such as teriparatide can induce drug toxicity, are associated with a risk of inducing tumors, and increase the incidence of cardiovascular disease and stroke7.
Oral bisphosphonates, once a week, are used to treat osteoporosis in clinical practice. However, the compliance of oral bisphosphonate therapy is poor, such that more than half of patients stop taking treatment within 1 year of initiation8, 9. Low compliance is associated with more fragility fracture outcomes7. Zoledronic acid (Aclasta, Novartis Pharma), a bisphosphonate administered intravenously which reduces fracture risk, is, therefore, likely to benefit the health of the population and impact favorably on public health expenditure and the burden of society and family10. At a dose of 5 mg once a year, it has anti‐fracture efficacy in postmenopausal women with osteoporosis and positive effects on bone mineral density in men. The intravenous bisphosphonate, zoledronate, reduces fracture risk when administered annually11, 12. Therefore, zoledronate has good efficacy and treatment compliance.
High bone turnover level is a typical characteristic in most postmenopausal women; bone mass decreases dramatically in this phase13. Then, as menopause progresses, bone mass loss can become increasingly serious. Therefore, we believe that if we can prevent and treat osteoporosis in the early period, it will be beneficial to the maintenance of bone mass. Greater bone mass will significantly reduce the risk of fracture in postmenopausal women. Bone turnover markers combined with bone mineral density (BMD) are important in monitoring the curative effect of osteoporosis and predicting risk of fracture. Markers such as procollagen I N‐terminal pro‐peptide (PINP) and serum C‐terminal peptide of type I collagen (S‐CTX) can be used as a clinical tool to identify postmenopausal women with high levels of bone turnover.
Thus, we assume that early intervention with intravenous zoledronic acid 5 mg/year will be beneficial by improving BMD and bone turnover markers, and reducing the risk of fractures in postmenopausal women. To test this hypothesis, we conducted a 24‐month randomized, double‐blind, placebo‐controlled trial of intravenous zoledronic acid 5 mg/year in women in East China with newly diagnosed osteoporosis at high risk of fracture, thereby assessing the necessity of early intervention, and the safety and efficacy of intravenous zoledronic acid 5 mg/year. This report also presents the first 2‐year clinical study of intravenous zoledronic acid 5 mg/year treatment in subjects in East China.
Materials and Methods
Subjects
The primary purpose of this study was to assess the response to 24 months of 5 mg/year intravenous zoledronic acid compared with placebo in subjects in east China with newly diagnosed osteoporosis and at high risk of fracture. The primary efficacy variable was the percent change in lumbar spine BMD from baseline to last measurement through 24 months. Other efficacy analyses included changes from baseline to 6, 12, and 18 months in lumbar spine BMD, and changes from baseline to 6, 12, 18, and 24 months in total hip BMD and bone turnover markers. The study was conducted at Xinhua Hospital in Zhejiang.
Chinese postmenopausal women (with newly diagnosed osteoporosis) were eligible to participate if they were 50–65 years of age, at high risk of fracture, and met the following diagnostic and exclusion criteria.
The diagnosis of osteoporosis was based on the following recommended criteria of the World Health Organization (WHO)14: Survey the lumbar vertebra normal position bone density by using dual energy X‐ray absorptiometry, T score −2.5 to −3.3 could be diagnosed as osteoporosis [T = the standard deviation of (measured value‐peak bone mass)/(normal adult bone density)].
Patients were excluded from the study based on the following criteria: (i) those that also had diseases that severely affect the metabolism of bone or calcium, such as diabetes, Cushing's syndrome, changes in function of the thyroid or parathyroid, osteomalacia, rheumatoid arthritis, multiple myeloma, bone tumor, osteoarthrosis, Paget's disease, and osteogenesis imperfecta; (ii) those that also had severe primary cardiac diseases, or diseases of the cerebral vessels or hematopoietic system; (iii) those that also had severe liver function or renal insufficiencies; (iv) those taking drugs within the past 6 months that affect bone metabolism, such as estrogen, steroid hormones, calcitonin, parathyroid hormones, bisphosphonates, fluoride, vitamin D, anticonvulsant drugs, and diuretics; (v) those who had a medical history of mental illness; and (vi) patients with Alzheimer's disease.
We screened 800 postmenopausal women in the Zhejiang area. Of these, 600 women were interested and were eligible for further screening. Of these women, 315 were deemed ineligible because their bone density was too high or low, and on the basis of screening blood tests, leaving 285 women who were eligible for the study. Thus, the final cohort consisted of 285 women.
The flow of trial participants is shown in Fig. 1.
Figure 1.
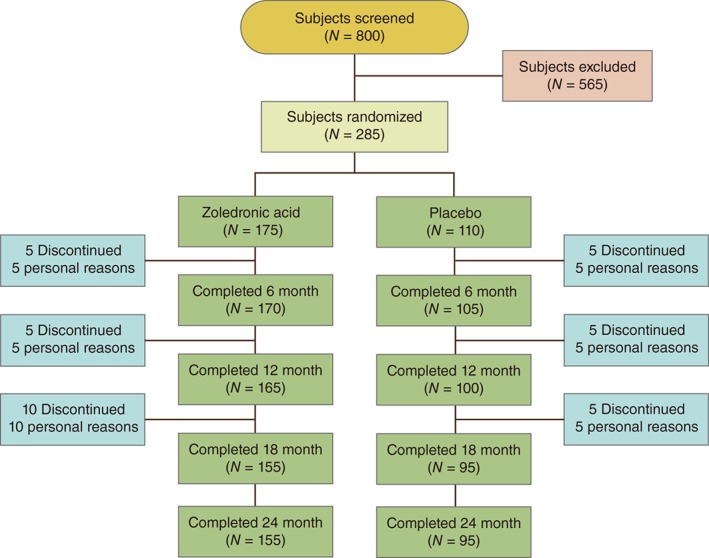
Flow of participants through the study.
This study (ClinicalTrials.gov identifier ChiCTR‐POC‐16008026) was conducted in compliance with the ethical principles stated in the Declaration of Helsinki, and was approved by the local Ethics Committee of The Second Affiliated Hospital of Zhejiang Chinese Medicine University. All subjects provided written informed consent for their participation before they enrolled in this study.
Study Design
As part of the standard therapy for subjects with osteoporosis and to provide protection for subjects receiving placebo, all subjects received daily calcium and vitamin D supplementation (1 tab Caltrate‐D per day) from the start of the screening phase and throughout the study.
The 285 subjects (57 [52–62] years old) were randomized 3:2 to zoledronic acid versus placebo (randomized at baseline, zoledronic acid [175 cases] and placebo‐zoledronic acid [110 cases]).
Blood was collected at baseline and at 6, 12, 18, and 24 months for routine chemical and bone turnover markers analysis.
Bone mineral density was measured with using dual‐energy X‐ray absorptiometry at baseline and every 6 months thereafter. BMD and T‐score were measured on the PA lumbar spine (L1–L4) and total hip at baseline and at 6, 12, 18, and 24 months using dual‐energy X‐ray absorptiometry on an Osteocore II Bone Densitometer (Osteocore II Osteodensitometer, Medilink, France). BMD of other regions was not measured.
A standardized questionnaire was administered at each visit to assess side effects. Adverse events were recorded and coded with the use of the Medical Dictionary for Regulatory Activities system. Events meeting criteria for a maxillofacial adverse event or for cardiac arrhythmia classified as a serious adverse event were adjudicated by a committee of independent external experts who were unaware of the group assignments.
This 24‐month, randomized, double‐blind, placebo‐controlled study was conducted in east China from November 2010 to December 2014.
Between January 2011 and November 2012, participants were randomly assigned to receive zoledronic acid at a dose of 5 mg or placebo, administered as a 15‐min to 30‐min intravenous infusion at baseline and month 12. All study participants and researchers were unaware of the study‐drug assignments throughout the trial.
Measurements of Bone Mineral Density and Bone Turnover Markers
To minimize the assay variability within and between the patients, all samples were shipped in batches from Xinhua Hospital laboratory for analysis at the end of the study. Samples from the same patient were analyzed in the same batch.
The markers of bone turnover, β‐C‐terminal telopeptide of type I collagen (β‐CTX) and procollagen type I N‐terminal propeptide (P1NP), were measured at baseline and thereafter every 6 months. Blood was collected after overnight fasting at 8.00–8.30 hours, prior to administration of Zoledronic acid, and serum was stored at −70 °C prior to shipping on dry ice. Measurements were performed at an internationally accredited local laboratory using the automated Roche Cobas e601 immunoassay analyzer (DIAN Diagnostics, Hangzhou, China). BMD was measured at baseline and thereafter every 6 months at lumbar spine (L1–L4) and left total hip, using dual‐energy X‐ray absorptiometry on an Osteocore II Bone Densitometer (Osteocore II Osteodensitometer; Medilink, France). An independent investigator of BMD was responsible for the supervision of quality control for these measurements and notified the investigators of this study if any patient had a decrease in bone density of more than 5% from the baseline values.
Statistical Analysis
The primary end point was percent change from baseline at month 24 in lumbar spine BMD. The secondary end points were relative change from baseline at months 6 and 24 in the two markers of bone turnover. All primary randomized or completed baseline characteristic analyses were based on both intention to treat and protocol, such that data from all 285 participants randomized at the inception of the trial or 250 subjects completed the trial were included. All other analyses were performed according to the per‐protocol principle with the use of all comparable and reliable data from all patients who completed the study. The per‐protocol population included all subjects who completed the study; this population was defined as the completed analysis set (CAS). Efficacy and safety analyses were conducted on the CAS. All analyses were performed on raw data, but BMD and bone turnover marker (BTM) data are presented as percent change from baseline, for ease of interpretation. The two independent‐samples t‐test or t′‐test (if data were found not to be normally distributed) were used to compare baseline measurements between two groups. Changes in BMD (expressed as percentage changes from baseline) between groups were compared using a t‐test or t′‐test, adjusting for baseline BMD. Baseline subject characteristics were compared between the treatment groups using a two‐sample t‐test for continuous variables and Fisher's exact test for categorical variables. Comparison between the completed treatment groups for percent change in BMD and BTM at the last measurement point during the placebo‐controlled phase (24 months) was assessed using a two‐sample t‐test or t′‐test. The frequency of adverse events (AE) was compared between randomized treatment groups using Fisher's exact test15. Descriptive statistics for percent change from baseline were presented as mean ± standard deviation for BMD and markers of bone turnover. All analyses were performed with the SPSS 22.0 software package. For all tests, a P value ≤0.05 was considered to indicate statistical significance. All Statistical testing was performed at a two‐sided significance level of 0.05 unless otherwise noted.
Results
Subjects
Of the 800 patients who were screened for the study, 285 were randomized to double‐blind treatment. Thirty‐five (12.5%) discontinued from the study: 20 who received zoledronic acid 5 mg and 15 who received placebo, most commonly for personal reasons (Fig. 1). Thus, 250 women completed the study. In all the women, naturally menopause had occurred at least 5 years previously. Baseline characteristics were comparable between treatment groups at Randomized or completed period of the study (Table 1).
Table 1.
Randomized or completed baseline characteristics of postmenopausal women with osteoporosis treated with Intravenous zoledronic acid 5 mg or placebo
| Characteristic | ZOL group | Placebo group | P‐value | |
|---|---|---|---|---|
| Age (years) | Randomized | 57.22 ± 2.81 | 57.48 ± 3.18 | 0.482 |
| Completed | 57.11 ± 2.75 | 57.12 ± 3.16 | 0.987 | |
| Height (cm) | Randomized | 159.91 ± 5.99 | 159.70 ± 5.55 | 0.771 |
| Completed | 164.01 ± 5.91 | 160.05 ± 5.71 | 0.957 | |
| Weight (kg) | Randomized | 59.31 ± 2.64 | 58.77 ± 2.56 | 0.087 |
| Completed | 59.27 ± 2.61 | 58.95 ± 2.58 | 0.344 | |
| Body‐mass index (kg/m2) | Randomized | 22.65 ± 1.90 | 23.07 ± 2.17 | 0.840 |
| Completed | 21.82 ± 1.08 | 21.58 ± 0.96 | 0.077 | |
| Years since natural menopause (years) | Randomized | 9.73 ± 1.43 | 10.07 ± 1.52 | 0.090 |
| Completed | 9.78 ± 1.45 | 9.98 ± 1.55 | 0.304 | |
| Alcohol consumption [Yes, cases (%)] | Randomized | 35(22.6) | 45(47.4) | 0.053 |
| Completed | 40(22.8) | 45(40.9) | 0.14 | |
| Serum t‐P1NP level (ng/mL) | Randomized | 58.171 ± 8.367 | 59.459 ± 7.601 | 0.191 |
| Completed | 58.489 ± 8.574 | 58.542 ± 7.781 | 0.961 | |
| Serum β‐CTX level (ng/mL) | Randomized | 0.551 ± 0.095 | 0.545 ± 0.089 | 0.605 |
| Completed | 0.534 ± 0.088 | 0.530 ± 0.078 | 0.668 | |
| Total hip BMD (g/cm2) | Randomized | 0.759 ± 0.043 | 0.751 ± 0.042 | 0.085 |
| Completed | 0.754 ± 0.042 | 0.748 ± 0.043 | 0.324 | |
| Posteroanterior spine BMD (g/cm2) | Randomized | 0.636 ± 0.043 | 0.634 ± 0.044 | 0.759 |
| Completed | 0.631 ± 0.041 | 0.628 ± 0.043 | 0.564 | |
Subjects randomized: Zoledronic acid group, n = 175; placebo group, n = 110. Subjects completed: Zoledronic acid group, n = 155, placebo group, n = 95.
The data shown are for the postmenopausal women in the randomized and completed population. P‐values are for the two‐way comparisons and were determined by analysis of t‐test. The body‐mass index is the weight in kilograms divided by the square of the height in meters. Values are mean ± standard deviation or n (%). A two‐sample t‐test for continuous variables and Fisher exact test for categorical variables.
β‐CTX, C‐telopeptide of type I collagen; PINP, N‐terminal propeptide of type I collagen; ZOL, zoledronic acid.
Bone Mineral Density
Only small increases from baseline occurred in the BMD values of both treatment groups (Table 2). For the CAS population, the percent change from baseline to month 24 was 5.390% ± 0.854% in the zoledronic acid 5 mg group and −1.038% ± 0.599% in the placebo group (P < 0.001).
Table 2.
Mean percent changes in bone mineral density at month 24 and rates of change among women treated with zoledronic acid or placebo (g/cm2)
| Bone site | Zoledronic acid group (n = 155) | Placebo group (n = 95) | P‐value |
|---|---|---|---|
| Posteroanterior spine | 5.390 ± 0.854 | −1.038 ± 0.599 | <0.001 |
| Total hip | 1.900 ± 0.262 | −1.631 ± 0.649 | <0.001 |
Values are means ± standard deviation. P‐values for the two‐way comparisons were calculated by analysis of t′‐test.
The primary efficacy endpoint was percent change in BMD at the lumbar spine (L1–L4) at last observation through 24 months; the mean percent change (±SD) was 5.39% ± 0.854% in the zoledronic acid group versus −1.038% ± 0.599% in the placebo group (P < 0.001, Table 2). The results are shown in Fig. 2A. The mean percent change in BMD at the total hip at last observation through 24 months was 1.900% ± 0.262% in the zoledronic acid group versus −1.631% ± 0.649% in the placebo group (P < 0.001, Table 2). The results are shown in Fig. 2B.
Figure 2.
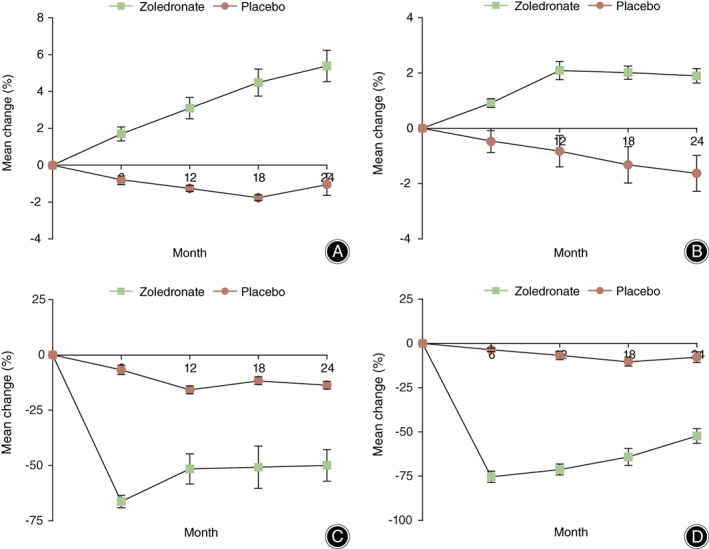
Mean percent changes in the bone mineral density of the posteroanterior spine (A) and total hip (B) as determined with dual‐energy X‐ray absorptiometry and in serum P1NP (C) and β‐CTX (D) level (ng/mL). I bars represent the standard deviation (error bars that are not seen are contained within the data‐point symbols).
The BMD at the posteroanterior spine increased continuously in subjects treated with zoledronic acid, while decreased in those treated with the placebo. As shown in Fig. 2A, the BMD at the total hip also increased continuously in the zoledronic acid group from baseline to month 12, while decreased continuously in the placebo group. The BMD at the total hip basically keep steady in the zoledronic acid group from month 12 to month 24, while it decreased continuously in the placebo group (Fig. 2B).
P‐values for the two‐way comparisons were calculated by analysis of t′‐test.
Markers of Bone Turnover
Overall, zoledronic acid treatment was associated with a rapid decrease in mean percent change of PINP (Fig. 2C) and CTX (Fig. 2D). Within the zoledronic acid treatment group, mean percent change of PINP decreased significantly by 6 months of treatment (mean 66.348%, P < 0.001) and elevated slowly throughout the 6‐month and 12‐month treatment periods, then basically remained steady in the zoledronic acid group from month 12 to month 24. Within the placebo treatment group, the mean percent change of PINP slowly decreased throughout the placebo treatment period.
The mean percent change in PINP at month 6 and month 24 was −66.348% ± 2.825% and −49.950% ± 7.168%, respectively, in the zoledronic acid group versus −6.800% ± 2.131% and −13.725% ± 1.745%, respectively, in the Placebo group (P < 0.001, Table 3, Fig. 2C). The mean percent change in CTX at month 6 and month 24 was −75.375% ± 3.203% and −52.325% ± 4.151%, respectively, in the zoledronic acid group versus −3.600% ± 1.039% and −7.791% ± 3.006%, respectively, in the placebo group (P < 0.001, Table 3, Fig. 2D).
Table 3.
Mean percent changes in serum β‐CTX level and serum t‐P1NP level at 6 or 24 months
| Zoledronic acid group (n = 155) | Placebo group (n = 95) | |||
|---|---|---|---|---|
| BTMs | Month 6 | Month 24 | Month 6 | Month 24 |
| Serum t‐P1NP level | −66.348 ± 2.825 | −49.950 ± 7.168 | −6.800 ± 2.131* | −13.725 ± 1.745* |
| Serum β‐CTX level | −75.375 ± 3.203 | −52.325 ± 4.151 | −3.600 ± 1.039# | −7.791 ± 3.006# |
BTM, bone turnover markers. Values are means ± standard deviation. Comparisons of serum t‐P1NP level at the same month between the zoledronic acid group and the placebo group,
P < 0.001. Comparisons of serum β‐CTX level at the same month between the zoledronic acid group and the placebo group,
P < 0.001.
Adverse Events or Safety
Safety was assessed through the collection of AE at all visits. No cases of serious AE, such as osteonecrosis of the jaw, atrial fibrillation, ocular inflammation, symptomatic hypocalcemia, or fragility fractures, were observed in the zoledronate group or the placebo group. AE that occurred in >5.0% of patients within 3 days of initial dosing were shown in Table 3. The subjects who received zoledronic acid reported more common adverse events of pyrexia, myalgia, or headache (Table 4). There were no significant differences between the groups in the incidence of arthralgia or back pain (Table 4). Both treatments were well‐tolerated, albeit with a higher incidence of influenza‐like illness and pyrexia events occurring within 3 days post‐infusion with zoledronic acid. The most common post‐dose symptom AE were generally mild to moderate in intensity and were of short duration (the majority lasting 3 days or less).
Table 4.
Percentage of visits at which subjects reported side effects [n (%)]
| Side effect | ZOL group (n = 155) | Placebo group (n = 95) | P‐value |
|---|---|---|---|
| Headache (influenza‐like illness) | 21 (13.5) | 2 (2.1) | 0.003 |
| Pyrexia | 43 (27.7) | 3 (3.2) | <0.001 |
| Myalgia | 34 (21.9) | 4 (4.2) | <0.001 |
| Arthralgia | 29 (18.7) | 11 (11.6) | 0.157 |
| Back pain | 24 (15.4) | 14 (14.7) | 1 |
Adverse events (AE) that occurred in >5.0% of patients within 3 days of initial dosing.
Five most common adverse events in zoledronic acid group.
P‐values were calculated with the use of Fisher's exact test. The output data of 2‐Tail P‐values were collected as the last P‐values. The two‐tail P‐value is calculated.
Patients with more than one AE may appear in both occurrence categories. A participant with multiple occurrences of an adverse event within a preferred term (according to codes used in the Medical Dictionary for Regulatory Activities) was counted only once.
Discussion
We found that the administration of intravenous zoledronic acid could increase the bone mineral density at the spine and the femoral neck. Zoledronic acid also reduced the P1NP and CTX levels, which reflect the stimulation of osteoclast activity.
This finding demonstrates that early application of zoledronic acid 5 mg/year was well stimulated and tolerated for bone mass in newly diagnosed subjects in east China with osteoporosis in a 24‐month treatment.
The results of this study were similar to the results for past research in other countries and regions10, 16, 17. Because the early treatment of osteoporosis is very important18, 19, the changes in BMD and bone turnover observed during the early period of osteoporosis are likely to be more important than those observed during later period of osteoporosis.
Taken together, these data suggest that administration of zoledronate may prevent fragility fractures in patients with osteoporosis. The current data have potentially important implications for patient care and for future research in fracture prevention. If very early zoledronate administration reduces fracture risk, it would facilitate greater availability of an effective treatment without increasing drug costs and health‐care costs20, 21.
No cases of serious adverse events, such as osteonecrosis of the jaw, atrial fibrillation, ocular inflammation, symptomatic hypocalcemia, or fragility fractures, were observed in this early use of zoledronate group22, 23, 24, 25. Our findings also suggest that early treatment with zoledronate might be an effective strategy for osteoporosis prevention.
In addition, the long‐term administration of zoledronate as well as estrogen, calcium supplements, with or without vitamin D, and alendronate may lead to atypical fractures26, 27, 28. The administration of anti‐osteoporosis also may lead to a higher risk of cardiovascular events in the older subjects29.
Finally, the strategy of early treatment with zoledronate may be effective, safe, inexpensive, and well tolerated.
Oral bisphosphonates prevent bone loss in early postmenopausal women, but their utility for fracture prevention over extended periods is questionable because of high rates of treatment discontinuation30. However, patients showed good adherence in this study.
Our study has particular strengths, such as being double‐blind, randomized, and placebo‐controlled, but it also has limitations. The sample size is small. To address the possibility that missing data influenced the results of the primary analysis, we conducted additional analyses using the intention‐to‐treat (ITT) analysis, which produced similar results to those for per‐protocol analysis. The consistency of these results suggests that the withdrawal of the 35 participants did not influence the outcome. Although the number of participants was small, the confidence intervals around the changes in bone turnover and BMD are compact, providing reassurance that the findings are valid.
In further research, we will evaluate the long‐term efficacy of zoledronate in China.
Disclosure: The authors declare no conflict of interest. No benefits in any form have been, or will be, received from commercial parties related directly or indirectly to the subject of this manuscript. All authors listed meet the authorship criteria according to the latest guidelines of the International Committee of Medical Journal Editors and are in agreement with the manuscript. The present paper was funded by Novartis Pharma; ClinicalTrials.gov number, ChiCTR‐POC‐16008026.
References
- 1. Kanis JA, McCloskey EV, Johansson H, et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int, 2013, 24: 23–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Si L, Winzenberg TM, Jiang Q, Chen M, Palmer AJ. Projection of incidence and economic burden of osteoporosis‐related fractures in China: 2010–2050. Osteoporos Int, 2015, 26: 1929–1937. [DOI] [PubMed] [Google Scholar]
- 3. Liu P, Yao Y, Liu MY, et al. Spinal trauma in mainland China from 2001 to 2007: an epidemiological study based on a nationwide database. Spine (Phila Pa 1976), 2012, 37: 1310–1315. [DOI] [PubMed] [Google Scholar]
- 4. Xia WB, He SL, Xu L, et al. Rapidly increasing rates of hip fracture in Beijing, China. J Bone Miner Res, 2012, 27: 125–129. [DOI] [PubMed] [Google Scholar]
- 5. Ganda K, Puech M, Chen JS, et al. Models of care for the secondary prevention of osteoporotic fractures: a systematic review and meta‐analysis. Osteoporos Int, 2013, 24: 393–406. [DOI] [PubMed] [Google Scholar]
- 6. Si L, Winzenberg T, Palmer A. A systematic review of models used in cost‐effectiveness analyses of preventing osteoporotic fractures. Osteoporos Int, 2014, 25: 51–60. [DOI] [PubMed] [Google Scholar]
- 7. Lewiecki EM, Miller PD, Harris ST, et al. Understanding and communicating the benefits and risks of denosumab, raloxifene, and teriparatide for the treatment of osteoporosis. J Clin Densitom, 2014, 17: 490–495. [DOI] [PubMed] [Google Scholar]
- 8. Hadji P, Claus V, Ziller V, Intorcia M, Kostev K, Steinle T. GRAND: the German retrospective cohort analysis on compliance and persistence and the associated risk of fractures in osteoporotic women treated with oral bisphosphonates. Osteoporos Int, 2012, 23: 223–231. [DOI] [PubMed] [Google Scholar]
- 9. Lo S. Compliance with oral osteoporosis drugs. Osteoporos Int, 2010, 21 (Suppl 1): S25–S388. [Google Scholar]
- 10. Black DM, Reid IR, Boonen S, et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON‐Pivotal Fracture Trial (PFT). J Bone Miner Res, 2012, 27: 243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Black DM, Reid IR, Cauley JA, et al. The effect of 6 versus 9 years of zoledronic acid treatment in osteoporosis: a randomized second extension to the HORIZON‐Pivotal Fracture Trial (PFT). J Bone Miner Res, 2015, 30: 934–944. [DOI] [PubMed] [Google Scholar]
- 12. Greenspan SL, Perera S, Ferchak MA, Nace DA, Resnick NM. Efficacy and safety of single‐dose zoledronic acid for osteoporosis in frail elderly women: a randomized clinical trial. JAMA Intern Med, 2015, 175: 913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cavalier E, Bergmann P, Bruyère O, et al. The role of biochemical of bone turnover markers in osteoporosis and metabolic bone disease: a consensus paper of the Belgian Bone Club. Osteoporos Int, 2016, 27: 2181–2195. [DOI] [PubMed] [Google Scholar]
- 14. Lupsa BC, Insogna K. Bone health and osteoporosis. Endocrinol Metab Clin North Am, 2015, 44: 517–530. [DOI] [PubMed] [Google Scholar]
- 15. Agresti A. A survey of exact inference for contingency tables. Stat Sci, 1992, 7: 131–153. [Google Scholar]
- 16. Black DM, Delmas PD, Eastell R, et al. Once‐yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med, 2007, 356: 1809–1822. [DOI] [PubMed] [Google Scholar]
- 17. Reid IR, Brown JP, Burckhardt P, et al. Intravenous zoledronic acid in postmenopausal women with low bone mineral density. N Engl J Med, 2002, 346: 653–661. [DOI] [PubMed] [Google Scholar]
- 18. Delaney MF. Strategies for the prevention and treatment of osteoporosis during early postmenopause. Am J Obstet Gynecol, 2006, 194 (2 Suppl): S12–S23. [DOI] [PubMed] [Google Scholar]
- 19. Riggs BL, Melton LJ 3rd. The prevention and treatment of osteoporosis. N Engl J Med, 1992, 327: 620–627. [DOI] [PubMed] [Google Scholar]
- 20. Grey A, Bolland MJ, Horne A, et al. Five years of anti‐resorptive activity after a single dose of zoledronate—results from a randomized double‐blind placebo‐controlled trial. Bone, 2012, 50: 1389–1393. [DOI] [PubMed] [Google Scholar]
- 21. Watson R. Health spending rising faster than GDP in most rich countries. BMJ, 2006, 333: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kennel KA, Drake MT. Adverse effects of bisphosphonates: implications for osteoporosis management. Mayo Clin Proc, 2009, 84: 632–637, quiz 638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Solomon DH, Rekedal L, Cadarette SM. Osteoporosis treatments and adverse events. Curr Opin Rheumatol, 2009, 21: 363–368. [DOI] [PubMed] [Google Scholar]
- 24. McClung M, Harris ST, Miller PD, et al. Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. Am J Med, 2013, 126: 13–20. [DOI] [PubMed] [Google Scholar]
- 25. Samelson EJ, Miller PD, Christiansen C, et al. RANKL inhibition with denosumab does not influence 3‐year progression of aortic calcification or incidence of adverse cardiovascular events in postmenopausal women with osteoporosis and high cardiovascular risk. J Bone Miner Res, 2014, 29: 450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schilcher J, Koeppen V, Aspenberg P, et al. Risk of atypical femoral fracture during and after bisphosphonate use. N Engl J Med, 2014, 371: 974–976. [DOI] [PubMed] [Google Scholar]
- 27. Chiang CY, Zebaze RM, Ghasem‐Zadeh A, et al. Teriparatide improves bone quality and healing of atypical femoral fractures associated with bisphosphonate therapy. Bone, 2013, 52: 360–365. [DOI] [PubMed] [Google Scholar]
- 28. Hough F, Brown SL, Cassim B, et al. The safety of osteoporosis medication. S Afr Med J, 2014, 104: 279–282. [DOI] [PubMed] [Google Scholar]
- 29. Weaver CM. Calcium supplementation: is protecting against osteoporosis counter to protecting against cardiovascular disease?. Curr Osteoporos Rep, 2014, 12: 211–218. [DOI] [PubMed] [Google Scholar]
- 30. Seeman E, Compston J, Adachi J, et al. Non‐compliance: the Achilles’ heel of anti‐fracture efficacy. Osteoporos Int, 2007, 18: 711–719. [DOI] [PubMed] [Google Scholar]


