Abstract
On the standard perspective, anorexia nervosa and other eating disorders are caused by genetically determined, neurochemically mediated mental illnesses. Standard treatment, cognitive behavioral therapy (CBT), targets cognitive processes thought to maintain the disorders. Effective neurochemically based treatments are not available and the rate of remission is ≤25% 1 year after CBT, with unknown outcomes in the long-term. With starvation as the major threat in biological history, the evolutionary perspective focuses on foraging for food and eating behavior. A neural network, including hypothalamic arcuate peptide-neurons, brainstem serotonin- and dopamine-neurons and their prefrontal cortical projections, mediates (rather than controls) the behavioral adaptations to variations in food availability; activation of the network is associated with opposing behavioral outcomes depending upon external variations. In the clinic, the control of eating behavior is therefore outsourced to a machine that provides feedback on how to eat. Hundreds of eating disorders patients have recovered by practicing eating; the rate of remission is 75% in on average 1 year of treatment, the rate of relapse is 10% over 5 years of follow-up and no patient has died. A two-parameter asymptotic exponential growth curve modeled the eating behavior of 17 healthy women but not that of 17 women with anorexia nervosa. When in remission, the eating behavior of the anorexic women approached that of the healthy women. It is suggested that the treatment of eating disorders should focus on eating behavior.
Keywords: evolution, anorexia, eating, hypothalamus, brainstem, prefrontal cortex, treatment, mathematical models
Introduction
“Anorexia nervosa is a psychiatric disorder characterized by fear of weight gain and dangerously low body weight … mortality rate exceeds that of other psychiatric disorders … finding comprehensive brain-based models … has been difficult" (Frank et al., 2018). Thus start most accounts. But it was recently suggested that this standard perspective needs to be modified because the treatment of anorexia is at a standstill (Gutierrez and Birmingham, 2018). We will describe the standard perspective and its translation into clinical practice first and then we will describe the evolutionary perspective, with eating behavior in clinical practice.
The Standard Perspective
On the standard perspective, anorexia is caused by a pre-existing, neurochemically mediated, genetically determined mental disorder as outlined some time ago as: “We hypothesize that people with anorexia nervosa have a trait-related increase in 5-HT neuronal transmission that occurs in the premorbid state and persists after recovery” (Kaye et al., 2003) and: “Childhood anxiety represents one important genetically mediated pathway toward the development of anorexia nervosa and bulimia nervosa” (Kaye et al., 2004). The perspective is similar today (Treasure et al., 2015).
Clinical Translation of Neurochemistry and Genetics
If anorexia is caused by an increase of 5-HT synthesis an inhibitor or an antagonist should be used, but paradoxically, indirect agonists are used, and although useful in patients with mental disorders (Locher et al., 2017), these drugs are not useful in patients with anorexia (Walsh et al., 2006; Zandian et al., 2007). But neither are other drugs, including neuroleptics, which are valuable for patients with mental disorders (Lieberman et al., 2005), useful in patients with eating disorders (Attia et al., 2019). This differential effectiveness of psychopharmacological intervention may be because the “mental disorders” of eating disorders differ from those of patients with mental disorders. Thus, a rating scale that dissociates anxiety from other mental disorders in patients with mental disorders did not dissociate these disorders in 358 patients with anorexia nervosa [PS and others, manuscript submitted for Gutierrez and Birmingham (2018)].
The discovery that mental disorders are not distinct categories but vary along continuous dimensions was made long ago (Fisher, 1918; Porter, 2018), emphasized not long ago (Borsboom et al., 2011) and recently re-discovered (Brainstorm Consortium, Anttila et al., 2018; Plana-Ripoll et al., 2019; Schork et al., 2019). Hence, attempts to find genotype-phenotype correlations among eating disorders and mental disorders have yielded inconsistent results (Borsboom et al., 2011). Translating these results into treatments for eating disorders will be difficult (Breithaupt et al., 2018). This approach, which was launched 20 years ago in other contexts, has been marginally successful (Joyner and Paneth, 2019).
The Standard Treatment
The standard treatment, cognitive behavioral therapy (CBT), assumes that eating disorders are maintained by cognitive processes. Even though CBT does not address the cause of eating disorders, it recognizes that the patients’ problems start with dieting (Slade et al., 2018). Launched for bulimia nervosa in 1981 (Fairburn, 1981), CBT is now recommended in the treatment guidelines for all eating disorders throughout the world [e.g., (NICE, 2017)].
Rather few patients have been treated with CBT in randomized controlled trials (RCT) (Slade et al., 2018). With a dropout rate ≈30%, which is generally expected and included in the power calculations of RCTs (Zipfel et al., 2014), a rate of remission <50% and a rate of relapse ≥30% within 1 year, ≤25% of the patients remain in remission at this point in time (Södersten et al., 2017).
Many more patients have been treated with CBT in general practice. For example, out of 683 patients referred to primary care for the treatment of bulimia within the United Kingdom healthcare system, 135 completed the treatment but although they improved, these patients did not remit (Knott et al., 2015). In Sweden, 15,411 patients were similarly treated in years 2012–2017 with a rate of remission of 18.4% at one year follow-up (Birgegård and Norring, 2019). There are no major differences between these outcomes and the outcomes in the specialized clinics in Sweden and other countries (Södersten et al., 2017, 2019).
What explains these low remission rates? Consider the most recent RCT in which 15 out of 36 patients (42%) went into remission from bulimia but not from anxiety (Poulsen et al., 2014). On the standard perspective, anxiety causes bulimia (Kaye et al., 2004) and it is unsurprising, therefore, that 5 of the 15 patients (33%) relapsed within 19 months. A new review found no “relevant new RCTs” and concluded that CBT is “an effective approach” (Slade et al., 2018), despite the fact that 22.2% of the patients dropped out, 33% relapsed and 39.3% received additional treatment during follow-up in the trial (Poulsen et al., 2014). Considering that there is no information of long-term outcomes, it should be possible to improve the effectiveness of CBT (Södersten et al., 2017; Slade et al., 2018). A new perspective might offer a start.
The Evolutionary Perspective
A framework for anorexia nervosa, the prototypical eating disorder from which the other eating disorders emerge, was launched in 1996, with food restriction as the main cause (Bergh and Södersten, 1996). The neuroendocrine changes associated with this brain-based model have been reviewed (Bergh et al., 2002, 2013; Zandian et al., 2007; Södersten et al., 2008, 2016, 2017) and can be briefly updated as follows.
Because starvation has been the main threat in evolution it is fitting to paraphrase Dobzhansky: ”Nothing in the biology of anorexia makes sense except in the light of evolution” (Dobzhansky, 1973). And 36 years ago, it was realized that the conspicuous high physical activity of anorexia is a normal, evolutionary conserved response, i.e., foraging for food when food is in short supply (Epling et al., 1983). Later on, the evolutionary perspective was presented twice more (Guisinger, 2003; Södersten et al., 2008).
In fact, anorexia provides an example of the human homeostatic phenotype, as this concept emerged from the clinical observations and hypotheses of Bernard and the subsequent experimental verifications of Cannon (Södersten et al., 2008). This perspective has now been validated for brain function. Thus, the signaling molecules of the hypothalamic arcuate nucleus support the search for food, rather than eating (Ammar et al., 2000; Nergårdh et al., 2007; Chen et al., 2015; Dietrich et al., 2015; Burnett et al., 2016). The agouti-related protein neurons of this nucleus can monitor the availability of food in the environment, changing energy utilization from fat to carbohydrate (Chen et al., 2015; Burke et al., 2017; Cavalcanti-de-Albuquerque et al., 2019). Silencing these neurons eliminates the search for food but leaves chewing and swallowing unaffected (Thomas et al., 2018), replicating the effect of dopamine receptor blockade or depletion (Berridge et al., 1989; Bednar et al., 1992; Qian et al., 1998).
The search for food and eating behavior, chewing in particular, have dominated the evolution of the behavior and the anatomy of the individual, including the head and the brain (Lieberman, 2011, 2014; Ungar, 2017; Smith, 2018). “You are How you eat,” suggests the evolutionary biologist and even that we should “encourage [our children] to chew more gum” (Lieberman, 2011). And since it was first reported that chewing gum is relaxing 80 years ago (Hollingworth, 1939), it is now recognized that chewing gum promotes both physical and mental health (Fukushima-Nakayama et al., 2017). The neural engagement in these beneficial effects of chewing include the serotonin cells in the dorsal raphe nucleus in the brainstem and their projections to the prefrontal and orbitofrontal cortex (Ioakimidis et al., 2011). These serotonin neurons and the hypothalamic agouti-related protein neurons also activate dopamine neurons in the ventral tegmental area in the brainstem (Davis et al., 2011; Browne et al., 2019). Interestingly, activity in these mesolimbic dopamine neurons can functionally rearrange the connections within the prefrontal cortex (Kahnt and Tobler, 2017). Foraging for food has shaped these cortical and subcortical areas into an extended neural network, parts of which are differentially engaged dependent upon environmental conditions (Kolling et al., 2012; Pearson et al., 2014; Carlén, 2017; Korn and Bach, 2018). Dopamine, of course, plays roles in addition to the one(s) discussed here, some of which are important in evolution, including the management of threats (Miller et al., 2019).
It is well known that in evolution “men hunt and women gather” (Fessler, 2002; Gilby et al., 2017), but it is not yet known how these behavioral sex differences are related to the neural network of foraging. Research on the neuroscience of foraging often use economic rewards and choices, food rewards are less common (Kolling et al., 2012; Shenhav et al., 2016). However, it was observed long ago that the emergence of the prefrontal cortex in primate evolution coincided with improvement of the strategies for food foraging (overview in Genovesio et al., 2014). Gonadal hormone sensitive sex differences have since been demonstrated in the anatomy of the prefrontal cortex and these can be related to sexually dimorphic behavior (Clark and Goldman-Rakic, 1989; Evans and Hampson, 2015). On the evolutionary perspective, it is tempting, therefore, to speculate that these findings are related to the marked sex difference in the prevalence of anorexia nervosa.
The neurobehavioral responses to food deprivation and the corresponding genotype are evolutionarily conserved and consistent with the evolutionary perspective of anorexia nervosa (Södersten et al., 2008; Alvergne et al., 2010; Itskov et al., 2014; Gibson et al., 2015; Sato and Kawata, 2018).
The Elusive Clinical Translation of the Neurobiology of Foraging
But rather than controlling behavior, the neural network just outlined is permissive; the cause of changes in eating behavior is outside of the individual (Södersten et al., 2011; Zandian et al., 2015). For example, the behavioral effects of experimental activation of the brainstem to prefrontal cortex part of the network in one environment are the opposite to the behavioral effect of the same experimental maneuver in another environment (Warden et al., 2012; Seo et al., 2019). Similarly, stimulating the brain with neuropeptide tyrosine makes a rat eat more food when food is continuously available but makes the rat forage for food and eat less food when the availability of food is restricted (Ammar et al., 2000; Nergårdh et al., 2007). These results support the proposed causal role of the environment in body weight regulation and suggest that neuropharmacological intervention may remain ineffective.
In normal circumstances, our biological propensity to eat as much as possible is counterbalanced by the need to forage for food (Södersten et al., 2011). But today the effort to find food is minimal and in the absence of internal controls people need external support in order not to lose control over body weight (Södersten et al., 2008).
Eating Behavior in Treatment
In the clinic, we have therefore outsourced the control of eating behavior and body weight to a machine first described in 1996 (Bergh et al., 1996; Södersten and Bergh, 2014). The patients learn to eat assisted by visual feedback from a computer screen as described many times already and recently in an open access video (Esfandiari et al., 2018). But they are also treated with warmth, their physical activity is reduced and they are supported to resume their social activities (Bergh et al., 2002). An RCT demonstrated the treatments effectiveness (Bergh et al., 2002), which was confirmed by a description of the outcomes at 3 months intervals during treatment and 1, 2, 3, 6, 9, 12, 18, 24, 26, 48, and 60 months after remission in 1,428 patients treated in six clinics in four countries (Bergh et al., 2013). The rate of remission was estimated to 75% within on average 1 year of treatment and the rate of relapse was estimated to 10% (Bergh et al., 2013). Psychoactive drugs that had been prescribed prior to admission to treat mental symptoms were withdrawn while the patients remitted from these symptoms by re-learning how to eat (PS and others, manuscript submitted for Gutierrez and Birmingham, 2018).
The Paradox of Standard Treatment
More patients go into remission in the long-term by re-learning how to eat than if treated with CBT (Södersten et al., 2017, 2019). The difference in outcome is unlikely due to difference in the state of the patients at admission. The published literature suggests the opposite; patients who are treated with standards of care are less serious ill at admission than patients whose eating behavior is treated (Södersten et al., 2016).
Considering the difference in outcomes, it is paradoxical that “the single most effective procedure in CBT” has long been recognized as “the prescription of a pattern of regular eating” (Fairburn et al., 1993). But because is unclear how this is achieved we have invited CBT-clinicians to use our method for treating eating behavior (Södersten et al., 2017).
How to Eat
The biological, default pattern of eating behavior, a gradual decrease in the rate of eating over the course of a meal, was first described in experimental animals as: N = Ktn; where N = amount of food eaten at time t and K and n are constants (Skinner, 1930) and then modified as an exponential growth curve: f = c(l-e-mt); where f = amount of food eaten, c and m constants and t = time (Bousfield, 1933). A model of human eating behavior was presented as: y = kx2+lx; where y = amount of food eaten, k = change in the rate of eating over the course of the meal and l = initial rate of eating (Pudel, 1971). This model was subsequently confirmed (Kissileff et al., 1982). The recent suggestion that the model should predict outcomes and disclose mechanisms is based on 40 year old experimental results rather than the recent biology of foraging (Thomas et al., 2017). At present, the model remains descriptive, but as outlined here, it can be used in the treatment of eating behavior in patients with eating disorders.
With k < 0 in the model, Westerterp-Plantenga launched the term decelerated eating and with k≈0, she launched the term linear eating (Westerterp-Plantenga et al., 1990). If rats are deprived of food for 4 days, food intake decreases the linearity of eating increases (Bousfield and Elliott, 1934). Women respond in the same manner after merely skipping dinner (Levitsky and DeRosimo, 2010; Zandian et al., 2011).
Linear eaters eat less food yet feel increasingly full when eating at a reduced rate experimentally and they eat more food yet feel less full when eating at an increased rate experimentally (Zandian et al., 2009a). Thus, dieting, the main cause of anorexia, causes linear eating very rapidly and puts women at risk of losing control over food intake. These undesirable effects can be prevented by practicing eating at a decelerated rate (Zandian et al., 2009b). And when women transit from linear eating to decelerated eating their mental state normalizes (Zandian et al., 2009b), just as 737 patients remitted from their mental symptoms by re-learning how to eat (Bergh et al., 2013).
The Eating Behavior of Anorexic Patients Treated to Remission
The derivative of the old model is a line but growth, including cumulative food intake, tapers off. We therefore re-launch the two-parameter asymptotic exponential curve as a minimally redundant model of eating behavior: y = a(1-e-bt), where y = amount of food eaten, a = hypothetical maximal food intake, b = change in the rate of eating and t = time (Bousfield, 1933).
Using non-linear regression (Bates and Chambers, 1992; R Nonlinear Regression, 2019), we describe the eating behavior of 17 women who were treated to remission from anorexia nervosa by practicing how to eat. Their mean (SD) age was 18.8 (3.7) years, they had been ill for 3.3 (2.2) years and had a Body Mass Index, BMI = 14.9 (1.0) at admission. The women went into remission in 359 (78) days, at a BMI = 19.8 (0.9). For a complete list of remission criteria, see (Bergh et al., 2002). Their eating behavior was compared to that of 17 healthy women, who were 23.6 (2.0) years old and had a BMI = 23.5 (1.5). The choice of 5 years older healthy women for comparison was based on the fact that patients who have been treated to remission are followed up for 5 years before they are considered cured (Bergh et al., 2002).
Table 1 shows that the patients ate only little food, slowly, at admission, but when in remission, they ate somewhat more food than the healthy women and the duration of their meal was a little shorter. While the initial rate of eating among the anorexics in remission and the healthy women was similar, the rate of eating decreased over the course of the meal significantly more among the healthy women than among the women in remission. These differences in eating behavior emerge clearly in Figure 1. One of the patients continued eating for 37 min at admission, i.e., beyond the 20 min limit displayed (bottom graph in panel A). Three patients ate in a linear manner at remission and their curves are therefore omitted in panel B.
Table 1.
Food intake, meal duration, initial rate of eating, change in rate of eating over the course of the meal (b) and hypothetical maximal food intake (a) in 17 women at admission for the treatment of Anorexia Nervosa (Adm AN) and at remission after treatment (Rem AN) and in 17 healthy women (Healthy).
| Group | Food intake (g) | Meal duration (min) | Initial rate of eating (g/min)a | Change in rate of eating (b) | Hypothetical maximal food intake (a) |
|---|---|---|---|---|---|
| Adm AN | 100 (72–160)b | 10.4 (7.7–13.6) | 8.4 (6.2–16.3)b | – | |
| Rem AN | 307 (266–351)c | 9.6 (8.0–10.6)c | 44.3 (34.4–46.9) | 0.06 (0–0.22)d | 586 (377–684) |
| Healthy | 268 (208–389) | 10.7 (10.1–12.2) | 39.1 (35.8–46.9) | 0.12 (0.05–0.23) | 374 (333–444) |
Values are median (quartile range). See text for model of eating behavior. aDerivative at time=1 min of the estimated function; bp < 0.01 vs. Healthy or Rem AN; cp = 0.06 vs. Healthy; dp = 0.015 (Mann-Whitney U-test).
FIGURE 1.
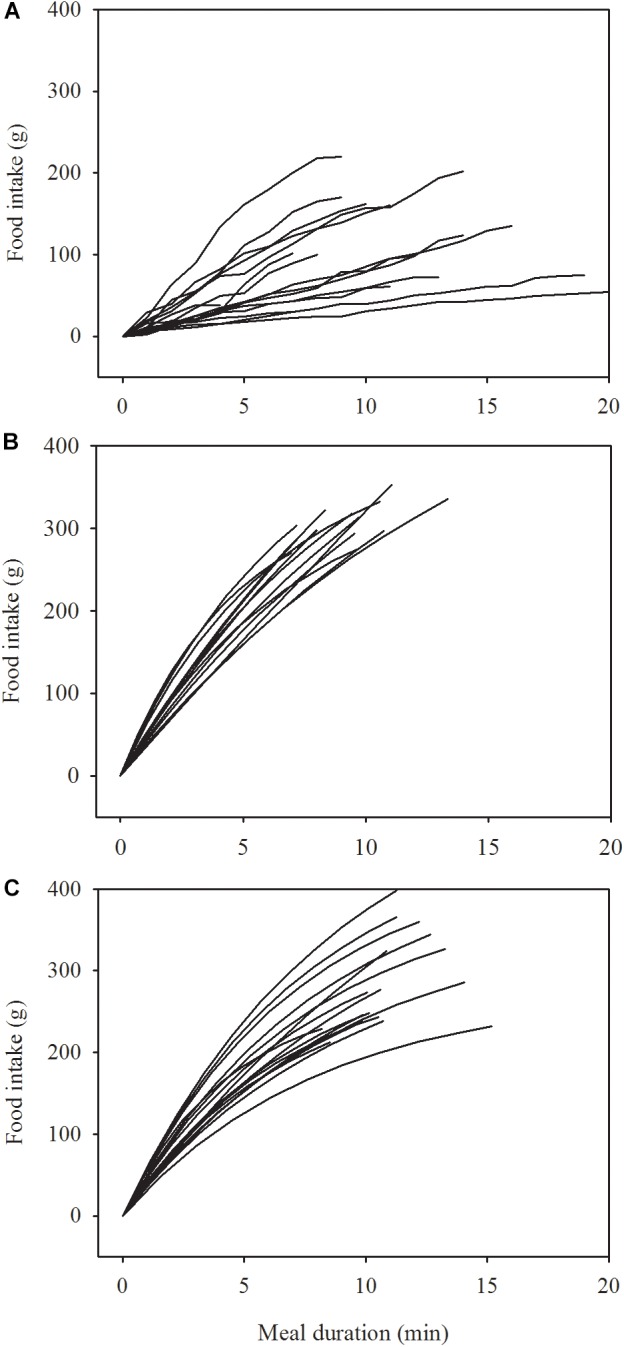
Change in the rate of eating in 17 women at admission (A) and at remission (B) after treatment of anorexia nervosa and in 17 healthy women (C). Data in A are raw data collected at 1 min intervals. Data in B,C are modeled by a two-parameter asymptotic exponential curve, see text for details.
Comments, Personal Insights and Opinions
While the anorexic women who practiced eating reached a BMI within the normal range and consumed a normal amount of food, their weight and their eating behavior was not the same as those of the healthy women. Our patients are followed for 5 years after treatment, including eleven appointments (Bergh et al., 2002, 2013) and, at present, we are examining if their physical characteristics and their eating behavior more closely resembles those of healthy women once they have completed the follow-up program. Yet, at the present state of knowledge, it is reasonable to suggest that patients with eating disorders should be offered the chance to practice eating using the device that has now restored the physical and mental health of hundreds of patients (Bergh et al., 2013; Södersten et al., 2017, 2019). Eating behavior thus treated makes it less important, albeit perhaps not unimportant, to treat cognitive processes (Södersten et al., 2017), although evidence that these interventions are redundant was presented 31 years ago (Freeman et al., 1988).
Practicing eating restores the levels of hormones thought to cause weight problems in obesity (Galhardo et al., 2012; Södersten et al., 2015), suggesting eating behavior control of hormonal secretion, i.e., the opposite causal relationship to the conventional homeostatic relationship (Lowell, 2019). The bidirectional relationship among brain and behavior, suggested by Darwin (1872) and confirmed in recent years (Woods, 1991; Ramsay and Woods, 2014), provides support for clinical translation of the present perspective.
In 1996 we suggested that eating disorders are eating disorders, rather than mental disorders, and that the patients therefore should practice eating (Bergh and Södersten, 1996; Bergh et al., 1996). At the time, it was thought that this was misplaced and even dangerous (Crisp, 1995), but today, 23 years later, no-one can treat patients with eating disorders in the Region of Stockholm unless a program for restoring their eating behavior is included in the treatment. Such overly long delays before evidence-based interventions are introduced into clinical practice are common (Kim et al., 2013). Policies to shorten the delay would be useful.
Data Availability
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
Data on eating behavior is part of treatment and the clinical files are kept in the register of the Mandometer Clinic, approved by the Regional Ethical Review Board of Stockholm. Patients entering treatment are informed verbally and in writing that their data might be used in research and if so, it will be anonymized. They are also informed that they can request that their data is not used and that they can leave the treatment any time without giving a reason. Written consent by the patients is not required for analysis of data collected in registries. The data from the healthy women were re-analyzed from a previous, ethically approved study (Zandian et al., 2009a).
Author Contributions
PS launched the idea with all authors. UB undertook the statistical analysis and reviewed these with PS in detail and repeatedly. MZ was keeper of databases and clinical files and quality controller at the clinic. CB was a clinical director and supervised all treatments. All authors contributed intellectually to the content and to completing the final version of the manuscript which has been approved accordingly and wrote and reviewed the manuscript repeatedly.
Conflict of Interest Statement
Complete openness concerning financial arrangements is intended here. UB and MZ declare that they have no financial interests related to this study. Our research is carried out at the Karolinska Institute, where PS is an emeritus professor. The research is translated clinically by Mando Group AB, a company started by PS and CB, who have 47.5% of the stock each. Professor Michael Leon of the University of California at Irvine has 5%. Mando Group AB contracts with the County Council of Stockholm every fifth year to treat patients with eating disorders. Mando Groups AB signed its first contract in 1997 with the County Council of Stockholm and, since then, its treatment is one of the standards of care treatments offered to the citizens of Stockholm. This arrangement is the same as when the County Council of Stockholm contracts with its own clinics to treat patients with all kinds of disease, including eating disorders. That is to say, the County Council of Stockholm provides eating-disorder services to the citizens of Stockholm both through a clinic of its own and through Mando Group AB. Until recently, there was a third provider of care for patients with eating disorders in Stockholm, which was a private clinic. Mando Group AB is the biggest provider of eating disorders services in Sweden as of 2019. All health care in Sweden is funded through the tax system; private pay is extremely uncommon. It should be added firstly, that Mando Group AB is in compliance with the recommendation of the International Committee of Medical Journal Editors on “Author Responsibilities-Conflicts of Interest” http://www.icmje.org/recommendations/browse/roles-and-responsibilities/author-responsibilities--conflicts-of-interest.html. Secondly, it should also be added that all profit that Mando Group AB has made has been re-invested in research and development and that there have been no dividends to stock owners. All of the above is declared in all manuscript submissions and thus far, journals have judged it necessary to publish only some of the details. It seems, however, that the potential ethical problem when scientists translate their research findings into the clinic in a company is not unlike that which arises when any scientist, in an academic setting is developing a theory and needs further economic funding for her/his work and may receive recognition and financial benefits for the work. The incentive is, in part, economic in this case as well and the ethical “problem” is similar in both cases. However, the more important incentive is the improvement of the treatment of patients with eating disorders. We are researchers working in an academic setting and like many other medical research institutes today, the Karolinska Institute encourages scientists to translate their research into the clinic in companies that aim to generate financial profits to be used for research and development (see https://issuu.com/karolinska_institutet/docs/ki_strategy2030_eng).
Footnotes
Funding. This work was supported by Mando Group AB.
References
- Alvergne A., Jokela M., Lummaa V. (2010). Personality and reproductive success in a high-fertility human population. Proc. Natl. Acad. Sci. U.S.A. 107 11745–11750. 10.1073/pnas.1001752107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammar A. A., Sederholm F., Saito T. R., Scheurink A. J., Johnson A. E., Södersten P. (2000). NPY-leptin: opposing effects on appetitive and consummatory ingestive behavior and sexual behavior. Am. J. Physiol. Regul. Integr. Comp. Physiol. 278 R1627–R1633. [DOI] [PubMed] [Google Scholar]
- Attia E., Steinglass J. E., Walsh B. T., Wang Y., Wu P., Schreyer C., et al. (2019). Olanzapine versus placebo in adult outpatients with anorexia nervosa: a randomized clinical trial. Am. J. Psychiatry [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D. M., Chambers J. M. (1992). Nonlinear models. in Statistical Models in S. eds Chambers J., Hastie Boca Raton T. FL: CRC Press, 421–453. [Google Scholar]
- Bednar I., Qureshi G. A., Södersten P. (1992). A comparison between the effect of cholecystokinin octapeptide and apomorphine on ingestion of intraorally administered sucrose in male rats. J. Neuroendocrinol. 4 727–734. 10.1111/j.1365-2826.1992.tb00224.x [DOI] [PubMed] [Google Scholar]
- Bergh C., Brodin U., Lindberg G., Södersten P. (2002). Randomized controlled trial of a treatment for anorexia and bulimia nervosa. PNAS 99 9486–9491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergh C., Callmar M., Danemar S., Hölcke M., Isberg S., Leon M., et al. (2013). Effective treatment of eating disorders: results at multiple sites. Behav. Neurosci. 127 878–889. 10.1037/a0034921 [DOI] [PubMed] [Google Scholar]
- Bergh C., Eklund S., Eriksson M., Lindberg G., Södersten P. (1996). A new treatment of anorexia nervosa. Lancet 348 611–612. [DOI] [PubMed] [Google Scholar]
- Bergh C., Södersten P. (1996). Anorexia nervosa, self–starvation and the reward of stress. Nat. Med. 2 21–22. 10.1038/nm0196-21 [DOI] [PubMed] [Google Scholar]
- Berridge K. C., Venier I. L., Robinson T. E. (1989). Taste reactivity analysis of 6-hydroxydopamine-induced aphagia: implications for arousal and anhedonia hypotheses of dopamine function. Behav. Neurosci. 103 36–45. 10.1037//0735-7044.103.1.36 [DOI] [PubMed] [Google Scholar]
- Birgegård A., Norring C. (2019). Nationellt kvalitetsregister för ätstörningsbehandling. J. Eat. Disord. 7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsboom D., Cramer A. O. J., Schmittmann V. D., Epskamp S., Waldorp L. J. (2011). The small world of psychopathology. PLoS One 6:e27407. 10.1371/journal.pone.0027407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousfield W. A. (1933). Certain quantitatve aspects of the food-behavior of cats. J. Gen. Psychol. 8 446–454. 10.1080/00221309.1933.9713197 [DOI] [Google Scholar]
- Bousfield W. A., Elliott M. H. (1934). The effect of fasting on the eating-behavior of rats. Pedagog. Semin. J. Genet. Psychol. 45 227–237. 10.1080/08856559.1934.10534256 [DOI] [Google Scholar]
- Brainstorm Consortium Anttila V., Bulik-Sullivan B., Finucane H. K., Walters R. K., Bras J. et al. (2018). Analysis of shared heritability in common disorders of the brain. Science 360:eaa8757 10.1126/science.8757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breithaupt L., Hubel C., Bulik C. M. (2018). Updates on genome-wide association findings in eating disorders and future application to precision medicine. Curr. Neuropharmacol. 16 1102–1110. 10.2174/1570159X16666180222163450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne C. J., Abela A. R., Chu D., Li Z., Ji X., Lambe E. K., et al. (2019). Dorsal raphe serotonin neurons inhibit operant responding for reward via inputs to the ventral tegmental area but not the nucleus accumbens: evidence from studies combining optogenetic stimulation and serotonin reuptake inhibition. Neuropsychopharmacology 44 793–804. 10.1038/s41386-018-0271-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke L. K., Darwish T., Cavanaugh A. R., Virtue S., Roth E., Morro J., et al. (2017). mTORC1 in AGRP neurons integrates exteroceptive and interoceptive food-related cues in the modulation of adaptive energy expenditure in mice. eLife 6:e22848. 10.7554/eLife.22848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett C. J., Li C., Webber E., Tsaousidou E., Xue S. Y., Brüning J. C., et al. (2016). Hunger-driven motivational state competition. Neuron 92 187–201. 10.1016/j.neuron.2016.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlén M. (2017). What constitutes the prefrontal cortex? Science 358 478–482. 10.1126/science.aan8868 [DOI] [PubMed] [Google Scholar]
- Cavalcanti-de-Albuquerque J. P., Bober J., Zimmer M. R., Dietrich M. O. (2019). Regulation of substrate utilization and adiposity by agrp neurons. Nat. Commun. 10:311. 10.1038/s41467-018-08239-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Lin Y.-C., Kuo T.-W., Knight Z. A. (2015). Sensory detection of food rapidly modulates arcuate feeding circuits. Cell 160 829–841. 10.1016/j.cell.2015.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. S., Goldman-Rakic P. S. (1989). Gonadal hormones influence the emergence of cortical function in nonhuman primates. Behav. Neurosci. 103 1287–1295. 10.1037//0735-7044.103.6.1287 [DOI] [PubMed] [Google Scholar]
- Crisp A. H. (1995). The dyslipophobias: a view of the psychopathologies involved and the hazards of construing anorexia nervosa and bulimia nervosa as “eating disorders.”. Proc. Nutr. Soc. 54 701–709. 10.1079/pns19950069 [DOI] [PubMed] [Google Scholar]
- Darwin C. (1872). The Expression of the Emotions in Man and Animals. London: John Murray. [Google Scholar]
- Davis J. F., Choi D. L., Shurdak J. D., Krause E. G., Fitzgerald M. F., Lipton J. W., et al. (2011). Central melanocortins modulate mesocorticolimbic activity and food seeking behavior in the rat. Physiol. Behav. 102 491–495. 10.1016/j.physbeh.2010.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich M. O., Zimmer M. R., Bober J., Horvath T. L. (2015). Hypothalamic Agrp neurons drive stereotypic behaviors beyond feeding. Cell 160 1222–1232. 10.1016/j.cell.2015.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky T. (1973). Nothing in biology makes sense except in the light of evolution. Am. Biol. Teach. 35 125–129. 10.2307/4444260 [DOI] [Google Scholar]
- Epling W. F., Pierce W. D., Stefan L. (1983). A theory of activity-based anorexia. Int. J. Eat. Disord. 3 27–46. [DOI] [Google Scholar]
- Esfandiari M., Papapanagiotou V., Diou C., Zandian M., Nolstam J., Södersten P., et al. (2018). Control of eating behavior using a novel feedback system. J. Vis. Exp. 135:57432. 10.3791/57432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans K. L., Hampson E. (2015). Sex differences on prefrontally-dependent cognitive tasks. Brain Cogn. 93 42–53. 10.1016/j.bandc.2014.11.006 [DOI] [PubMed] [Google Scholar]
- Fairburn C. (1981). A cognitive behavioural approach to the treatment of bulimia. Psychol. Med. 11 707–711. 10.1017/s0033291700041209 [DOI] [PubMed] [Google Scholar]
- Fairburn C. G., Marcus M. D., Wilson G. T. (1993). Cognitive-behavioral therapy for binge eating and bulimia nervosa: a comprehensive treatment manual. in Binge Eating, Nature, Assessment and Treatment. ed. Wilson G. New York NY: Guilford Press, 361–404. [Google Scholar]
- Fessler D. M. (2002). Dimorphic foraging behaviors and the evolution of hominid hunting. Riv. Biol. 95 429–453. [PubMed] [Google Scholar]
- Fisher R. A. (1918). The correlation between relatives on the supposition of mendelian inheritance. Trans. R. Soc. Edinb. 52 399–433. 10.1017/s0080456800012163 [DOI] [Google Scholar]
- Frank G. K. W., DeGuzman M. C., Shott M. E., Laudenslager M. L., Rossi B., Pryor T. (2018). Association of brain reward learning response with harm avoidance, weight gain, and hypothalamic effective connectivity in adolescent anorexia nervosa. JAMA Psychiatry 75 1071–1080. 10.1001/jamapsychiatry.2018.2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman C. P. L., Barry F., Dunkeld-Turnbull J., Henderson A. (1988). Controlled trial of psychotherapy for bulimia nervosa. Br. Med. J. 296 521–525. 10.1136/bmj.296.6621.521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima-Nakayama Y., Ono T., Hayashi M., Inoue M., Wake H., Ono T., et al. (2017). Reduced mastication impairs memory function. J. Dent. Res. 96 1058–1066. 10.1177/0022034517708771 [DOI] [PubMed] [Google Scholar]
- Galhardo J., Hunt L. P., Lightman S. L., Sabin M. A., Bergh C., Sodersten P., et al. (2012). Normalizing eating behavior reduces body weight and improves gastrointestinal hormonal secretion in obese adolescents. J. Clin. Endocrinol. Metab. 97 E193–E201. 10.1210/jc.2011-1999 [DOI] [PubMed] [Google Scholar]
- Genovesio A., Wise S. P., Passingham R. E. (2014). Prefrontal-parietal function: from foraging to foresight. Trends Cogn. Sci. 18 72–81. 10.1016/j.tics.2013.11.007 [DOI] [PubMed] [Google Scholar]
- Gibson W. T., Gonzalez C. R., Fernandez C., Ramasamy L., Tabachnik T., Du R. R., et al. (2015). Behavioral responses to a repetitive visual threat stimulus express a persistent state of defensive arousal in Drosophila. Curr. Biol. 25 1401–1415. 10.1016/j.cub.2015.03.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilby I. C., Machanda Z. P., O’Malley R. C., Murray C. M., Lonsdorf E. V., Walker K., et al. (2017). Predation by female chimpanzees: toward an understanding of sex differences in meat acquisition in the last common ancestor of pan and homo. J. Hum. Evol. 110 82–94. 10.1016/j.jhevol.2017.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guisinger S. (2003). Adapted to flee famine: adding an evolutionary perspective on anorexia nervosa. Psychol. Rev. 110 745–761. 10.1037/0033-295x.110.4.745 [DOI] [PubMed] [Google Scholar]
- Gutierrez E., Birmingham C. L. (2018). New perspectives to unlock the current impasse in treating anorexia nervosa. Front. Psychol. (in press). Available at: https://www.frontiersin.org/research-topics/7656/new-perspectives-to-unlock-the-current-impasse-in-treating-anorexia-nervosa [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth H. L. (1939). Chewing as a techning of relaxation. Science 90 385–387. 10.1126/science.90.2339.385 [DOI] [PubMed] [Google Scholar]
- Ioakimidis I., Zandian M., Ulbl F., Bergh C., Leon M., Södersten P. (2011). How eating affects mood. Physiol. Behav. 103 290–294. 10.1016/j.physbeh.2011.01.025 [DOI] [PubMed] [Google Scholar]
- Itskov P. M., Moreira J.-M., Vinnik E., Lopes G., Safarik S., Dickinson M. H., et al. (2014). Automated monitoring and quantitative analysis of feeding behaviour in Drosophila. Nat. Commun. 5:4560. 10.1038/ncomms5560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner M. J., Paneth N. (2019). Promises, promises, and precision medicine. J. Clin. Invest. 29 946–948. 10.1172/jci126119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahnt T., Tobler P. N. (2017). Dopamine modulates the functional organization of the orbitofrontal cortex. J. Neurosci. 37 1493–1504. 10.1523/JNEUROSCI.2827-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye W. H., Barbarich N. C., Putnam K., Gendall K. A., Fernstrom J., Fernstrom M., et al. (2003). Anxiolytic effects of acute tryptophan depletion in anorexia nervosa. Int. J. Eat. Disord. 33 257–267; discussion268–270. [DOI] [PubMed] [Google Scholar]
- Kaye W. H., Bulik C. M., Thornton L., Barbarich N., Masters K. (2004). Comorbidity of anxiety disorders with anorexia and bulimia nervosa. Am. J. Psychiatry 161 2215–2221. [DOI] [PubMed] [Google Scholar]
- Kim J. Y., Farmer P., Porter M. E. (2013). Redefining global health-care delivery. Lancet 382 1060–1069. 10.1016/s0140-6736(13)61047-8 [DOI] [PubMed] [Google Scholar]
- Kissileff H. R., Thornton J., Becker E. (1982). A quadratic equation adequately describes the cumulative food intake curve in man. Appetite 3 255–272. 10.1016/s0195-6663(82)80022-6 [DOI] [PubMed] [Google Scholar]
- Knott S., Woodward D., Hoefkens A., Limbert C. (2015). Cognitive behaviour therapy for bulimia nervosa and eating disorders not otherwise specified: translation from randomized controlled trial to a clinical setting. Behav. Cogn. Psychother. 43 641–654. 10.1017/s1352465814000393 [DOI] [PubMed] [Google Scholar]
- Kolling N., Behrens T. E., Mars R. B., Rushworth M. F. (2012). Neural mechanisms of foraging. Science 336 95–98. 10.1126/science.1216930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn C. W., Bach D. R. (2018). Heuristic and optimal policy computations in the human brain during sequential decision-making. Nat. Commun. 9:325. 10.1038/s41467-017-02750-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitsky D. A., DeRosimo L. (2010). One day of food restriction does not result in an increase in subsequent daily food intake in humans. Physiol. Behav. 99 495–499. 10.1016/j.physbeh.2009.12.020 [DOI] [PubMed] [Google Scholar]
- Lieberman D. E. (2011). Evolution of the Human Head. Cambridge, MA: Harvard University Press. [Google Scholar]
- Lieberman D. E. (2014). The Story of the Human Body: Evolution, Health and Disease. New York, NY: Random House LLC. [PubMed] [Google Scholar]
- Lieberman J. A., Stroup T. S., McEvoy J. P., Swartz M. S., Rosenheck R. A., Perkins D. O., et al. (2005). Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N. Engl. J. Med. 353 1209–1223. [DOI] [PubMed] [Google Scholar]
- Locher C., Koechlin H., Zion S. R., Werner C., Pine D. S., Kirsch I., et al. (2017). Efficacy and safety of selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and placebo for common psychiatric disorders among children and adolescents: a systematic review and meta-analysis. JAMA Psychiatry 74 1011–1020. 10.1001/jamapsychiatry.2017.2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell B. B. (2019). New neuroscience of homeostasis and drives for food, water, and salt. N. Engl. J. Med. 380 459–471. 10.1056/NEJMra1812053 [DOI] [PubMed] [Google Scholar]
- Miller S. M., Marcotulli D., Shen A., Zweifel L. S. (2019). Divergent medial amygdala projections regulate approach–avoidance conflict behavior. Nat. Neurosci. 22:565. 10.1038/s41593-019-0337-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nergårdh R., Ammar A., Brodin U., Bergström J., Scheurink A., Södersten P. (2007). Neuropeptide Y facilitates activity-based-anorexia. Psychoneuroendocrinology 32 493–502. 10.1016/j.psyneuen.2007.03.002 [DOI] [PubMed] [Google Scholar]
- NICE (2017). Eating disorders: recognition and treatment. NICE Clin. Guide. 69 1–40. [Google Scholar]
- Pearson J. M., Watson K. K., Platt M. L. (2014). Decision making: the neuroethological turn. Neuron 82 950–965. 10.1016/j.neuron.2014.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plana-Ripoll O., Pedersen C. B., Holtz Y., Benros M. E., Dalsgaard S., de Jonge P., et al. (2019). Exploring comorbidity within mental disorders among a danish national population. JAMA Psychiatry [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter T. M. (2018). Genetics in the Madhouse: The Unknown History of Human Heredity. Princeton, N.J: Princeton Univ. Press. [Google Scholar]
- Poulsen S., Lunn S., Daniel S. I. F., Folke S., Mathiesen B. B., Katznelson H., et al. (2014). A randomized controlled trial of psychoanalytic psychotherapy or cognitive-behavioral therapy for bulimia nervosa. Am. J. Psychiatry 171 109–116. 10.1176/appi.ajp.2013.12121511 [DOI] [PubMed] [Google Scholar]
- Pudel V. (1971). Food-dispenser, eine methode zur untersuchung des,spontanen’ appetitverhaltens. Zeitschrift Fü Ehrnärungswissenschaft 10 833–893. [DOI] [PubMed] [Google Scholar]
- Qian M., Johnson A. E., Södersten P. (1998). CCK-8 inhibits ingestive behavior in rats with lateral hypothalamic 6-OHDA lesions. Neuroreport 9 2763–2767. 10.1097/00001756-199808240-00015 [DOI] [PubMed] [Google Scholar]
- R Nonlinear Regression (2019). R: R Nonlinear Regression. Available at: http://r-statistics.co/Linear-Regression.html (accessed May 30 2019). [Google Scholar]
- Ramsay D. S., Woods S. C. (2014). Clarifying the roles of homeostasis and allostasis in physiological regulation. Psychol. Rev. 121 225–247. 10.1037/a0035942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato D. X., Kawata M. (2018). Positive and balancing selection on SLC18A1 gene associated with psychiatric disorders and human-unique personality traits. Evol. Lett. 2 499–510. 10.1002/evl3.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schork A. J., Won H., Appadurai V., Nudel R., Gandal M., Delaneau O., et al. (2019). A genome-wide association study of shared risk across psychiatric disorders implicates gene regulation during fetal neurodevelopment. Nat. Neurosci. 22:353. 10.1038/s41593-018-0320-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo C., Guru A., Jin M., Ito B., Sleezer B. J., Ho Y.-Y., et al. (2019). Intense threat switches dorsal raphe serotonin neurons to a paradoxical operational mode. Science 363 538–542. 10.1126/science.aau8722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A., Straccia M. A., Botvinick M. M., Cohen J. D. (2016). Dorsal anterior cingulate and ventromedial prefrontal cortex have inverse roles in both foraging and economic choice. Cogn. Affect. Behav. Neurosci. 16 1127–1139. 10.3758/s13415-016-0458-8 [DOI] [PubMed] [Google Scholar]
- Skinner B. F. (1930). On the conditions of elicitation of certain eating reflexes. Proc. Natl. Acad. Sci. U.S.A. 16 433–438. 10.1073/pnas.16.6.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade E., Keeney E., Mavranezouli I., Dias S., Fou L., Stockton S., et al. (2018). Treatments for bulimia nervosa: a network meta-analysis. Psychol. Med. 48 2629–2636. 10.1017/S0033291718001071 [DOI] [PubMed] [Google Scholar]
- Smith T. M. (2018). The Tales Teeth Tell. Cambridge, MA: MIT Press. [Google Scholar]
- Södersten P., Bergh C. (2014). Recovering from anorexia nervosa by machine. J. Neuroendocrinol. 26 750–751. 10.1111/jne.12187 [DOI] [PubMed] [Google Scholar]
- Södersten P., Bergh C., Leon M., Brodin U., Zandian M. (2017). Cognitive behavior therapy for eating disorders versus normalization of eating behavior. Physiol. Behav. 174 178–190. 10.1016/j.physbeh.2017.03.016 [DOI] [PubMed] [Google Scholar]
- Södersten P., Bergh C., Leon M., Zandian M. (2016). Dopamine and anorexia nervosa. Neurosci. Biobehav. Rev. 60 26–30. 10.1016/j.neubiorev.2015.11.003 [DOI] [PubMed] [Google Scholar]
- Södersten P., Bergh C., Shield J., Lightman S. (2015). Reversible biological adaptations in obesity. Lancet Diabetes Endocrinol. 3:314 10.1016/s2213-8587(15)00090-x [DOI] [PubMed] [Google Scholar]
- Södersten P., Bergh C., Zandian M., Ioakimidis I. (2011). Obesity and the brain. Med. Hypotheses 77 371–373. [DOI] [PubMed] [Google Scholar]
- Södersten P., Brodin U., Sjöberg J., Zandian M., Bergh C. (2019). Treatment outcomes for eating disorders in Sweden: data from the national quality registry. BMJ Open 9:e024179. 10.1136/bmjopen-2018-024179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Södersten P., Nergårdh R., Bergh C., Zandian M., Scheurink A. (2008). Behavioral neuroendocrinology and treatment of anorexia nervosa. Front. Neuroendocrinol. 29:445–462. 10.1016/j.yfrne.2008.06.001 [DOI] [PubMed] [Google Scholar]
- Thomas D. M., Paynter J., Peterson C. M., Heymsfield S. B., Nduati A., Apolzan J. W., et al. (2017). A new universal dynamic model to describe eating rate and cumulative intake curves. Am. J. Clin. Nutr. 105 323–331. 10.3945/ajcn.115.127811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. A., Tran V., Ryu V., Xue B., Bartness T. J. (2018). AgRP knockdown blocks long-term appetitive, but not consummatory, feeding behaviors in Siberian hamsters. Physiol. Behav. 190 61–70. 10.1016/j.physbeh.2017.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treasure J., Zipfel S., Micali N., Wade T., Stice E., Claudino A., et al. (2015). Anorexia nervosa. Nat. Rev. Dis. Primers 1:15074. 10.1038/nrdp.2015.74 [DOI] [PubMed] [Google Scholar]
- Ungar P. S. (2017). Evolution’s Bite: A Story of Teeth, Diet, and Human Origins. Princeton: Princeton University Press. [Google Scholar]
- Walsh B. T., Kaplan A. S., Attia E., Olmsted M., Parides M., Carter J. C., et al. (2006). Fluoxetine after weight restoration in anorexia nervosa: a randomized controlled trial. JAMA 295 2605–2612. [DOI] [PubMed] [Google Scholar]
- Warden M. R., Selimbeyoglu A., Mirzabekov J. J., Lo M., Thompson K. R., Kim S.-Y., et al. (2012). A prefrontal cortex–brainstem neuronal projection that controls response to behavioural challenge. Nature 492 428–432. 10.1038/nature11617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerterp-Plantenga M. S., Westerterp K. R., Nicolson N. A., Mordant A., Schoffelen P. F. M., ten Hoor F. (1990). The shape of the cumulative food intake curve in humans, during basic and manipulated meals. Physiol. Behav. 47 569–576. 10.1016/0031-9384(90)90128-q [DOI] [PubMed] [Google Scholar]
- Woods S. C. (1991). The eating paradox: how we tolerate food. Psychol. Rev. 98 488–505. 10.1037//0033-295x.98.4.488 [DOI] [PubMed] [Google Scholar]
- Zandian M., Bergh C., Ioakimidis I., Esfandiari M., Shield J., Lightman S., et al. (2015). Control of body weight by eating behavior in children. Front. Pediatr. 3:89. 10.3389/fped.2015.00089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandian M., Ioakimidis I., Bergh C., Brodin U., Södersten P. (2009a). Decelerated and linear eaters: effect of eating rate on food intake and satiety. Physiol. Behav. 96 270–275. 10.1016/j.physbeh.2008.10.011 [DOI] [PubMed] [Google Scholar]
- Zandian M., Ioakimidis I., Bergh C., Södersten P. (2009b). Linear eaters turned decelerated: reduction of a risk for disordered eating? Physiol. Behav. 96 518–521. 10.1016/j.physbeh.2008.11.017 [DOI] [PubMed] [Google Scholar]
- Zandian M., Ioakimidis I., Bergh C., Leon M., Södersten P. (2011). A sex difference in the response to fasting. Physiol. Behav. 103 530–534. 10.1016/j.physbeh.2011.04.009 [DOI] [PubMed] [Google Scholar]
- Zandian M., Ioakimidis I., Bergh C., Södersten P. (2007). Cause and treatment of anorexia nervosa. Physiol. Behav. 92 283–290. 10.1016/j.physbeh.2007.05.052 [DOI] [PubMed] [Google Scholar]
- Zipfel S., Wild B., Groß G., Friederich H.-C., Teufel M., Schellberg D., et al. (2014). Focal psychodynamic therapy, cognitive behaviour therapy, and optimised treatment as usual in outpatients with anorexia nervosa (ANTOP study): randomised controlled trial. Lancet 383 127–137. 10.1016/S0140-6736(13)61746-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.


