Abstract
Background: Phylloquinone is the most abundant form of vitamin K in US diets. Green vegetables are considered the predominant dietary source of phylloquinone. As our food supply diversifies and expands, the food groups that contribute to phylloquinone intake are also changing, which may change absolute intakes. Thus, it is important to identify the contributors to dietary vitamin K estimates to guide recommendations on intakes and food sources.
Objective: The purpose of this study was to estimate 1) the amount of phylloquinone consumed in the diet of US adults, 2) to estimate the contribution of different food groups to phylloquinone intake in individuals with a high or low vegetable intake (≥2 or <2 cups vegetables/d), and 3) to characterize the contribution of different mixed dishes to phylloquinone intake.
Methods: Usual phylloquinone intake was determined from NHANES 2011–2012 (≥20 y old; 2092 men and 2214 women) and the National Cancer Institute Method by utilizing a complex, stratified, multistage probability-cluster sampling design.
Results: On average, 43.0% of men and 62.5% of women met the adequate intake (120 and 90 μg/d, respectively) for phylloquinone, with the lowest self-reported intakes noted among men, especially in the older age groups (51–70 and ≥71 y). Vegetables were the highest contributor to phylloquinone intake, contributing 60.0% in the high-vegetable-intake group and 36.1% in the low-vegetable-intake group. Mixed dishes were the second-highest contributor to phylloquinone intake, contributing 16.0% in the high-vegetable-intake group and 28.0% in the low-vegetable-intake group.
Conclusion: Self-reported phylloquinone intakes from updated food composition data applied to NHANES 2011–2012 reveal that fewer men than women are meeting the current adequate intake. Application of current food composition data confirms that vegetables continue to be the primary dietary source of phylloquinone in the US diet. However, mixed dishes and convenience foods have emerged as previously unrecognized but important contributors to phylloquinone intake in the United States, which challenges the assumption that phylloquinone intake is a marker of a healthy diet. These findings emphasize the need for the expansion of food composition databases that consider how mixed dishes are compiled and defined.
Keywords: dietary assessment, NHANES, nutrition counseling, phylloquinone, vitamin K, warfarin
Introduction
Vitamin K is a fat-soluble vitamin that is required for the posttranslational carboxylation of vitamin K–dependent proteins involved in multiple physiological processes, including clotting, bone formation, and regulation of vascular calcification (1). The primary dietary form of vitamin K is phylloquinone. In the US diet, phylloquinone is predominantly found in dark-green leafy vegetables and vegetable oils (2). Menaquinones are secondary forms that differ in structure and dietary sources when compared with phylloquinone (3).
The current US DRIs recommend an adequate intake (AI) for vitamin K based on our current knowledge of phylloquinone intakes (4). The current AI for adult men and women is 120 and 90 μg/d, respectively (4). An RDA was not established because there is insufficient knowledge regarding vitamin K's bioavailability, transport, and excretion. Further, there is no single biomarker that reflects vitamin K status or adequate physiological function. Analysis of NHANES III (1988–1994) indicated that median intakes of dietary vitamin K ranged from 89 to 117 μg/d for men (≥19 y), and 79 to 88 μg/d for women (≥19 y), thus providing a reference for setting the current AI recommendations (Supplemental Table 1) (4). More recently, it was reported that phylloquinone intakes have declined among Irish adults, particularly among younger adults (5). Estimated intakes of phylloquinone among US adults have not been examined since the analysis of NHANES III so it is not known if these shifts are unique to Ireland or a more global phenomenon.
Food composition databases are a robust source of dietary pattern information and nutritional content, and they play an integral role in diet assessment and recommendation. Over time, the databases have expanded or updated the nutrient profiles of combination meals, restaurant foods, and culturally diverse foods. This provides a better resource to accurately capture an individual's dietary pattern and change over time. Advances in methodology have allowed for broader characterization of vitamin K in the food supply, including many of these mixed dishes. With the diversification of the food supply and changes in food preparation techniques, plant-based oils have become a more noticeable food source of vitamin K (6). Characterizing the contribution of these additional food sources of vitamin K is critical for developing dietary recommendations for the US population as a whole but also for clinical populations, such as individuals receiving vitamin K antagonist anticoagulant therapy (coumarin-based oral anticoagulants). The direct drug-nutrient interaction influences drug efficacy and dosing (7), and changes in dietary vitamin K intake can result in adverse events, including over-anticoagulation or severe clotting (8). Individuals initiating or continuing therapy receive counseling to maintain a diet with consistent vitamin K intake to ensure anticoagulant stability, demonstrating the importance of accurately identifying dietary sources of vitamin K.
The purpose of this study was to 1) characterize the usual intake of phylloquinone consumed in the diet of US adults, 2) characterize dietary patterns by phylloquinone intake in individuals with a high vegetable intake (≥2 cups or cup equivalents of vegetables/d) or a low vegetable intake (<2 cups or cup equivalents of vegetables/d), and 3) identify the contribution of mixed dishes to phylloquinone intake in the most recently available data on the current diets of American adults.
Methods
NHANES is a nationally representative, cross-sectional survey that samples civilian, noninstitutionalized US residents by using a complex, multistage, probability-cluster design. The survey is conducted by the US CDC National Center for Health Statistics, who obtain written, informed consent for all participants and proxies. The survey protocol was approved by the National Center of Health Statistics research ethics review board.
In this study, demographic, health examination, and dietary survey data from 2011 to 2012 NHANES were assessed for adults (aged ≥20 y; n = 5560). Individuals without 2 reliable dietary recalls (n = 1209) and extreme outliers [records with <400 kcal/d (n = 44) or >6000 μg/d of phylloquinone (n = 1)] were excluded. Thus this analysis includes data for 4306 participants. Pregnant and lactating women were not excluded from the analysis because the recommendations for phylloquinone intake are the same for women and individuals in these life stage groups.
NHANES participants were asked to complete an in-person household interview, during which demographic data (including age, race, sex, income, and education) were obtained by using the Computer-Assisted Personal Interviewing system. After the in-home interview, participants underwent a health examination in the Mobile Examination Center (MEC) by a trained health technician. Physical examination data recorded by the trained health technician and utilized in our analyses include BMI (in kg/m2) only. The first 24-h dietary recall was collected in the MEC and was conducted in-person via the validated Automated Multiple-Pass Method, which has been described elsewhere (9, 10). The second dietary recall occurred 3–10 d after the MEC examination and was conducted via telephone by using the same methodology.
The phylloquinone content of foods was obtained from the USDA 2011–2012 Food and Nutrition Database for Dietary Studies (FNDDS). The vitamin K values in the FNDDS are from the USDA National Nutrient Database for Standard Reference. These values are determined through routine analysis by the Vitamin K Laboratory at Tufts University as part of the USDA National Food and Nutrient Analysis Project (11). Classification of vegetable intake was determined by using the USDA Food Patterns Equivalents Database 2011–2012, which classifies reported foods into 37 corresponding food-pattern components and totals daily intake in cups, ounces, teaspoons, grams, or counts of specified food patterns. In this study, cups or cup equivalents of total vegetable intake were used to determine if a participant had a high or low vegetable intake on any given day. A study participant was classified as having a high vegetable intake if he or she consumed an average of ≥2 cups or cup equivalents of vegetables/d and a low vegetable intake if he or she consumed an average of <2 cups or cup equivalents of vegetables/d. Two cups of vegetables per day is the lowest amount of recommended daily vegetable intake in this study's population (12). Food group classifications were determined according to the USDA What We Eat in America 15 food groupings. Alcoholic beverages, water, sugars, and “other” food groups were combined into one “other” food group category, and baby food and infant formula were not analyzed because no participants in this study population consumed these foods. Eleven food groups are reported (Supplemental Tables 2 and 3). The “mixed dishes” food group was further expanded for subgroup analysis. Because of the interaction between vitamin K and coumarin-based anticoagulant therapy stability, inclusion of substantial amounts of vitamin K in dietary supplements carry a high risk of severe clinical implications, including over-anticoagulation or severe clotting for individuals prescribed these medications (13). Thus, availability of vitamin K supplements is very limited, and because they do not contribute substantially to vitamin K intakes, supplements were not included in these estimates (14).
NHANES dietary recalls report 2 d of a person's intake, and therefore these data represent the participant's intake on any given day and not the long-term habitual or usual intake (15). To overcome this limitation, the National Cancer Institute developed a mixed-effects model and quantile estimation procedure (16) to predict usual intake from repeated dietary recalls. The method also produces valid SE estimates for complex survey data, allows for the incorporation of subgroup effects as covariates, and uses a Box-Cox parameter for data, which are difficult to transform to approximate normality (16).
The means and percentages presented in Table 1 reflect an adjustment for within-person variability with the use of the amount-only part of the National Cancer Institute Method to estimate usual-intake distributions. Covariates included are weekend compared with weekday, order of dietary recall, and total calories for nutrient estimation. SEs were approximated by Fay's Modified Balanced Repeated Replication technique by using 16 sets of replicate weights with an initial perturbation factor of 0.7 to calculate the appropriate weights for both the full and subgroup analyses. All weights remained as integers throughout the analysis. Replication weights were poststratified to control totals computed from the initial sample weights.
TABLE 1.
Dietary phylloquinone and energy intakes adjusted for day-to-day variability in the diets of US adults (aged ≥20 y) by age, BMI, race/ethnicity, income, education level, and vegetable intake, NHANES 2011–20121
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| n | Energy, kcal/d | Phylloquinone, μg/d | >120 μg phylloquinone/d, % | n | Energy, kcal/d | Phylloquinone, μg/d | >90 μg phylloquinone/d, % | |
| All adults | 2092 | 2456 ± 33.9 | 117 ± 10.2 | 43.0 | 2214 | 1798 ± 28.6 | 117 ± 8.49 | 62.5 |
| Age, y | ||||||||
| 20–30 | 413 | 2495 ± 63.7a,b | 107 ± 8.86b | 45.4 | 419 | 1874 ± 30.9a | 101 ± 8.36b | 66.1 |
| 31–50 | 703 | 2695 ± 158a | 124 ± 5.59a | 48.7 | 748 | 1865 ± 24.3a | 109 ± 11.9b | 63.4 |
| 51–70 | 681 | 2304 ± 74.2b | 117 ± 26.6a,b | 38.6 | 755 | 1757 ± 72.5b | 136 ± 9.30a | 61.8 |
| ≥71 | 295 | 1992 ± 46.3c | 111 ± 11.9a,b | 31.8 | 292 | 1587 ± 42.8c | 111 ± 12.1b | 56.2 |
| BMI, kg/m2 | ||||||||
| Underweight (<18.5) | 54 | 2348 ± 215 | 87.6 ± 16.9c | 47.2 | 77 | 1814 ± 120 | 93.2 ± 16.9c | 60.3 |
| Normal (18.5–24.9) | 593 | 2501 ± 45.7 | 117 ± 24.3a,b | 42.8 | 632 | 1834 ± 40.2 | 122 ± 12.7a,b | 63.7 |
| Overweight (25–29.9) | 771 | 2416 ± 34.7 | 124 ± 12.1a | 42.2 | 597 | 1765 ± 38.8 | 123 ± 12.2a | 61.4 |
| Obese (≥30) | 674 | 2472 ± 97.2 | 108 ± 3.77b | 43.9 | 908 | 1790 ± 29.3 | 110 ± 6.04b | 62.5 |
| Race/ethnicity | ||||||||
| Non-Hispanic white | 831 | 2494 ± 83.5a | 98.0 ± 14.9b | 43.6 | 848 | 1791 ± 54.4b,c | 85.3 ± 8.93c | 62.4 |
| Non-Hispanic black | 514 | 2479 ± 40.2a | 115 ± 13.7b | 42.2 | 626 | 1796 ± 34.5a,b | 121 ± 10.0a,b | 64.0 |
| Non-Hispanic Asian | 271 | 2372 ± 48.2a,b | 131 ± 13.3b | 36.1 | 267 | 1849 ± 24.7a | 122 ± 16.6a,b | 59.3 |
| Mexican American/other Hispanic | 407 | 2219 ± 59.8b | 158 ± 22.1a | 44.2 | 417 | 1678 ± 39.0c | 140 ± 17.8a | 61.5 |
| Other race (multiracial) | 69 | 2398 ± 97.8a,b | 109 ± 14.6b | 36.7 | 56 | 1841 ± 100a | 97.1 ± 13.9b | 66.4 |
| Annual household income | ||||||||
| Below <$25,000 | 577 | 2380 ± 57.5a,b | 94.0 ± 9.05b | 41.7 | 692 | 1780 ± 50.4 | 101 ± 5.23b | 61.8 |
| Above ≥$25,000 | 1420 | 2485 ± 57.4a | 123 ± 10.6a | 43.4 | 1441 | 1801 ± 28.4 | 122 ± 12.0a | 62.6 |
| Not available | 95 | 2137 ± 259b | 97.9 ± 43.4b | 40.6 | 81 | 1872 ± 127 | 124 ± 19.4a | 64.3 |
| Education level2 | ||||||||
| Did not complete high school | 495 | 2379 ± 84.5 | 95.9 ± 12.1c | 43.9 | 460 | 1658 ± 54.3b | 83.4 ± 3.60c | 58.8 |
| High school graduate | 457 | 2488 ± 116 | 104 ± 9.04b,c | 44.1 | 430 | 1770 ± 46.9a | 99.4 ± 8.07b,c | 61.2 |
| Some college | 590 | 2462 ± 39.9 | 108 ± 4.41b | 42.3 | 729 | 1803 ± 23.1a | 114 ± 11.3a,b | 63.0 |
| College graduate | 548 | 2472 ± 43.3 | 146 ± 29.3a | 42.4 | 594 | 1869 ± 56.4a | 145 ± 10.8a | 64.3 |
| Vegetable intake3 | ||||||||
| High (≥2 cups/d) | 625 | 2805 ± 61.3a | 195 ± 31.8a | 50.9 | 546 | 2043 ± 33.5a | 223 ± 16.7a | 69.0 |
| Low (<2 cups/d) | 1467 | 2295 ± 36.1b | 80.8 ± 3.36b | 39.3 | 1668 | 1706 ± 33.3b | 78.0 ± 3.47b | 60.1 |
Values are means ± SEs unless otherwise indicated. Means in a column within a group without a common superscript letter are significantly different as a > b > c, P ≤ 0.01. Multiple pairwise t tests were used for comparisons between population groups with df dictated by the NHANES sample design.
Three participants (2 men, 1 woman) did not report education level.
High vegetable intake defined as ≥2 cups or cup equivalents/d; low vegetable intake defined as <2 cups or cup equivalents/d. Examples include 1 cup or cup equivalent = 2 cups raw leafy vegetables or 1 cup chopped, cooked, or raw broccoli, peppers, or carrots. The Food and Nutrition Database for Dietary Studies defines cups and cup equivalents for various foods, and they are based on food pattern definitions used in the Dietary Guidelines for Americans (17). Because of the variability in foods, the weight in grams varies by cooking method and food item. For vegetables, 1 cup in grams varies from 70 to 245 g. Thus, we categorized by cup or cup equivalents.
All statistical analyses were performed by using SAS v 9.4. Sample weights were used to account for the unequal probabilities of selection, nonparticipation by selected sample persons, dietary recall nonresponse, and differential allocation by day of the week for the dietary recall (16). Means and SEs were estimated for phylloquinone and are presented as micrograms per day for multiple demographic categories within men and women separately. Multiple pairwise t tests were used for all comparisons between population groups with df dictated by the NHANES sample design. The percentages and SEs of phylloquinone supplied by each food group, vegetables, and mixed dishes was compared between individuals with high or low vegetable intake on any given day by multiple pairwise t tests with df dictated by the NHANES sample design. Significance was determined by P < 0.01.
Results
Men and women consumed 2456 and 1798 kcal/d, respectively, and 117 μg phylloquinone/d for both sexes. Overall, usual phylloquinone intake was consistent among men and women by demographic category (Table 1). On average, 43% of men and 62.5% of women were meeting or exceeding the AI of 120 and 90 μg/d, respectively. In men, the prevalence of phylloquinone intakes >120 μg/d decreased with age; 45.4% of men aged 20–30 y meet or exceed the AI compared with 31.8% of men aged ≥71 y. There was no difference in AI prevalence by all other demographic categories. Phylloquinone intake in men ranged from 80.0 to 195 μg/d, representing men who eat <2 cups or cup equivalents of vegetables/d and men who eat ≥2 cups or cup equivalents of vegetables/d. A similar relation was observed in women, in whom phylloquinone intake ranged from 78 to 223 μg/d, with the lowest intake observed in individuals who consumed <2 cups or cup equivalents of vegetables/d, and highest among individuals who ate >2 cups or cup equivalents of vegetables/d.
Comparisons of energy and phylloquinone intakes between vegetable intake groups were significantly different in men and women. In men, the mean phylloquinone intake in the high- and low-vegetable-intake groups was 195 and 80.8 μg/d (P < 0.01), respectively. In women, the mean phylloquinone intake in the high- and low-vegetable-intake groups was 223 and 78 μg/d (P < 0.01), respectively.
To characterize dietary sources of vitamin K, we examined the distribution of various food groups contributing to phylloquinone intake by high- and low-vegetable-intake groups (Table 2). For both vegetable-intake groups, vegetables, followed by mixed dishes, contributed the most phylloquinone in the diet. In the high-vegetable group, vegetables and mixed dishes contributed 60.0% and 16.1% of total phylloquinone, respectively. For the low-vegetable group, the contributions were 36.1% and 27.7% for vegetables and mixed dishes, respectively. Fats and oils and snacks and sweets were the next highest contributors with 5–10% of phylloquinone contribution in both groups. In the low-vegetable-intake group, protein foods also contributed 5.4% to total phylloquinone. All other food groups contributed <5% to total phylloquinone.
TABLE 2.
Reported food group contributions to dietary phylloquinone in the diets of US adults (aged ≥20 y) by high and low vegetable intake, NHANES 2011–20121
| Contribution to total phylloquinone, % | ||
|---|---|---|
| High vegetable intake, ≥2 cups/d (n = 1117) | Low vegetable intake, <2 cups/d (n = 3133) | |
| Vegetables2 | 60.0 ± 3.0 | 36.1 ± 2.0* |
| Mixed dishes3 | 16.1 ± 1.8 | 27.7 ± 1.5* |
| Fats and oils2 | 6.4 ± 0.6 | 8.0 ± 0.6 |
| Snacks and sweets2 | 5.0 ± 0.6 | 8.7 ± 0.4* |
| Beverages (nonalcoholic)2 | 3.4 ± 1.4 | 1.9 ± 0.4 |
| Protein foods2 | 2.7 ± 0.2 | 5.4 ± 0.3* |
| Grains2 | 2.1 ± 0.3 | 4.6 ± 0.3* |
| Fruits2 | 1.8 ± 0.2 | 2.7 ± 0.3 |
| Condiments and sauces2 | 1.6 ± 0.3 | 3.3 ± 1.0 |
| Other2 | 0.5 ± 0.2 | 0.6 ± 0.1 |
| Milk and dairy2 | 0.5 ± 0.1 | 0.9 ± 0.1* |
Values are means ± SEs. High vegetable intake defined as ≥2 cups or cup equivalents/d; low vegetable intake defined as <2 cups or cup equivalents/d. Examples include 1 cup or cup equivalent = 2 cups raw leafy vegetables or 1 cup chopped, cooked, or raw broccoli, peppers, or carrots. The Food and Nutrition Database for Dietary Studies defines cups and cup equivalents for various foods, and they are based on food pattern definitions used in the Dietary Guidelines for Americans (17). Because of the variability in foods, the weight in grams varies by cooking method and food item. For vegetables, 1 cup in grams varies from 70 to 245 g. Thus, we categorized by cup or cup equivalents. Unlike the National Cancer Institute–adjusted statistics that represent long-term, usual dietary intake estimates presented in Table 1, the food intake statistics here are estimates on any given day. The above sample sizes reflect a categorization of the NHANES respondents based on their daily vegetable intake and describe the sample, not the population. Multiple pairwise t tests were used to compare the phylloquinone contribution between high- or low-vegetable-intake eaters within each food group. *Different from high vegetable intake, P < 0.01.
See Supplemental Table 2 for food groups.
See Supplemental Table 3 for mixed-dish subgroups.
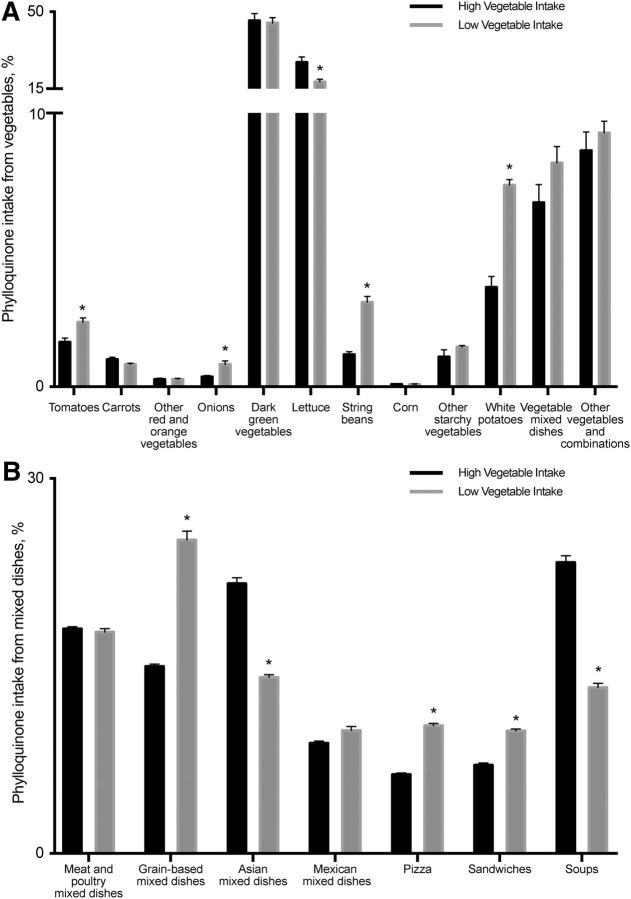
Vegetables are considered the predominant source of vitamin K in the diet and continue to be the greatest contributor to phylloquinone intake in both high- and low-vegetable-intake groups. We examined the distribution of vegetable groups contributing to phylloquinone intake (Figure 1). In both the high- and low-vegetable intake groups, dark-green vegetables and lettuce were the top foods to contribute to phylloquinone intake. Unique to the low-vegetable-intake group, the contribution of white potatoes and string beans to phylloquinone intake from vegetables was 2 times greater in the low-vegetable-intake group than in the high-vegetable-intake group (Figure 1A).
FIGURE 1.
Contribution (%) of vegetable (A) and mixed-dish (B) subgroups to dietary vitamin K intake in the diets of US adults (aged ≥20 y; high vegetable intake, n = 1117; low vegetable intake, n = 3133) in NHANES 2011–2012 by food group categories. Data are presented as means ± SEs. *Different from high vegetable intake, P < 0.01.
Mixed dishes were found to be the second largest food group contributing to dietary phylloquinone intake; thus, we examined mixed-dish food subgroups in greater detail that revealed a unique distribution within the categories by vegetable intake (Figure 1B). In individuals with a high vegetable intake, soups accounted for 23.0% of mixed dishes' phylloquinone contribution, followed by Asian dishes (such as fried rice with soy-based sauce mixtures, lo mein or chow mein, egg rolls, dumplings, and sushi; 21.5%) and meat and poultry (17.9%). In individuals with a low vegetable intake, grain-based dishes (pasta and rice dishes, turnovers, and macaroni and cheese) contributed the highest phylloquinone contribution from mixed dishes (25.3%), which also had the largest percentage of difference between the vegetable-intake groups. Sandwiches (burgers, chicken and turkey sandwiches, egg and breakfast sandwiches, and frankfurter sandwiches, 9.7%) and pizza (10%) were the next-largest contributing food groups to phylloquinone from mixed dishes in individuals with a low vegetable intake.
Discussion
This analysis revealed that the usual intake of phylloquinone across all demographic categories of Americans is consistent with the current recommendations, although fewer men achieve the current AI, particularly in the older age groups, compared with the original estimates conducted in 1998–1994 (4, 18). Compared with a recent report from national surveys of Irish adults (5), we did not observe declines in overall phylloquinone intakes among younger US adults or in women over the last 15 y. However, a decline in phylloquinone intake was also reported in the United Kingdom among British adults in 1986–1987 and 2000–2001. The percentage of adults meeting the recommended intake and average phylloquinone intake decreased from 1986–1987 to 2000–2001, which was attributed to a decline in total vegetable intake (19). Vegetables remain the predominant dietary source of phylloquinone in our analysis, although we did determine the novel observation that mixed dishes are important food sources of phylloquinone among US adults. Given that most of these food sources were not captured in earlier surveys because of gaps in the nutrient databases at the time, it was unexpected that the inclusion of more comprehensive phylloquinone data from mixed dishes did not result in an overall net increase in phylloquinone intakes.
Categorizing individuals by vegetable intake further demonstrated vegetables are a major dietary source of phylloquinone; more specifically, dark-green vegetables and lettuce were the top 2 vegetable groups contributing most to phylloquinone intake. However, in the low-vegetable-intake group, white potatoes were a main source of phylloquinone intake. The white potato food group includes baked or boiled white potatoes, French fries and other fried white potatoes, and mashed potatoes. These foods are often accompanied by additional calories from oil, which is likely the source of phylloquinone in these dishes (20).
Our observations have identified mixed dishes as a significant contributor to dietary phylloquinone. Among those with a low vegetable intake, grain-based dishes, pizza, and sandwiches were major sources. Unique to these mixed-dish groups are the moderate to high contributions to phylloquinone intake without any apparent phylloquinone-rich vegetables as a main ingredient, e.g., macaroni and cheese and burgers, further demonstrating the substantial role of food preparation methods and ingredients in mixed dishes in terms of manipulating phylloquinone content in foods. Trends in dietary patterns from the 2015–2020 Dietary Guidelines are similar to our observations in the low-vegetable-intake group. The Dietary Guidelines for Americans report states that ~75% of the population is not meeting the recommended intake for vegetables, and more than half of the population is meeting or exceeding the total grain and protein-food recommendations (12). Further demonstrating that although phylloquinone intake has not changed for the majority of US adults, the distribution of foods contributing to total intake are shifting with the current eating patterns observed in the United States.
In development of dietary recommendations, bioavailability of the nutrient is of consideration. Studies have shown the absorption of the fat-soluble phylloquinone, as measured by postprandial serum concentration of phylloquinone, is significantly higher after intake of phylloquinone-fortified oil or vegetables with added fat than vegetables alone (21, 22). Jones et al. (23) showed that the bioavailability of phylloquinone in meals containing fast food and refined cereals, with lower-than-average intakes of fruits, vegetables, and whole grains, have more than twice the bioavailability than meal patterns with higher-than-average intakes of fruits, vegetables, whole grains, fish, and dairy. Collectively these studies suggest that the phylloquinone obtained from a meal with phylloquinone-rich oil may have greater bioavailability than phylloquinone obtained from fruits and vegetables.
Surprisingly, among those with a low vegetable intake, foods that may be classified as “fast foods” or “convenience meals,” such as burgers, pizza, frankfurter sandwiches, and macaroni and cheese, were major sources of phylloquinone, likely because of the addition of phylloquinone-rich oils, such as soybean and canola, used during food preparation. This challenges the assumption that phylloquinone intakes are markers of a healthy diet (24). Characterizing dietary patterns of phylloquinone intake and identifying uncommon food sources of vitamin K is important when setting recommendations for special populations, particularly individuals receiving vitamin K antagonist anticoagulants.
Current recommendations state that patients receiving vitamin K antagonist anticoagulation treatment should maintain a consistent intake of phylloquinone (25). In practice, this often translates to avoidance of phylloquinone-rich vegetables (26–28). Studies have shown that this results in a 35–46% lower mean intake of vitamin K as a result of a lower green-vegetable intake (26). The contribution of alternative sources of phylloquinone in this population is not well characterized although dietary stability is critical to maintaining therapeutic control. The characterization of phylloquinone in mixed dishes, the continued use of phylloquinone-rich plant oils in food preparation, and the possible increased bioavailability of phylloquinone from these sources warrants reevaluation of current dietary recommendations and for individuals receiving vitamin K antagonist anticoagulants.
Strengths of this study include the use of a large, nationally representative cohort of adults and examination of both nutrient and food-level contributions of vitamin K to the diet. Moreover, we estimated usual intakes that account for measurement error, day-to-day variability, and within-subject variability (as random error) in dietary assessment to the extent possible. This study has some limitations. Dietary data are limited by the accuracy and currency of the databases used to estimate nutrient intakes from food. As noted, phylloquinone is the most abundant form of vitamin K in the diet, but complete analysis of vitamin K intake was limited because menaquinones are not quantified in the FNDDS database. Some animal products are moderate sources of vitamin K from menaquinones content alone and will need to be considered when examining total vitamin K intake in future analyses (29). In addition, some foods with a high vitamin K content may be episodically consumed and not captured in the 2 d of dietary recall. This study obtained food intake data via 24-h recall, which has inherit errors in underreporting. However, the Automated Multiple-Pass Method has been validated for energy (total calories) and documented elsewhere (10, 30). As previously stated, dietary supplements were not examined as part of this analysis.
This study demonstrates that as the US diet continues to evolve and technological advances in food analysis continue, it is important to reevaluate dietary sources of nutrients and corresponding changes in nutrient intakes. Green vegetables remain the predominant and rich source of dietary vitamin K, but the contribution of mixed dishes and convenience foods also has an important role with respect to dietary vitamin K, which may have led to previous underestimates in dietary vitamin K intakes in dietary surveys. The data presented here reinforce the value of continuing to update and improve data in our food composition databases to better capture vitamin K intake and provide the necessary information for individuals to achieve a more consistent dietary vitamin K intake pattern. This also has important clinical practice implications for dietitians and other health care providers who counsel patients initiating or continuing vitamin K antagonist anticoagulant therapy to consume a diet with a consistent vitamin K intake. Future expansion of food composition databases must consider how mixed dishes are compiled and defined to better understand differences in current standardized mixed dishes compared with entering individual recipes. Within mixed dishes, oil is an extremely variable ingredient and can be difficult to capture; therefore, the effect on vitamin K content may be altered substantially, demonstrating the need for a more comprehensive analysis of mixed dishes. Additional research is required to quantify all vitamin K forms, including menaquinones in the US food supply, and to determine the bioavailability of multiple vitamin K forms in conjunction with varied meal composition, providing insight into dietary requirements and the physiological function of vitamin K.
Acknowledgments
The authors' responsibilities were as follows—EGF and SGH: conducted the research; EGF, SGH, and KJB: analyzed the data; SGH and EGF: wrote the paper; SLB: had primary responsibility for the final content; and all authors: reviewed the data, aided in interpretation of the results, reviewed the manuscript, and read and approved the final manuscript.
Abbreviations
- AI
adequate intake
- FNDDS
Food and Nutrient Database of Dietary Studies
- MEC
Mobile Examination Center
References
- 1. Booth SL. Vitamin K: food composition and dietary intakes. Food Nutr Res 2012April 2 (Epub ahead of print; DOI: 10.1182/blood-2011-11-390849). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shearer MJ, Fu X, Booth SL. Vitamin K nutrition, metabolism, and requirements: current concepts and future research. Adv Nutr 2012;3:182–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walther B, Karl JP, Booth SL, Boyaval P. Menaquinones, bacteria, and the food supply: the relevance of dairy and fermented food products to vitamin K requirements. Adv Nutr 2013;4:463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Institute of Medicine Panel on Micronutrients Dietary references intakes for vitamin K, arsenic, chromium, copper, iodine, iron, managanses, molybeum, silicon, vanadium, and zinc. Washington (DC): National Academies Press; 2001. [PubMed] [Google Scholar]
- 5. Hayes A, Hennessy A, Walton J, McNulty BA, Lucey AJ, Kiely M, Flynn A, Cashman KD. Phylloquinone intakes and food sources and vitamin K status in a nationally representative sample of Irish adults. J Nutr 2016;146:2274–80. [DOI] [PubMed] [Google Scholar]
- 6. Kearney J. Food consumption trends and drivers. Philos Trans R Soc Lond B Biol Sci 2010;365:2793–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kramps M, Flanagan A, Smaldone A. The use of vitamin K supplementation to achieve INR stability: a systematic review and meta-analysis. J Am Assoc Nurse Pract 2013;25:535–44. [DOI] [PubMed] [Google Scholar]
- 8. Sato Y, Murata M, Chiba T, Umegaki K. A systematic review of the acceptable intake level of vitamin K among warfarin users. Shokuhin Eiseigaku Zasshi 2015;56:157–65. [DOI] [PubMed] [Google Scholar]
- 9. CDC National health and nutrition examination survey MEC in-person dietary interviewers procedures manual [Internet]. 2009[cited 2016 May 22]. Available from: https://www.cdc.gov/nchs/data/nhanes/nhanes_09_10/DietaryInterviewers_Inperson.pdf.
- 10. Moshfegh AJ, Rhodes DG, Baer DJ, Murayi T, Clemens JC, Rumpler WV, Paul DR, Sebastian RS, Kuczynski KJ, Ingwersen LA, et al. The US Department of Agriculture automated multiple-pass method reduces bias in the collection of energy intakes. Am J Clin Nutr 2008;88:324–32. [DOI] [PubMed] [Google Scholar]
- 11. Haytowitz DB, Pehrsson PR. USDA's National Food and Nutrient Analysis Program (NFNAP) produces high-quality data for USDA food composition databases: two decades of collaboration. Food Chem 2016Nov 19 (Epub ahead of print; DOI: 10.1016/j.foodchem.2016.11.082). [DOI] [PubMed] [Google Scholar]
- 12. US Department of Health and Human Services, USDA Dietary Guidelines for Americans 2015–2020 [Internet]. 2015 [cited 2016 May 21]. Available from: http://health.gov/dietaryguidelines/2015/guidelines/.
- 13. Hirsh J, Fuster V, Ansell J, Halperin JL. American Heart Association/American College of Cardiology Foundation guide to warfarin therapy. Circulation 2003;107:1692–711. [DOI] [PubMed] [Google Scholar]
- 14. Booth SL, Al Rajabi A. Determinants of vitamin K status in humans. Vitam Horm 2008;78:1–22. [DOI] [PubMed] [Google Scholar]
- 15. Freedman LS, Midthune D, Carroll RJ, Krebs-Smith S, Subar AF, Troiano RP, Dodd K, Schatzkin A, Bingham SA, Ferrari P, et al. Adjustments to improve the estimation of usual dietary intake distributions in the population. J Nutr 2004;134:1836–43. Erratum in: J Nutr 2005;135(6):1524. [DOI] [PubMed] [Google Scholar]
- 16. Tooze JA, Kipnis V, Buckman DW, Carroll RJ, Freedman LS, Guenther PM, Krebs-Smith SM, Subar AF, Dodd KW. A mixed-effects model approach for estimating the distribution of usual intake of nutrients: the NCI method. Stat Med 2010;29:2857–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bowman S, Clemens J, Friday J, Thoerig R, Moshfegh A. Food patterns equivalents database 2011–12: methodology and user guide [Internet]. Beltsville (MD); 2014. [cited 2016 May 21]. Available from: http://www.ars.usda.gov/nea/bhnrc/fsrg. [Google Scholar]
- 18. Institute of Medicine Using the adequate intake for nutrient assessment of groups. Dietary reference intakes: applications in dietary assessment [Internet]. Washington (DC): National Academies Press; 2000. p. 106–12. Available from: https://www.nap.edu/read/9956/chapter/11. [Google Scholar]
- 19. Thane CW, Bolton-Smith C, Coward WA. Comparative dietary intake and sources of phylloquinone (vitamin K1) among British adults in 1986–7 and 2000–1. Br J Nutr 2006;96:1105–15. [DOI] [PubMed] [Google Scholar]
- 20. King JC, Slavin JL. White potatoes, human health, and dietary guidance. Adv Nutr 2013;4:393S–401S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Booth SL, Lichtenstein AH, Dallal GE. Phylloquinone absorption from phylloquinone-fortified oil is greater than from a vegetable in younger and older men and women. J Nutr 2002;132:2609–12. [DOI] [PubMed] [Google Scholar]
- 22. Gijsbers BL, Jie KS, Vermeer C. Effect of food composition on vitamin K absorption in human volunteers. Br J Nutr 1996;76:223–9. [DOI] [PubMed] [Google Scholar]
- 23. Jones KS, Bluck LJC, Wang LY, Stephen AM, Prynne CJ, Coward WA. The effect of different meals on the absorption of stable isotope-labelled phylloquinone. Br J Nutr 2009;102:1195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shea MK, Booth SL. Concepts and controversies in evaluating vitamin K status in population-based studies. Nutrients 2016;8E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Academy of Nutrition and Dietetics Nutrition care manual [Internet]. 2013[cited 2016 May 21]. Available from: http://www.nutritioncaremanual.org. [DOI] [PubMed]
- 26. Leblanc C, Dubé M-P, Presse N, Dumas S, Nguyen M, Rouleau-Mailloux É, Perreault S, Ferland G, Ageno W, Gallus AS, et al. Avoidance of vitamin K−rich foods is common among warfarin users and translates into lower usual vitamin K intakes. J Acad Nutr Diet 2016;116:1000–7. [DOI] [PubMed] [Google Scholar]
- 27. Li RC, Finkelman BS, Chen J, Booth SL, Bershaw L, Brensinger C, Kimmel SE. Dietary vitamin K intake and anticoagulation control during the initiation phase of warfarin therapy: a prospective cohort study. Thromb Haemost 2013;110:195–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harshman SG, Saltzman E, Booth SL. Vitamin K: dietary intake and requirements in different clinical conditions. Curr Opin Clin Nutr Metab Care 2014;17:531–8. [DOI] [PubMed] [Google Scholar]
- 29. Fu X, Shen X, Finnan EG, Haytowitz DB, Booth SL. Measurement of multiple vitamin K forms in processed and fresh-cut pork products in the U.S. food supply. J Agric Food Chem 2016;64:4531–5. [DOI] [PubMed] [Google Scholar]
- 30. Archer E, Hand GA, Blair SN. Validity of U.S. nutritional surveillance: National Health and Nutrition Examination Survey caloric energy intake data, 1971–2010. PLoS One 2013;8:e76632. [DOI] [PMC free article] [PubMed] [Google Scholar]