Abstract
Context
Type 1 diabetes in adolescence is characterized by insulin deficiency and insulin resistance (IR), both thought to increase cardiovascular disease risk. We previously demonstrated that adolescents with type 1 diabetes have adipose, hepatic, and muscle IR, and that metformin lowers daily insulin dose, suggesting improved IR. However, whether metformin improves IR in muscle, hepatic, or adipose tissues in type 1 diabetes was unknown.
Objective
Measure peripheral, hepatic, and adipose insulin sensitivity before and after metformin or placebo therapy in youth with obesity with type 1 diabetes.
Design
Double-blind, placebo-controlled clinical trial.
Setting
Multi-center at eight sites of the T1D Exchange Clinic Network.
Participants
A subset of 12- to 19-year-olds with type 1 diabetes (inclusion criteria: body mass index ≥85th percentile, HbA1c 7.5% to 9.9%, insulin dosing ≥0.8 U/kg/d) from a larger trial (NCT02045290) were enrolled.
Intervention
Participants were randomized to 3 months of metformin (N = 19) or placebo (N = 18) and underwent a three-phase hyperinsulinemic euglycemic clamp with glucose and glycerol isotope tracers to assess tissue-specific IR before and after treatment.
Main Outcome Measures
Peripheral insulin sensitivity, endogenous glucose release, rate of lipolysis.
Results
Between-group differences in change in insulin sensitivity favored metformin regarding whole-body IR [change in glucose infusion rate 1.3 (0.1, 2.4) mg/kg/min, P = 0.03] and peripheral IR [change in metabolic clearance rate 0.923 (−0.002, 1.867) dL/kg/min, P = 0.05]. Metformin did not impact insulin suppression of endogenous glucose release (P = 0.12). Adipose IR was not assessable with traditional methods in this highly IR population.
Conclusions
Metformin appears to improve whole-body and peripheral IR in youth who are overweight/obese with type 1 diabetes.
Adolescents with obesity with type 1 diabetes were randomized to 3 months of metformin or placebo. Youth who received metformin had an improvement in peripheral, but not hepatic, insulin sensitivity.
The incidence of youth-onset type 1 diabetes is increasing worldwide (1, 2), translating to a lifetime of diabetes exposure and increased risk of early-onset cardiovascular disease (CVD) (3, 4). Although controlling hyperglycemia, hypertension, and hyperlipidemia is beneficial, this only partially reduces CVD risk (5). CVD is associated with insulin resistance (IR) in youth and adults with type 2 diabetes (6, 7). Moreover, increasing evidence implicates IR in the pathophysiology of CVD in youth and adults with type 1 diabetes (8–15). Using gold-standard techniques, we and others demonstrated that IR is prominent in normal-weight adolescents (16, 17) and adults (10) with type 1 diabetes, in the adipose, liver, and skeletal muscle tissue (10, 18–20). Moreover, obesity worsens IR and CVD risk, and obesity is now increasing in youth with type 1 diabetes (16, 21–26). Furthermore, the Diabetes Control and Complications Trial emphasized that intensive glycemic control reduces, but does not eliminate, diabetes complications, and it was associated with weight gain in a subset of participants, along with worsening lipid profiles, blood pressure, central adiposity, and inflammation, all of which may negate the positive effects of improved HbA1c (27). Thus, interventions targeting IR may be necessary to reduce CVD risk in type 1 diabetes.
Metformin is a safe medication that improves IR and BMI in youth with prediabetes and type 2 diabetes and decreases CVD risk and mortality in adults with type 2 diabetes (28–30). We previously demonstrated that metformin lowers insulin dose in adolescents who are overweight/obese with type 1 diabetes and in adolescents with poorly controlled type 1 diabetes, implying improved IR, as well as improving BMI and body composition (31, 32). However, metformin’s direct effect on insulin sensitivity in muscle, liver, and adipose tissue in type 1 diabetes was unknown. Therefore, we performed an ancillary study to a 3-month, double-blind, placebo-controlled, randomized trial to determine the effect of the addition of metformin to insulin treatment on the primary outcome of insulin-stimulated peripheral glucose uptake and the secondary outcomes of endogenous glucose production (EGP) and adipose lipolysis, measured by a gold-standard hyperinsulinemic-euglycemic clamp with stable isotope tracers in youth who are overweight/obese with type 1 diabetes.
Methods
Our study was a multicenter, double-blind, randomized trial conducted at eight clinical sites of the T1D Exchange Clinic Network as a preplanned substudy of a larger metformin trial with the primary outcome of change in HbA1c. Detailed methods from the parent study have already been published (31). The protocol was approved by local Institutional Review Boards. Written informed consent was obtained from participants aged 18 years and older or from a parent or guardian for younger participants who also assented. Study oversight was provided by an independent data and safety monitoring committee. The number of participants per site were as follows: University of Colorado Anschutz Medical Campus, 11; Yale University, 6; Nemours Children’s Specialty Care, 3; Indiana University, 5; University of Minnesota, 3; University of Iowa, 6; Children’s Hospital of Pittsburgh, 3.
Study participants
Major inclusion criteria included presumed autoimmune type 1 diabetes (diagnosis prior to age 10 years and/or positive diabetes-related autoantibodies) for at least 1 year treated on either an insulin pump or three or more daily injections of insulin, age of 12 to <20 years, HbA1c 7.5% to 9.9% (58 to 85 mmol/mol), BMI ≥85th percentile for age and sex, total daily insulin dose (TDI) of ≥0.8 U/kg/d, and frequency of self-monitoring of blood glucose three or more times per day. Fasting C-peptide was undetectable in 26 participants, <0.3 nmol/L in an additional 10 participants, and was equal to 1 nmol/L in one participant.
Treatment
Each participant was randomly assigned using a computer-generated sequence to either metformin or placebo with equal probability using a permuted block design. A central pharmacy compounded the placebo to match the 500-mg metformin tablets. Study medication was titrated during 4 weeks up to 1000 mg twice daily for the remainder of the 3-month treatment period. Study drug dosage could be titrated at a slower rate and/or adjusted due to adverse effects per investigator discretion. Clinician judgment was used to adjust insulin doses. Pill counts of bottles returned by study participants were used to assess compliance. All adverse events were reported regardless of whether the event was considered treatment-related and coded using the Medical Dictionary for Regulatory Activities. Reportable severe hypoglycemia was defined as low blood glucose requiring assistance of another person to administer carbohydrate, glucagon, or other resuscitative actions to treat altered consciousness.
Follow-up visits
During the first 4 weeks after randomization, participants received weekly phone calls to adjust study drug and insulin dosages, and to assess for adverse events. An interim office visit was conducted after 6 weeks of study drug therapy.
Study procedures and assessments
At baseline and 12 weeks, height, weight, blood pressure, waist circumference, and TDI/kg of participants were assessed; Tanner staging was performed at randomization (pubic hair for both sexes, breasts for girls, genitalia for boys); dual-energy x-ray absorptiometry scans were performed to assess body composition.
Participants were admitted to the inpatient clinical translational research center for 12 hours of overnight monitored fasting and overnight glucose control. Subcutaneous insulin was replaced with a variable-rate algorithm-driven overnight IV insulin infusion to normalize blood sugar concentrations (goal of 100 mg/dL). The last dose of medication (metformin or placebo) was given in the hospital, the night prior to the clamp. The following morning, fasting blood samples to measure background enrichment and concentrations of glucose and glycerol were obtained. A bolus of 4.5 mg/kg [6,6-2H2]glucose (Isotec, Miamisburg, OH) was then given, followed by a continuous infusion of 0.03 mg/kg/min [6,6-2H2]glucose paired with a primed (1.6 µmol/kg) then constant (0.11 µmol/kg/min) infusion of [2H5]glycerol. Following a 2-hour basal phase during which the overnight insulin infusion was continued to maintain normoglycemia, a 3-phase (2-hour basal phase, 1.5-hour 16 mU/m2/min insulin phase, and a 2-hour 80 mU/m2/min insulin phase) hyperinsulinemic euglycemic clamp was performed (7, 16) to assess adipose, hepatic, and peripheral IR. Insulin doses were based on ours and others’ (7, 16, 20) previous experience with insulin requirements in pubertal youth. Twenty percent dextrose (spiked with [6,6-2H2]glucose) was titrated to maintain arterialized plasma glucose at ∼95 mg/dL, monitored every 5 minutes at the bedside using the Yellow Springs Instrument (YSI, Yellow Springs, OH). Glucose infusion rate [GIR; mg/kg/min and mg/kg of fat-free mass (FFM)/min] was measured based on steady-state glucose values from the last 30 minutes of the 80 mU/m2/min (highest dose) phase. During the last 30 minutes of each of the three clamp phases (basal, 16 mU/m2/min and 80 mU/m2/min), four samples, each 10 minutes apart, were drawn for glucose, glycerol, free fatty acid (FFA) and insulin concentrations, and glucose and glycerol tracer enrichments (33).
Indirect calorimetry was performed at the end of each clamp phase using a metabolic cart system with a hood attachment for ≥20 minutes per phase. Carbohydrate oxidation was calculated as 4.55 × volume CO2 − 3.21 volume O2, and fat oxidation was calculated as 1.67 × volume CO2 − 1.67 × volume O2, with O2 and CO2 in liters per minute (34).
Sample analysis
All tracer samples were analyzed at the University of Colorado. Analysis of glycerol and glucose were performed using gas chromatography–mass spectrometry as described (18, 33, 35). Serum laboratory analyses were performed at a central laboratory (Northwest Lipid Research Laboratories, University of Washington, Seattle, WA) for HbA1c (automated glycohemoglobin analyzer HLC-723G8, Tosoh Bioscience, San Francisco, CA), FFA (Wako Diagnostics, Richmond, VA) on a Roche Modular P autoanalyzer (Roche Diagnostics, Indianapolis, IN), and insulin concentrations (two-site immune-enzymometric assay performed on the Tosoh 2000 autoanalyzer (Tosoh Bioscience).
All isotopic enrichments were corrected for background enrichments. The glucose and glycerol rate of appearance (Ra), rate of disappearance (Rd), and metabolic clearance rate (MCR) during the last 30 minutes of each clamp phase were calculated using Steele non–steady-state equations, accounting for “spiked” glucose in the 20% dextrose infusion (18). To describe changes in Ra across the insulin concentrations of each phase, the intercept and slope of the log-transformed regression line for each individual’s data were also used to calculate the predicted glycerol and glucose Ra at the average insulin concentration for all participants during the 16 mU/m2/min phase (36).
Outcomes
The primary analysis was treatment group comparison of mean change in GIR (mg/kg/min) at 13 weeks, adjusted for baseline GIR using analysis of covariance and an intent-to-treat approach. Multiple imputation using Rubin’s method, in which each missing value is imputed multiple times and the resulting data sets are combined per standard procedures, was used to impute missing IR outcomes. The primary analysis was repeated without imputation and compared with the results using imputation in a sensitivity analysis.
Statistical analysis
Descriptive statistics are presented as mean ± SD, median (Q1, Q3), or frequencies and percentages, as appropriate. For single time point variables, group comparisons were made using a χ2 or Fisher’s exact test and the t test or Kruskal–Wallis test.
Repeated-measures mixed models compared outcomes measured at multiple time points during the clamp. The repeated-measures models for glucose and insulin contained terms for group (type 1 diabetes or control), clamp phase, and the interaction of group and phase. For repeated-measures outcomes (glucose Ra, glycerol Ra, glycerol, FFAs) potentially impacted by serum insulin concentrations, insulin was added to the model and the Aikaike information criterion, an estimate of model fit to a particular set of data, was used to test whether a random effect of insulin should be included (37). The three-way interactions of group, phase, and insulin were tested for significance, but all two-way interactions were retained in the models. For glucose Ra, glycerol Ra, and insulin concentrations, there were two repeat factors: phase of the clamp (basal, 16 mU/m2/min, and 80 mU/m2/min phase) and visit (baseline and 13 weeks). Nested repeated mixed models were used to assess for significant differences in the change in glucose Ra and glycerol Ra from baseline to 13 weeks between treatment groups. To accommodate nested repeated measures data, a model with a composite Kronecker product covariance structure, which essentially combines two types of covariance matrices, accounted for the separate covariance structures for each repeat factor (phase of clamp, visit).
P values <0.05 were considered significant for main effects; P values <0.25 were considered significant for interactions. All statistical analyses were performed with SAS software, version 9.4 (SAS Institute, Cary, NC).
Results
Thirty-seven youth with type 1 diabetes were randomized to either 3 months of metformin (N = 19) or placebo (N = 18) in addition to their insulin therapy (Table 1). Table 2 displays adverse events by treatment group. Side effects were minimal and were limited to gastrointestinal symptoms, which did not differ between groups, and to one episode of diabetic ketoacidosis in the metformin group. No participants experienced severe hypoglycemia.
Table 1.
Baseline Characteristics by Treatment Group
| Placebo (N = 18) | Metformin (N = 19) | |
|---|---|---|
| Age, y | 15.5 (2.2) | 15.8 (2.1) |
| Sex: female, n (%) | 8 (44) | 12 (63) |
| Race/ethnicity, n (%) | ||
| White | 13 (72) | 16 (84) |
| Non-Hispanic black | 1 (6.3) | 1 (5.3) |
| Hispanic white | 3 (18.8) | 2 (10.5) |
| Other | 1 (6) | |
| Tanner stage (4 or 5), n (%) | 15 (81) | 17 (91) |
| Duration of diabetes, y | 7.2 ± 3.1 | 6.9 ± 3.7 |
| Weight, kg | 85.2 ± 17.7 | 86.7 ± 17.0 |
| FFM, kg | 49.8 ± 9.0 | 49.8 ± 9.9 |
| Waist circumference, cm | 98.3 ± 13.3 | 97.0 ± 13.0 |
| BMI, percentile | 96.7 ± 2.3 | 96.3 ± 2.5 |
| BMI z score | 1.9 ± 0.3 | 1.9 ± 0.4 |
| HbA1c, % | 8.4 ± 1.0 | 9.2 ± 1.1a |
| HbA1c, mmol/mol | 68.3 ± 8.6 | 77 ± 9.7a |
| TDI/kg | 1.03 (0.87, 1.16) | 1.03 (0.91, 1.31) |
| Steady-state GIR, corrected for serum glucose, mg/kg/min | 5.0 ± 2.5 | 4.8 ± 2.8 |
Data are shown as mean ± SD or median (25th percentile, 75th percentile).
P < 0.05 to 0.01.
Table 2.
Adverse Events Separated by Treatment Group
| Placebo (N = 18) | Metformin (N = 19) | |
|---|---|---|
| Total no. of adverse events reported | 12 | 8 |
| Total no. of serious adverse events reported | 2 | 1 |
| Total no. of hospitalizations | 4 | 3 |
| Proportion reporting at least one adverse event, no. [% (95% CI)] | 7 [39 (14–64)] | 8 [42 (18–67)] |
| Proportion reporting at least one serious adverse event, no. [% (95% CI)] | 1 [6 (<1–17)] | 1 [5 (<1–16)] |
| Proportion with adverse event related to treatment, no. [% (95% CI)] | 3 [17 (<1–36)] | 3 [16 (<1–34)] |
| Severe hypoglycemia | ||
| Subjects with at least one event, no. [% (95% CI)] | 0 | 0 |
| Diabetic ketoacidosis | ||
| Subjects with at least one event, no. [% (95% CI)] | 0 | 1 [5 (<1–16)] |
| Gastrointestinal events | ||
| Subjects with at least one event, no. [% (95% CI)] | 5 [28 (5–51)] | 5 [26 (5–48)] |
| Liver enzymes, % (95% CI) | ||
| ALT, % >1.5-fold upper limit of normal | 0 | 0 |
| Serum creatinine, % (95% CI) | ||
| Proportion above upper limit of normal | 0 | 0 |
| Lactic acidosis, % (95% CI) | ||
| Proportion with detected lactic acidosis | 0 | 0 |
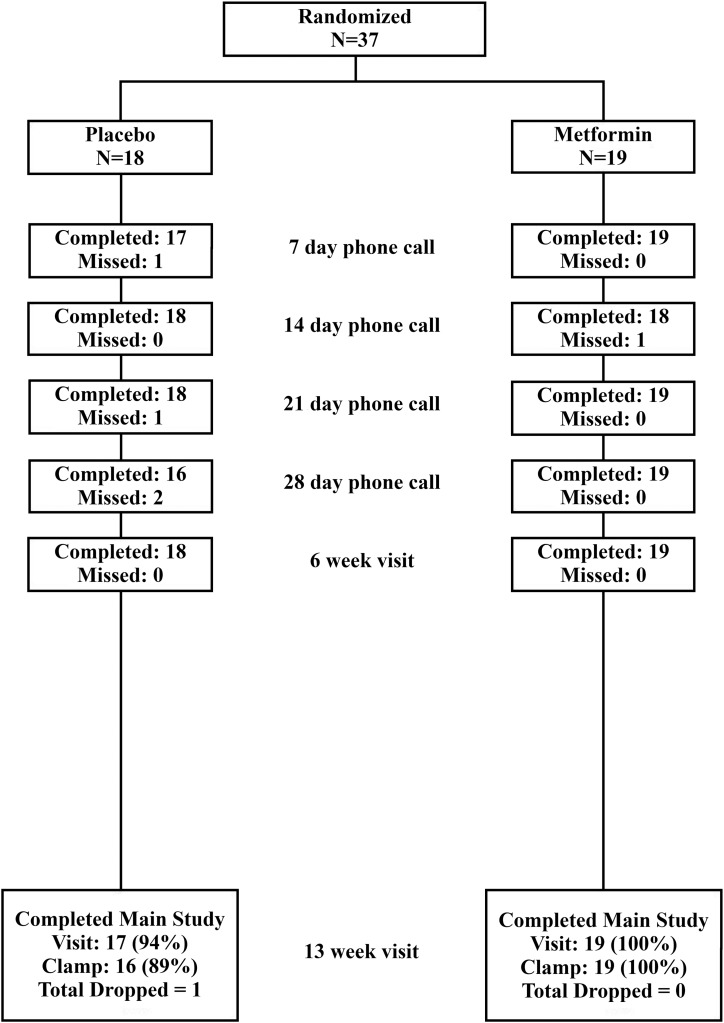
The two groups were similar for age, sex, race/ethnicity, Tanner stage, weight, lean mass, BMI percentile, BMI z score, TDI/kg, and GIR (Table 1). At 6 weeks, all participants reported taking at least 1500 mg a day in the metformin group. By 13 weeks, one of the metformin participants discontinued medication, and a second reported taking only 1000 mg a day. All participants in the metformin group and 16 of 18 in the placebo group completed the final visit (Fig. 1).
Figure 1.
Consort diagram. Participant visit and call follow up after randomization is shown above.
Baseline clamp assessments
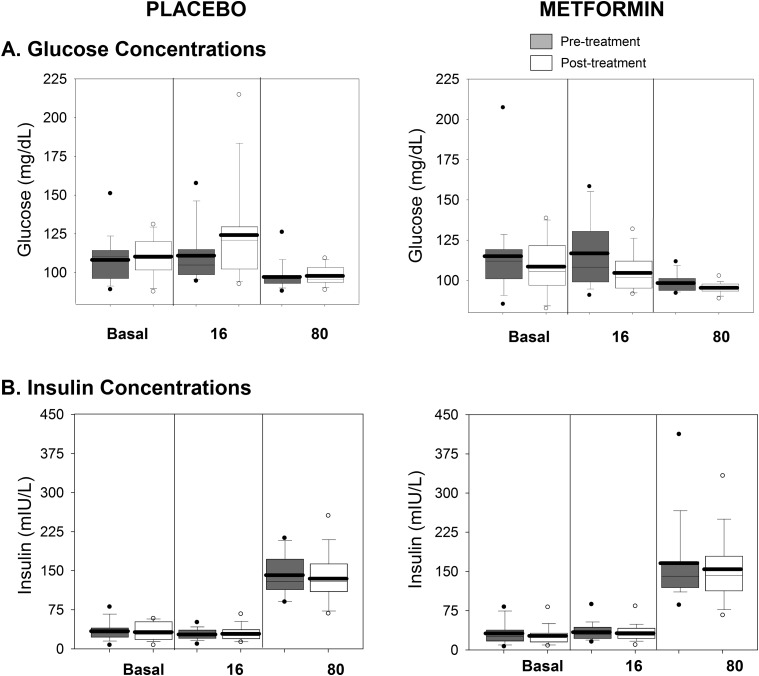
By design, the glucose concentrations throughout the three clamp phases were similar between groups (Fig. 2A). The insulin concentrations (Fig. 2B) increased in both groups with the administration of increasing doses of insulin and were similar between groups. Owing to the significant insulin requirements to maintain glycemia overnight, resulting insulin concentrations at the initial am basal phase were similar to those achieved at the 16 mU/m2/min phase.
Figure 2.
Glucose and insulin concentrations during each phase of the clamp. Glucose (A) and insulin (B) concentrations are shown. The placebo-treated groups are shown on the left, and the metformin treated-group is shown on the right, with pretreatment in gray and posttreatment in white. Data are shown from each phase of the clamp (basal, 16 mIU/m2/min, and 80 mIU/m2/min). Box plots are 25th to 75th percentile, with the group mean as the dark line and outliers shown as circles.
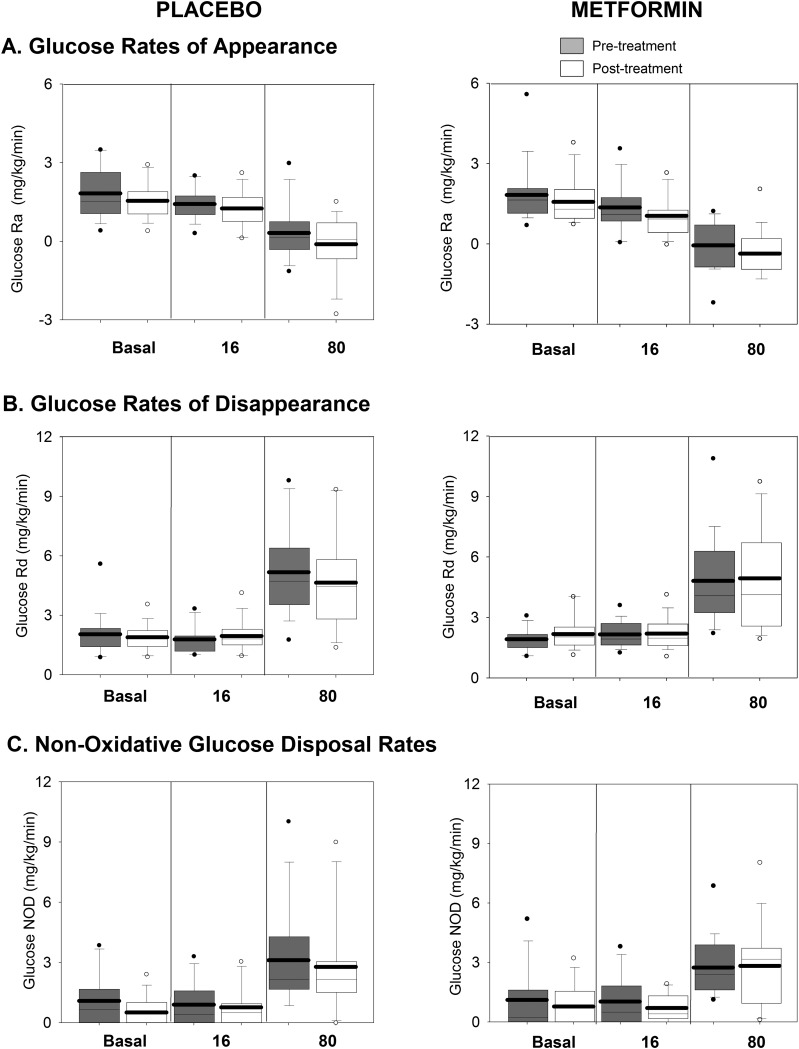
Glucose Ra (Fig. 3A) in the basal and 16 mU/m2/min phases was similar, reflecting excess insulin in the basal period and abnormal incomplete suppression in the 16 mU/m2/min phase. Glucose Ra was further suppressed during the 80 mU/m2/min phase in both groups, although not to 0. Glucose Rd (Fig. 3B) increased as expected equally during the 80 mU/m2/min phase in both groups. Nonoxidative glucose disposal increased during the 80 mU/m2/min phase (Fig. 3C) similarly in both treatment groups.
Figure 3.
Glucose-related measures during the clamp, before and after intervention. Rates of glucose Ra (A), glucose (B), and glucose nonoxidative disposal (NOD) (C) are shown. The placebo-treated groups are shown on the left, and the metformin-treated group is shown on the right, with pretreatment in gray and posttreatment in white. Data are shown from each phase of the clamp (basal, 16 mIU/m2/min, and 80 mIU/m2/min). Box plots are 25th to 75th percentile, with the group mean as the dark line and outliers shown as circles.
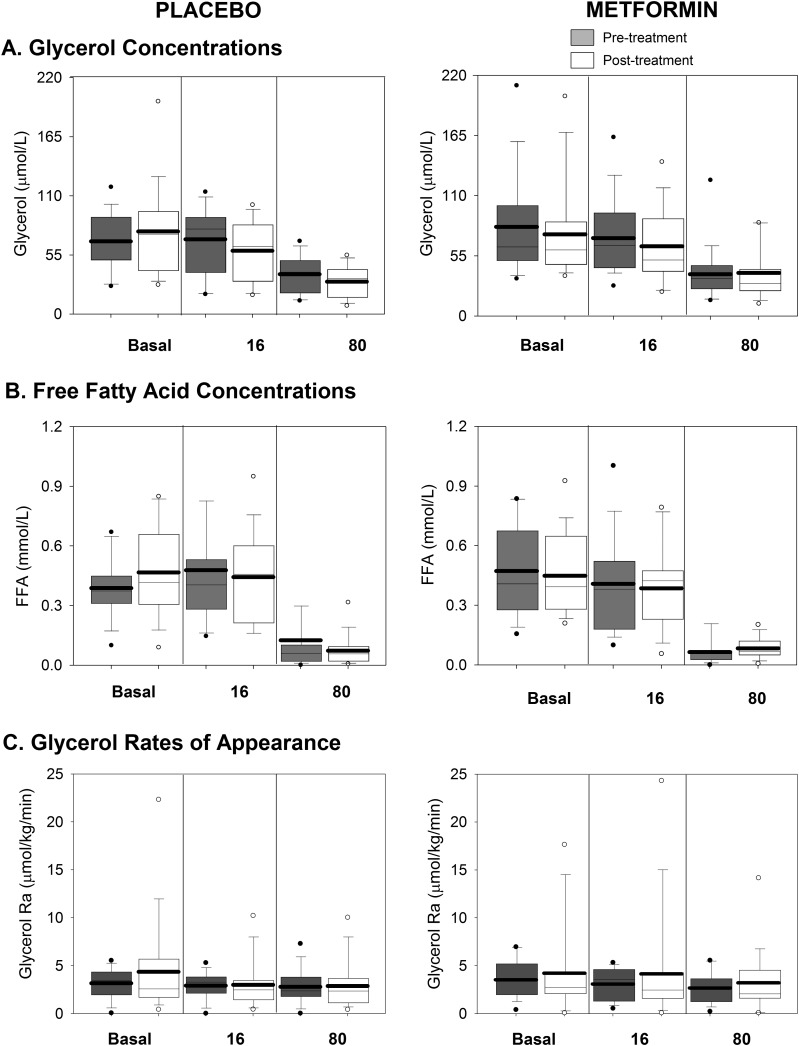
Glycerol (Fig. 4A) and FFA (Fig. 4B) concentrations were similar but inappropriately incompletely suppressed between the basal and 16 mU/m2/min phases and suppressed further during the 80 mU/m2/min phase. Glycerol Ra (Fig. 4C) was suppressed at baseline by the insulin required for overnight glucose control and remained similarly suppressed at all phases of the clamps.
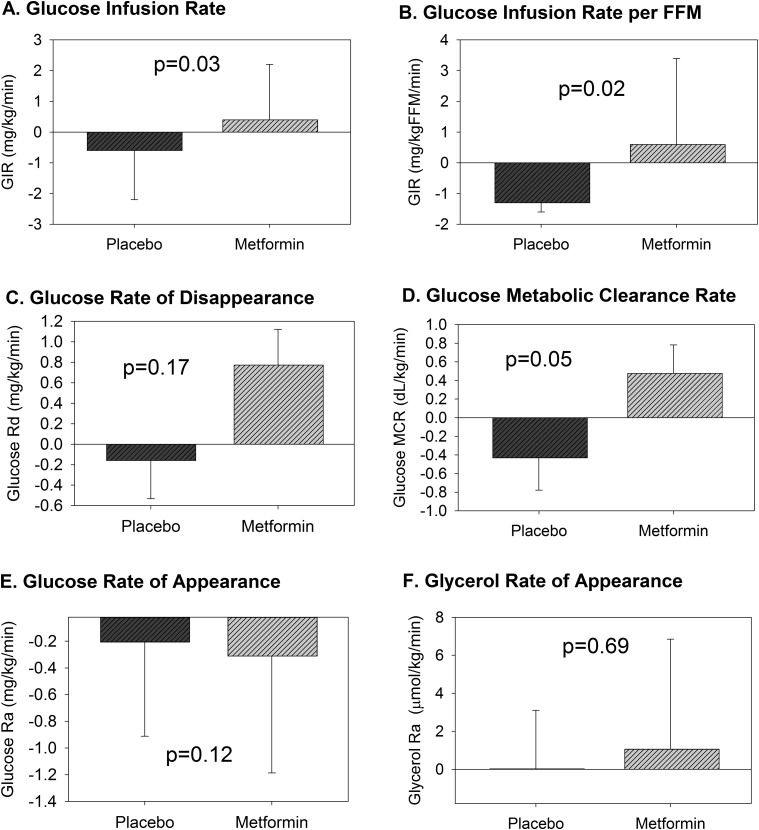
Figure 4.
Change in clamp measures of insulin sensitivity. The change in each measure of insulin sensitivity from baseline to 13 weeks for the placebo or metformin treatment group are shown above. GIR (A) and GIR per FFM (B) representing whole-body sensitivity were different between groups after treatment. Peripheral insulin sensitivity from the 80 mU/m2/min phase, represented as change in glucose Rd (C), was not different between groups, whereas glucose MCR (D), which controls for serum glucose, trended to be different. There was no difference in change in glucose Ra (E) during the 16 mU/m2/min phase, representing endogenous glucose production, nor glycerol Ra (F) during the 10 mU/m2/min phase, representing lipolysis. Data are means and standard errors of the means.
Posttreatment clamp assessments
Glucose (Fig. 2A) and insulin (Fig. 2B) concentrations after treatment were similar to the values before treatment. Posttreatment glucose Ra (Fig. 3A) was again similar but inappropriately incompletely suppressed during the basal and 16 mU/m2/min phase and was further suppressed during the 80 mU/m2/min phase in both groups. Posttreatment glycerol (Fig. 5A) and FFA (Fig. 5B) concentrations were again similar but inappropriately incompletely suppressed between the basal and 16 mU/m2/min phases and suppressed further during the 80 mU/m2/min phase.
Figure 5.
Lipid-related measures during the clamp and before and after intervention. Serum glycerol concentrations (A), FFA concentrations (B), and glycerol Ra (C) are shown. The placebo-treated groups are shown on the left, and the metformin-treated group is shown on the right, with pretreatment in gray and posttreatment in white. Data are shown from each phase of the clamp (basal, 16 mIU/m2/min, and 80 mIU/m2/min). Box plots are 25th to 75th percentile, with the group mean as the dark line and outliers shown as circles.
Change between groups with intervention
Change in body weight and BMI z score were significantly different between groups postintervention (Table 3). The groups were not different postintervention regarding change in FFM, BMI percentile, waist circumference, HbA1c, or TDI/kg. Change in tissue-specific insulin sensitivity with each treatment is shown in Fig. 4. Between-group difference in change in the primary outcome of whole-body insulin sensitivity during the 80 mU/m2/min phase, expressed as GIR adjusted for baseline GIR, sex, and random center effects, was significant and favored the metformin group [1.3 (0.1, 2.4) mg/kg/min, P = 0.03) at 3 months (Fig. 4A). Results remained significant when GIR was expressed per kilogram of FFM [2.3 (0.03, 4.2), P = 0.02] (Fig. 4B). Change in related glucose Rd during the 80 mU/m2/min phase did not significantly favor metformin postmedication intervention, but the change in glucose MCR from the 80 mU/m2/min phase was 0.923 (−0.002, 1.867) dL/kg/min (P = 0.05, Fig. 4D) and favored metformin. The posttreatment change in glucose Ra during the 16 mU/m2/min phase representing hepatic insulin sensitivity (Fig. 4E) was not significantly different between groups (P = 0.12). The change in glycerol Ra (Fig. 2F), representing adipose insulin sensitivity, was not different (P = 0.69).
Table 3.
Change in Clinical Variables With Treatment From Baseline to Study End (3 mo)
| Placebo (N = 16) | Metformin (N = 19) | Unadjusted P Value | Adjusted P Value | |
|---|---|---|---|---|
| Change in weight, kg | 1.1 ± 1.8 | −0.7 ± 2.6 | 0.025a | 0.030a |
| Change in FFM, kg | 1.3 ± 2.2 | 0.13 ± 1.2 | 0.085 | 0.386 |
| Change in BMI percentile | 0.09 ± 0.88 | −0.20 ± 0.88 | 0.343 | 0.252 |
| Change in BMI z score | 0.02 ± 0.08 | −0.04 ± 0.10 | 0.082 | 0.041a |
| Change in waist circumference, cm | −0.31 ± 7.2 | −1.03 ± 6.6 | 0.775 | 0.860 |
| Change in HbA1c, % | 0.37 ± 0.59 | 0.15 ± 1.24 | 0.512 | 0.562 |
| Change in HbA1c, mol/mmol | 2.9 ± 4.1 | 1.4 ± 11.2 | ||
| Change in TDI/kg | −0.05 ± 0.16 | −0.06 ± 0.32 | 0.934 | 0.917 |
Data are shown as mean ± SD. Data are adjusted for baseline values, sex, and random center effects.
P < 0.05–0.01.
Table 4 shows the results of the repeated-measures mixed models for glucose and glycerol Ra. The least-squares estimate is the estimated difference between groups in the change in the outcome from baseline to week 13, at the overall group mean insulin concentration during each phase. There were no significant differences between the groups in either the change in glycerol Ra or glucose Ra during any of the stages postintervention.
Table 4.
Results of Repeated-Measures Models for Glucose and Glycerol Ra
| Estimate | P Value | |
|---|---|---|
| Glucose Ra | ||
| Basal phase; mean insulin concentration 30 IU/mL | −0.295 ± 0.321 | 0.36 |
| Phase 16 mU/m2/min; mean insulin concentration 30 IU/mL | 0.146 ± 0.256 | 0.57 |
| Phase 80 mU/m2/min; mean insulin concentration 149 IU/mL | 0.053 ± 0.373 | 0.89 |
| Glycerol Ra | ||
| Basal phase; mean insulin concentration 30 IU/mL | 0.435 ± 1.728 | 0.80 |
| Phase 16 mU/m2/min; mean insulin concentration 30 IU/mL | −1.013 ± 1.598 | 0.53 |
| Phase 80 mU/m2/min; mean insulin concentration 149 IU/mL | −0.272 ± 1.072 | 0.81 |
For each measurement, data shown are metformin vs placebo and the change from baseline to 13 wks.
Discussion
We previously established that IR is present in the muscle, liver, and adipose tissue in adolescents with type 1 diabetes (19). We also previously reported in a large cohort of youth who were overweight/obese with type 1 diabetes that despite no change in HbA1c, 6 months of adjunctive metformin decreased BMI, adiposity, and TDI/kg, suggesting improved insulin sensitivity. We now demonstrate in a subgroup of the same study, utilizing gold-standard measures of tissue-specific insulin sensitivity, the novel finding that 3 months of metformin significantly improved whole-body and related peripheral insulin sensitivity in youth who were overweight/obese with type 1 diabetes. We also now show, to our knowledge for the first time, that metformin does not significantly improve the abnormal failure of suppression of EGP in this population. Although not directly compared in the same study, we also report a greater degree of muscle IR in youth who were overweight/obese with type 1 diabetes than that previously reported in normal-weight youth with type 1 diabetes, yet a similar degree of hepatic IR (16, 19). Furthermore, owing to high insulin requirements to control overnight glycemia, adipose IR was unable to be fully assessed by traditional methods in the adolescent population with obesity with type 1 diabetes, as fasting glycerol Ra was suppressed.
Most metformin studies in individuals with type 1 diabetes, including two pediatric studies (31, 32) and a Cochrane Systematic Review (38), found that metformin decreases TDI and BMI with no improvement in HbA1c, suggesting improved insulin sensitivity. A smaller study in Swedish adolescents found a significant improvement in whole-body IR within 11 participants in the metformin group (39). However, this study used a lower insulin clamp dose than typical for adolescents and a brief insulin infusion, lacked overnight glucose control, was not powered to compare effects between metformin and placebo and did not focus on participants who were overweight/obese (39). Our data now demonstrate that metformin improves whole-body IR in adolescents who are overweight/obese with type 1 diabetes and adds the novel finding of specifically improving peripheral IR in this population.
Altered muscle mitochondrial function, pubertal hormones, and unknown factors unique to type 1 diabetes, confounded by obesity and obesity-related hormones, likely play a role in the significant peripheral IR found in our participants (16, 19). We previously demonstrated decreased repletion of ATP from ADP following exercise in normal-weight youth with diabetes, and this mitochondrial dysfunction related to peripheral IR (17). Pubertal changes in growth hormone and sex steroids also contribute to the unique IR seen in adolescents (40). Whereas more extreme hyperglycemia typical in historic diabetes therapy related to IR, we showed that in an HbA1c range more consistent with modern therapies, similar to the youth now presented, hyperglycemia did not explain the whole-body IR (20, 41). Alternations in blood flow, insulin diffusion across blood vessels and epithelial layers, elevated FFAs from adipose IR, and nonphysiologic mode of insulin delivery are other likely contributors (42). Obesity is also a well-established cause of peripheral IR and likely worsens the IR of type 1 diabetes, potentially secondary to excess influx of FFAs, adipose-related inflammatory cytokines, and hormones such as leptin (19, 42). Further research is needed to completely understand peripheral IR in type 1 diabetes.
The cause of persistent EGP during hyperinsulinemia in adolescents with type 1 diabetes is likely multifactorial and potentially related to hepatic IR, lack of portal insulin delivery secondary to peripheral insulin administration, insulin omission, and/or altered α-cell function/glucagon release. Normally significant hepatic extraction of secreted insulin occurs via the portal circulation and likely meditates EGP prior to entering the peripheral circulation, but this process is bypassed with peripheral delivery of insulin (43, 44). Furthermore, glucagon secretion is altered in type 1 diabetes, likely secondary to lack of paracrine effects of secreted insulin and primary loss of α-cells (43, 44), and may contribute to persistent hepatic EGP. Alterations in pancreatic and hepatic insulin delivery due to decreased pancreatic and/or portal blood flow may also impact EGP (45). Thus, multiple factors might explain why EGP was similarly not suppressed by peripheral hyperinsulinemia between previously published normal weight youth and our current cohort of youth who were overweight, and was not affected by metformin therapy.
In youth who were overweight/obese with type 1 diabetes, we were unable to show whether metformin therapy improved adipose IR. Although we were not able to fully model suppression of lipolysis with glycerol Ra as discussed below, neither glycerol nor FFA concentrations decreased following metformin therapy during any phase of clamp. Previous work in normal weight youth and adults with type 1 diabetes indicate significant adipose IR when studied with a similar protocol, and lack of full suppression of glycerol and FFAs during the 16 mU/m2/min phase in the current study indicates that adipose IR is also present in members of our population who are overweight/obese (19, 35). Unlike participants without diabetes, adults with type 1 diabetes lacked a relationship between palmitoleic acid and IR, indicating that mechanisms of adipose IR in type 1 diabetes may be unique (35). Adipose IR in type 1 diabetes requires further investigation, including local tissue effects.
Owing to the extreme peripheral IR in our adolescent population with obesity and type 1 diabetes, performance of clamp studies to assess hepatic and adipose IR were challenging. For the assessment of adipose IR, glycerol and FFA concentrations were already partially suppressed during the basal phase. Compared with normal-weight youth or adults with type 1 diabetes who underwent protocols similar to our current study, basal glycerol and FFA concentrations were lower in our those who were overweight/obese with type 1 diabetes (18, 19, 35). Similarly, glycerol Ra was already suppressed in the basal state in those who were overweight/obese, a finding not present in our normal-weight type 1 group (19), indicating that the high overnight insulin requirements suppressed lipolysis before starting the clamp. These findings suggest that the reduction in peripheral insulin sensitivity that occurs when overweight/obesity is superimposed on adolescent type 1 diabetes is extreme, making traditional adipose IR methods uninterpretable. In contrast, the basal glucose Ra in members of our population who were overweight/obese with type 1 diabetes was similar to our previous normal-weight population, indicating that overweight/obesity did not worsen the already existing hepatic IR in type 1 diabetes (19).
In normal-weight youth with type 1 diabetes, we were previously able to perform a four-phase insulin clamp that included a 10 mU/m2/min phase between the basal and 16 mU/m2/min phase (basal, 10, 16, and 80 mU/m2/min) (19). This sequence allowed initially measurable glycerol and glucose Ra without rising glycemia, followed by an eventual response to increasing insulin doses, but markedly reduced insulin responses compared with nondiabetic controls. Similarly, the CACTI study in adults with type 1 diabetes successfully employed even lower insulin doses (basal, 4, 8, and 40 mU/m2/min) than required in normal-weight adolescents without hyperglycemia (18). However, in our adolescents with obesity with type 1 diabetes, it was challenging to maintain normoglycemia (∼95 mg/dL) even during the 16 mU/m2/min phase, as some participants had rising blood glucose despite no infused dextrose at this dose. Thus, using a lower insulin dose in this patient population was not feasible. Another approach would be an FFA tracer such as palmitate. However, there are inherent limitations with FFA tracers due to FFA recycling, as well as sterility and tolerability limitations in youth. Another approach would be allowing higher target blood sugars during the clamp, but this would introduce confounders, including renal glucose losses and noninsulin-mediated glucose disposal, among others. Further work is therefore needed to develop novel methods for assessing adipose IR in people with severe IR in other tissues such as our population.
The mechanisms of action of metformin are not fully understood, but its impacts on hepatic IR are typically thought to be primary. Metformin is thought to lower adenosine monophosphate, decrease gluconeogenesis, and inhibit the effect of glucagon (46). A systematic review of 19 clinical studies investigating metformin’s effect on endogenous glucose production in adults with type 2 diabetes used similar methodology as the current study (47). Results indicated that metformin primarily improved hepatic IR by enhancing insulin suppression of EGP and fasting plasma glucose clearance in this population. To a lesser degree, metformin increased insulin-stimulated glucose utilization; however, the estimated treatment-induced change in insulin-stimulated glucose utilization did not meet statistical significance in the pooled placebo-controlled studies. Our study population differs, as we performed investigations in adolescents with type 1 diabetes. Whole-body insulin sensitivity is known to be significantly lower in youth vs adults (48), including youth with type 1 diabetes (16, 19), and, as a result, insulin requirements are dramatically higher than in adults (42). The phenotype of IR in type 1 diabetes is also unique from type 2 diabetes, as many people with type 1 diabetes lack metabolic syndrome components despite being insulin resistant (7), which also may explain the different responses seen to metformin in our study vs those on type 2 diabetes. Moreover, the basal insulin delivery required to achieve fasting euglycemia was greater than expected in our population, and it may have limited our ability to assess some aspects of IR.
Our study has several strengths. First, to our knowledge, it was the first multicenter, double-blind, randomized control trial of metformin in youth who were overweight/obese with type 1 diabetes with insulin sensitivity assessed using isotope tracers and a multiphase clamp. All of the sites included investigators and pharmacists who were trained in the performance of pediatric hyperinsulinemic clamps with tracers using standardized protocols to limit site-to-site variation, and overnight glucose control was performed with a centralized algorithm. The multicenter nature also allowed for greater geographic diversity. The overall reported adherence to medication and study procedures was excellent for a clinical trial, with a low dropout or discontinuation rate. Additionally, all isotope samples were processed in one central laboratory and all clinical laboratory assays were performed at a central laboratory.
The major limitation of our study was the relatively small sample size, which did not allow us to examine the effect of sex, ethnicity, or different home insulin regimens. A few of the secondary study measures, such as change in GDR, are close to significance, suggesting a possible type 2 error due to smaller sample size. Furthermore, the inclusion criteria were fairly strict in terms of HbA1c and weight, which may limit the generalizability of the results to a broader type 1 diabetes population. Moreover, the basal insulin requirements to control glycemia were so high in members of this population who were overweight/obese that the basal (overnight) insulin infusions were elevated enough to suppress glycerol and FFAs as discussed. Whereas improvement of insulin sensitivity was found at 13 weeks of metformin therapy, longer studies in adults indicate that metformin adherence wanes over time with a 26% discontinuation rate, which may limit the utility of this therapy, and thus longer-term studies are needed (15).
In summary, we demonstrated that the addition of metformin to insulin therapy in youth who were overweight or obese with type 1 diabetes improves whole-body and specifically peripheral muscle IR during a 13-week period. However, alternative approaches are likely needed to target the hepatic IR of youth who are overweight/obese with type 1 diabetes. Moreover, new method development is needed in this population to assess adipose IR. Improvements in IR during metformin therapy have been tied to improvements in CVD risk markers in adults and youth with type 1 diabetes (15, 49). Further work is now needed to determine whether this modified risk translates to long-term improvements in morbidity and mortality to warrant widespread use of metformin in youth who are obese with type 1 diabetes.
Acknowledgments
A listing of the T1D Exchange Clinic Network Metformin Clamp RCT Study Group sites with participating principal investigators (PI), coinvestigators (I), and coordinators (C) ordered by the number of participants recruited per site is as follows: Indianapolis, IN; Indiana University: Tamara Hannon (PI), Stephanie Woerner (C), Bonnie Jagielo (C). Aurora, CO; Barbara Davis Center for Childhood Diabetes: Kristen Nadeau (PI), Melanie Cree-Green (I), Katherine Manseau (C), Amy Baumgartner (C). Pittsburgh, PA; Children’s Hospital of Pittsburgh of UPMC: Ingrid Libman (PI), Ana Diaz (C). New Haven, CT; Yale Children’s Diabetes Program: Eda Cengiz (PI), Eileen Tichy (I), Amy Steffen (C), Melinda Zgorski (C). Minneapolis, MN; University of Minnesota: Brandon Nathan (PI), Anne Street (C), Janice Leschyshyn (C). Iowa City, IA; University of Iowa Children’s Hospital: Eva Tsalikian (PI), Michael Tansey (I), Julie Coffey (C). Jacksonville, FL; Nemours Children’s Specialty Care: Larry Fox (PI), Kim Englert (C), Kaitlin Sikes (C).
Financial Support: This work was supported by the Juvenile Diabetes Research Foundation International and by National Institutes of Health/National Center for Advancing Translational Sciences Colorado Clinical and Translational Science Awards (CTSA) Grant UL1TR002535, Pittsburgh CTSA Grant UL1TR001857, and Iowa CTSA Grant UL1TR002537.
Author Contributions: Study concept and design: M.C.-G., I.L., K.M., and K.J.N. Acquisition, analysis, or interpretation of data: all authors. Drafting of the manuscript: M.C.-G. and K.J.N. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: L.P. and K.M. Obtained funding: K.M. and K.J.N. Administrative, technical, or material support: K.M. Study supervision: M.C.-G., K.J.N., I.L., and K.M. Guarantor statement: K.J.N. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Clinical Trial Information: ClinicalTrials.gov no. NCT02045290 (registered 15 January 2014).
Disclosure Summary: I.L. has received grants to her institution, Children’s Hospital of Pittsburgh at University of Pittsburgh Medical Center, from Novo Nordisk. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- BMI
body mass index
- CVD
cardiovascular disease
- EGP
endogenous glucose production
- FFA
free fatty acid
- FFM
fat-free mass
- GIR
glucose infusion rate
- IR
insulin resistance
- MCR
metabolic clearance rate
- Ra
rate of appearance
- Rd
rate of disappearance
- TDI
total daily insulin dose
References
- 1. Soltesz G, Patterson CC, Dahlquist G; EURODIAB Study Group. Worldwide childhood type 1 diabetes incidence—what can we learn from epidemiology? Pediatr Diabetes. 2007;8(Suppl 6):6–14. [DOI] [PubMed] [Google Scholar]
- 2. Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, Imperatore G, Linder B, Marcovina S, Pettitt DJ, Pihoker C, Saydah S, Wagenknecht L; SEARCH for Diabetes in Youth Study. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N Engl J Med. 2017;376(15):1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rewers M, Zimmet P. The rising tide of childhood type 1 diabetes—what is the elusive environmental trigger? Lancet. 2004;364(9446):1645–1647. [DOI] [PubMed] [Google Scholar]
- 4. Vehik K, Hamman RF, Lezotte D, Norris JM, Klingensmith G, Bloch C, Rewers M, Dabelea D. Increasing incidence of type 1 diabetes in 0- to 17-year-old Colorado youth. Diabetes Care. 2007;30(3):503–509. [DOI] [PubMed] [Google Scholar]
- 5. Maahs DM, Daniels SR, de Ferranti SD, Dichek HL, Flynn J, Goldstein BI, Kelly AS, Nadeau KJ, Martyn-Nemeth P, Osganian SK, Quinn L, Shah AS, Urbina E; American Heart Association Atherosclerosis, Hypertension and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular and Stroke Nursing, Council for High Blood Pressure Research, and Council on Lifestyle and Cardiometabolic Health. Cardiovascular disease risk factors in youth with diabetes mellitus: a scientific statement from the American Heart Association. Circulation. 2014;130(17):1532–1558. [DOI] [PubMed] [Google Scholar]
- 6. Dabelea D, Kinney G, Snell-Bergeon JK, Hokanson JE, Eckel RH, Ehrlich J, Garg S, Hamman RF, Rewers M; Coronary Artery Calcification in Type 1 Diabetes Study. Effect of type 1 diabetes on the gender difference in coronary artery calcification: a role for insulin resistance? The Coronary Artery Calcification in Type 1 Diabetes (CACTI) study. Diabetes. 2003;52(11):2833–2839. [DOI] [PubMed] [Google Scholar]
- 7. Nadeau KJ, Zeitler PS, Bauer TA, Brown MS, Dorosz JL, Draznin B, Reusch JE, Regensteiner JG. Insulin resistance in adolescents with type 2 diabetes is associated with impaired exercise capacity. J Clin Endocrinol Metab. 2009;94(10):3687–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bacha F, Klinepeter Bartz S. Insulin resistance, role of metformin and other non-insulin therapies in pediatric type 1 diabetes. Pediatr Diabetes. 2016;17(8):545–558. [DOI] [PubMed] [Google Scholar]
- 9. Pereira RI, Snell-Bergeon JK, Erickson C, Schauer IE, Bergman BC, Rewers M, Maahs DM. Adiponectin dysregulation and insulin resistance in type 1 diabetes. J Clin Endocrinol Metab. 2012;97(4):E642–E647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schauer IE, Snell-Bergeon JK, Bergman BC, Maahs DM, Kretowski A, Eckel RH, Rewers M. Insulin resistance, defective insulin-mediated fatty acid suppression, and coronary artery calcification in subjects with and without type 1 diabetes: the CACTI study. Diabetes. 2011;60(1):306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Specht BJ, Wadwa RP, Snell-Bergeon JK, Nadeau KJ, Bishop FK, Maahs DM. Estimated insulin sensitivity and cardiovascular disease risk factors in adolescents with and without type 1 diabetes. J Pediatr. 2013;162(2):297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Orchard TJ, Olson JC, Erbey JR, Williams K, Forrest KY, Smithline Kinder L, Ellis D, Becker DJ. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care. 2003;26(5):1374–1379. [DOI] [PubMed] [Google Scholar]
- 13. Cree-Green M, Maahs DM, Ferland A, Hokanson JE, Wang H, Pyle L, Kinney GL, King M, Eckel RH, Nadeau KJ. Lipoprotein subfraction cholesterol distribution is more atherogenic in insulin resistant adolescents with type 1 diabetes. Pediatr Diabetes. 2016;17(4):257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maahs DM, Hokanson JE, Wang H, Kinney GL, Snell-Bergeon JK, East A, Bergman BC, Schauer IE, Rewers M, Eckel RH. Lipoprotein subfraction cholesterol distribution is proatherogenic in women with type 1 diabetes and insulin resistance. Diabetes. 2010;59(7):1771–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Petrie JR, Chaturvedi N, Ford I, Brouwers MC, Greenlaw N, Tillin T, Hramiak I, Hughes AD, Jenkins AJ, Klein BE, Klein R, Ooi TC, Rossing P, Stehouwer CD, Sattar N, Colhoun HM; REMOVAL Study Group. Cardiovascular and metabolic effects of metformin in patients with type 1 diabetes (REMOVAL): a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5(8):597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nadeau KJ, Regensteiner JG, Bauer TA, Brown MS, Dorosz JL, Hull A, Zeitler P, Draznin B, Reusch JE. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab. 2010;95(2):513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cree-Green M, Newcomer BR, Brown MS, Baumgartner AD, Bergman B, Drew B, Regensteiner JG, Pyle L, Reusch JE, Nadeau KJ. Delayed skeletal muscle mitochondrial ADP recovery in youth with type 1 diabetes relates to muscle insulin resistance. Diabetes. 2015;64(2):383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bergman BC, Howard D, Schauer IE, Maahs DM, Snell-Bergeon JK, Eckel RH, Perreault L, Rewers M. Features of hepatic and skeletal muscle insulin resistance unique to type 1 diabetes. J Clin Endocrinol Metab. 2012;97(5):1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cree-Green M, Stuppy JJ, Thurston J, Bergman BC, Coe GV, Baumgartner AD, Bacon S, Scherzinger A, Pyle L, Nadeau KJ. Youth with type 1 diabetes have adipose, hepatic, and peripheral insulin resistance. J Clin Endocrinol Metab. 2018;103(10):3647–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arslanian S, Heil BV, Kalhan SC. Hepatic insulin action in adolescents with insulin-dependent diabetes mellitus: relationship with long-term glycemic control. Metabolism. 1993;42(3):283–290. [DOI] [PubMed] [Google Scholar]
- 21. Järvisalo MJ, Raitakari M, Toikka JO, Putto-Laurila A, Rontu R, Laine S, Lehtimäki T, Rönnemaa T, Viikari J, Raitakari OT. Endothelial dysfunction and increased arterial intima-media thickness in children with type 1 diabetes. Circulation. 2004;109(14):1750–1755. [DOI] [PubMed] [Google Scholar]
- 22. Urbina EM, Wadwa RP, Davis C, Snively BM, Dolan LM, Daniels SR, Hamman RF, Dabelea D. Prevalence of increased arterial stiffness in children with type 1 diabetes mellitus differs by measurement site and sex: the SEARCH for Diabetes in Youth Study. J Pediatr. 2010;156(5):731–737.e1. [DOI] [PubMed] [Google Scholar]
- 23. Margeirsdottir HD, Stensaeth KH, Larsen JR, Brunborg C, Dahl-Jørgensen K. Early signs of atherosclerosis in diabetic children on intensive insulin treatment: a population-based study. Diabetes Care. 2010;33(9):2043–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bjornstad P, Truong U, Pyle L, Dorosz JL, Cree-Green M, Baumgartner A, Coe G, Regensteiner JG, Reusch JE, Nadeau KJ. Youth with type 1 diabetes have worse strain and less pronounced sex differences in early echocardiographic markers of diabetic cardiomyopathy compared to their normoglycemic peers: a Resistance to Insulin in Type 1 and Type 2 Diabetes (RESISTANT) study. J Diabetes Complications. 2016;30(6):1103–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. DuBose SN, Hermann JM, Tamborlane WV, Beck RW, Dost A, DiMeglio LA, Schwab KO, Holl RW, Hofer SE, Maahs DM. Obesity in youth with type 1 diabetes in Germany, Austria, and the United States. J Pediatr. 2015;167(3):627–632.e1–4. [DOI] [PubMed] [Google Scholar]
- 26. Libman IM, Pietropaolo M, Arslanian SA, LaPorte RE, Becker DJ. Changing prevalence of overweight children and adolescents at onset of insulin-treated diabetes. Diabetes Care. 2003;26(10):2871–2875. [DOI] [PubMed] [Google Scholar]
- 27. Purnell JQ, Hokanson JE, Marcovina SM, Steffes MW, Cleary PA, Brunzell JD. Effect of excessive weight gain with intensive therapy of type 1 diabetes on lipid levels and blood pressure: results from the DCCT. Diabetes Control and Complications Trial. JAMA. 1998;280(2):140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352(9131):854–865. [PubMed] [Google Scholar]
- 29. Basu R, Basu A, Chandramouli V, Norby B, Dicke B, Shah P, Cohen O, Landau BR, Rizza RA. Effects of pioglitazone and metformin on NEFA-induced insulin resistance in type 2 diabetes. Diabetologia. 2008;51(11):2031–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. RISE Consortium. Impact of insulin and metformin versus metformin alone on β-cell function in youth with impaired glucose tolerance or recently diagnosed type 2 diabetes. Diabetes Care. 2018;41(8):1717–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Libman IM, Miller KM, DiMeglio LA, Bethin KE, Katz ML, Shah A, Simmons JH, Haller MJ, Raman S, Tamborlane WV, Coffey JK, Saenz AM, Beck RW, Nadeau KJ; T1D Exchange Clinic Network Metformin RCT Study Group. Effect of metformin added to insulin on glycemic control among overweight/obese adolescents with type 1 diabetes: a randomized clinical trial. JAMA. 2015;314(21):2241–2250. [DOI] [PubMed] [Google Scholar]
- 32. Nadeau KJ, Chow K, Alam S, Lindquist K, Campbell S, McFann K, Klingensmith G, Walravens P. Effects of low dose metformin in adolescents with type I diabetes mellitus: a randomized, double-blinded placebo-controlled study. Pediatr Diabetes. 2015;16(3):196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gilker CD, Pesola GR, Matthews DE. A mass spectrometric method for measuring glycerol levels and enrichments in plasma using 13C and 2H stable isotopic tracers. Anal Biochem. 1992;205(1):172–178. [DOI] [PubMed] [Google Scholar]
- 34. Wolfe RR, Chinkes DL. Isotope Tracers in Metabolic Research. Principles and Practice of Kinetic Analysis. Hoboken, NJ: Wiley-Liss; 2005. [Google Scholar]
- 35. Bergman BC, Howard D, Schauer IE, Maahs DM, Snell-Bergeon JK, Clement TW, Eckel RH, Perreault L, Rewers M. The importance of palmitoleic acid to adipocyte insulin resistance and whole-body insulin sensitivity in type 1 diabetes. J Clin Endocrinol Metab. 2013;98(1):E40–E50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pyle L, Bergman BC, Nadeau KJ, Cree-Green M. Modeling changes in glucose and glycerol rates of appearance when true basal rates of appearance cannot be readily determined. Am J Physiol Endocrinol Metab. 2016;310(5):E323–E331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cree-Green M, Bergman BC, Coe GV, Newnes L, Baumgartner AD, Bacon S, Sherzinger A, Pyle L, Nadeau KJ. Hepatic steatosis is common in adolescents with obesity and PCOS and relates to de novo lipogenesis but not insulin resistance. Obesity (Silver Spring). 2016;24(11):2399–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Al Khalifah RA, Alnhdi A, Alghar H, Alanazi M, Florez ID. The effect of adding metformin to insulin therapy for type 1 diabetes mellitus children: a systematic review and meta-analysis. Pediatr Diabetes. 2017;18(7):664–673. [DOI] [PubMed] [Google Scholar]
- 39. Särnblad S, Kroon M, Aman J. Metformin as additional therapy in adolescents with poorly controlled type 1 diabetes: randomised placebo-controlled trial with aspects on insulin sensitivity. Eur J Endocrinol. 2003;149(4):323–329. [DOI] [PubMed] [Google Scholar]
- 40. Kelsey MM, Zeitler PS. Insulin resistance of puberty. Curr Diab Rep. 2016;16(7):64. [DOI] [PubMed] [Google Scholar]
- 41. Chan CL, Pyle L, Morehead R, Baumgartner A, Cree-Green M, Nadeau KJ. The role of glycemia in insulin resistance in youth with type 1 and type 2 diabetes. Pediatr Diabetes. 2017;18(6):470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cree-Green M, Triolo TM, Nadeau KJ. Etiology of insulin resistance in youth with type 2 diabetes. Curr Diab Rep. 2013;13(1):81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Unger RH. Glucagon and the insulin: glucagon ratio in diabetes and other catabolic illnesses. Diabetes. 1971;20(12):834–838. [DOI] [PubMed] [Google Scholar]
- 44. Charlton MR, Nair KS. Role of hyperglucagonemia in catabolism associated with type 1 diabetes: effects on leucine metabolism and the resting metabolic rate. Diabetes. 1998;47(11):1748–1756. [DOI] [PubMed] [Google Scholar]
- 45. Carlbom L, Espes D, Lubberink M, Eriksson O, Johansson L, Jansson L, Korsgren O, Ahlström H, Carlsson PO. Pancreatic perfusion and subsequent response to glucose in healthy individuals and patients with type 1 diabetes. Diabetologia. 2016;59(9):1968–1972. [DOI] [PubMed] [Google Scholar]
- 46. Viollet B, Foretz M. Revisiting the mechanisms of metformin action in the liver. Ann Endocrinol (Paris). 2013;74(2):123–129. [DOI] [PubMed] [Google Scholar]
- 47. Natali A, Ferrannini E. Effects of metformin and thiazolidinediones on suppression of hepatic glucose production and stimulation of glucose uptake in type 2 diabetes: a systematic review. Diabetologia. 2006;49(3):434–441. [DOI] [PubMed] [Google Scholar]
- 48. RISE Consortium. Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: I. Observations using the hyperglycemic clamp. Diabetes Care. 2018;41(8):1696–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bjornstad P, Schäfer M, Truong U, Cree-Green M, Pyle L, Baumgartner A, Garcia Reyes Y, Maniatis A, Nayak S, Wadwa RP, Browne LP, Reusch JE, Nadeau KJ. Metformin improves insulin sensitivity and vascular health in youth with type 1 diabetes mellitus. Circulation. 2018;138(25):2895–2907. [DOI] [PMC free article] [PubMed] [Google Scholar]