Abstract Abstract
Four species of Haploporus, H.angustisporus, H.crassus, H.gilbertsonii and H.microsporus are described as new and H.pirongia is proposed as a new combination, based on morphological characteristics and molecular phylogenetic analyses inferred from internal transcribed spacer (ITS) and large subunit nuclear ribosomal RNA gene (nLSU) sequences. Haploporusangustisporus, H.crassus and H.microsporus occur in China, H.gilbertsonii occurs in the USA, and the distribution of H.pirongia is extended from New Zealand to Australia. Haploporusangustisporus is characterized by the distinct narrow oblong basidiospores measuring 10.5–13.5 × 3.9–5 µm. Haploporuscrassus is characterized by the presence of ventricose cystidioles occasionally with a simple septum, dissepimental hyphae usually with a simple septum, unique thick-walled basidia and distinctly wide oblong basidiospores measuring 13.5–16.5 × 7.5–9.5 µm. Haploporusgilbertsonii is characterized by its large pores (2–3 per mm), a dimitic hyphal structure with non-dextrinoid skeletal hyphae and wide oblong basidiospores measuring 12–15 × 6–8 µm. Haploporusmicrosporus is characterized by distinctly small pores (7–9 per mm), the presence of dendrohyphidia, and distinctly small ellipsoid basidiospores measuring 5.3–6.7 × 3–4.1 µm. Haploporuspirongia is proposed as a new combination. Haploporusamarus is shown to be a synonym of H.odorus and Pachykytosporawasseri is considered a synonym of H.subtrameteus.
Keywords: Polyporales , taxonomy, wood-inhabiting fungi
Introduction
Haploporus Bondartsev & Singer (Polyporales, Basidiomycota) is characterized by annual to perennial, resupinate to pileate basidiocarps, a di- to trimitic hyphal system with clamped connections on the generative hyphae, cyanophilous skeletal hyphae, cylindrical to subglobose, hyaline, thick-walled, cyanophilous and ornamented basidiospores, and formation of a white rot (Singer 1944, Dai et al. 2002, Piątek 2005, Li et al. 2007, Shen et al. 2016). Pachykytospora was shown to be, micro-morphologically, similar to Haploporus, differing only in having resupinate basidiocarps; both names were treated as synonyms (Dai et al. 2002) and consequently, all Pachykytospora species have been transferred to Haploporus (Dai et al. 2002, Piątek 2005, Shen et al. 2016), but P.major G.Y.Zheng&Z.S.Bi (add lit.), which belong to Megasporia because of its thin-walled and smooth basidiospores (Dai and Li 2002). The monophyly of Pachykytospora was confirmed later on by molecular analysis (Shen et al. 2016). So far 13 species have been accepted in Haploporus (Dai et al. 2002, Hattori et al. 2002, Piątek 2005, Li et al. 2007, Dai and Kashiwadani 2009, Shen et al. 2016).
During a study on taxonomy of Polyporaceae, several specimens of Haploporus from USA, Australia and China were studied. After morphological examinations and phylogenetic analysis of ITS and nLSU sequences, four new species were confirmed to be members of the Haploporus lineage. In this paper, we describe and illustrate these new species. In addition, Poriapirongia G. Cunn. was originally described from New Zealand (Cunningham 1947), and treated as a synonym of Poriapapyracea (Schwein.) Cooke (= Haploporuspapyraceus (Schwein.) Y.C.Dai&Niemelä (Cunningham 1965, Lowe 1966 and Buchanan and Ryvarden 1988) is shown to represent an independent species, based on new specimens and both morphology and phylogenetic evidences. Therefore, a new combination (H.pirongia) is proposed.
Materials and methods
Morphological studies
Sections were studied microscopically according to Dai (2010) at magnifications ≤1000× using a Nikon Eclipse 80i microscope with phase contrast illumination. Drawings were made with the aid of a drawing tube. Microscopic features, measurements, and drawings were made from sections stained with Cotton Blue and Melzer’s reagent. Spores were measured from sections cut from the tubes. To present spore size variation, the 5% of measurements excluded from each end of the range are given in parentheses. Basidiospore spine lengths were not included in the measurements. Abbreviations include: IKI = Melzer’s reagent, IKI– = negative in Melzer’s reagent, KOH = 5% potassium hydroxide, CB = Cotton Blue, CB+ = cyanophilous, L = mean spore length (arithmetic average of all spores), W = mean spore width (arithmetic average of all spores), Q = the L/W ratio, and n = number of spores measured / from given number of specimens. Color terms follow Petersen (1996). Herbarium abbreviations follow Thiers (2018).
Molecular study and phylogenetic analysis
A CTAB rapid plant genome extraction kit (Aidlab Biotechnologies, Beijing) was used to obtain PCR products from dried specimens, according to the manufacturer’s instructions with some modifications (Cao et al. 2012, Zhao et al. 2013). The DNA was amplified with the primers: ITS5 and ITS4 for ITS (White et al. 1990), and LR0R and LR7 (http://www.biology.duke.edu/fungi/mycolab/primers.htm) for nLSU (Vilgalys and Hester 1990). The PCR procedure for ITS was as follows: initial denaturation at 95 °C for 3 min, followed by 34 cycles at 94 °C for 40 s, 54 °C for 45 s and 72 °C for 1 min, and a final extension of 72 °C for 10 min. The PCR procedure for nLSU was as follows: initial denaturation at 94 °C for 1 min, followed by 34 cycles at 94 °C for 30 s, 50 °C for 1 min and 72 °C for 1.5 min, and a final extension of 72 °C for 10 min. The PCR products were purified and sequenced at the Beijing Genomics Institute, China with the same primers.
Phylogenetic analyses. New sequences, deposited in GenBank (http://www.ncbi.nlm.nih.gov/genbank/) (Table 1), were aligned with additional sequences retrieved from GenBank (Table 1) using BioEdit 7.0.5.3 (Hall 1999) and ClustalX 1.83 (Thompson et al. 1997). The sequence quality were checked followed Nilsson et al. (2012). Perenniporiahainaniana B.K.Cui&C.L.Zhao and P.medulla-panis (Jacq.) Donk were used as outgroups, following Shen et al. (2016). Prior to phylogenetic analysis, ambiguous regions at the start and the end of the alignment were trimmed and gaps were manually adjusted to optimize the alignment were trimmed. The edited alignment was deposited at TreeBase (http://purl.org/phylo/treebase; submission ID 24089).
Table 1.
Information on the sequences used in this study.
| Species | Sample no. | Location | GenBank accession no. | |
|---|---|---|---|---|
| ITS | nLSU | |||
| Haploporus alabamae | JV_0610_K16-Kout | Belize | KY264039 | |
| Dollinger 895 | USA | KY264038 | MK433606 | |
| JV 1704/75 | Costa Rica | MK429754 | MK433607 | |
| H. angustisporus | Cui 9046 | China | KU941862 | KU941887 |
| Dai 10951 | China | KX900634 | KX900681 | |
| H. crassus | Dai 13580 | China | FJ627252 | KU941886 |
| H. cylindrosporus | Dai 15643 | China | KU941853 | KU941877 |
| Dai 15664 | China | KU941854 | KU941878 | |
| H. gilbertsonii | JV 1209/63-J | USA | MK429755 | MK433608 |
| JV 1611/5-J | USA | MK429756 | MK433609 | |
| H. latisporus | Dai 11873 | China | KU941847 | KU941871 |
| Dai 10562 | China | KU941848 | KU941872 | |
| H. microsporus | Dai 12147 | China | KU941861 | KU941885 |
| H. nanosporus | LYAD 2044a | Gabon | KU941859 | KU941883 |
| LYAD 2044b | Gabon | KU941860 | KU941884 | |
| H. nepalensis | Dai 12937 | China | KU941855 | KU941879 |
| Cui 10729 | China | KU941856 | KU941880 | |
| H. odorus | Dai 11296 | China | KU941845 | KU941869 |
| Yuan 2365 | China | KU941846 | KU941870 | |
| H. cf. odorus | KUC20121123-29 | Republic of Korea | KJ668537 | KJ668390 |
| H. papyraceus | Dai 10778 | China | KU941839 | KU941863 |
| Cui 8706 | China | KU941840 | KU941864 | |
| KUC20130719-04 | Republic of Korea | KJ668535 | KJ668388 | |
| H. pirongia | Dai 18659 | Australia | MH631017 | MH631021 |
| Dai 18660 | Australia | MH631018 | MH631022 | |
| Dai 18661 | Australia | MH631019 | MH631023 | |
| Dai 18662 | Australia | MH631020 | MH631024 | |
| PDD 95714 | New Zealand | MK429757 | ||
| H. septatus | Dai 13581 | China | KU941843 | KU941867 |
| Cui 4100 | China | KU941844 | KU941868 | |
| H. sp. | KUC20080606-35 | Republic of Korea | KJ668534 | KJ668387 |
| H. subpapyraceus | Dai 9324 | China | KU941841 | KU941865 |
| Cui 2651 | China | KU941842 | KU941866 | |
| H. subtrameteus | Dai 4222 | China | KU941849 | KU941873 |
| Cui 10656 | China | KU941850 | KU941874 | |
| Dai11270 | China | KY264042 | ||
| H. cf. subtrameteus | KUC20121102-36 | Republic of Korea | KJ668536 | KJ668389 |
| H. thindii | Cui 9373 | China | KU941851 | KU941875 |
| Cui 9682 | China | KU941852 | KU941876 | |
| H. tuberculosus | 15559 | Sweden | KU941857 | KU941881 |
| 15560 | Austria | KU941858 | KU941882 | |
| H.tuberculosus (as Pachykytospora) | KA11 (GB) | Sweden | JX124705 | |
| JV 9610/20 | Slovakia | KY264040 | MK433610 | |
| JV 0509/19 | Czech Republic | KY264041 | MK433611 | |
| Pachykytospora wasseri | LE814872 (T) | Russia | KM411456 | KM411472 |
| Perenniporia hainaniana | Cui 6364 | China | JQ861743 | JQ861759 |
| P. medulla-panis | Cui 3274 | China | JN112792 | JN112793 |
Maximum parsimony (MP) and Bayesian inference (BI) were employed to perform phylogenetic analysis of the two aligned datasets. The two phylogenetic analysis algorithms generated nearly identical topologies for each dataset, and, thus only the topology from the MP analysis is presented along with statistical values from the MP and BI algorithms. Most parsimonious phylogenies were inferred from the ITS + nLSU, and their combinability was evaluated with the incongruence length difference (ILD) test (Farris et al. 1994) implemented in PAUP* 4.0b10 (Swofford 2002), under a heuristic search and 1000 homogeneity replicates giving a P value of 1.000, much greater than 0.01, which means there is no discrepancy among the two loci in reconstructing phylogenetic trees. Phylogenetic analysis approaches followed Zhao et al. (2015). The tree construction procedure was performed in PAUP* version 4.0b10 (Swofford 2002). All characters were equally weighted, and gaps were treated as missing data. Trees were inferred using the heuristic search option with TBR branch swapping and 1000 random sequence additions. Max-trees were set to 5000, branches of zero length were collapsed and all parsimonious trees were saved. Clade robustness was assessed using a bootstrap (BT) analysis with 1000 replicates (Felsenstein 1985). Descriptive tree statistics tree length (TL), consistency index (CI), retention index (RI), rescaled consistency index (RC), and homoplasy index (HI) were calculated for each maximum parsimonious tree (MPT) generated. jModeltest v.2.17 (Darriba et al. 2012) was used to determine the best-fit evolution model of the combined dataset for Bayesian inference (BI). The Bayesian inference (BI) was conducted with MrBayes 3.2.6 (Ronquist et al. 2012) in two independent runs, each of which had four chains for 10 million generations and started from random trees. Trees were sampled every 1000th generation. The first 25% of sampled trees were discarded as burn-in, whereas other trees were used to construct a 50 % majority consensus tree and for calculating Bayesian posterior probabilities (BPPs).
Phylogenetic trees were visualized using Treeview (Page 1996). Nodes that received Bootstrap support ≥50% and Bayesian posterior probabilities (BPP) ≥0.90 are considered as significantly supported.
Results
Molecular phylogeny
The combined ITS and 28S dataset included sequences from 46 fungal collections representing 21 species. The dataset had an aligned length of 2054 characters, of which 1399 characters are constant, 98 are variable and parsimony-uninformative, and 557 are parsimony-informative. MP analysis yielded 4 equally parsimonious trees (TL = 1370, CI = 0. 639, RI = 0.870, RC = 0.556, HI = 0.361). The best model for the combined ITS and 28S sequences dataset estimated and applied in the BI was GTR+I+G. BI resulted in a similar topology with an average standard deviation of split frequencies = 0.004515 to MP analysis, and thus only the MP tree is provided. Both BT values (≥50%) and BPPs (≥0.90) are shown at the nodes (Fig. 1). The ITS-based phylogenies included ITS sequences from 47 fungal collections representing 21 species. The dataset had an aligned length of 711 characters, of which 317 characters are constant, 54 are variable and parsimony-uninformative, and 340 are parsimony-informative. MP analysis yielded 4 equally parsimonious trees (TL = 927, CI = 0. 653, RI = 0.888, RC = 0.580, HI = 0.347). The best model for the ITS sequences dataset estimated and applied in the BI was GTR+I+G. BI resulted in a similar topology with an average standard deviation of split frequencies = 0.005040 to MP analysis, and thus only the MP tree is provided. Both BT values (≥50%) and BPPs (≥0.90) are shown at the nodes (Fig. 2).
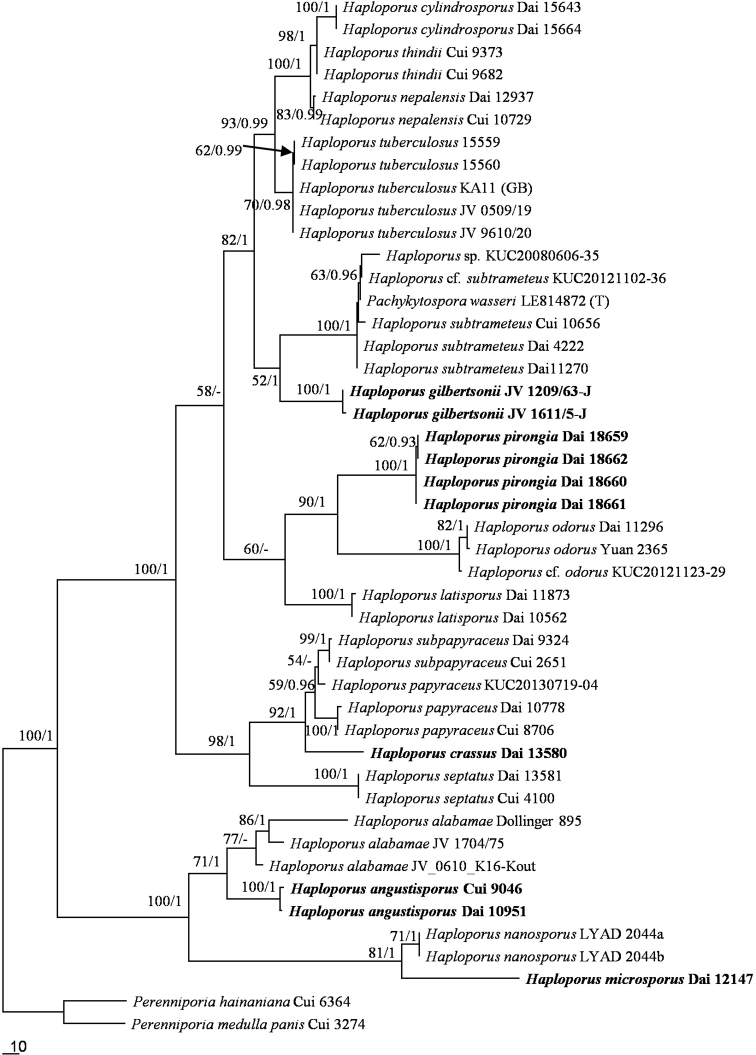
Figure 1.
Maximum parsimony strict consensus tree illustrating the phylogeny of Haploporus based on ITS+nLSU sequences. Branches are labeled with parsimony bootstrap proportions (before slanting line) greater than 50% and bayesian posterior probabilities (after slanting line) greater than 0.90.
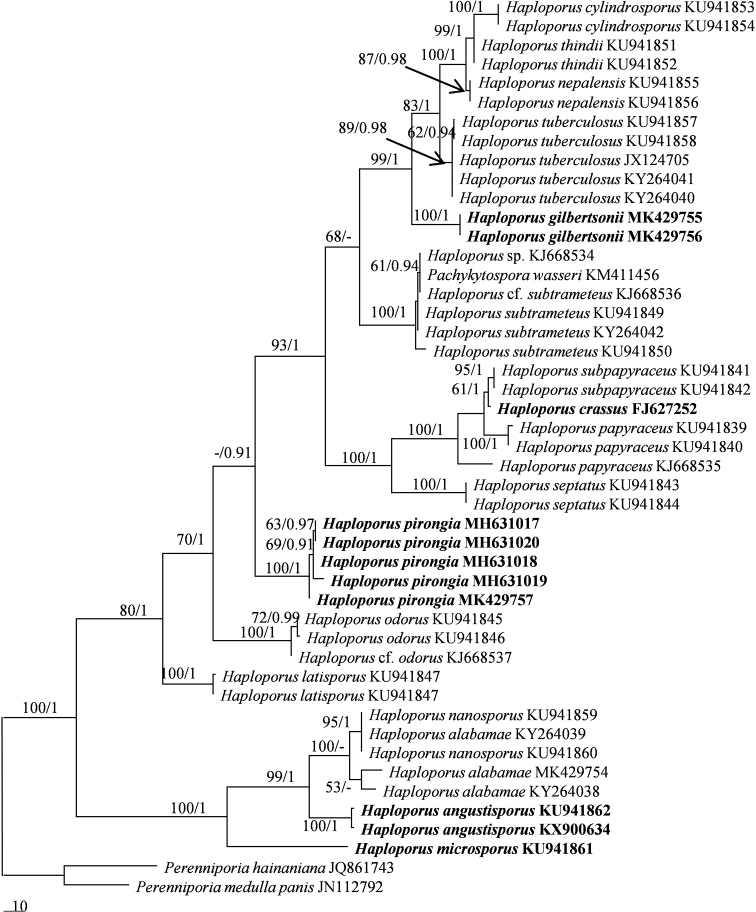
Figure 2.
Maximum parsimony strict consensus tree illustrating the phylogeny of Haploporus based on ITS sequences. Branches are labeled with parsimony bootstrap proportions (before slanting line) greater than 50% and bayesian posterior probabilities (after slanting line) greater than 0.90.
In both 28S+ITS- and ITS-based phylogenies (Figs 1–2), five new well-supported lineages were identified. Among them three well-supported terminal clades and two isolated branches (100% MP and 1.00 BI). Haploporusangustisporus is sister to H.alabamae (Berk. & Cooke) Y.C.Dai&Niemelä and this two species clade is related to H.nanosporus (A.David&Rajchenb.) Piątek, whereas H.gilbertsonii clustered with H.cylindrosporus L.L. Shen, Y.C.Dai&B.K.Cui, H.thindii (Natarajan & Koland.) Y.C.Dai, H.nepalensis (T. Hatt.) Piątek and H.tuberculosus (Fr.) Niemelä&Y.C.Dai. Four Australian specimens and a specimen of Poriapirongia from New Zealand formed a well-supported clade (100% MP and 1.00 BI), sister to the H.odorus clade. In addition, the other two lineages formed two distinct sublineages; Haploporuscrassus is closely related to H.papyraceus and H.subpapyraceus L.L.Shen, Y.C.Dai&B.K.Cui; whereas The H.nanosporus and H.microsporus clades are sister clades.
Taxonomy
Haploporus angustisporus
Meng Zhou&Y.C.Dai sp. nov.
MB829583
Figure 3.
A basidiocarp of Haploporusangustisporus (Holotype). Scale bar: 1.0 cm.
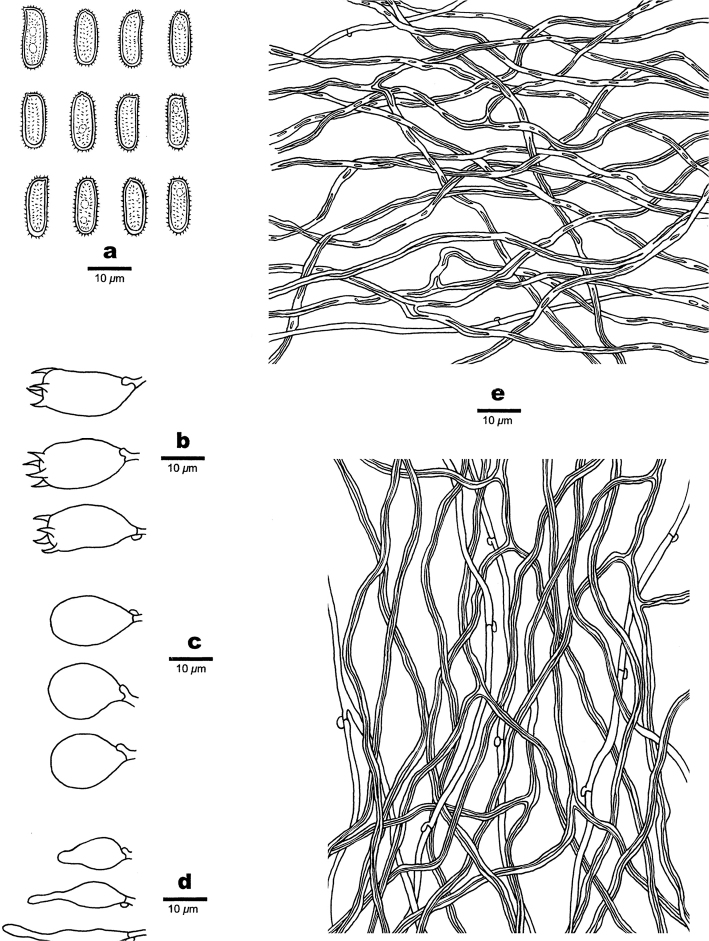
Figure 4.
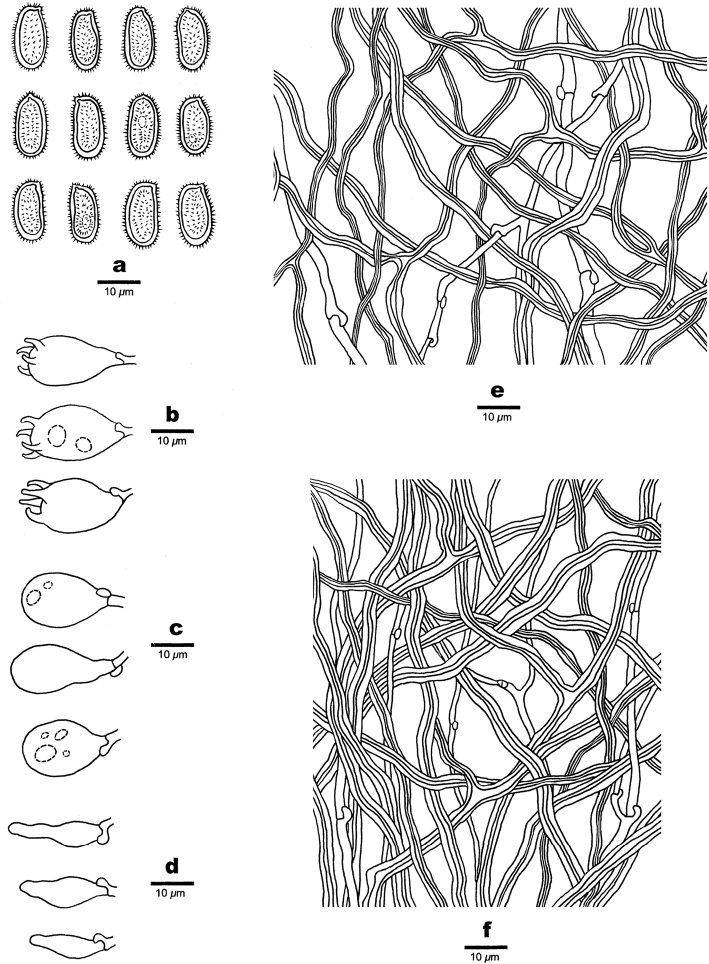
Microscopic structures of Haploporusangustisporus (Holotype). a Basidiospores b Basidia c Basidioles d Cystidioles e Hyphae from subiculum f Hyphae from trama.
Diagnosis.
Differs from other Haploporus species by the combination of its resupinate habit, a dimitic hyphal structure with dextrinoid skeletal hyphae, the absence of dendrohyphidia, and distinct narrow oblong basidiospores measuring 10–13.5 × 4–5 µm.
Holotype.
CHINA. Guangdong Prov., Lianzhou County, Nanling Nat. Res., on fallen angiosperm branch, 15 May 2009, Dai 10951 (Holotype in BJFC).
Etymology.
Angustisporus (Lat.): referring to the species having narrow basidiospores.
Fruitbody.
Basidiocarps annual, resupinate, adnate, soft corky when fresh, become corky upon drying, without odor or tasteless when fresh, up to 3 cm long, 2.5 cm wide, 2 mm thick at center. Pore surface cream to pale yellowish brown when fresh, brownish when bruised, olivaceous buff to pale brown upon drying; sterile margin indistinct, very narrow to almost lacking; pores angular, 3–5 per mm; dissepiments thick, entire. Subiculum cream, corky, thin, about 0.1 mm thick. Tubes light buff, corky, about 1.9 mm long.
Hyphal structure.
Hyphal system dimitic: generative hyphae bearing clamp connections, hyaline, thin-walled; skeletal hyphae dominant, thick-walled, frequently branched, dextrinoid, CB+, tissues unchanging in KOH.
Subiculum.
Generative hyphae infrequent, hyaline, thin-walled, rarely branched, 1.5–2.5 µm in diam; skeletal hyphae dominant, hyaline, thick-walled with a narrow lumen to subsolid, frequently branched, interwoven, 1–2.5 µm in diam.
Tubes.
Generative hyphae frequent, hyaline, thin-walled, occasionally branched, 1.5–2.5 µm in diam; skeletal hyphae distinctly thick-walled with a narrow to wide lumen, frequently branched, interwoven, 1.2–2.5 µm in diam. Cystidia absent; cystidioles present, fusiform, 23–35 × 4–7 µm. Basidioles dominant, pear-shaped to subglobose, basidia barrel-shaped with 4-sterigmata and a basal clamp connection, 21–26 × 8–11 µm; . Dendrohyphidia absent. Some irregular-shaped crystals present among tube tramal structures.
Spores.
Basidiospores oblong, hyaline, thick-walled, with short tuberculate ornamentation, IKI–, CB+, 10–13.5(–14) × (3.5–)4–5 µm, L = 11.25 µm, W = 4.44 µm, Q = 2.38–2.70 (n = 60/2).
Additional specimen examined (paratype).
CHINA. Guangdong Prov., Fengkai County, Heishiding Nat. Res., on fallen angiosperm branch, 1 July 2010, Cui 9046 (in BJFC).
Haploporus crassus
Meng Zhou&Y.C.Dai sp. nov.
MB829584
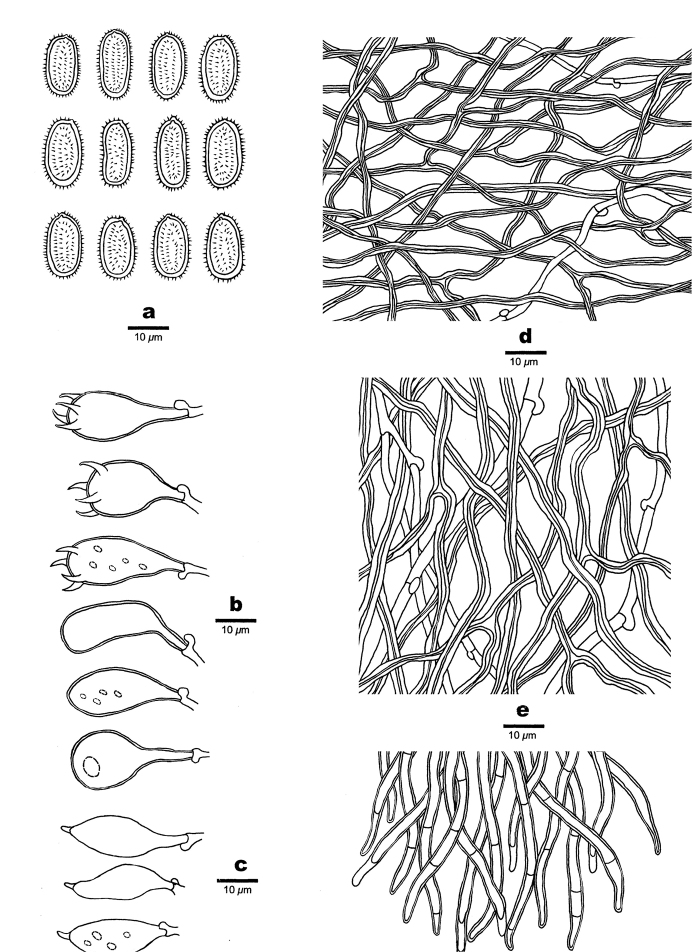
Figure 5.
Microscopic structures of Haploporuscrassus (Holotype). a Basidiospores b Basidia and Basidioles c Cystidioles d Hyphae from subiculum e Hyphae from trama f Hyphae at dissepiment.
Diagnosis.
Differs from other Haploporus species by the combination of a resupinate habit, a dimitic hyphal structure with non-dextrinoid skeletal hyphae, the presence of ventricose cystidioles occasionally with a simple septum, dissepimental hyphae usually with a simple septum, unique thick-walled basidia and distinct wide oblong basidiospores measuring 13.5–16.5 × 7.5–9.5 µm.
Holotype.
CHINA. Yunnan Prov., Xinping County, Ailaoshan Nat. Res., on rotten angiosperm wood, 15 Oct. 2013, Dai 13580 (Holotype in BJFC).
Etymology.
Crassus (Lat.): referring to the species having wide basidiospores.
Fruitbody.
Basidiocarps annual, resupinate, adnate, soft corky when fresh, become corky and cracked upon drying, without odor or taste when fresh, up to 35 cm long, 3 cm wide and 1 mm thick at center. Pore surface white to cream when fresh, becoming buff-yellow upon drying; sterile margin indistinct, very narrow to almost lacking; pores round, 3–5 per mm; dissepiments thin, mostly entire, sometimes lacerate. Subiculum cream, corky, thin, about 0.1 mm thick. Tubes light buff, corky, about 0.9 mm long.
Hyphal structure.
Hyphal system dimitic: generative hyphae bearing clamp connections, hyaline, thin-walled; skeletal hyphae dominant, thick-walled, frequently branched, IKI–, CB+, tissues unchanging in KOH.
Subiculum.
Generative hyphae infrequent hyaline, thin-walled, rarely branched, 1.5–2.5 µm in diam; skeletal hyphae dominant, hyaline, thick-walled with a narrow lumen, frequently branched, interwoven, 1–2 µm in diam.
Tubes.
Generative hyphae frequent, hyaline, thin-walled, occasionally branched, 1.5–3 µm in diam; skeletal hyphae dominant, distinctly thick-walled with a narrow to wide lumen, frequently branched, interwoven, 1.5–2.5 µm in diam; dissepimental hyphae usually with a simple septum. Cystidia absent; cystidioles present, ventricose, usually with a small umbo having a simple septum, occasionally with a few small guttules, 21–31× 8–10 µm. Basidioles thick-walled, dominant, similar in shape to basidia, but smaller; basidia thick-walled, pear-shaped to barrel-shaped with 4-sterigmata and a basal clamp connection, occasionally with some small guttules, 22–31 × 8–13 µm; dendrohyphidia absent. Some irregular-shaped crystals present among tube tramal stru ctures.
Spores.
Basidiospores oblong, hyaline, thick-walled, with tuberculate ornamentation, IKI–, CB+, 13.5–16.5(–17) × (7–)7.5–9.5 µm, L = 15.06 µm, W = 8.15 µm, Q = 1.85 (n = 30/1).
Haploporus gilbertsonii
Meng Zhou, Vlasák&Y.C.Dai sp. nov.
MB829649
Figure 6.
A basidiocarp of Haploporusgilbertsonii (Holotype). Scale bar: 1.0 cm.
Figure 7.
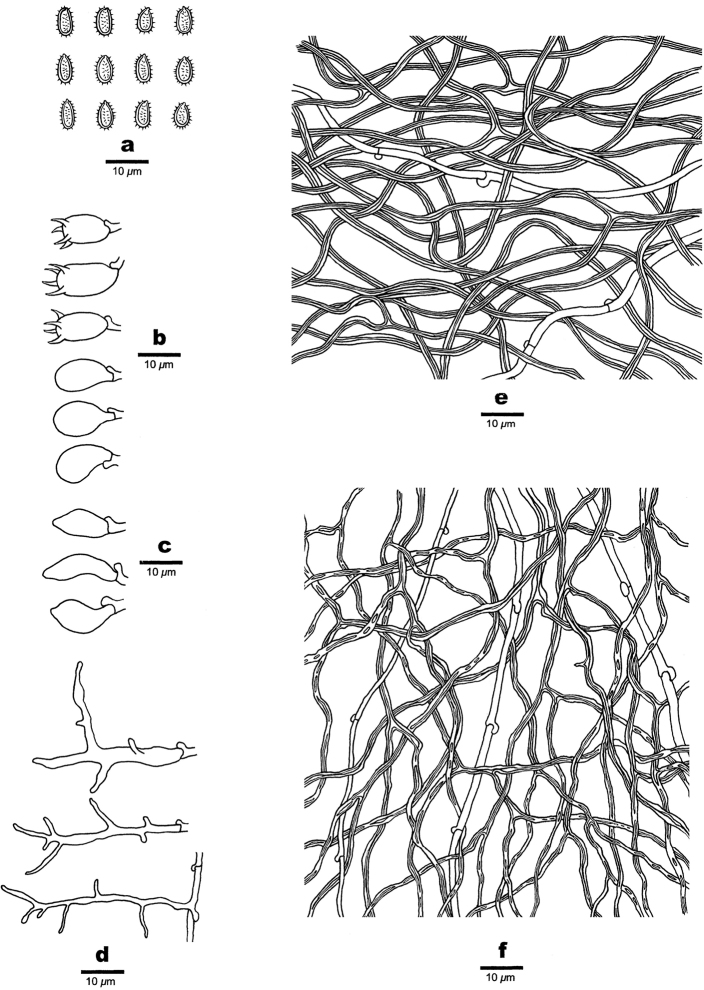
Microscopic structures of Haploporusgilbertsonii (Holotype). a Basidiospores b Basidia c Basidioles d Cystidioles e Hyphae from subiculum f Hyphae from trama.
Diagnosis.
Differs from other Haploporus species by its relatively large pores, 2–3 per mm, a dimitic hyphal structure with non-dextrinoid skeletal hyphae, the absence of dendrohyphidia, and wide oblong basidiospores measuring 12–15 × 6–8 µm.
Holotype.
USA. Arizona, Santa Rita Mt., Madera Canyon, on dead tree of Quercus, 20 Nov. 2016, Vlasák Jr. 1611/5-J (Holotype in PRM, isotype in JV and BJFC).
Etymology.
Gilbertsonii (Lat.): in honor of Prof. R.L. Gilbertson, the American mycologist.
Fruitbody.
Basidiocarps annual, resupinate, difficult to separate from the substrate, corky when dry, up to 10 cm long, 8 cm wide and 0.8 mm thick at center. Pore surface pale buff to buff when dry; sterile margin indistinct, very narrow to almost lacking; pores round to angular, 2–3 per mm; dissepiments thick, entire. Subiculum cream, corky, thin, about 0.3 mm thick. Tubes light buff, corky, about 0.5 mm long.
Hyphal structure.
Hyphal system dimitic: generative hyphae bearing clamp connections, hyaline, thin-walled; skeletal hyphae dominant, thick-walled, frequently branched, IKI–, CB–, tissues unchanging in KOH.
Subiculum.
Generative hyphae infrequent, hyaline, thin-walled, occasionally branched, 2–3 µm in diam; skeletal hyphae dominant, hyaline, distinctly thick-walled, frequently branched, interwoven, 1.5–3 µm in diam.
Tubes.
Generative hyphae infrequent, hyaline, thin-walled, occasionally branched, 1–3 µm in diam; skeletal hyphae dominant, distinctly thick-walled, frequently branched, interwoven, 2–4 µm in diam. Cystidia absent; cystidioles present, fusiform, hyaline, thin-walled, 13–23 × 4.5–6 µm. Basidia pear-shaped to barrel-shaped with 4-sterigmata and a basal clamp connection, occasionally with a few large guttules, 21–25 × 10–14 µm; basidioles dominant, similar in shape to basidia, but slightly smaller. Dendrohyphidia absent. Some irregular-shaped crystals present among tube tramal structures.
Spores.
Basidiospores oblong, hyaline, thick-walled, with tuberculate ornamentation, IKI–, CB+, 12–15(–16) × (5.5–)6–8 µm, L = 14.07 µm, W = 6.9 µm, Q = 1.83–2.15 (n = 60/2).
Additional specimen examined (paratype).
USA. Arizona, Chiricahua Mt., Turkey Canyon, on dead tree of Quercus, 5 Sep. 2012, Vlasák Jr. 1209/63-J (JV, dupl. in BJFC).
Haploporus microsporus
Meng Zhou&Y.C.Dai sp. nov.
MB829585
Figure 8.
A basidiocarp of Haploporusmicrosporus (Holotype). Scale bar: 1.0 cm.
Figure 9.
Microscopic structures of Haploporusmicrosporus (Holotype). a Basidiospores b Basidia and Basidioles c Cystidioles d Dendrohyphidia e Hyphae from subiculum f Hyphae from trama.
Diagnosis.
Differs from other Haploporus species by the combination of a resupinate habit, a dimitic hyphal structure with dextrinoid skeletal hyphae, distinct small pores, 7–9 per mm, the presence of dendrohyphidia, and distinct small ellipsoid basidiospores measuring 5.3–6.7 × 3–4.1 µm.
Holotype.
CHINA. Hainan Prov., Ledong County, Jianfengling Nat. Res., on dead angiosperm tree, 23 March 2011, Dai 12147 (Holotype in BJFC).
Etymology.
Microsporus (Lat.): referring to the small basidiospores of this species.
Fruitbody.
Basidiocarps annual, resupinate, adnate, soft corky when fresh, become corky upon drying, odor- or tasteless when fresh, up to 20 cm long, 4.5 cm wide and 2 mm thick at center. Pore surface pinkish buff to clay-buff when dry; sterile margin indistinct, very narrow to almost lacking; pores angular, 7–9 per mm; dissepiments thick, entire. Subiculum cream, corky, thin, about 0.2 mm thick. Tubes light buff, corky, about 1.8 mm long.
Hyphal structure.
Hyphal system dimitic: generative hyphae bearing clamp connections, hyaline, thin-walled; skeletal hyphae dominant, thick-walled, frequently branched, dextrinoid, CB–, skeletal hyphae swollen in KOH.
Subiculum.
Generative hyphae infrequent, hyaline, thin-walled, rarely branched, 1.5–2.5 µm in diam; skeletal hyphae dominant, hyaline, thick-walled with a narrow to wide lumen, frequently branched, interwoven, 1.5–3 µm in diam.
Tubes.
Generative hyphae infrequent, hyaline, thin-walled, rarely branched, 1.5–3 µm in diam; skeletal hyphae distinctly thick-walled with a narrow lumen to subsolid, frequently branched, interwoven, 1–2 µm in diam. Cystidioles present, fusiform, 10–20 × 3.5–6 µm. Basidia barrel-shaped with 4-sterigmata and a basal clamp connection, 11–16 × 5.5–6.5 µm; basidioles dominant, similar in shape to basidia, but slightly smaller. Dendrohyphidia abundant, frequently branched. Some irregular-shaped crystals present among tube tramal structures
Spores.
Basidiospores ellipsoid, hyaline, thick-walled, with tuberculate ornamentation, dextrinoid, CB+, 5.3–6.7(–7) × (2.9–)3–4.1 µm, L = 5.98 µm, W = 3.90 µm, Q = 1.78 (n = 30/1).
Haploporus pirongia
(G. Cunn.) Meng Zhou, Y.C.Dai&T.W. May comb. nov.
MB829650
Figure 10.
Basidiocarps of Haploporuspirongia. Scale bar: 1.0 cm.
Figure 11.
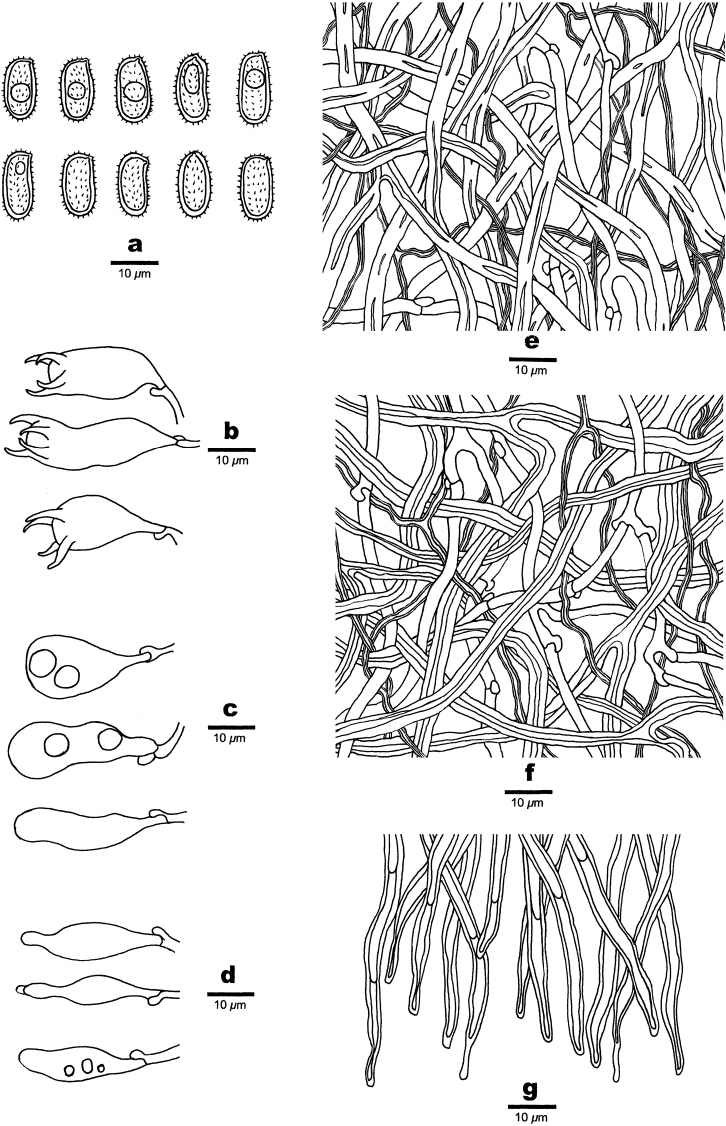
Microscopic structures of Haploporuspirongia. a Basidiospores b Basidia c Basidioles d Cystidioles e Hyphae from subiculum f Hyphae from trama g Hyphae at dissepiment.
Poria pirongia G. Cunn., Bull. N.Z. Dept. Sci. Industr. Res., Pl. Dis. Div. 72: 39 (1947) (Basionym)
Etymology.
the epithet pirongia, derived from the type locality, Mount Pirongia, is a noun in apposition, and therefore remains spelt the same when transferred from Poria to Haploporus, despite the latter genus being masculine in gender.
Fruitbody.
Basidiocarps annual, resupinate, difficult to separate from the substrate, soft corky when fresh, corky upon drying, odor- or tasteless when fresh, up to 8 cm long, 2 cm wide and 1.7 mm thick at center. Pore surface white to cream when fresh, pale brownish when bruised, pinkish buff to clay-buff upon drying; sterile margin very narrow to almost lacking; pores round to angular, 3–4 per mm; dissepiments thick, entire. Subiculum cream, corky, thin, about 0.3 mm thick. Tubes light buff, corky, about 1.4 mm long.
Hyphal structure.
Hyphal system trimitic: generative hyphae bearing clamp connections, hyaline, thin-walled, frequently branched; skeletal hyphae dominant, thick-walled to subsolid, hyaline to slightly yellowish, frequently branched; binding hyphae abundant, slightly thick-walled, IKI–, CB+, tissues unchanging in KOH.
Subiculum.
Generative hyphae frequent, hyaline, thin-walled, frequently branched, 2.3–3.5 µm in diam; skeletal hyphae dominant, hyaline, distinctly thick-walled with a narrow lumen to subsolid, occasionally branched, interwoven, 2.5–4 µm in diam; binding hyphae abundant, slightly thick-walled,1–2 µm in diam.
Tubes.
Generative hyphae frequent, hyaline, thin-walled, frequently branched, 1.7–3.5 µm in diam; skeletal hyphae distinctly thick-walled with a narrow to wide lumen, frequently branched, interwoven, 2.5–4 µm in diam; binding hyphae slightly thick-walled,1–2.5 µm in diam. Cystidia absent; cystidioles present, fusiform, occasionally with an apical simple septum, sometimes with a few small guttules, 21–28 × 5–7 µm. Basidioles dominant, similar in shape to basidia, but slightly smaller, occasionally with a few large guttules; basidia pear-shaped to barrel-shaped with 4-sterigmata and a basal clamp connection, 21–35 × 8–11 µm. Hyphae at dissepiment usually thick-walled with simple septum. Dendrohyphidia absent. Some irregular-shaped crystals present among tube tramal structures.
Spores.
Basidiospores oblong-ellipsoid to cylindrical, hyaline, thick-walled, with tuberculate ornamentations, some with a guttule, IKI–, CB+, 11–14(–15) × (4.8–)5.2–7 µm, L = 12.35 µm, W = 6.11 µm, Q = 1.83–2.15 (n = 90/3).
Specimens examined.
AUSTRALIA. Victoria, Melbourne, Dandenong Ranges Botanical Garden, on dead branch of Rhododendron, 12 May 2018, Dai 18659, 18660 & 18661 (MEL, dupl. in BJFC); on dead branch of Eucalyptus, 12 May 2018, Dai 18662 (MEL, dupl. in BJFC). NEW ZEALAND. Omahu Bush, on Melicytus, 15 Feb 2010, Cooper (PDD 95714, dupl. in BJFC).
Haploporus odorus
(Sommerf.) Bondartsev & Singer in Singer, Mycologia 36: 68 (1944)
=Haploporusamarus X.L. Zeng & Y.P. Bai, Acta Mycol. Sin. 12(1): 13 (1993). Holotype: China, Jilin Province, Northeast Normal University, Changchun, NENU, Zeng 1931.
Notes.
Haploporusamarus was described from NE China (Zeng and Bai 1993). The type was studied, and its morphology is in agreement with that of H.odorus.
Haploporus subtrameteus
(Pilát) Y.C.Dai&Niemelä, in Dai, Niemelä and Kinnunen, Ann. bot. fenn. 39(3): 181 (2002)
=Pachykytosporawasseri Zmitr., Malysheva & Spirin, Ukrainskiy Botanichnyi Zhurnal 64(1): 42 (2007) Holotypus: Russia, Samara Reg., Stavropol Dist., Zhiguli Nat. Res., Padus avium, 12.09.2006, V.F. Malysheva, E.F. Malysheva, I.V. Zmitrovich, isotypus, LE 214872.
Notes.
In our phylogenies (Figs 1 and 2), P.wasseri (Zmitrovich et al. 2007) nested within H.subtrameteus clade. In addition, there are not major morphological differences between the two taxa (Zmitrovich et al. 2007).
Discussion
In the ITS-based phylogeny (Fig. 2), Haploporusangustisporus is closely related to H.alabamae and H.nanosporus. Morphologically, Haploporusangustisporus may be confused with H.alabamae in having approximately the same basidiospores size (9.5–12.5 × 4–5.5 µm vs. 10–13.5 × 4–5 µm) but H.alabamae has a trimitic hyphal system and lacks cystidioles (Gilbertson and Ryvarden 1986–1987). Haploporusnanosporus differs from H.angustisporus by its smaller pores (9–12 per mm vs. 3–5 per mm), non-dextrinoid skeletal hyphae, and smaller basidiospores (5–6 × 3–4 µm vs. 10–13.5 × 4–5 µm, Piątek 2005).
Haploporusgilbertsonii is closely related to H.cylindrosporus, H.thindii, H.nepalensis and H.tuberculosus. However, Haploporusthindii differs from H.gilbertsonii by its distinctly slimmer basidia (20–37 × 6.5–9.1 µm vs. 21–25 × 10–14 µm) and the absence of cystidioles (Yu et al. 2005). Haploporusnepalensis is distinguished by its smaller basidiospores (5.5–11.5 × 4.5–6.5 µm vs. 12–15 × 6–8 µm) and the absence of cystidioles (Piątek 2003). Whereas Haploporustuberculosus is distinguished from H.gilbertsonii by its trimitic hyphal system and longer basidia (30–43 × 11–13.5 µm vs. 21–25 × 10–14 µm, Ryvarden and Gilbertson 1994).
The Haploporusnanosporus and H.microsporus clades are sister clades and Haploporusnanosporus is closely related to H.alabamae and H.angustisporus. Haploporus and H.nanosporus both have small basidiospores and occurs in tropical ecosystems,and all other differing in having larger basidiospores. However, H.nanosporus differs from H.microsporus by the absence of dendrohyphidia at the dissepiments, a trimitic hyphal system and absence of cystidioles (Piątek 2005). In addition, Haploporusalabamae differs from H.microsporus through a trimitic hyphal system and absence of cystidioles (Gilbertson and Ryvarden 1986–1987). Haploporusangustisporus differs from H.microsporus by its longer basidiospores (10–13.5 × 4–5 µm vs. 5.3–6.7 × 3–4.1 µm).
In the ITS-LSU based phylogeny (Fig. 1), Haploporuscrassus is closely related to H.papyraceus and H.subpapyraceus. However, morphologically Haploporuspapyraceus differs from H.crassus by the presence of dendrohyphidia at the dissepiments, absence of cystidioles and thin-walled basidioles (Ryvarden and Johansen 1980). Haploporussubpapyraceus also differs from H.crassus in having dextrinoid skeletal hyphae and thin-walled basidioles (Shen et al. 2016).
Haploporuspirongia is related to H.odorus, but the latter has a perennial and pileate basidiocarp with strong anise odor, ovoid basidiospores and lacks cystidioles (Niemelä 1971). Haploporuspirongia resembles H.thindii and H.subpapyraceus by sharing resupinate basidiocarps with approximately the same pore size. However, Haploporusthindii has a dimitic hyphal structure, lacks cystidioles, and has a distribution in subtropical India and valley of Tibet of China (Natarajan and Kolandavelu 1993, Dai et al. 2007). Moreover, H.subpapyraceus has ellipsoid basidiospores (9–12 × 5.5–8 μm, Shen et al. 2016).
Gilbertson and Ryvarden (1987) reported Haploporustuberculosus (as Pachykytosporatuberculosa) from the USA, but only in a small region of southern Arizona where it should be “quite common on oaks, especially in Chiricahua Mountains”. Locally, we have collected in this region only H.gilbertsonii and believe that, in most cases, this species was mistaken for H.tuberculosus in Arizona. The presence of H.tuberculosus in America is questionable.
Supplementary Material
Acknowledgements
The research is supported by the National Natural Science Foundation of China (Project No. U1802231). We thank the curator of PDD for making material available on loan, and Shaun Pennycook for advice on the spelling of epithets.
Citation
Zhou M, Wang L, May TW, Vlasák J, Chen J-J, Dai Y-C (2019) Phylogeny and diversity of Haploporus (Polyporaceae, Basidiomycota). MycoKeys 54: 77–98. https://doi.org/10.3897/mycokeys.54.34362
References
- Buchanan PK, Ryvarden L. (1988) Type studies in the Polyporaceae 18. Species described by GH Cunningham. Mycotaxon 33: 1–38. [Google Scholar]
- Cao Y, Wu SH, Dai YC. (2012) Species clarification of the prize medicinal Ganoderma mushroom “Lingzhi”. Fungal Diversity 56: 49–62. 10.1007/s13225-012-0178-5 [DOI] [Google Scholar]
- Cunningham GH. (1947) New Zealand Polyporaceae 1. The genus Poria. Bulletin of the New Zealand Department of Industrial Research 72: 1–43. [Google Scholar]
- Cunningham GH. (1965) Polyporaceae of New Zealand. Bulletin of the New Zealand Department Scientific and Industrial Research 64: 1–304. [Google Scholar]
- Dai YC. (2010) Hymenochaetaceae (Basidiomycota) in China. Fungal Diversity 45: 131–343. 10.1007/s13225-010-0066-9 [DOI] [Google Scholar]
- Dai YC, Cui BK, Liu XY. (2010) Bondarzewiapodocarpi, a new and remarkable polypore from tropical China. Mycologia 102: 881–886. 10.3852/09-050 [DOI] [PubMed] [Google Scholar]
- Dai YC, Kashiwadani H. (2009) Haploporussubtrameteus (Polyporaceae, Basidiomycota) found in Japan. Mycoscience 50: 452–454. 10.1007/s10267-009-0498-9 [DOI] [Google Scholar]
- Dai YC, Li TH. (2002) Megasporoporiamajor (Basidiomycota), a new combination. Mycosystema 21: 519–521. [Google Scholar]
- Dai YC, Niemelä T, Kinnunen J. (2002) The polypore genera Abundisporus and Perenniporia (Basidiomycota) in China, with notes on Haploporus. Annales Botanicci Fennici 39: 169–182. [Google Scholar]
- Dai YC, Yu CJ, Wang HC. (2007) Polypores from eastern Xizang (Tibet), western China. Annales Botanicci Fennici 44: 135–145. [Google Scholar]
- Darriba D, Taboada GL, Doallo R, Posada D. (2012) jModelTest 2: more models, new heuristics and parallel computing. Nature Methods 9: 772. 10.1038/nmeth.2109 [DOI] [PMC free article] [PubMed]
- Donk MA. (1967) Notes on European polypores 2. Persoonia 5: 47–130. [PubMed] [Google Scholar]
- Farris JS, Mari Källersjö, Kluge AG, Bult C. (1994) Testing significance of incongruence. Cladistics 10: 315–319. 10.1111/j.1096-0031.1994.tb00181.x [DOI] [Google Scholar]
- Felsenstein J. (1985) Confidence intervals on phylogenetics: An approach using the bootstrap. Evolution 39: 783–791. 10.1111/j.1558-5646.1985.tb00420.x [DOI] [PubMed] [Google Scholar]
- Gilbertson RL, Ryvarden L. (1986–1987) North American polypores 1–2. Fungiflora, Oslo, 1–885.
- Hall TA. (1999) Bioedit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98. [Google Scholar]
- Hattori T, Adhikari MK, Suda T, Doi Y. (2002) A list of polypores (Basidiomycotina, Aphyllophorales) collected in Jumla. Nepal. Bulletin of the National Science Museum 28: 27–38. [Google Scholar]
- Li J, Dai YC, Yuan HS. (2007) A new species of Haploporus (Basidiomycotina) from China. Mycotaxon 99: 181–187. [Google Scholar]
- Lowe JL. (1966) Polyporaceae of North America. The genus Poria. Technical Bulletin of State University College, Syracuse University Forestry 90: 1–183. [Google Scholar]
- Niemelä T. (1971) On Fennoscandian polypores 1. Haploporusodorus (Sommerf.) Bond. & Sing. Annales Botanicci Fennici 8: 237–244. [Google Scholar]
- Natarajan K, Kolandavelu K. (1993) A new species of Pachykytospora Kotl. et Pouz. from india. Cryptogamic Botany 3: 195–196. [Google Scholar]
- Nilsson RH, Tedersoo L, Abarenkov K, Ryberg M, Kristiansson E, Hartmann M, Schoch CL, Nylander JAA, Bergsten J, Porter TM, Jumpponen A, Vaishampayan P, Ovaskainen O, Hallenberg N, Bengtsson-Palme J, Eriksson KM, Larsson KH, Larsson E, Kõljalg U. (2012) Five simple guidelines for establishing basic authenticity and reliability of newly generated fungal ITS sequences. MycoKeys 4: 37–63. 10.3897/mycokeys.4.3606 [DOI] [Google Scholar]
- Page RDM. (1996) TreeView: Application to display phylogenetic trees on personal computers. Computer Applications in the Biosciences Cabios 12: 357–358. [DOI] [PubMed] [Google Scholar]
- Petersen JH. (1996) Farvekort. The Danish Mycological Society´s colour-chart. Foreningen til Svampekundskabens Fremme, Greve, 1–6.
- Piątek M. (2003) Haploporustuberculosus, a new polypore genus and species in Belarus, with a new combination in Haploporus. Polish Botanical Journal 48: 81–83. [Google Scholar]
- Piątek M. (2005) Taxonomic position and world distribution of Pachykytosporananospora (Polyporaceae). Annales Botanicci Fennici 42: 23–25. [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Hőhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. (2012) MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryvarden L, Gilbertson RL. (1994) European polypores. Part 2. Synopsis Fungorum 7: 394–743. [Google Scholar]
- Ryvarden L, Johansen I. (1980) A preliminary polypore flora of East Africa. Fungifora. Oslo, 1–636.
- Singer R. (1944) Notes on taxonomy and nomenclature of the polypores. Mycologia 36: 65–69. 10.2307/3754880 [DOI] [Google Scholar]
- Shen LL, Chen JJ, Wang M, Cui BK. (2016) Taxonomy and multi-gene phylogeny of Haploporus (Polyporales, Basidiomycota) Mycological Progress 15: 731–742. 10.1007/s11557-016-1203-y [DOI]
- Swofford DL. (2002) PAUP*: Phylogenetic analysis using parsimony (*and other methods). Sinauer Associates, Massachusetts.
- Thiers B. (2018) Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium, New York. http://sweetgum.nybg.org/science/ih/
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. (1997) The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 25: 4876–4882. 10.1093/nar/25.24.4876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilgalys R, Hester M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. 10.1128/jb.172.8.4238-4246.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gefand DH, Sninsky JJ, White JT. (Eds) .Academic Press, San Diego, 315–322. 10.1016/B978-0-12-372180-8.50042-1 [DOI]
- Yu CJ, Zuo L, Dai YC. (2005) Three polypores from Xizang new to China. Fungal Science 20: 61–68. [Google Scholar]
- Zeng XL, Bai YP. (1993) The genus Haploporus in China. Mycosystema 12: 12–15. [Google Scholar]
- Zhao CL, Chen H, Song J, Cui BK. (2015) Phylogeny and taxonomy of the genus Abundisporus (Polyporales, Basidiomycota). Mycological Progress 14: 38. 10.1007/s11557-015-1062-y [DOI]
- Zhao CL, Cui BK, Dai YC. (2013) New species and phylogeny of Perenniporia based on morphological and molecular characters. Fungal Diversity 58: 47–60. 10.1007/s13225-012-0177-6 [DOI] [Google Scholar]
- Zmitrovich IV, Malysheva VF, Spirin WA. (2007) A new Pachykytospora species (Basidiomycota, Polyporales) from Zhiguli, European Russia. Ukrayins’kyi Botanichnyi Zhurnal 64: 42–46. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.