Abstract
Degenerative disc disease (DDD) is the major cause of low back pain, which usually leads to work absenteeism, medical visits and hospitalization. Because the current conservative procedures and surgical approaches to treatment of DDD only aim to relieve the symptoms of disease but not to regenerate the diseased disc, their long‐term efficiency is limited. With the rapid developments in medical science, tissue engineering techniques have progressed markedly in recent years, providing a novel regenerative strategy for managing intervertebral disc disease. However, there are as yet no ideal methods for constructing tissue‐engineered intervertebral discs. This paper reviews published reports pertaining to intervertebral disc tissue engineering and summarizes data concerning the seed cells and scaffold materials for tissue‐engineered intervertebral discs, construction of tissue‐engineered whole intervertebral discs, relevant animal experiments and effects of mechanics on the construction of tissue‐engineered intervertebral disc and outlines the existing problems and future directions. Although the perfect regenerative strategy for treating DDD has not yet been developed, great progress has been achieved in the construction of tissue‐engineered intervertebral discs. It is believed that ongoing research on intervertebral disc tissue engineering will result in revolutionary progress in the treatment of DDD.
Keywords: Intervertebral disc, Progress, Tissue engineering
Introduction
Intervertebral discs (IVDs) are cartilaginous structures located between two vertebral bodies that can resist compression and support body weight, allow some movement in the vertebral trunk and join the vertebral bodies.1 Degenerative disc disease (DDD) develops gradually with age and is one of the commonest diseases to present clinically. DDD has been linked with aging and is thought to be caused by a number of factors, including excess mechanical loading, biological factors, smoking and changes in cell nutrition. In addition to the direct costs of medical treatment, DDD also causes great economic losses in work and life because of pain and loss of mobility.2, 3, 4 Surgical treatment for DDD, such as discectomy, fusion, artificial disc replacement (nucleus replacement or allogeneic disc transplantation), has been reported in recent years; however, the long‐term results are still not satisfactory.5, 6, 7, 8 An ideal treatment that can permanently repair the diseased intervertebral disc is needed.
In recent years, tissue engineering techniques have emerged as an ideal means of permanently repairing tissue defects. Significant achievements that have been applied in clinic have been made in seed cells, scaffolds and signal factors, especially for tissue‐engineered skin, heart valves and cartilage.9, 10, 11, 12 Tissue engineering techniques also provide a novel regenerative strategy for constructing functional discs to treat disc disease.13, 14, 15, 16 Recent studies have focused mainly on seed cells, scaffolds and construction of tissue‐engineered disc and achieved some gains. In this paper, we review the construction strategies and problems in intervertebral disc tissue engineering and aim to provide information to support the construction of tissue‐engineered intervertebral disc.
Materials and Methods
Using the PubMed database, we conducted an electronic search of articles about intervertebral disc tissue engineering published in the English language within the last 20 years. We used the search terms “tissue engineering” and “intervertebral disc” as simple text searches and identified various types of article, including research and reviews. We included articles involving seed cells, scaffolds and mechanics of the annulus fibrosus (AF), nucleus pulposus (NP) and intervertebral disc tissue engineering. Duplicate reports and research with insufficient data were excluded for the purposes of this review. We classified abstracts as definitely include, unsure or definitely exclude and reassessed these classifications once the full text of the article was available.
Scaffolds for Intervertebral Disc Tissue Engineering
The intervertebral disc consists of the AF and NP (Fig. 1). The AF encircles the NP and is highly organized and oriented in concentric rings composed if collagen fibers that form lamellar layers. The collagen fibers enable the disc to return to its original position after a load charge. The AF are firmly attached to the endplates and inserted into the anterior and posterior longitudinal ligaments, being strongly attached to the anterior longitudinal ligament, whereas their attachment to the posterior ligament is weaker. This fact may explain why posterior protrusions of the disc occur more frequently than anterior bulging. The AF can be divided into inner and outer layers, the outer layer being mainly composed of type I collagen, whereas the inner AF is mainly type II collagen. From outer to inner AF, the content of water and proteoglycans increases, whereas the content of type I collagen gradually decreases.17 The NP, which is located in the central region of the IVD and has a jelly‐like structure, is rich in proteoglycan, type II collagen and water.18, 19 The content of type I collagen decreases from the AF to the central NP, whereas the content of type II collagen increases.20 IVD degeneration is thought to originate in the NP, where there is a loss of normal matrix, increased matrix metalloproteinase (1, 3, 7, 9, 10 and 13), and A disintegrin and metalloproteinase with thrombospondin motifs (1, 4, 5, 9 and 15) activity being responsible for matrix catabolism. There is also a shift from type II to type I collagen expression by NP cells and a decrease in aggrecan synthesis, leading to dehydration of the matrix of the NP. Dehydration leads to a loss of the swelling pressures that are responsible for maintaining mechanical integrity, ultimately leading to local spinal instability and mechanical trauma. In parallel, the diminished amounts of aggrecan and increased amounts of catabolic cytokine allow the in‐growth of neurites, resulting in pain. The structure of the IVD is complex and extremely heterogeneous. The NP and AF consist of different cells and extracellular matrix (ECM) components, making it difficult to construct tissue‐engineered intervertebral discs.
Figure 1.
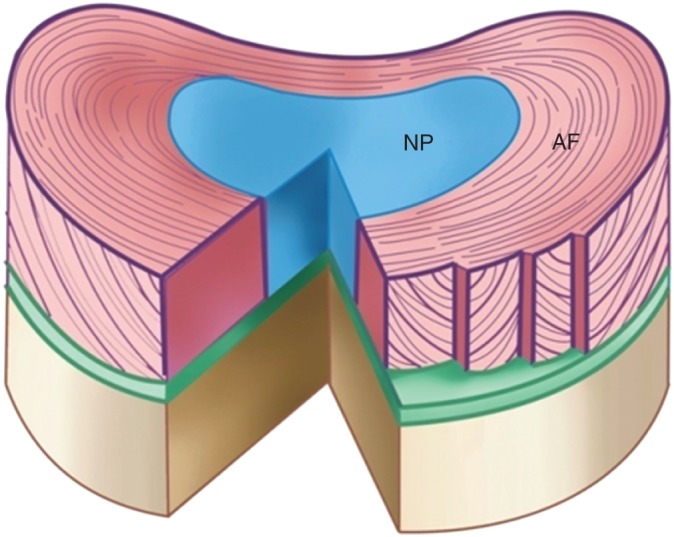
Schematic diagram of an intervertebral disc. The intervertebral disc comprises the AF, which encircles the NP and is highly organized and oriented in concentric rings and the NP, which is located in the central region of the IVD and has a jelly‐like structure.
Scaffold is a critical factor in the construction of engineered disc tissue. It must act as a temporary 3‐D matrix for supporting cellular functions. Its ultimate purpose is to provide a support with the appropriate biological and mechanical stimuli for live cells to regenerate, restore disc height and, therefore, mimic the anatomical functions of the IVD.1 Thus, the ideal disc scaffold should possess good biocompatibility, proper pores and an appropriate rate of degradation. Additionally, the scaffold should be similar to the disc ECM in component, shape, structure and mechanical properties. As we all know, the disc possesses an avascular sealed environment.21 The diffusion of nutrients and signal factors should be taken into account during scaffold design.
Scaffold of NP Tissue Engineering
Loss of natural NP materials causes collapse of the disc space because the inner region cannot fully resist applied loads. NP tissue engineering aims to replace the lost materials so that the IVD can adequately sustain pressure.22 Most implantable scaffolds aim to mimic the mechanical and biochemical properties of the native NP. Scaffolds previously used for NP tissue engineering include natural materials (such as alginate, agarose, hyaluronic acid and silk) and chemical synthetic polymers (such as polylactic acid and polyglycolic acid copolymer)23 (Fig. 2). These scaffolds have deficiencies in biocompatibility, biomechanical properties and degradation rates. Recently, materials containing collagen and proteoglycan, which are the main ECM components of intervertebral discs, have been widely studied. Alini et al. constructed tissue‐engineered discs with composite scaffolds that were fabricated with type I collagen and hyaluronic acid and seeded with NP and AF cells, respectively.24 The cells in the scaffold can secrete proteoglycan and types I and II collagen. Huang et al. fabricated composite scaffold with type II collagen, hyaluronic acid and 6‐chondroitin sulfate.25 Rabbit NP cells were seeded into such scaffolds and implanted into allogenic rabbits to repair intervertebral disc degeneration. The disc heights were maintained and the signals on MRI T2‐weighted images found to have been restored 24 weeks later. Rowland et al. found that cartilage‐derived matrix scaffolds contract during in vitro culture, which unpredictably alters their shape.26 They analyzed the effects of dehydrothermal treatment, UV light irradiation and the chemical crosslinker carbodiimide on scaffold contraction and found that both physical and chemical crosslinking treatments can prevent cell‐mediated contraction of cartilage‐derived matrix scaffolds, resulting in retention of the original scaffold dimensions. Additionally, crosslinking treatments influence chondrogenic differentiation. Dehydrothermal and UV treatments produced significantly greater glycosaminoglycans (GAGs) and collagen content than did carbodiimide crosslinked and non‐crosslinked constructs. Mercuri et al. innovatively made NP scaffolds with acellular porcine NP matrix by decellularization by chemical wash, sonication and nucleic acidase digestion.27 The antigen α‐Gal was removed from the acellular NP matrix, which possesses components similar to the ECM of natural NP (including aggrecan, 6‐chondroitin sulfate and collagen II, IX and XI), with a good expansion rate, appropriate biomechanical properties and good biocompatibility. An acellular NP matrix would be an appropriate biological material for NP tissue engineering. Further study should be concentrated on seeding cells into scaffolds and constructing tissue‐engineered NP in vitro and in vivo.
Figure 2.
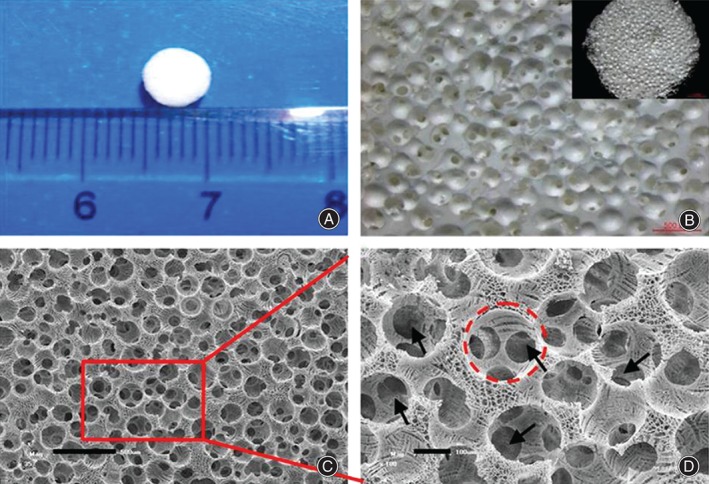
NP scaffold made of silk fibroin through paraffin‐sphere‐leaching methods and freeze‐drying. (A) Optical stereomicroscopy images of scaffold, (B) optical microscopy images of scaffold, (C) representative scanning electron microscope (SEM) images of cross‐sectional morphology of scaffold; the scale is 500 μm; and (D) representative SEM images of cross‐sectional morphology of scaffold; the scale is 100 μm. This is a magnified view of the area surrounded by a red line in (C) and shows interconnections between the macropores (black arrows).
Scaffolds for AF Tissue Engineering
Natural AF has a unique angle‐ply laminated structure (Fig. 1), the outer layer being rich in type I collagen and the inner layer in proteoglycans and type II collagen. The AF performs many roles, including resisting fluid flow, which helps in pressurizing the NP and directly bear created by torsion and bending; these are disrupted when the AF is torn or punctured. In view of the native structure of AF tissue, restoration of the function and properties of the AF requires the use of biomaterials that are easy to process and have good mechanical properties.5 In recent years, scaffolds used for tissue engineered AF have included silk protein, an alginate/chitosan composite material, demineralized bone matrix and synthetic polymer materials (such as poly‐ɛ‐caprolactone [PCL]).28, 29, 30
Scaffolds can be divided into three categories as follows:
Single‐phase scaffolds with or without fiber orientation structure (Fig. 3). Porous silk scaffolds, which encourage AF cell attachment and collagen type synthesis, have been investigated.31 Modification of porous silk scaffolds with arginine‐glycine‐aspartic acid peptide encourages cell growth, as well as collagen and GAG synthesis.32 Combinations of silk and fibrin have been used to create ordered scaffolds, which increase GAG, collagen content, and compressive modulus when seeded with cells over 4 weeks.33 Using an electro‐spinning technique, Nerurkar et al. constructed anisotropic multilayered scaffolds with PCL, which was seeded with mesenchymal stem cells (MSCs) and cultured for 10 weeks in vitro.34 ECM rich in collagen was found to have accumulated in the scaffold. This cell scaffold construct is similar to the natural annulus in its multi‐layer structure and mechanical properties. Additionally, alginate/chitosan can be wet‐spun and freeze‐dried to create fibrous scaffolds that support AF cell growth.29 Collagen–fibrin gels made from demineralized bone matrix and seeded with AF cells show increased biochemical synthesis and shape fidelity.35
Scaffolds with biphasic materials that can mimic the inner and outer structure of natural AF. Wan et al. fabricated biphasic AF scaffolds with poly (polycaprolactone triol malate) and demineralized bone matrix gelatin (PPCLM/BMG).36 The outer layer (BMG) is mainly composed of type I collagen, mimicking the outer layer of AF. The inner layer is concentric circular PPCLM seeded with rabbit chondrocytes, mimicking the inner structure of the AF. The tensile stress of the cell‐scaffold construct is reportedly 3.37 MPa, which is 50‐fold that of PPCLM material and similar to the mechanical properties of the rabbit normal AF.
Acellular AF matrix. Xu et al. compared the effects of Triton X‐100, sodium dodecyl sulfate and trypsin on natural porcine AF37 and found that Triton X‐100 group completely removes the cells, preserves the structure well, retains an orderly arrangement of collagen and preserves the biomechanical properties. Because acellular AF matrix can mimic angle‐ply laminated structures and retain the main ECM component of natural AF, it may be the ideal candidate for scaffolds for AF tissue engineering. However, acellular AF matrix is compactly arranged with insufficient porosity: seeding cells evenly into the matrix and ensuring they are supplied with sufficient nutrients in vitro and in vivo are the main problems that need to be overcome by further research.
Figure 3.
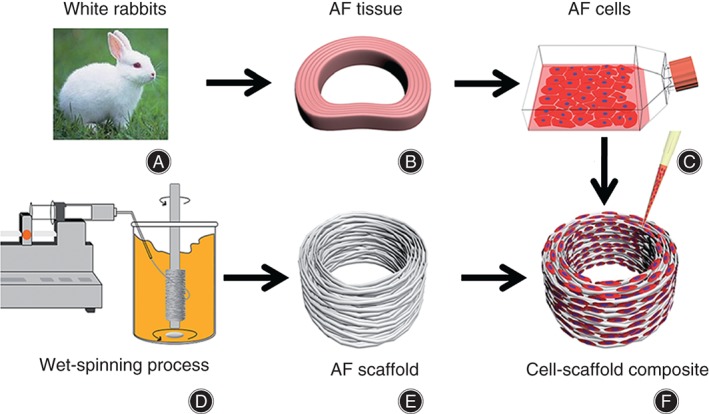
Schematic illustration of fabrication of scaffold and AF cells‐scaffold composite. (A) Material obtained from New Zealand white rabbits is used to (B) separate AF tissue followed by (C) digesting to obtain primary AF cells. (D) Wet‐spun samples are cut to yield (E) oriented micro‐fibrous 3D scaffolds of 6 mm outer diameter, 3 mm inner diameter and 2 mm thickness. (F) AF cells‐scaffold composite are formed by seeding AF cells onto an AF scaffold.
Scaffolds for Whole IVD Tissue Engineering
The focus of previous studies has been concentrated on fabricating AF or NP scaffolds separately; however, separate scaffolds cannot mimic the structure and mechanical properties of discs. There has been little research on scaffolds for integrated whole IVDs. The methods for constructing whole IVD can be divided into the following three categories:
Integrated biphasic AF‐NP scaffolds (Fig. 4). Wu et al. fabricated integrated biphasic AF–NP scaffolds from BMG and acellular cartilage matrix, achieving appropriately sized pore and close junctions between the AF and NP.38 Additionally, these scaffolds are similar to IVD in structure, biochemical components and biomechanical properties with good biocompatibility. Choy et al. fabricated biphasic scaffolds from collagen and GAGs, two of the most abundant extracellular matrix components in the IVD.14 These biphasic scaffolds are composed of a collagen‐GAG co‐precipitate making up the NP‐like core and this is encapsulated in multiple lamellae of photochemically crosslinked collagen membranes, which comprise the AF‐like lamellae. On mechanical testing, the heights of engineered discs recovered by 82%–89% in an annulus‐independent manner, compared with a recovery rate of 99% for native discs. Biphasic scaffolds comprised of 10 AF‐like lamellae had the best overall mechanical performance because of their similarity in most respects to native discs, including elastic compliance during creep and recovery and viscous compliance during recovery.
AF and NP scaffolds have been prepared separately, seeded with cells and assembled into composite AF‐NP constructs. Nesti et al. seeded induced bone marrow‐derived mesenchymal stem cells into poly‐L‐lactic acid (PLLA) electrospun scaffolds and hyaluronic acid gel separately and combined them to fabricate composite constructs that are similar to autologous IVDs (outer layer similar to the AF, inner layer similar to the NP).39 Similarly, embedding of an agarose NP into an electrospun PCL AF significantly enhances the mechanical performance of constructs over that of agarose gels alone.40 The electrospinning technique can recreate the lamellar structure of the native AF, which not only enhances the mechanical properties of composite constructs but also guides the formation of oriented extracellular matrix by MSCs to mimic the organization of the native AF.41
Scaffolds made of acellular natural IVD. Chan et al. made IVD scaffolds containing an end plate structure with acellular bovine IVDs.42 They reported that up to 70% of the cells could be removed by adjusting the chemistry and physics decellularization variables. These relatively acellular discs retain GAG content, the structure of collagen fibers and biomechanical properties. NP cells implanted into acellular IVD scaffolds survive more than 7 days, demonstrating that these scaffolds have good cell permeability.
Figure 4.
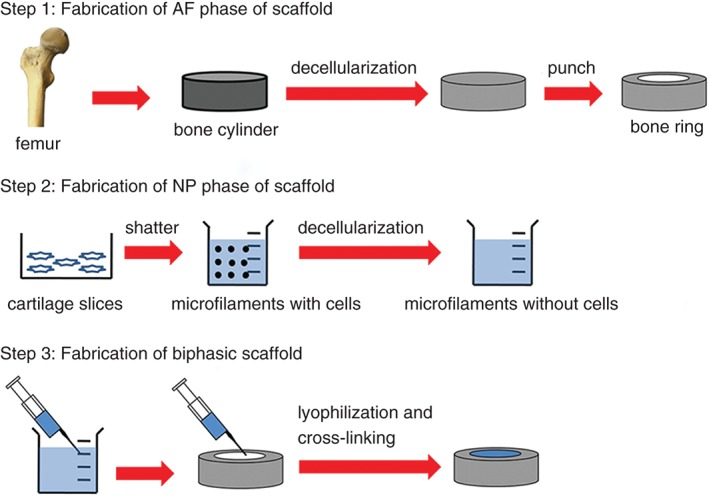
The fabrication process for biphasic IVD scaffolds, which are made using a simple freeze‐drying and cross‐linking technique using pig BMG for the outer AF phase and pig acellular cartilage extracellular matrix for the inner NP phase.
Seed Cells for IVD Tissue Engineering
Seed cells used to construct IVDs include NP cells, AF cells, chondrocytes, MSCs, induced pluripotent stem cells (iPSCs) and so on.43, 44, 45, 46 Currently, NP and AF cells are the most commonly used seed cells for animal experiments and IVD tissue engineering in vitro. Growth factors are sometimes used in conjunction with NP and AF cells to encourage greater ECM deposition. Of the myriad used, the most common are transforming growth factor‐β and bone morphogenetic protein‐2. Although these do increase accumulation of collagen and GAGs, they have been shown to cause ossification of the AF region.22 Additionally, many problems hinder the clinical application of NP and AF cells, such as cell sampling difficulties, limited cell sources, de‐differentiation and markedly reduced cell function.
Recent studies have confirmed that iPSCs can overcome the problems of finite proliferation and differentiation ability of MSCs and be induced to develop into chondrocytes, AF and NP cells, thus avoiding the ethical controversy around using embryonic stem cells.43 iPSCs are therefore good candidates for seed cells for IVD tissue engineering. MSCs have also been proposed as an appropriate cell source for IVD regeneration, an increasing number of studies having demonstrated the ability of both bone marrow‐derived MSCs (BM‐MSCs) and adipose‐derived stem cells (ADSCs) to differentiate into disc‐like phenotypes. In vivo studies have also demonstrated the ability of implanted MSCs to enhance matrix production, particularly GAG synthesis, and increase disc height and hydration.47 Because ADSCs can be obtained easily in large quantities, proliferate rapidly in vitro, cause little damage to donor areas, and can be induced to develop into disc‐like cells under appropriate conditions (such as induction by transforming growth factor‐β1 or co‐culture with NP cells), they are suitable candidates for seed cells for IVD tissue engineering.48 Zhang et al. reported that ADSCs grew well in a chitosan‐alginate gel scaffold and produce more proteoglycan and type II collagen in a hypoxic state than in a normoxic state.49 Furthermore, several types of stem cells have successfully been isolated from AF, NP and cartilage endplate. Using MRI, X‐rays, histologic examination and so on, Wang et al. compared the regenerative potentials of the above‐mentioned three types of disc‐derived stem cells with that of the classic BM‐MSCs in a rabbit disc degeneration model50 and found that cartilage endplate‐derived stem cells have better regenerative capacity than AF‐or NP‐derived stem cells and BM‐MSCs. This study demonstrated that cartilage endplate‐derived stem cell‐seeded alginate constructs have the most powerful ability for NP regeneration. To sum up, the main trend of future research will likely be that stem cells are induced to develop into disc‐like cells and seeded into scaffolds to form tissue‐engineered IVDs.
Construction of Tissue Engineered Whole IVDs and Animal Experiments
Previous studies have mainly focused on constructing separate NP or AF tissue. In recent years, tissue‐engineered whole IVDs have gradually been developed by combining constructed tissue engineered AF and NP. A series of relevant studies has been performed in vitro and in vivo in a nude mice, small animal model of IVD regeneration.
Mizuno et al. were the first to construct whole IVDs with oval AF scaffolds made of polyglycolic acid‐PLLA seeded with goat AF cells, their centers being injected with alginate gel seeded with NP cells.51 The constructed whole IVDs were implanted and cultured subcutaneously in nude mice and it was found that the gross morphology of the constructed disc tissue was similar to that of natural IVDs. The outer AF‐like tissue contained more collagen type I and the central NP‐like tissue more type II collagen, the distribution of these components thus being similar to that in natural IVDs. Furthermore, the ECM increased with time, the DNA content being 50% greater and the elastic modulus fourfold that in natural tissue at 16 weeks. Nesti et al. seeded induced bone marrow stem cells into PLLA electrospinning scaffolds and hyaluronic acid gel, cultured for 28 days and constructed IVD‐like composite tissue.39 The seeded cells differentiated towards chondrocytes with time, as confirmed by histology, biochemical analysis, immunohistochemistry and gene expression. The outer layer was similar to the AF and the center to the NP. Zhuang et al. seeded rabbit AF and NP cells into scaffolds made of demineralized bone matrix gelatin and type II collagen/hyaluronic acid/chondroitin‐6‐sulfate to construct tissue‐engineered IVDs that they then implanted and cultured subcutaneously in nude mice.52 The gross morphology and biochemical components of the constructs (DNA, GAG and collagen content) were similar to those of natural discs 12 weeks later and the content of biochemical components increased with time.
Bowles et al. fabricated anatomy‐matching tissue‐engineered discs according to the anatomical parameters of the rat tail measured by microCT and MRI.53 The AF phase was made of oriented type collagen seeded with goat AF cells and the NP phase of alginate gel seeded with goat NP cells. These tissue‐engineered IVDs were implanted into disc defects in athymic rats’ tails and survived for up to 6 months in the intervertebral space. They maintained disc space height and secreted ECM, integrated well with the spine and ultimately developed mechanical and biochemical characteristics similar to those of autologous IVDs. This study may provide a basis for clinical applications of tissue engineered IVD. Subsequently, Bowles et al. reconstructed L4–5 disc defects in athymic mice with tissue engineered IVDs using the same technology (n = 5).54 Sixteen weeks later, three animals had fully or partially maintained the intervertebral height and biochemical components similar to those of autologous IVDs were confirmed by histological staining. Martin et al. constructed acellular electrospinning PCL scaffolds with a disc‐like angle‐ply structure to repair disc defects in rats’ tails.55 They showed that the dense PCL formed a disc‐like angle‐ply structure that was unable to guide the ingrowth of endogenous cells and achieve disc repair. After the interlaminar space had been increased by adding a water‐soluble polyethylene oxide, endogenous cells grew into these scaffolds and produced a collagen network structure.
The above studies confirm that constructing tissue‐engineered composite AF‐NP tissue offers a feasible method for the construction of integrated IVDs and provides a basis for the further study of constructing tissue‐engineered whole IVDs.
Effect of Mechanics on ECM Secretion and Construction of Tissue Engineered IVD
Discs are the largest avascular organs in human bodies. The exchange of nutrients and metabolites is achieved by infiltration and convective diffusion and the mechanics can directly produce convective transport. It has been found that the mechanics have direct and indirect effects on the metabolism and growth of disc cells, and cyclic force and appropriate dynamic loading can promote the metabolism, gene expression and matrix secretion of IVD cells.56, 57, 58, 59 Varying results can be achieved by changing the magnitude and frequency of loads.60, 61 For example, 2% cyclic strain can cause up‐regulation of GAG gene expression and down‐regulation of matrix metalloproteinase gene expression in human AF cells.62 By culturing isolated IVDs, it was found that GAG content was better retained under appropriate loads (0.2–1 MPa, 1 Hz) than by static culture.63 The magnitude, frequency and duration of dynamic compression affects the physiological function of discs, physiological magnitude, frequency and duration of load being beneficial to the metabolism of synthetic disc cells. Barbir et al. found that cyclic compression and torsion had different biological roles during culture of isolated rabbit IVDs.58 Cyclic compression increases the NP metabolism, whereas cyclic torsion (1 Hz, 90 min, ± 5°, ± 15°) increases the gene expression of elastose, which can be remodeled under shear stress. However, a large magnitude of torsion (± 30o) increases gene expression of tumor necrosis factor‐α and interleukin‐1β, indicating the effects of damage.
Chik et al. used MSC and collagen to build tissue‐engineered spinal motion segment constructs that included two subunits (an NP core and surrounding AF multilayer structure).64 The constructs were cultured in a bioreactor under compressive, torsional or compressive/torsional stress and it was found that chondrocyte culture medium could be used to stabilize the osteochondral subunits, cyclic compressive stress promoted optimization of fiber matrix structure, cyclic torsional loading promoted optimization of the cell arrangement on the layered AF and the number of AF layers affected the mechanical properties of spinal motion segment constructs. This study can be regarded as a milestone in the construction of functional whole IVDs, providing a 3‐D model for studying tissue maturation and function reconstruction.
Existing Problems and Future Directions
Analysis of previous research leads to the conclusion that disc degeneration requiring discectomy or artificial disc replacement is an indication for repair by tissue‐engineered whole IVDs. Functional whole discs implanted into human must possess good biomechanical strength and function; the following problems needs to be further studied:
Seed cells: the characteristics of AF and NP cells need to be further clarified and the differences between them identified. Limited cell sources hinder the wide application of IVD tissue engineering and needs to be expanded.
Scaffold material: an ideal biomimetic IVD scaffold that contains AF, NP and cartilage endplate has not yet been developed.
Growth factor: there is still little research on the effects of growth factors (such as growth differentiation factor‐5) and/or gene therapy on disc cells; this topic needs to be studied further.
Bioreactors: bioreactors that can create a mechanical environment similar to that in vivo are not yet available, hindering research on IVD tissue engineering in vitro.
It is possible that the problem of limited cell sources will be solved by MSCs with their capacity for multipotent differentiation. Designing and fabricating biomimetic NP, AF and cartilage endplate scaffolds that can be assembled easily and integrate well or developing integrated biomimetic disc scaffolds that function well and have similar components and structure to native discs may become new research trends. To mimic the mechanical environment in vivo, development of a bioreactor capable of applying biomimetic mechanical loads (axial compression and torsion) that can promote differentiation of seed cells, ECM secretion and arrangement, structural optimization and functional maturation is needed.
Summary
DDD is recognized as one of the most serious degenerative disorders that dramatically affect quality of life. Because both conservative and surgical treatments target the relief of symptoms but not tissue regeneration, satisfactory long‐term result cannot be achieved with these modalities. Regenerative therapy for DDD is the most advanced type of research in spinal surgery. Although a perfect regenerative strategy for treating DDD has not yet been developed, great progress has been made in the construction of tissue‐engineered IVDs. We believe that IVD tissue engineering will result in revolutionary progress in the treatment of DDD with the development of relevant research.
Disclosure: This study was supported by the National Natural Science Foundation of China (Nos. 81272046, 31500781, 31470937 and 31300798), Tianjin Science and Technology Committee (No. 15JCYBJC25300), the key scientific and technical problems of the Tianjin Health Bureau (No. 14KG121, 15KG125) and China Postdoctoral Science Foundation funded project (No. 2012T50235).
References
- 1. Silva‐Correia J, Correia SI, Oliveira JM, Reis RL. Tissue engineering strategies applied in the regeneration of the human intervertebral disk. Biotechnol Adv, 2013, 31: 1514–1531. [DOI] [PubMed] [Google Scholar]
- 2. Bian Z, Sun J. Development of a KLD‐12 polypeptide/TGF‐β1‐tissue scaffold promoting the differentiation of mesenchymal stem cell into nucleus pulposus‐like cells for treatment of intervertebral disc degeneration. Int J Clin Exp Pathol, 2015, 8: 1093–1103. [PMC free article] [PubMed] [Google Scholar]
- 3. Petit A, Roquelaure Y. Low back pain, intervertebral disc and occupational diseases. Int J Occup Saf Ergon, 2015, 21: 15–19. [DOI] [PubMed] [Google Scholar]
- 4. Yang H, Cao C, Wu C, et al TGF‐βl suppresses inflammation in cell therapy for intervertebral disc degeneration. Sci Rep, 2015, 5: 13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blanquer SB, Grijpma DW, Poot AA. Delivery systems for the treatment of degenerated intervertebral discs. Adv Drug Deliv Rev, 2015, 84: 172–187. [DOI] [PubMed] [Google Scholar]
- 6. Weber KT, Jacobsen TD, Maidhof R, et al Developments in intervertebral disc disease research: pathophysiology, mechanobiology, and therapeutics. Curr Rev Musculoskelet Med, 2015, 8: 18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee WT, Liu G, Thambiah J, Wong HK. Clinical outcomes of single‐level lumbar artificial disc replacement compared with transforaminal lumbar interbody fusion in an Asian population. Singapore Med J, 2015, 56: 208–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xia LZ, Zheng YP, Xu HG, Liu P. Effect of anterior cervical discectomy and fusion on adjacent segments in rabbits. Int J Clin Exp Med, 2014, 7: 4291–4299. [PMC free article] [PubMed] [Google Scholar]
- 9. Varkey M, Ding J, Tredget EE. Advances in skin substitutes‐potential of tissue engineered skin for facilitating anti‐fibrotic healing. J Funct Biomater, 2015, 6: 547–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paré B, Touzel‐Deschênes L, Lamontagne R, et al Early detection of structural abnormalities and cytoplasmic accumulation of TDP‐43 in tissue‐engineered skins derived from ALS patients. Acta Neuropathol Commun, 2015, 3: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alavi SH, Kheradvar A. A hybrid tissue‐engineered heart valve. Ann Thorac Surg, 2015, 99: 2183–2187. [DOI] [PubMed] [Google Scholar]
- 12. Nover AB, Stefani RM, Lee SL, et al Long‐term storage and preservation of tissue engineered articular cartilage. J Orthop Res, 2015; doi: 10.1002/jor.23034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang Q, Zhao YH, Xia Q, et al Novel cartilage‐derived biomimetic scaffold for human nucleus pulposus regeneration: a promising therapeutic strategy for symptomatic degenerative disc diseases. Orthop Surg, 2013, 5: 60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Choy AT, Chan BP. A structurally and functionally biomimetic biphasic scaffold for intervertebral disc tissue engineering. PLoS One, 2015, 10: e0131827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu B, Xu H, Wu Y, et al Intervertebral disc tissue engineering with natural extracellular matrix‐derived biphasic composite scaffolds. PLoS One, 2015, 10: e0124774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu B, Du L, Zhang J, et al Circumferentially oriented microfiber scaffold prepared by wet‐spinning for tissue engineering of annulus fibrosus. RSC Adv, 2015, 5: 42705–42713. [Google Scholar]
- 17. Wu LC, CJ C, ZH L, et al Fabrication and properties of acellular porcine anulus fibrosus for tissue engineering in spine surgery. J Orthop Surg Res, 2014, 9: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nair MB, Baranwal G, Vijayan P, et al Composite hydrogel of chitosan‐poly(hydroxybutyrate‐co‐valerate) with chondroitin sulfate nanoparticles for nucleus pulposus tissue engineering. Colloids Surf B Biointerfaces, 2015, 136: 84–92. [DOI] [PubMed] [Google Scholar]
- 19. Tao H, Wu Y, Li H, et al BMP7‐based functionalized self‐assembling peptides for nucleus pulposus tissue engineering. ACS Appl Mater Interfaces, 2015, 7: 17076–17087. [DOI] [PubMed] [Google Scholar]
- 20. Raj PP. Intervertebral disc: anatomy‐physiology‐pathophysiology‐treatment. Pain Pract, 2008, 8: 18–44. [DOI] [PubMed] [Google Scholar]
- 21. Maerz T, Herkowitz H, Baker K. Molecular and genetic advances in the regeneration of the intervertebral disc. Surg Neurol Int, 2013, 4: S94–S105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hudson KD, Alimi M, Grunert P, Härtl R, Bonassar LJ. Recent advances in biological therapies for disc degeneration: tissue engineering of the annulus fibrosus, nucleus pulposus and whole intervertebral discs. Curr Opin Biotechnol, 2013, 24: 872–879. [DOI] [PubMed] [Google Scholar]
- 23. Neo PY, Shi P, Goh JC, Toh SL. Characterization and mechanical performance study of silk/PVA cryogels: towards nucleus pulposus tissue engineering. Biomed Mater, 2014, 9: 065002. [DOI] [PubMed] [Google Scholar]
- 24. Alini M, Li W, Markovic P, Aebi M, Spiro RC, Roughley PJ. The potential and limitations of a cell‐seeded collagen/hyaluronan scaffold to engineer an intervertebral disc‐like matrix. Spine (Phila Pa 1976), 2003, 28: 446–454, discussion 453. [DOI] [PubMed] [Google Scholar]
- 25. Huang B, Zhuang Y, Li CQ, Liu LT, Zhou Y. Regeneration of the intervertebral disc with nucleus pulposus cell‐seeded collagen II/hyaluronan/chondroitin‐6‐sulfate tri‐copolymer constructs in a rabbit disc degeneration model. Spine (Phila Pa 1976), 2011, 36: 2252–2259. [DOI] [PubMed] [Google Scholar]
- 26. Rowland CR, Lennon DP, Caplan AI, Guilak F. The effects of crosslinking of scaffolds engineered from cartilage ECM on the chondrogenic differentiation of MSCs. Biomaterials, 2013, 34: 5802–5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mercuri JJ, Gill SS, Simionescu DT. Novel tissue‐derived biomimetic scaffold for regenerating the human nucleus pulposus. J Biomed Mater Res A, 2011, 96: 422–435. [DOI] [PubMed] [Google Scholar]
- 28. Park SH, Gil ES, Cho H, et al Intervertebral disk tissue engineering using biphasic silk composite scaffolds. Tissue Eng Part A, 2012, 18: 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shao X, Hunter CJ. Developing an alginate/chitosan hybrid fiber scaffold for annulus fibrosus cells. J Biomed Mater Res A, 2007, 82: 701–710. [DOI] [PubMed] [Google Scholar]
- 30. Jin L, Shimmer AL, Li X. The challenge and advancement of annulus fibrosus tissue engineering. Eur Spine J, 2013, 22: 1090–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chang G, Kim HJ, Vunjak‐Novakovic G, Kaplan DL, Kandel R. Enhancing annulus fibrosus tissue formation in porous silk scaffolds. J Biomed Mater Res A, 2010, 92: 43–51. [DOI] [PubMed] [Google Scholar]
- 32. Chang G, Kim HJ, Kaplan D, Vunjak‐Novakovic G, Kandel RA. Porous silk scaffolds can be used for tissue engineering annulus fibrosus. Eur Spine J, 2007, 16: 1848–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bhattacharjee M, Miot S, Gorecka A, et al Oriented lamellar silk fibrous scaffolds to drive cartilage matrix orientation: towards annulus fibrosus tissue engineering. Acta Biomater, 2012, 8: 3313–3325. [DOI] [PubMed] [Google Scholar]
- 34. Nerurkar NL, Baker BM, Sen S, Wible EE, Elliott DM, Mauck RL. Nanofibrous biologic laminates replicate the form and function of the annulus fibrosus. Nat Mater, 2009, 8: 986–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pan Y, Chu T, Dong S, et al Cells scaffold complex for intervertebral disc anulus fibrosus tissue engineering: in vitro culture and product analysis. Mol Biol Rep, 2012, 39: 8581–8594. [DOI] [PubMed] [Google Scholar]
- 36. Wan Y, Feng G, Shen FH, Laurencin CT, Li X. Biphasic scaffold for annulus fibrosus tissue regeneration. Biomaterials, 2008, 29: 643–652. [DOI] [PubMed] [Google Scholar]
- 37. Xu H, Xu B, Yang Q, et al Comparison of decellularization protocols for preparing a decellularized porcine annulus fibrosus scaffold. PLoS One, 2014, 9: E86723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wu Y, Xu B, Yang Q, et al A novel natural ECM‐derived biphasic scaffold for intervertebral disc tissue engineering. Mater Lett, 2013, 105: 102–105. [Google Scholar]
- 39. Nesti LJ, Li WJ, Shanti RM, et al Intervertebral disc tissue engineering using a novel hyaluronic acid‐nanofibrous scaffold (HANFS) amalgam. Tissue Eng Part A, 2008, 14: 1527–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lazebnik M, Singh M, Glatt P, Friis LA, Berkland CJ, Detamore MS. Biomimetic method for combining the nucleus pulposus and annulus fibrosus for intervertebral disc tissue engineering. J Tissue Eng Regen Med, 2011, 5: e179–e187. [DOI] [PubMed] [Google Scholar]
- 41. Nerurkar NL, Sen S, Huang AH, Elliott DM, Mauck RL. Engineered disc‐like angle‐ply structures for intervertebral disc replacement. Spine (Phila Pa 1976), 2010, 35: 867–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chan LK, Leung VY, Tam V, Lu WW, Sze KY, Cheung KM. Decellularized bovine intervertebral disc as a natural scaffold for xenogenic cell studies. Acta Biomater, 2013, 9: 5262–5272. [DOI] [PubMed] [Google Scholar]
- 43. Chen J, Lee EJ, Jing L, Christoforou N, Leong KW, Setton LA. Differentiation of mouse induced pluripotent stem cells (iPSCs) into nucleus pulposus‐like cells in vitro . PLoS One, 2013, 8: E75548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu Y, Fu S, Rahaman MN, Mao JJ, Bal BS. Native nucleus pulposus tissue matrix promotes notochordal differentiation of human induced pluripotent stem cells with potential for treating intervertebral disc degeneration. J Biomed Mater Res A, 2015, 103: 1053–1059. [DOI] [PubMed] [Google Scholar]
- 45. Jin ES, Min J, Jeon SR, Choi KH, Jeong JH. Analysis of molecular expression in adipose tissue‐derived mesenchymal stem cells: prospects for use in the treatment of intervertebral disc degeneration. J Korean Soc, 2013, 53: 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Frith JE, Cameron AR, Menzies DJ, et al An injectable hydrogel incorporating mesenchymal precursor cells and pentosan polysulphate for intervertebral disc regeneration. Biomaterials, 2013, 34: 9430–9440. [DOI] [PubMed] [Google Scholar]
- 47. Richardson SM, Kalamegam G, Pushparaj PN, et al Mesenchymal stem cells in regenerative medicine: focus on articular cartilage and intervertebral disc regeneration. Methods, 2015, doi: 10.1016/j.ymeth.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 48. Dai J, Wang H, Liu G, Xu Z, Li F, Fang H. Dynamic compression and co‐culture with nucleus pulposus cells promotes proliferation and differentiation of adipose‐derived mesenchymal stem cells. J Biomech, 2014, 47: 966–972. [DOI] [PubMed] [Google Scholar]
- 49. Zhang Z, Li F, Tian H, et al Differentiation of adipose‐derived stem cells toward nucleus pulposus‐like cells induced by hypoxia and a three‐dimensional chitosan‐alginate gel scaffold in vitro. Chin Med J (Engl), 2014, 127: 314–321. [PubMed] [Google Scholar]
- 50. Wang H, Zhou Y, Huang B, et al Utilization of stem cells in alginate for nucleus pulposus tissue engineering. Tissue Eng Part A, 2014, 20: 908–920. [DOI] [PubMed] [Google Scholar]
- 51. Mizuno H, Roy AK, Vacanti CA, Kojima K, Ueda M, Bonassar LJ. Tissue‐engineered composites of anulus fibrosus and nucleus pulposus for intervertebral disc replacement. Spine (Phila Pa 1976), 2004, 29: 1290–1297. [DOI] [PubMed] [Google Scholar]
- 52. Zhuang Y, Huang B, Li CQ, et al Construction of tissue‐engineered composite intervertebral disc and preliminary morphological and biochemical evaluation. Biochem Biophys Res Commun, 2011, 407: 327–332. [DOI] [PubMed] [Google Scholar]
- 53. Bowles RD, Gebhard HH, Hartl R, Bonassar LJ. Tissue‐engineered intervertebral discs produce new matrix, maintain disc height, and restore biomechanical function to the rodent spine. Proc Natl Acad Sci U S A, 2011, 108: 13106–13111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bowles RD, Gebhard HH, Dyke JP, et al Image‐based tissue engineering of a total intervertebral disc implant for restoration of function to the rat lumbar spine. NMR Biomed, 2012, 25: 443–451. [DOI] [PubMed] [Google Scholar]
- 55. Martin JT, Milby AH, Chiaro JA, et al Translation of an engineered nanofibrous disc‐like angle‐ply structure for intervertebral disc replacement in a small animal model. Acta Biomater, 2014, 10: 2473–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Malandrino A, Lacroix D, Hellmich C, Ito K, Ferguson SJ, Noailly J. The role of endplate poromechanical properties on the nutrient availability in the intervertebral disc. Osteoarthritis Cartilage, 2014, 22: 1053–1060. [DOI] [PubMed] [Google Scholar]
- 57. Vergroesen PP, Kingma I, Emanuel KS, et al Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthritis Cartilage, 2015, 23: 1057–1070. [DOI] [PubMed] [Google Scholar]
- 58. Barbir A, Godburn KE, Michalek AJ, Lai A, Monsey RD, Iatridis JC. Effects of torsion on intervertebral disc gene expression and biomechanics, using a rat tail model. Spine (Phila Pa 1976), 2011, 36: 607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hee HT, Zhang J, Wong HK. An in vitro study of dynamic cyclic compressive stress on human inner annulus fibrosus and nucleus pulposus cells. Spine J, 2010, 10: 795–801. [DOI] [PubMed] [Google Scholar]
- 60. Chan SC, Ferguson SJ, Wuertz K, Gantenbein‐Ritter B. Biological response of the intervertebral disc to repetitive short‐term cyclic torsion. Spine (Phila Pa 1976), 2011, 36: 2021–2030. [DOI] [PubMed] [Google Scholar]
- 61. Chan SC, Ferguson SJ, Gantenbein‐Ritter B. The effects of dynamic loading on the intervertebral disc. Eur Spine J, 2011, 20: 1796–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Neidlinger‐Wilke C, Würtz K, Liedert A, et al A three‐dimensional collagen matrix as a suitable culture system for the comparison of cyclic strain and hydrostatic pressure effects on intervertebral disc cells. J Neurosurg Spine, 2005, 2: 457–465. [DOI] [PubMed] [Google Scholar]
- 63. Korecki CL, MacLean JJ, Iatridis JC. Dynamic compression effects on intervertebral disc mechanics and biology. Spine (Phila Pa 1976), 2008, 33: 1403–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chik TK, Chooi WH, Li YY, et al Bioengineering a multicomponent spinal motion segment construct–a 3D model for complex tissue engineering. Adv Healthc Mater, 2015, 4: 99–112. [DOI] [PubMed] [Google Scholar]


