Abstract
Historically, a simple approach centered on palliation was applicable to the majority of patients with metastatic spinal disease. With advances in diagnosis and treatment, a more complicated algorithm has devolved requiring a multidisciplinary approach with institutional commitment and support. We performed a database review including pertinent articles exploring the multidisciplinary management of spinal metastatic disease. The wide variation in clinical presentation and tumor response to treatment necessitates a multidisciplinary approach that integrates the diagnosis and treatment of the cancer, symptom management, and rehabilitation for optimal care of patients with spinal metastases. Advances in the field of radiology have led to earlier and more focused diagnosis of spinal metastasis and acts to guide therapy. Advances in surgical techniques, neurophysiologic monitoring, and anesthetic expertise have allowed surgeons to perform more extensive procedures leading to improved outcomes and reduced morbidity. Radiation oncology input that is essential as external beam radiation therapy can provide significant pain relief. Non‐operative measures may include bisphosphonate infusions, management of complications (e.g. hypercalcemia of malignancy), monoclonal antibody infusions, and chemotherapy if indicated in the treatment of the primary malignancy. Input from psychology services is necessary to address the biopsychosocial ramifications of spinal metastasis. Allied health professionals in the form of physiotherapists, social workers, and dieticians also contribute in maximizing patients’ quality of life and well‐being.
Keywords: Diagnosis, Metastasis, Multidisciplinary, Oncology, Spine
Introduction
Bone is the most frequent site of metastatic cancer and is responsible for a significant clinical burden and demand on health‐care resources1. Advances in cancer care have prolonged the survival of patients with metastatic disease to the spine. Metastases limited to the skeleton have a more favorable outcome than visceral metastases, reflected by a median survival of 20 months after first bone relapse in comparison with 3 months after first liver recurrence2, 3. The morbidity associated with metastatic spinal disease is significant: more than half of these patients will require radiotherapy or surgical intervention for spinal cord or nerve root compression4. The wide variation in clinical presentation and tumor response necessitates a multidisciplinary approach that integrates oncology, surgery, radiotherapy, rehabilitation, and palliative services for optimal care of patients with spinal metastasis. Furthermore, early identification of patients with a high likelihood of developing spinal metastases enables teams to be proactive in their diagnosis and treatment. With this in mind, the use of a multidisciplinary approach integrating the diagnosis and treatment of the disease with appropriate symptom management, palliation, and rehabilitation ensures optimal care. The purpose of our paper was to perform a database review of current treatment strategies for spinal metastatic disease, and to describe the importance of a multidisciplinary approach in its treatment.
Methods
A database review was undertaken. PubMed, OVID Medline, and the Cochrane database were searched. The search algorithm ([spine OR spinal OR vertebral] AND [tumor OR tumour OR neoplasm OR cancer OR metastases]) was used in the “Topic” field to identify articles of interest. The following search parameters were used: (i) articles published in the years 1990–2016; and (ii) English languages. Pertinent but not all articles were used to review the multidisciplinary management of spinal metastatic disease (Fig. 1). This article does not contain any studies with human participants or animals performed by any of the authors.
Figure 1.
Flow chart of literature searching.
Radiology
Radiology
Radiological investigations play a central role in the diagnosis and treatment planning of spinal metastases. Spinal metastases may be recognized based on imaging findings in patients without a known diagnosis of malignancy. If metastases are present, further imaging or imaging‐guided biopsy techniques may be necessary to confirm the diagnosis or stage of the tumor. Plain radiographs are a quick and inexpensive first‐line investigation; however, CT offers improved sensitivity and staging benefits. CT scanning is vastly superior to plain radiographs in the detection of trabecular and cortical bone destruction, soft‐tissue extension, and involvement of neurovascular structures, thereby allowing more accurate decision‐making5. Snyder et al. demonstrated that CT analysis was more sensitive and specific than radiographic criteria (59% vs 24% accurate) in predicting fractures in breast cancer patients with spinal metastases5.
Bone scintigraphy is an effective means of assessing the metabolic activity of the spine, while plain radiographs can only demonstrate lesions with a loss of 30%–50% of bone mineral content6, 7. Technetium‐99m (99m Tc) planar bone scintiscans detect metastatic bone deposits through increased osteoblastic activity, considered to be an indirect marker of an oncological process. For this reason, it is considered to be the most efficient modality for screening the whole body for metastasis8, 9. 18F‐fluoro‐deoxy‐D‐glucose positron emission tomography (18FDG PET) offers superior spatial resolution and improved sensitivity, which is superior to bone scintigraphy in the detection of osteolytic metastases, while osteoblastic metastases show lower metabolic activity and are frequently undetectable by PET9, 10.
It is worth noting that scintigraphy is non‐specific in determining the origin of lesions identified, and is best used in conjunction with other modalities, such as CT or MRI11, 12. Positron emission tomography (PET) identifies early marrow infiltration through aberrancies in glucose metabolism in neoplastic cells13, 14. Daldrup‐Link et al. report sensitivities for 18FDG‐PET scanning, whole‐body MRI, and 99mTc bone scintiscanning as 90%, 82%, and 71% respectively, in a comparative study of the three modalities13.
MRI is useful in delineating soft tissue involvement in spinal metastases and is particularly useful in diagnosing spinal cord compression, which can be a devastating consequence of spinal metastatic disease. MRI depicts early hematogenous dissemination of the tumor to the bone marrow before metabolic bone reactions are detectable on scintigraphy15. Eustace et al. report respective sensitivities of 96.5% and 72%, specificities of 100% and 98%, and positive predictive values of 100% and 95% for MRI and scintigraphy 15, 16.
Surgery
Developments in surgical techniques, neurophysiologic monitoring, and anesthetic expertise have allowed surgeons to perform safer and more extensive procedures with improved outcomes and reduced morbidity4, 17. Surgery in patients with spinal metastases may be required to provide a tissue diagnosis but is more often required to provide spinal stability or neural element decompression4. The most common and significant outcome of surgical intervention is pain relief. This is generally attributed to removal of the metastatic deposit and prevention or correction of deformity with stabilization18. The Spinal Oncology Study Group (SOSG) developed the Spinal Instability Neoplastic Score (SINS), a standardized framework to help physicians assess and categorize spinal instability. This score has a sensitivity and specificity for potentially unstable or unstable lesions of 95.7% and 79.5%, respectively, with near‐perfect inter‐observer and intra‐observer reliability for differentiating three clinical categories of stability (stable, potentially unstable, and unstable)19, 20.
Surgery remains the standard treatment for patients with rapidly progressive spinal cord compression or the presence of a significant osteolytic lesion, implying a high risk of fracture21. In such cases, the goal of treatment is palliative rather than curative. Historically, surgery was only considered in patients with a large tumor burden that progressed despite optimal oncological treatment with radiotherapy, as well as in patients with radiotherapy‐resistant lesions. Now, many surgeons advocate vertebral‐body resection and stabilization more as a preventive measure for spinal instability and as a supplementation for external beam radiation therapy. A meta‐analysis by Klimo and Schmidt concluded that surgery should be the primary treatment in patients with spinal epidural disease, with radiotherapy used as an adjunct17.
Prior to the introduction and evolution of instrumentation, the mainstay of surgery for spinal metastatic disease with neural compromise was decompressive laminectomy22. Laminectomy alone may compromise the stability of the spine in an individual who already has reduced functional capacity from a systemic disease23. Subsequent radiotherapy can further compromise the soft tissues, including muscle, leading to myopathy24 and late kyphosis at the site of decompression25. Pedicle screw constructs placed across the decompression site allow stabilization and can prevent late deformity. They also allow more aggressive tumor debulking or even resection in selected cases26.
While posterior approaches to the spine allow decompression with removal of the posterior elements, it is more difficult to address disease of the middle and anterior columns. A variety of approaches can be utilized, such as a transpedicular, to access the middle and anterior column from a posterior incision27. Alternatively, anterior approaches may also be used in addressing lumbar and thoracic lesions in selected cases where there is a desire to achieve complete resection or in addressing a lesion that is both radio‐resistant and chemo‐resistant28. The anterior approach is more commonly utilized in the cervical spine where there is a need to use a construct that resists the compressive forces present in the anterior column29.
Kyphoplasty or vertebroplasty are minimally invasive modalities used primarily for pain relief. Multiple studies have demonstrated the potential for significant pain relief in patients with osteolytic lesions30, 31, 32. New “minimally invasive” techniques have also been developed, such as percutaneous fixation and posterior element resection to achieve immediate stabilization and decompression while reducing the morbidity of the approach to the spine33.
It must be acknowledged that performing spinal surgery upon this cohort of patients with metastatic disease carries a significant risk34. These patients are at high risk of a plethora of medical problems, including dehydration, hypercalcemia, coagulopathies, and anemia. In all cases, the aims of the surgical intervention need to be considered and the invasiveness of surgery needs to be weighed against the patients’ physiologic condition and prognosis.
Radiation Oncology
All patients with symptomatic bone metastases and lesions in long bones should be evaluated by Radiation Oncologists prior to surgery. Radiation therapy (RT) provides successful palliation of painful bone metastasis that is time efficient and associated with few morbidities35. External beam radiation therapy (EBRT) provides significant palliation of painful bone metastases in 50%–80% of patients, with up to 30% achieving near total pain relief at the treated site36. Modern technological advances in radiotherapy delivery (e.g. stereotactic body radiotherapy [SBRT]) may augment the results of the primary treatment of metastatic spine lesions37. SBRT delivers a high dose of radiation to metastatic lesions with a steep dose gradient that may spare adjacent neural structures, notably the cord and conus38, 39. MRI‐guided robotic linear accelerator (LINAC) radiotherapy is under development, and capable of focusing beams to within 1 mm of spatial accuracy40, 41. Similarly, advances in the realm of CT and MRI‐based planning has vastly improved the precision of information pertaining to the location of the metastatic deposit and its relationship to surrounding tissues42. The traditional treatment plan is to irradiate two vertebral bodies above and two below the lesion with single‐fraction image‐guided intensity‐modulated RT, in light of the fact that recurrence is seen most commonly in vertebral bodies neighboring the site of involvement43.
The Radiation Therapy Oncology Group reported that 6‐month treatment regimes with variable RT doses, such as 8 Gy in 1 fraction, 20 Gy in 5 fractions, and 30 Gy in 10 fractions, provide complete analgesic relief in 57% of patients44. It has also been demonstrated that a total RT dose of 30 Gy given in 10 fractions provides pain relief for 77%–82% of patients with multiple bone metastases after a year of treatment45, 46. The selection of fractionated or single fraction treatment seems to be patient and physician dependent. Fractionated treatment courses are associated with an 8% re‐treatment to the same anatomic site owing to recurrence of pain, as opposed to a 20% rate following a single fraction47. However, the single fraction treatment approach optimizes convenience for both the patient and the radiation oncologist: an important consideration in the palliation of patients with spinal metastases.
A prospective randomized study by Teshima et al. compared the addition of methylprednisolone with external beam radiation therapy to radiation therapy alone for bony metastases47. The combination group was found to experience more rapid and longer duration of pain relief. The evidence pertaining to the use of moderate‐dose dexamethasone (16 mg/day) plus radiation therapy for malignant cord compression is inconclusive48, 49.
The American Society for Radiation Oncology (ASTRO) stated that surgical decompression and post‐operative radiotherapy is appropriate for spinal cord compression or spinal instability in highly selected patients with adequate performance status and sufficient life expectancy34. Bisphosphonate use, radionuclide use, vertebroplasty, and kyphoplasty for the prevention or treatment of cancer‐related symptoms does not obviate the need for EBRT in appropriate patients35, 36.
Non‐operative Measures
Oral analgesia as titrated by the World Health Organization Analgesic Ladder is considered first line in the treatment of bony pain50. Morphine is the most commonly used opioid for moderate to severe pain and may be combined with adjuvant medications such as tricyclic antidepressants and corticosteroids. Side effects of medications can limit opioid dosage and cause significant morbidity. These include delirium, constipation, pruritus, nausea, vomiting, sedation, myoclonus, and respiratory depression51, 52, 53. Other non‐invasive methods of pain relief include cutaneous stimulation, continuous repositioning, spine cryoablation, and regional nerve blocks54, 55, 56.
Monthly infusions of bisphosphonates like zoledronic acid to patients with bone metastases reduces the frequency and delays the onset of skeletal‐related events57. Their administration also provides significant improvements in bone pain and quality of life58. Bisphosphonate use in patients with spinal metastases has increased in the past decade. Their use has decreased bone pain scores and reduced skeletal‐related events such as the need for local radiotherapy, hypocalcemia, pathologic fracture, and spinal cord compression59, 60. Once injected, bisphosphonates are internalized by osteoclasts. This leads to a decrease in osteoclast activity and viability61. Complications of bisphosphonate therapy include renal impairment and mandibular osteonecrosis62, 63. Bisphosphonate demonstrates maximal effectiveness and safety when combined with either single or multiple fraction radiotherapy64, 65. This synergism is due to the fact that radiotherapy is believed to reduce numbers of tumor‐produced osteoclast activating factors66. The American Society of Clinical Oncology guidelines and the International Expert Panel guidelines recommend starting bisphosphonates when the first radiographic indication of metastatic deposits in the spine is noted67, 68.
Denosumab, a fully human monoclonal antibody to RANK‐L, has demonstrated in clinical trials inhibition of osteoclast‐mediated bone destruction in breast, prostate, and myeloma tumors, and is considered non‐inferior to zoledronic acid69, 70. A meta‐analysis performed by Lipton et al. compared the efficacy of denosumab to zoledronic acid in preventing skeletal‐related events in people with prostate cancer, breast cancer, solid tumors, or multiple myeloma. Denosumab increased the time to first on‐study skeletal‐related event by 8.21 months, and reduced the risk of a first skeletal‐related event by 17%69.
Chemotherapy is very seldom considered in the targeted treatment of metastatic spinal tumors due to its systemic nature and also owing to the fact that it requires an extended course of administration prior to pain relief71. Complications are the source of morbidity and fear for the patient and include pain, gastrointestinal abnormalities, hematological disturbances, immunosupression, and biopsychosocial sequelae (alopecia and infertility)72.
Pain caused by bone metastases has multiple causes, including periosteal elevation and inflammation73. Radiopharmaceuticals may be used in the palliation of bony pain. Ethylenediamine tetramethylene phosphonic acid is an IV radioisotope that preferentially binds to osteoplastic metastases and osteosarcomas, and a significant analgesic effect may be achieved in 83%–93% of patients74. Strontium‐89 chloride infusions have also been trialed as a similar treatment. Response rates vary in the published literature from minimal to up to 77%75. Corticosteroids produce effects that include mood elevation, an anti‐inflammatory effect, and reduction of spinal cord edema in bony metastases76. There is good evidence supporting the use of high dose dexamethasone (64 mg/day) in the treatment of pain from spinal metastases, particularly if epidural compression is present. It is associated with significant pain relief and the ability to remain ambulatory in up to 81% of patients22.
Psychiatry
Involvement of the psychiatric services may be necessary in patients with a diagnosis of metastatic spinal disease. Psychological complications in this instance usually manifest as anxiety, depression, adjustment disorder, and loss of self‐esteem77, 78. Studies have shown that up to 50% of such patients may experience psychological issues following such a diagnosis79, 80. Early involvement and assessment of this cohort of patients by psychiatric services is essential in the multidisciplinary management and treatment of spinal metastases.
Nutrition
Nutritional support is another consideration in the approach to metastatic spinal disease81. The goals of nutrition support include preventing or reversing nutrient deficiencies, maximizing quality of life, aiding immunologic function, and preserving lean body mass.
This cohort are at risk of anorexia and cachexia82. Important considerations include dysphagia after radiation, oesophagitis, and reduced motility owing to pain medications83. Anorexia has been noted to be an almost universal side effect in individuals with widely metastatic disease84. The multidisciplinary team must also consider decreased caloric intake as a result of diminished appetite and malaise and tumor competition for nutrients. Constipation may be secondary to opiate analgesia or spinal cord involvement by tumors causing an upper motor neuron lesion. Malnutrition may also exacerbate this. The addition of dietary calcium and vitamin D for bone health in the patients at risk of therapy‐associated fractures is warranted.
Screening and nutrition assessment should be interdisciplinary. Physicians, nurses, dietitians, and social workers (as members of the health‐care team) should all participate in nutritional management throughout the continuum of the management of metastatic spinal disease. Such screening tests include the prognostic nutrition index85.
Suggestions for appetite improvement include keeping a daily menu, snacking between meals, eating small and frequent meals, and adding extra protein to meals86, 87. Progestational agents such as megestrol acetate and medroxyprogesterone can lead to appetite stimulation and subsequent weight gain88. The preferred method of nutritional support is via the oral route. If the GI tract is rendered dysfunctional, TPN may be indicated89.
Physiotherapy
Physiotherapists play a central role in the multidisciplinary approach to spinal metastasis. Their role is to maximize quality of life by maintaining patient mobility and facilitating their capacity to perform activities of daily living90, 91. Pain reduction therapies may also be employed, such as hot/cold packs, massage, and electrical stimulation. Assistive devices or orthotics, such as frames, canes, and thoracolumbosacral orthosis (TLSO), are provided by the physiotherapy department to patients with spinal metastasis when required91.
Discussion
To optimize the outcomes of patients with spinal metastatic disease, a multidisciplinary approach is necessary (Fig. 2). Advances in diagnosis and treatment of oncology patients have prolonged life expectancy and, in doing so, have altered the multifaceted treatment algorithm required. Yet, despite improved clinical approaches in all the elements of the multidisciplinary team, the complexity of the clinical problem and the need for a symbiotic input from a variety of health‐care providers can pose a logistical and clinical challenge.
Figure 2.
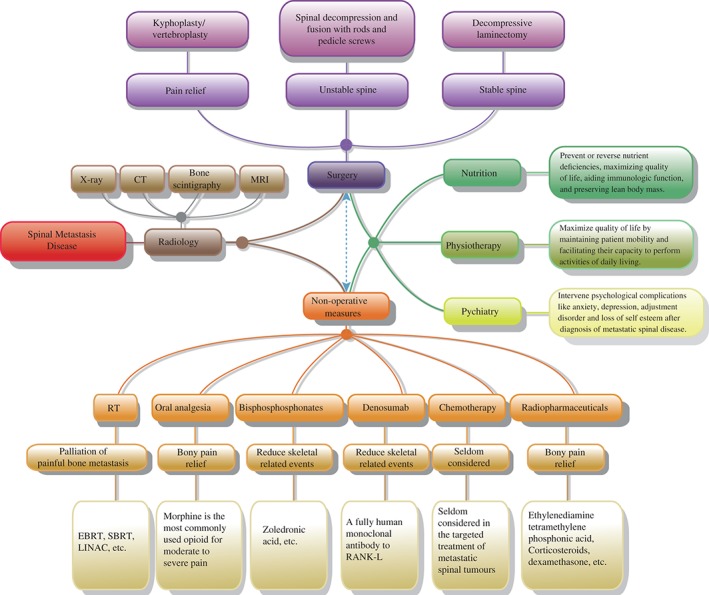
Flow chart showing multidisciplinary treatment of spinal metastatic disease.
Disclosure: The authors declare no conflicts of interest.
References
- 1. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res, 2006, 12(Pt 2): 6243s–6249s. [DOI] [PubMed] [Google Scholar]
- 2. Blanchard DK, Shetty PB, Hilsenbeck SG, Elledge RM. Association of surgery with improved survival in stage IV breast cancer patients. Ann Surg, 2008, 247: 732–738. [DOI] [PubMed] [Google Scholar]
- 3. Niikura N, Liu J, Hayashi N, et al. Treatment outcome and prognostic factors for patients with bone‐only metastases of breast cancer: a single‐institution retrospective analysis. Oncologist, 2011, 16: 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T. Surgical strategy for spinal metastases. Spine (Phila Pa 1976), 2001, 26: 298–306. [DOI] [PubMed] [Google Scholar]
- 5. Snyder BD, Cordio MA, Nazarian A, et al. Noninvasive prediction of fracture risk in patients with metastatic cancer to the spine. Clin Cancer Res, 2009, 15: 7676–7683. [DOI] [PubMed] [Google Scholar]
- 6. Dinter DJ, Neff WK, Klaus J, et al. Comparison of whole‐body MR imaging and conventional X‐ray examination in patients with multiple myeloma and implications for therapy. Ann Hematol, 2009, 88: 457–464. [DOI] [PubMed] [Google Scholar]
- 7. Lecouvet FE, El Mouedden J, Collette L, et al. Can whole‐body magnetic resonance imaging with diffusion‐weighted imaging replace Tc 99m bone scanning and computed tomography for single‐step detection of metastases in patients with high‐risk prostate cancer?. Eur Urol, 2012, 62: 68–75. [DOI] [PubMed] [Google Scholar]
- 8. Uchida K, Nakajima H, Miyazaki T, et al. 18F‐FDG PET/CT for diagnosis of osteosclerotic and osteolytic vertebral metastatic lesions: comparison with bone scintigraphy. Asian Spine J, 2013, 7: 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cook GJ, Houston S, Rubens R, Maisey MN, Fogelman I. Detection of bone metastases in breast cancer by 18FDG PET: differing metabolic activity in osteoblastic and osteolytic lesions. J Clin Oncol, 1998, 16: 3375–3379. [DOI] [PubMed] [Google Scholar]
- 10. Schuster DM, Nieh PT, Jani AB, et al. Anti‐3‐[18 F] FACBC positron emission tomography‐computerized tomography and 111 In‐capromab pendetide single photon emission computerized tomography‐computerized tomography for recurrent prostate carcinoma: results of a prospective clinical trial. J Urol, 2014, 191: 1446–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shen G, Deng H, Hu S, Jia Z. Comparison of choline‐PET/CT, MRI, SPECT, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: a meta‐analysis. Skeletal Radiol, 2014, 43: 1503–1513. [DOI] [PubMed] [Google Scholar]
- 12. Poulsen MH, Petersen H, Høilund‐Carlsen PF, et al. Spine metastases in prostate cancer: comparison of technetium‐99m‐MDP whole‐body bone scintigraphy, [(18) F] choline positron emission tomography (PET)/computed tomography (CT) and [(18) F] NaF PET/CT. BJU Int, 2014, 114: 818–823. [DOI] [PubMed] [Google Scholar]
- 13. Daldrup‐Link HE, Franzius C, Link TM, et al. Whole‐body MR imaging for detection of bone metastases in children and young adults: comparison with skeletal scintigraphy and FDG PET. AJR Am J Roentgenol, 2001, 177: 229–236. [DOI] [PubMed] [Google Scholar]
- 14. Even‐Sapir E. Imaging of malignant bone involvement by morphologic, scintigraphic, and hybrid modalities. J Nucl Med, 2005, 46: 1356–1367. [PubMed] [Google Scholar]
- 15. Eustace S, Tello R, DeCarvalho V, et al. A comparison of whole‐body turboSTIR MR imaging and planar 99mTc‐methylene diphosphonate scintigraphy in the examination of patients with suspected skeletal metastases. AJR Am J Roentgenol, 1997, 169: 1655–1661. [DOI] [PubMed] [Google Scholar]
- 16. Gavaret M, Jouve JL, Péréon Y, et al. Intraoperative neurophysiologic monitoring in spine surgery. Developments and state of the art in France in 2011. Orthop Traumatol Surg Res, 2013, 99 (6 Suppl): S319–S327. [DOI] [PubMed] [Google Scholar]
- 17. Klimo P, Schmidt MH. Surgical management of spinal metastases. Oncologist, 2004, 9: 188–196. [DOI] [PubMed] [Google Scholar]
- 18. Weber MH, Burch S, Buckley J, et al. Instability and impending instability of the thoracolumbar spine in patients with spinal metastases: a systematic review. Int J Oncol, 2011, 38: 5–12. [PubMed] [Google Scholar]
- 19. Fourney DR, Frangou EM, Ryken TC, et al. Spinal instability neoplastic score: an analysis of reliability and validity from the spine oncology study group. J Clin Oncol, 2011, 29: 3072–3077. [DOI] [PubMed] [Google Scholar]
- 20. Agarwal MG, Nayak P. Management of skeletal metastases: an orthopaedic surgeon's guide. Indian J Orthop, 2015, 49: 83–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sørensen PS, Børgesen SE, Rohde K, et al. Metastatic epidural spinal cord compression. Results of treatment and survival. Cancer, 1995, 65: 1502–1508. [DOI] [PubMed] [Google Scholar]
- 22. Delank KS, Wendtner C, Eich HT, Eysel P. The treatment of spinal metastases. Dtsch Arztebl Int, 2011, 108: 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ghosh PS, Milone M. Clinical and laboratory findings of 21 patients with radiation‐induced myopathy. J Neurol Neurosurg Psychiatry, 2015, 86: 152–158. [DOI] [PubMed] [Google Scholar]
- 24. McMullen K, Buchsbaum J, Douglas J, McDonald M, Johnstone P. Growth abnormalities of the spine after radiation therapy: respecting the past while moving forward in proton craniospinal irradiation. Pract Radiat Oncol, 2013, 3: 337–343. [DOI] [PubMed] [Google Scholar]
- 25. Wang M, Bünger CE, Li H, et al. Improved patient selection by stratified surgical intervention: Aarhus Spinal Metastases Algorithm. Spine J, 2015, 15: 1554–1562. [DOI] [PubMed] [Google Scholar]
- 26. Metcalfe S, Gbejuade H, Patel NR. The posterior transpedicular approach for circumferential decompression and instrumented stabilization with titanium cage vertebrectomy reconstruction for spinal tumors: consecutive case series of 50 patients. Spine (Phila Pa 1976), 2012, 37: 1375–1383. [DOI] [PubMed] [Google Scholar]
- 27. Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet, 2005, 366: 643–648. [DOI] [PubMed] [Google Scholar]
- 28. Cacciola F, La Fata G, Pino MA, et al. Cervical spine metastases: our experience. Global Spine J, 2015, 5: A306. [Google Scholar]
- 29. Anselmetti GC, Zoarski G, Manca A, et al. Percutaneous vertebroplasty and bone cement leakage: clinical experience with a new high‐viscosity bone cement and delivery system for vertebral augmentation in benign and malignant compression fractures. Cardiovasc Intervent Radiol, 2008, 31: 937–947. [DOI] [PubMed] [Google Scholar]
- 30. Ramos L, Las Heras D, Antonio J, et al. Medium‐term results of percutaneous vertebroplasty in multiple myeloma. Eur J Haematol, 2006, 77: 7–13. [DOI] [PubMed] [Google Scholar]
- 31. Zairi F, Marinho P, Allaoui M, Assaker R. New advances in the management of thoracolumbar spine metastasis. Bull Cancer, 2013, 100: 435–441. [DOI] [PubMed] [Google Scholar]
- 32. Dabravolski D, Eßer J, Lahm A, Merk H. Minimally invasive treatment of tumours and metastases in the spine by plasma field therapy (cavity coblation) and vertebro−/kyphoplasty with and without additional dorsal percutaneous instrumentation. Z Orthop Unfall, 2014, 152: 489–497. [DOI] [PubMed] [Google Scholar]
- 33. Gerszten PC, Mendel E, Yamada Y. Radiotherapy and radiosurgery for metastatic spine disease: what are the options, indications, and outcomes?. Spine (Phila Pa 1976), 2009, 34: S78–S92. [DOI] [PubMed] [Google Scholar]
- 34. Lutz S, Balboni T, Jones J, et al. Palliative radiotherapy for bone metastases: update of an ASTRO evidence‐based guideline. Pract Radiat Oncol, 2017, 7: 4–12. [DOI] [PubMed] [Google Scholar]
- 35. Ahmed KA, Stauder MC, Miller RC, et al. Stereotactic body radiation therapy in spinal metastases. Int J Radiat Oncol Biol Phys, 2012, 82: e803–e809. [DOI] [PubMed] [Google Scholar]
- 36. Nguyen QN, Shiu AS, Rhines LD, et al. Management of spinal metastases from renal cell carcinoma using stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys, 2010, 76: 1185–1192. [DOI] [PubMed] [Google Scholar]
- 37. Hsu SW, Chao HL, Lin KT, et al. Pain relief following spinal lesion treatment with stereotactic radiosurgery: clinical experience in 65 cases. J Med Sci, 2015, 35: 162. [Google Scholar]
- 38. Yang J, Ma L, Wang XS, et al. Dosimetric evaluation of 4 different treatment modalities for curative‐intent stereotactic body radiation therapy for isolated thoracic spinal metastases. Med Dosim, 2016, 41: 105–112. [DOI] [PubMed] [Google Scholar]
- 39. Wang XS, Rhines LD, Shiu AS, et al. Stereotactic body radiation therapy for management of spinal metastases in patients without spinal cord compression: a phase 1–2 trial. Lancet Oncol, 2012, 13: 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hoogcarspel SJ, Kontaxis C, van der Velden JM, et al. SU‐C‐17A‐07: the development of an MR accelerator‐enabled planning‐to‐delivery technique for stereotactic palliative radiotherapy treatment of spinal metastases. Med Phys, 2014, 41: 100. [Google Scholar]
- 41. Lau D, Than KD, La Marca F, Park P. Independent predictors for local recurrence following surgery for spinal metastasis. Acta Neurochir, 2014, 156: 277–282. [DOI] [PubMed] [Google Scholar]
- 42. Yamada Y, Bilsky MH, Lovelock DM, et al. High‐dose, single‐fraction image‐guided intensity‐modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys, 2008, 71: 484–490. [DOI] [PubMed] [Google Scholar]
- 43. Yarnold JR. 8 Gy single fraction radiotherapy for the treatment of metastatic skeletal pain: randomised comparison with a multifraction schedule over 12 months of patient follow‐up. On behalf of the Bone Pain Trial Working Party. Radiother Oncol, 1999, 52: 111–121. [PubMed] [Google Scholar]
- 44. Tong D, Gillick L, Hendrickson FR. The palliation of symptomatic osseous metastases final results of the study by the radiation therapy oncology group. Cancer, 1982, 50: 893–899. [DOI] [PubMed] [Google Scholar]
- 45. Maranzano E, Latini P. Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: final results from a prospective trial. Int J Radiat Oncol Biol Phys, 1995, 32: 959–967. [DOI] [PubMed] [Google Scholar]
- 46. Kapoor A, Singhal MK, Bagri PK, et al. Comparison of single versus multiple fractions for palliative treatment of painful bone metastasis: first study from north west India. Indian J Palliat Care, 2015, 21: 45–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Teshima T, Inoue T, Ikeda H, et al. Symptomatic relief for patients with osseous metastasis treated with radiation and methylprednisolone: a prospective randomized study. Radiat Med, 1996, 14: 185–188. [PubMed] [Google Scholar]
- 48. Wallace AN, Robinson CG, Meyer J, et al. The metastatic spine disease multidisciplinary working group algorithms. Oncologist, 2015, 20: 1205–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lussier D, Huskey AG, Portenoy RK. Adjuvant analgesics in cancer pain management. Oncologist, 2004, 9: 571–591. [DOI] [PubMed] [Google Scholar]
- 50. McNicol E, Horowicz‐Mehler N, Fisk RA, et al. Management of opioid side effects in cancer‐related and chronic noncancer pain: a systematic review. J Pain, 2003, 4: 231–256. [DOI] [PubMed] [Google Scholar]
- 51. Mercadante S. Opioid rotation for cancer pain: rationale and clinical aspects. Cancer, 1999, 86: 1856–1866. [DOI] [PubMed] [Google Scholar]
- 52. Cherny N, Ripamonti C, Pereira J, et al. Expert Working Group of the European Association of Palliative Care NetworkStrategies to manage the adverse effects of oral morphine: an evidence‐based report. J Clin Oncol, 2001, 19: 2542–2554. [DOI] [PubMed] [Google Scholar]
- 53. Singleton PA, Moss J, Karp DD, Atkins JT, Janku F. The mu opioid receptor: a new target for cancer therapy?. Cancer, 2015, 121: 2681–2688. [DOI] [PubMed] [Google Scholar]
- 54. Tomasian A, Wallace A, Northrup B, Hillen TJ, Jennings JW. Spine cryoablation: pain palliation and local tumor control for vertebral metastases. AJNR Am J Neuroradiol, 2016, 37: 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Public Health and Emerg . Interventional pain techniques in the treatment of cancer pain 2016. Available from: http://phe.amegroups.com/article/view/3559 (accessed 18 May 2016).
- 56. Smith MR, Coleman RE, Klotz L, et al. Denosumab for the prevention of skeletal complications in metastatic castration‐resistant prostate cancer: comparison of skeletal‐related events and symptomatic skeletal events. Ann Oncol, 2015, 26: 368–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Amadori D, Aglietta M, Alessi B, et al. Efficacy and safety of 12‐weekly versus 4‐weekly zoledronic acid for prolonged treatment of patients with bone metastases from breast cancer (ZOOM): a phase 3, open‐label, randomised, non‐inferiority trial. Lancet Oncol, 2013, 14: 663–670. [DOI] [PubMed] [Google Scholar]
- 58. Dearnaley DP, Mason MD, Parmar MK, Sanders K, Sydes MR. Adjuvant therapy with oral sodium clodronate in locally advanced and metastatic prostate cancer: long‐term overall survival results from the MRC PR04 and PR05 randomised controlled trials. Lancet Oncol, 2009, 10: 872–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pavlakis N, Schmidt RL, Stockler MR. Bisphosphonates for breast cancer. Cochrane Database Syst Rev, 2005, 3: CD003474. [DOI] [PubMed] [Google Scholar]
- 60. Gossiel F, Hoyle C, McCloskey EV, et al. The effect of bisphosphonate treatment on osteoclast precursor cells in postmenopausal osteoporosis: the TRIO study. Bone, 2016, 92: 94–99. [DOI] [PubMed] [Google Scholar]
- 61. Diel IJ, Bergner R, Grotz KA. Adverse effects of bisphosphonates: current issues. J Support Oncol, 2007, 5: 475–482. [PubMed] [Google Scholar]
- 62. Ruggiero SL, Fantasia J, Carlson E. Bisphosphonate‐related osteonecrosis of the jaw: background and guidelines for diagnosis, staging and management. Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 2006, 102: 433–441. [DOI] [PubMed] [Google Scholar]
- 63. Berning D, Schaefer U, Willich N, Horn K, Horst E, Micke O. Combination of radiotherapy and ibandronate in metastatic bone disease‐results of a randomized study. Int J Radiat Oncol Biol Phys, 2002, 54: 308–309. [Google Scholar]
- 64. Vassiliou V, Leotsinides M, Kalogeropoulou C, Kardamakis D. Concurrent application of bisphosphonates and external beam radiotherapy in patients with metastatic bone disease from renal cancer. BJU Int, 2009, 104: 417–418. [DOI] [PubMed] [Google Scholar]
- 65. Lipton A, Uzzo R, Amato RJ, et al. The science and practice of bone health in oncology: managing bone loss and metastasis in patients with solid tumors. J Natl Compr Canc Netw, 2009, 7 Suppl. 7: S1–S29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Aapro M, Abrahamsson PA, Body JJ, et al. Guidance on the use of bisphosphonates in solid tumours: recommendations of an international expert panel. Ann Oncol, 2008, 19: 420–432. [DOI] [PubMed] [Google Scholar]
- 67. Hillner BE, Ingle JN, Chlebowski RT, et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol, 2003, 21: 4042–4057. [DOI] [PubMed] [Google Scholar]
- 68. Lipton A. Denosumab in breast cancer. Curr Oncol Rep, 2011, 13: 1–4. [DOI] [PubMed] [Google Scholar]
- 69. Lipton A, Fizazi K, Stopeck AT, et al. Superiority of denosumab to zoledronic acid for prevention of skeletal‐related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer, 2012, 48: 3082–3092. [DOI] [PubMed] [Google Scholar]
- 70. Dunning EC, Butler JS, Morris S. Complications in the management of metastatic spinal disease. World J Orthop, 2012, 3: 114–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Verstappen CC, Heimans JJ, Hoekman K, Postma TJ. Neurotoxic complications of chemotherapy in patients with cancer. Drugs, 2003, 63: 1549–1563. [DOI] [PubMed] [Google Scholar]
- 72. Simmons CP, MacLeod N, Laird BJ. Clinical management of pain in advanced lung cancer. Clin Med Insights Oncol, 2012, 6: 331–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Deng H, Luo S, Tan T, et al. 153Sm‐EDTMP for moderate and severe bone cancer pain. Hua Xi Yi Ke Da Xue Xue Bao, 1995, 26: 391–394. [PubMed] [Google Scholar]
- 74. Lee CK, Aeppli DM, Unger J, Boudreau RJ, Levitt SH. Strontium‐89 chloride (Metastron) for palliative treatment of bony metastases: the University of Minnesota experience. Am J Clin Oncol, 1996, 19: 102–107. [DOI] [PubMed] [Google Scholar]
- 75. Kvale PA, Simoff M, Prakash UB, American College of Chest Physicians . Lung cancer. Palliative care. Chest, 2003, 123: 284S–311S. [DOI] [PubMed] [Google Scholar]
- 76. Sarafino EP, Smith TW. Health Psychology: Biopsychosocial Interactions. New York: John Wiley & Sons, 2014. [Google Scholar]
- 77. Molassiotis A, Boughton BJ, Burgoyne T, van den Akker OB. Comparison of the overall quality of life in 50 long‐term survivors of autologous and allogeneic bone marrow transplantation. J Adv Nurs, 1995, 22: 509–516. [DOI] [PubMed] [Google Scholar]
- 78. Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr, 2004, 32: 57–71. [DOI] [PubMed] [Google Scholar]
- 79. Burgess C, Cornelius V, Love S, Graham J, Richards M, Ramirez A. Depression and anxiety in women with early breast cancer: five year observational cohort study. BMJ, 2005, 330: 702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Reeves GK, Pirie K, Beral V, et al., Million Women Study Collaboration . Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ, 2007, 335: 1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Omlin A, Blum D, Wierecky J, Haile SR, Ottery FD, Strasser F. Nutrition impact symptoms in advanced cancer patients: frequency and specific interventions, a case–control study. J Cachexia Sarcopenia Muscle, 2013, 4: 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Szczesniak MM, Maclean J, Zhang T, Graham PH, Cook IJ. Persistent dysphagia after head and neck radiotherapy: a common and under‐reported complication with significant effect on non‐cancer‐related mortality. Clin Oncol (R Coll Radiol), 2014, 26: 697–703. [DOI] [PubMed] [Google Scholar]
- 83. Langstein HN, Norton JA. Mechanisms of cancer cachexia. Hematol Oncol Clin North Am, 1991, 5: 103–123. [PubMed] [Google Scholar]
- 84. Steiber AL, Handu DJ, Cataline DR, Deighton TR, Weatherspoon LJ. The impact of nutrition intervention on a reliable morbidity and mortality indicator: the hemodialysis‐prognostic nutrition index. J Ren Nutr, 2003, 13: 186–190. [DOI] [PubMed] [Google Scholar]
- 85. Porporato PE. Understanding cachexia as a cancer metabolism syndrome. Oncogene, 2016, 5: e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bruera E. ABC of palliative care. Anorexia, cachexia, and nutrition. BMJ, 1997, 315: 1219–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wen HS, Li X, Cao YZ, et al. Clinical studies on the treatment of cancer cachexia with megestrol acetate plus thalidomide. Chemotherapy, 2012, 58: 461–467. [DOI] [PubMed] [Google Scholar]
- 88. Torelli GF, Campos AC, Meguid MM. Use of TPN in terminally ill cancer patients. Nutrition, 1999, 15: 665–667. [DOI] [PubMed] [Google Scholar]
- 89. Ülger Ö, Yağlı NV. Effects of yoga on the quality of life in cancer patients. Complement Ther Clin Pract, 2010, 16: 60–63. [DOI] [PubMed] [Google Scholar]
- 90. Adamsen L, Quist M, Andersen C, et al. Effect of a multimodal high intensity exercise intervention in cancer patients undergoing chemotherapy: randomised controlled trial. BMJ, 2009, 339: b3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rief H, Förster R, Rieken S, et al. The influence of orthopedic corsets on the incidence of pathological fractures in patients with spinal bone metastases after radiotherapy. BMC Cancer, 2015, 15: 745. [DOI] [PMC free article] [PubMed] [Google Scholar]


