Abstract
Recently, the demand of transferable embryos in cattle industry is increasing, and the number of embryos produced in vitro is also increasing in the world. Although oocytes are collected from individual elite cattle by ovum-pick up (OPU) and used for in vitro production (IVP) of embryos, the cattle are mono-ovulatory animal. It means that most of oocytes collected from ovaries are destined to degenerate. To improve the IVP efficiency, we should predict the developmental competence of oocytes correctly and culture them by the suitable way. In addition, in vitro production of bovine oocytes by in vitro growth (IVG) culture system will become a candidate of supply source of oocytes for IVP. If we can produce high competent oocytes by IVG, IVP efficiency will be improved and the genetic improvement of cattle will be dramatically accelerated. In the review, I introduce our researches related to oocyte morphology, the developmental competence, and the production of oocytes having high developmental competence by IVG culture.
Keywords: Aging, Lipid droplet, Mitochondria, Oocyte morphology, Pseudomaturation
The development of an in vitro production (IVP) system of bovine embryo has provided important new information about oocyte maturation and fertilization processes, early embryo development and embryo quality. However, embryos produced in vitro are fundamentally different from their in vivo counterparts in terms of, for example, morphology [1, 2], gene expression patterns [3] and chromosomal abnormalities [4, 5]. IVP systems have also been utilized to produce a large number of embryos needed for scientific research, including efforts to produce cloned animals by somatic cell nuclear transfer, generating transgenic animals, embryonic stem cells [6] and for the rescue of irreplaceable genetic materials [7]. However, to improve the efficiency of IVP, it is necessary to evaluate the maturational ability and the functional status of oocytes before in vitro maturation (IVM).
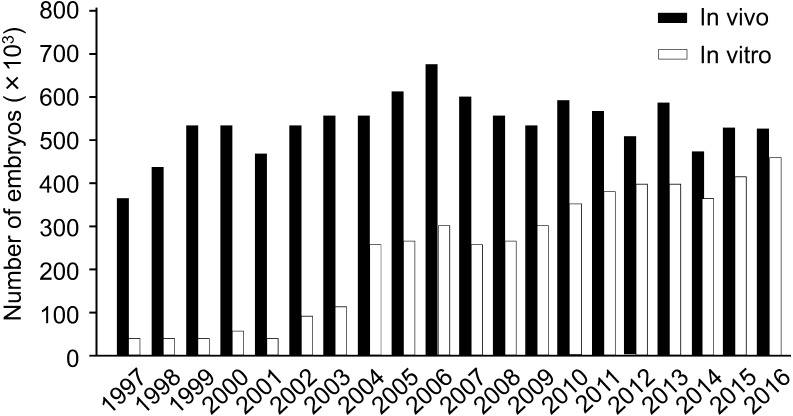
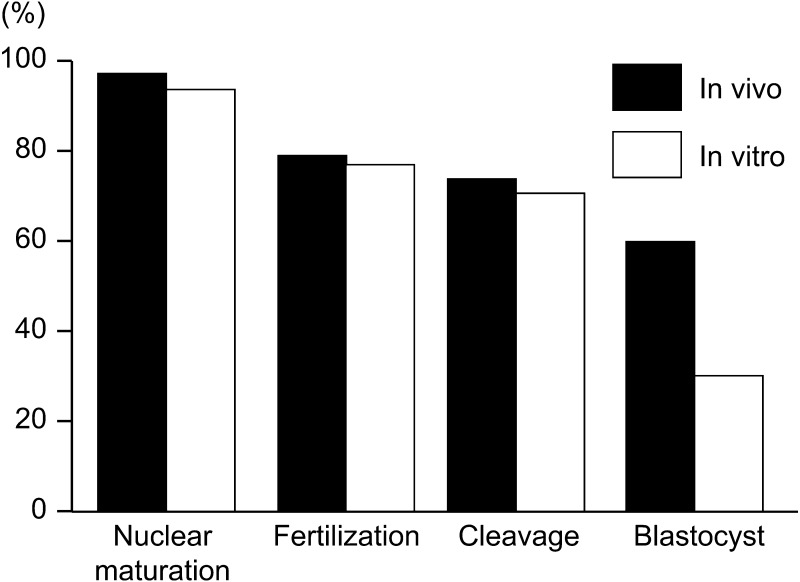
Nowadays IVP technology has been widely used commercially for producing embryos in cattle [8]. In addition, the bovine genome sequences and the variation of single nucleotide polymorphisms (SNPs) have already clarified, and the “genomic selection” based on SNPs has shortened the generation intervals dramatically [9]. Therefore, the production of embryos from younger heifers before the applicable stage of in vivo embryo production is strongly requested [8, 10, 11]. Ultrasound-guided ovum-pick up (OPU) combined with in vitro fertilization (IVF) is widely used to produce embryos in cattle for genetic improvement [8]. In addition, it is reported that the efficiency of embryo production by OPU-IVF is higher than that by in vivo embryo production [12, 13]. Therefore, the number of embryos produced in vitro has been increased internationally, and become similar to that produced in vivo (Fig. 1) [14]. On the other hand, it is well known that the developmental competence of in vitro matured (IVM) oocytes is lower than that of oocytes matured in vivo (Fig. 2) [15, 16]. Also, bovine immature oocytes transferred to pre-ovulatory follicles and induced maturation in follicles showed higher developmental competence than oocytes matured in vitro [17, 18]. These results indicate that further studies are necessary to investigate the acquisition of oocyte developmental competence in vivo and in vitro for the improvement of bovine IVP system.
Fig. 1.
Numbers of in vivo and in vitro derived embryos transferred [14]. The data was collected by national data collectors who volunteered to collect the information from the embryo transfer (ET) practitioners within their country, either directly from these practitioners or indirectly via the national ET association and entered on the IETS (International Embryo Transfer Society) database.
Fig. 2.
Developmental competence of bovine oocytes derived from in vivo and in vitro [15].
Factors Affecting the Developmental Competence of Bovine Oocytes
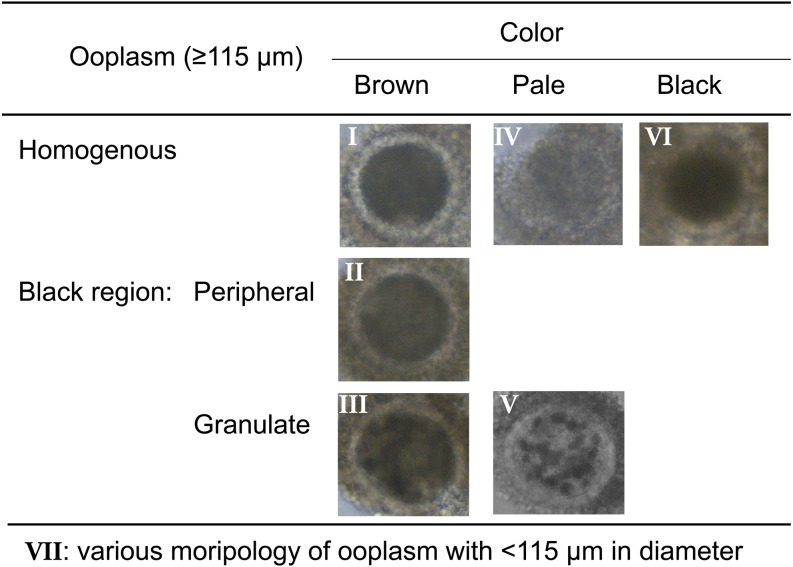
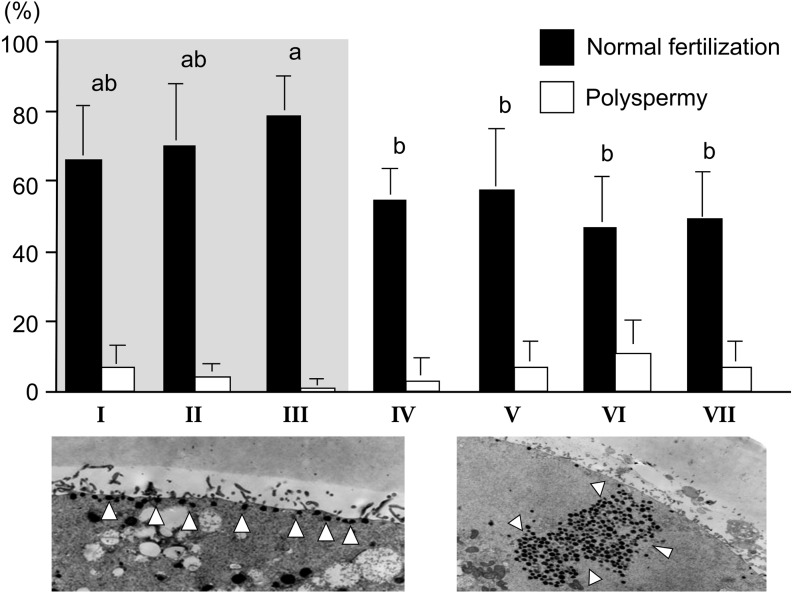
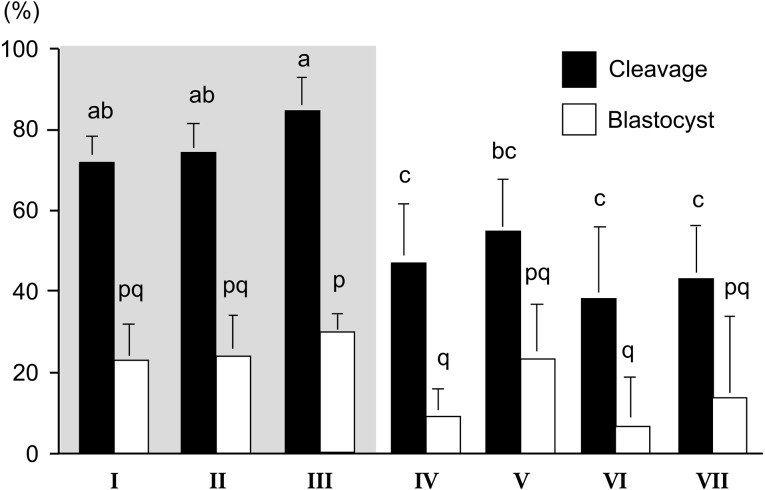
It has been widely accepted that oocytes with brown and homogeneous ooplasm surrounded by compact multi-layered cumulus investment are suitable for IVM [19]. However, we can collect a large number of bovine oocytes from slaughter house-derived ovaries and OPU. In addition, oocytes derived from antral follicles (2−8 mm in diameter) exhibit a wide variety of morphological characteristics [19, 20]. If we correctly estimate their developmental capacity before IVM culture, we can select only those oocytes capable of developing into blastocysts or develop the suitable culture system for each oocyte having various morphologies. Therefore, we investigated the relationship between the morphology of oocytes collected from small antral follicles and their developmental competences. Firstly, we divided immature oocytes derived from slaughter house materials into 7 groups (I–VII; Fig. 3) and they were submitted to IVM, IVF and in vitro culture (IVC) for development into blastocysts [21]. Furthermore, to clarify the cause of the difference in the appearance of oocytes, the ultrastructure of oocytes was also investigated [21]. After IVF, sperm penetration and normal fertilization rates were higher in the oocytes whose ooplasm appeared brown (Fig. 4), especially group III oocytes having dark cluster in the ooplasm showed few polyspermy. After IVC, the rates of cleavage and of development to the blastocyst stage were also higher in the brown oocytes. Although the oocytes with dark clusters in a pale cytoplasm showed lower cleavage rates, cleaved zygotes had high developmental rates the same as the oocytes with a brown ooplasm (Fig. 5). Transmission electron microscopy showed that the oocytes with a pale or black ooplasm had organelles arranged differently from other oocytes before IVM. Most of the oocytes with a brown, homogeneous ooplasm or small diameter had the characteristics of an immature ooplasm (large clusters of cortical granules) even after IVM (Fig. 4). On the other hand, the brown oocytes with a dark zone at the periphery or with dark clusters showed the similar arrangement of organelle as in vivo matured oocytes (Fig. 4). The oocytes with a pale or black ooplasm appeared to be degenerating and/or aging. A dark ooplasm indicates an accumulation of lipids (Fig. 6) and good developmental potential, while a pale ooplasm indicates a low density of organelles and poor developmental potential.
Fig. 3.
Morphology of bovine oocytes derived from antral follicles with 2−8 mm in diameter [21, 26].
Fig. 4.
Sperm penetration after IVF of bovine oocytes having various morphology and distribution of cortical granules [21]. ab Different letters indicate the significant difference between groups (P < 0.05). Group III oocytes had cortical granules lined up near oolemma frequently after IVM (left panel: white arrowheads indicate cortical granules). On the other hand, groups I, II, and VII oocytes had large clusters of cortical granules even after IVM (right panel). Gray area in the bar graph indicates the oocytes having brown ooplasm.
Fig. 5.
Development after IVF of bovine oocytes having various morphology [21]. abc, pq Different letters indicate the significant difference between groups (P < 0.05). Gray area indicates the oocytes having brown ooplasm.
Fig. 6.
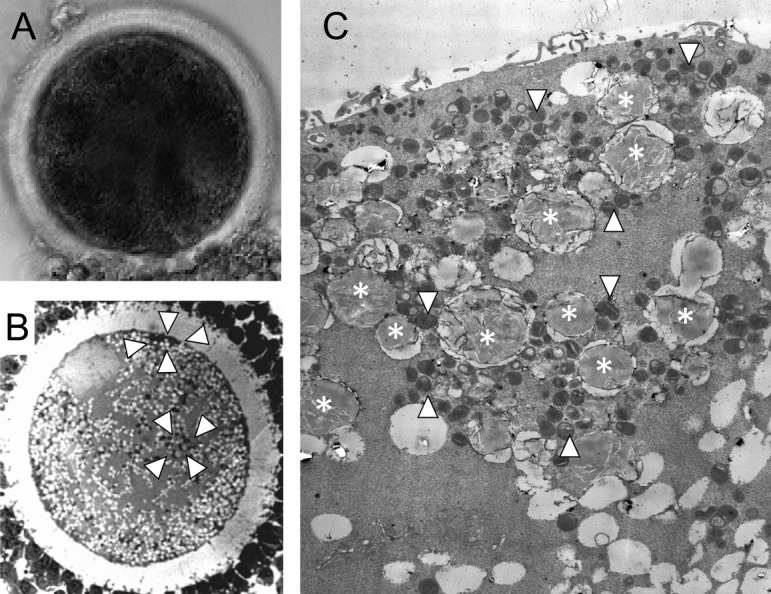
Dark area and lipid droplets in bovine oocytes [21]. A: Denuded oocytes having dark clusters in ooplasm. B: Oocyte at germinal vesicle stage has clusters of lipid droplets and mitochondria (surrounded by whit arrowheads). C: Many lipid droplets (asterisks) existed in ooplasm, and they are surrounded by mitochondria (white arrowheads).
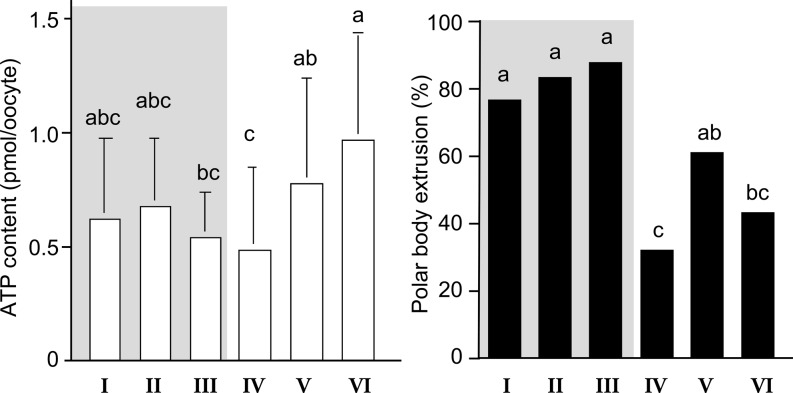
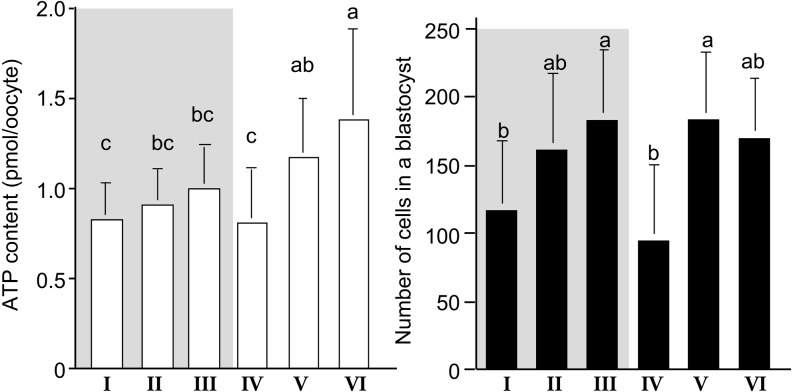
During nuclear and cytoplasmic maturation of oocytes, a rearrangement of organelles occurs. The organization and metabolic activity of mitochondria seems to be correlated with cytoplasmic maturation and a resumption of meiosis [22,23,24,25]. Therefore, we investigated the relationship between nuclear maturational ability of oocytes with various ooplasm appearances and ATP content of oocytes before IVM [26]. Also, we evaluated the relationship between ATP content and the cell numbers in blastocyst of oocytes with various ooplasm appearances [21, 26]. As shown in Fig. 7, the oocytes in groups I–III showed the highest polar body (PB) extrusion rate (P < 0.05), and had intermediate levels of ATP before IVM culture at the germinal vesicle (GV) stage. The PB extrusion rate and ATP levels of GV oocytes in group IV were the lowest, maybe due to low level of energy substrate (lipid droplets), and thus, they cannot produce the ATP required to complete nuclear maturation. GV oocytes having a pale ooplasm with dark clusters (group V) and those with a black ooplasm (group VI) had higher level of ATP but showed lower rates of PB extrusion than the oocytes with a brown ooplasm. GV oocytes in groups V and VI had a similar arrangement of organelles, including the distribution of mitochondria, to that of matured oocytes, and accumulated lipid droplets in their ooplasm [21]. Thus, GV oocytes in groups V and VI may have produced a larger amount of ATP before they were collected from ovarian follicles, or unusually high levels of ATP in ooplasm may indicate a disruption of oocyte functions for consuming ATP. After IVM, the ATP levels of matured oocytes were higher than those of GV oocytes in all groups (Fig. 8). Matured oocytes in groups II - III had intermediate levels of ATP. The ATP levels of matured oocytes were highest in group VI and lowest in groups I and IV. The ATP levels of matured oocytes in group V were between those in groups II - III and group VI. Oocytes in groups I and IV, which showed low rates of development to the blastocyst stage (Fig. 5) and small cell numbers in resulting blastocysts (Fig. 8), had fewer active mitochondria [26] and less ATP (Fig. 8). ATP is mainly used by microtubules and actin filaments during cell division [27]. Thus, matured oocytes with low levels of ATP may develop into blastocysts with a small number of cells. Matured oocytes in group VI had the highest levels of ATP, but a low rate of development into blastocysts. These results clearly indicated that storage of ATP at proper levels in matured oocytes is one of the key factors determining subsequent embryonic development and the quality of resulting blastocysts. More importantly, the results suggest that too high levels of ATP in matured oocytes may indicate an impaired developmental competence and disruption of the control of mitochondrial functions of oocytes. In our previous study, we showed the aged bovine oocytes had higher mitochondrial activity and ATP level [28]. The control of mitochondrial activity in oocytes should be investigate in further study.
Fig. 7.
ATP content and nuclear maturation rate of bovine oocytes having various morphology [26]. abc Different letters indicate the significant difference between groups (P < 0.05). Gray area indicates the oocytes having brown ooplasm.
Fig. 8.
ATP content after IVM and cell number in a blastocyst derived from bovine oocytes having various morphology [21, 26]. abc [ letters indicate the significant difference between groups (P < 0.05). Gray area indicates the oocytes having brown ooplasm.
Changes of Morphological Appearance of Bovine Oocytes during Follicular Development
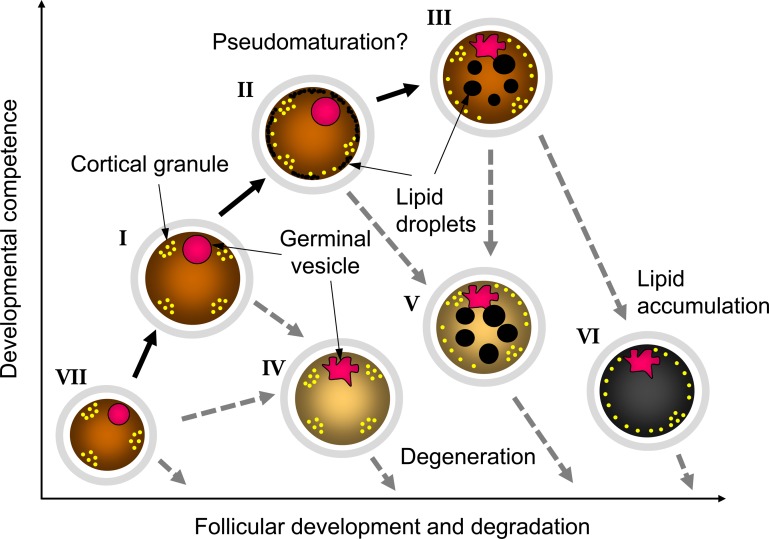
For the efficient production of transferable embryos in vitro, we should collect the oocytes with high developmental competence; however, in cattle, only one oocyte will be ovulated, and others are destined to degenerate. On the other hand, some oocytes acquire relatively high developmental competence before degeneration as mentioned above. Therefore, it is important to know when we can collect the oocytes having high developmental competence, and we investigate the relationship between estrous cycle (follicular wave stage) and oocyte morphology. As the results, it was clarified that we can collect many oocytes for submitting IVP at the recruit and the selection phases of follicular wave [29]. Although we can collect the larger number of oocytes at the recruit phase, many follicles under 3 mm in diameter, including oocytes with small diameter or pale ooplasm oocytes having low developmental competence, were existing at recruit phase. From our consecutive studies [21, 26, 29], we speculate the changes of oocyte morphology and developmental competence as described in Fig. 9. Briefly, small oocytes (group VII) included in small follicles grow with follicular development, and the size and developmental competence of oocytes increase (groups I and II). Then only one follicle is selected to develop to a dominant follicle and ovulates, but other follicles start to degenerate. During degeneration process, the accumulation of lipid droplets and undulation of nuclear membrane of GV start, and the developmental competence of oocytes also increase (pseudomaturation like change in group III). However, too much pseudomaturation like changes impair the developmental competence of oocytes (groups V and VI). If oocytes start to degenerate before pseudomaturation like changes, the oocytes may become group IV. Actually, the timing of oocyte recovery is clearly affected the developmental competence of oocytes. In our previous study [30], we showed the obvious change of the fertilizability of bovine oocytes collected at different duration of OPU interval. The result indicates the possibility, we can collect only high-quality oocytes by control the timing of OPU or by control the follicular development. In further study, we should examine the method for control of oocyte acquisition of developmental competence.
Fig. 9.
Schematic features of oocyte morphology, developmental competence, and follicular development in cattle.
Studies on the Improvement of Oocyte Developmental Competence
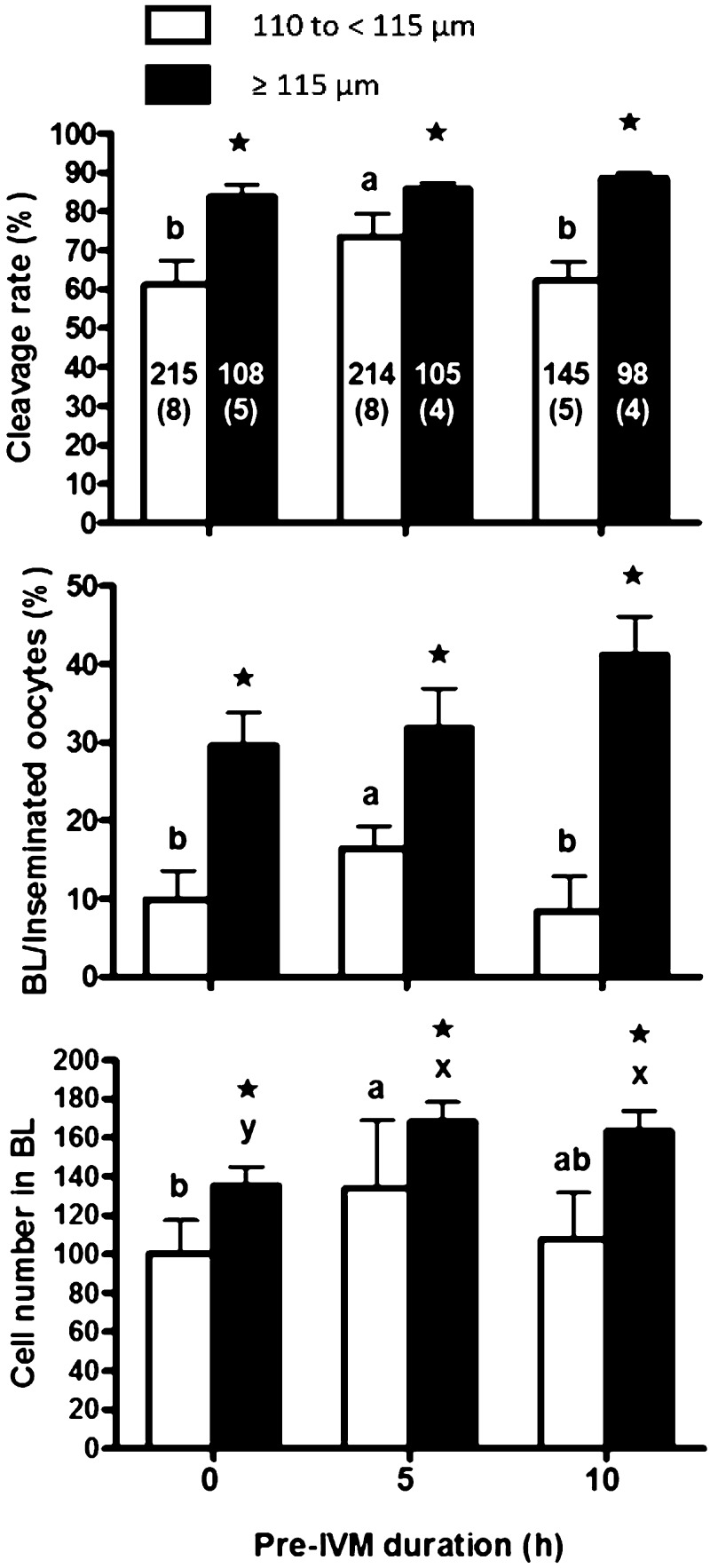
The oocytes collected from bovine ovaries vary in size, and development to the blastocyst stage is known to increase with larger follicular and oocyte diameters [31]. It was reported that the percentage of 115 to < 120 µm oocytes developing to blastocyst stage were higher than that of 110 to < 115 µm oocytes [32]. Furthermore, there was a positive correlation between oocyte diameter and follicular size [33]. When OPU is performed, follicles with more than 2 mm in diameter are generally aspirated, but the number of oocytes harvested is limited [34]. It was also reported that the mean diameter of oocytes collected from follicles with 2–3 mm in diameter was 112.9 µm [33], thus, the improvement of small-sized oocytes (110 to < 115 µm; group VII) should be attempted for the effective IVP [35]. We speculated that oocytes collected by OPU, especially small-sized oocytes, need time for cytoplasmic maturation prior to IVM to acquire developmental competence, and we divided oocytes into small-sized (110 to < 115 µm) and large-sized (≥ 115 µm) oocytes and cultured for 0, 5, or 10 h with 3-isobutyl-1-methylxanthine (IBMX), which prevented the meiotic resumption of bovine oocytes and improved the nuclear maturation and the blastocyst development [36, 37], before IVM culture (pre-IVM). As shown in Fig. 10, the cleavage rate of embryos derived from the small-sized oocytes with 5 h pre-IVM was higher than those with 0 and 10 h pre-IVM. The blastocyst rate, based on inseminated oocytes, of embryos derived from small-sized oocytes subjected to 5 h pre-IVM was higher than those with 0 and 10 h pre-IVM, but was lower than that of the large-sized oocytes. In addition, blastocysts derived from small-sized oocytes with 5 h pre-IVM had a higher mean cell number than those with 0 and 10 h pre-IVM. In addition, 5 and 10 h pre-IVM did not have any detrimental effects (aging) on cleavage and blastocyst rates in large-sized oocytes, and the cell number in blastocysts significantly increased with 5 and 10 h pre-IVM than without pre-IVM. These results may indicate that we can apply the pre-IVM to OPU-IVF and improve IVP efficiency by pre-IVM.
Fig. 10.
Cleavage rates, blastocyst rates, and blastocyst cell numbers after 0, 5, and 10 h of pre-IVM for small-sized oocytes (110 to < 115 µm in diameter) and large-sized oocytes (≥ 115 µm in diameter) [34]. The number in the bar is the number of oocytes and replicates in parentheses. * Asterisk indicates a significant difference between experimental groups (P < 0.05). abc Different letters indicate a significant difference in small-sized oocytes (P < 0.05). xyz Different letters indicate a significant difference in large-sized oocytes (P < 0.05).
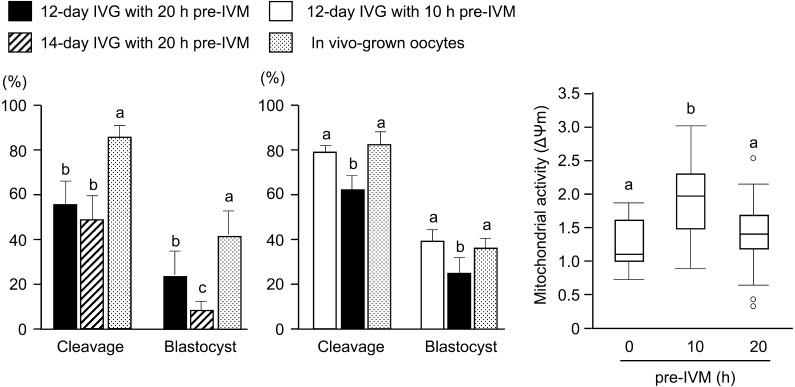
We have also tried to develop the in vitro growth (IVG) culture system for bovine small-sized oocytes (approximately 95 µm in diameter), which have no developmental competence without IVG [36,37,38,39,40,41,42,43,44,45]. In these studies, we attempted the pre-IVM culture of oocytes using IBMX after 12- or 14-day IVG culture [36, 37]. As shown in Fig. 11, the extension of culture period from 12 to 14 days had a negative effect on the development rate to blastocysts although the cleavage rate was similar. When we evaluated the mitochondrial activity in IVG oocytes derived from 12-day IVG during pre-IVM culture, the mitochondrial activity increased at 10 h pre-IVM, but decreased at 20 h pre-IVM. The result indicates that oocyte aging occurs during pre-IVM. In addition, oocytes derived from 12-day IVG and 10 h pre-IVM showed high developmental competence to blastocyst stage the same as in vivo-grown oocytes derived from antral follicles with 2−8 mm in diameter. We also succeeded to produce a healthy offspring derived from 12-day IVG and 10 h pre-IVM [36]. However, the examination of the optimal duration of pre-IVM culture is necessary.
Fig. 11.
Effect of IVG culture duration and pre-IVM on the development of IVG oocytes and mitochondrial activity [36, 37]. abc Different letters indicate the significant difference between groups (P < 0.05).
Our ultimate objectives are to establish the model of in vivo follicular development and to produce the oocytes with high developmental competence the same as in vivo matured (ovulated) oocytes; however, cultured oocyte-granulosa-cumulus complexes produce a large amount of progesterone during IVG like as degenerating follicles [41,42,43]. It means that the IVG system can be used as the model of degenerating follicles but not healthily developing follicles. Therefore, several modifications of IVG culture system is required. Recently, we reported that the addition of astaxanthin to IVG medium dramatically decreased the progesterone production during IVG culture [45]. Astaxanthin exhibits more powerful antioxidant activity than vitamin C, vitamin E, and β-carotene; the antioxidant activity of astaxanthin was shown to be 100- to 500-fold greater than that α-tocopherol and 15-fold greater than those of other carotenoids [46]. The antioxidant effects on the developmental competence of bovine IVP embryos have been attributed to the induction of antioxidant genes and suppression of apoptotic genes [47]. However, the mechanism of the inhibition of progesterone production is unknown.
Concluding Remarks
The demand of in vitro derived bovine embryos will increase more; therefore, the efficient IVP system should be developed more. The present IVG culture system is including oocyte and granulosa cells but not theca cells. Theca cells are essential to oocyte growth and support follicular structure and provide several factors to granulosa cells. In addition, to mimic in vivo follicular development, we should investigate the function of theca cells and perform the integrated study on in vivo and in vitro phenomena. There are so many things to investigate about in vivo and in vitro follicular development. We are continuing the investigation about them and we hope our research can contribute to the development of cattle industry and the basic research in reproduction.
Acknowledgments
I would like to express my gratitude to the Society for Reproduction and Development (SRD) for awarding me an SRD Outstanding Research Award in 2018. I am also very grateful to Dr Y Takahashi and Dr S Katagiri for valuable suggestions and kind advices concerning my research. I am thankful to my students in Hokkaido University and Tottori University for performing the researches with me. This work was supported by JSPS KAKENHI Grant Numbers 12760183, 17780206, 21780286, 25450441, and JP16K08043.
References
- 1.Crosier AE, Farin PW, Dykstra MJ, Alexander JE, Farin CE. Ultrastructural morphometry of bovine blastocysts produced in vivo or in vitro. Biol Reprod 2001; 64: 1375–1385. [DOI] [PubMed] [Google Scholar]
- 2.Maddox-Hyttell P, Gjørret JO, Vajta G, Alexopoulos NI, Lewis I, Trounson A, Viuff D, Laurincik J, Müller M, Tveden-Nyborg P, Thomsen PD. Morphological assessment of preimplantation embryo quality in cattle. Reprod Suppl 2003; 61: 103–116. [PubMed] [Google Scholar]
- 3.Lonergan P, Rizos D, Gutierrez-Adan A, Fair T, Boland MP. Oocyte and embryo quality: effect of origin, culture conditions and gene expression patterns. Reprod Domest Anim 2003; 38: 259–267. [DOI] [PubMed] [Google Scholar]
- 4.Viuff D, Hendriksen PJM, Vos PLAM, Dieleman SJ, Bibby BM, Greve T, Hyttel P, Thomsen PD. Chromosomal abnormalities and developmental kinetics in in vivo-developed cattle embryos at days 2 to 5 after ovulation. Biol Reprod 2001; 65: 204–208. [DOI] [PubMed] [Google Scholar]
- 5.Viuff D, Palsgaard A, Rickords L, Lawson LG, Greve T, Schmidt M, Avery B, Hyttel P, Thomsen PD. Bovine embryos contain a higher proportion of polyploid cells in the trophectoderm than in the embryonic disc. Mol Reprod Dev 2002; 62: 483–488. [DOI] [PubMed] [Google Scholar]
- 6.Niemann H, Kues WA. Application of transgenesis in livestock for agriculture and biomedicine. Anim Reprod Sci 2003; 79: 291–317. [DOI] [PubMed] [Google Scholar]
- 7.Hasler JF. The current status and future of commercial embryo transfer in cattle. Anim Reprod Sci 2003; 79: 245–264. [DOI] [PubMed] [Google Scholar]
- 8.Moore SG, Hasler JF. A 100-Year Review: Reproductive technologies in dairy science. J Dairy Sci 2017; 100: 10314–10331. [DOI] [PubMed] [Google Scholar]
- 9.García-Ruiz A, Cole JB, VanRaden PM, Wiggans GR, Ruiz-López FJ, Van Tassell CP. Changes in genetic selection differentials and generation intervals in US Holstein dairy cattle as a result of genomic selection. Proc Natl Acad Sci USA 2016; 113: E3995–E4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landry DA, Bellefleur AM, Labrecque R, Grand FX, Vigneault C, Blondin P, Sirard MA. Effect of cow age on the in vitro developmental competence of oocytes obtained after FSH stimulation and coasting treatments. Theriogenology 2016; 86: 1240–1246. [DOI] [PubMed] [Google Scholar]
- 11.Currin L, Michalovic L, Bellefleur AM, Gutierrez K, Glanzner W, Schuermann Y, Bohrer RC, Dicks N, da Rosa PR, De Cesaro MP, Lopez R, Grand FX, Vigneault C, Blondin P, Gourdon J, Baldassarre H, Bordignon V. The effect of age and length of gonadotropin stimulation on the in vitro embryo development of Holstein calf oocytes. Theriogenology 2017; 104: 87–93. [DOI] [PubMed] [Google Scholar]
- 12.Pontes JH, Nonato-Junior I, Sanches BV, Ereno-Junior JC, Uvo S, Barreiros TR, Oliveira JA, Hasler JF, Seneda MM. Comparison of embryo yield and pregnancy rate between in vivo and in vitro methods in the same Nelore (Bos indicus) donor cows. Theriogenology 2009; 71: 690–697. [DOI] [PubMed] [Google Scholar]
- 13.Blondin P. Logistics of large scale commercial IVF embryo production. Reprod Fertil Dev 2016; 29: 32–36. [DOI] [PubMed] [Google Scholar]
- 14.Perry G. 2016 Statistics of embryo collection and transfer in domestic farm animals. ET Newsletter 2017; 35: 8–23. [Google Scholar]
- 15.Sirard M, Blondin P. Oocyte maturation and IVF in cattle. Anim Reprod Sci 1996; 42: 417–426. [Google Scholar]
- 16.Matoba S, Yoshioka H, Matsuda H, Sugimura S, Aikawa Y, Ohtake M, Hashiyada Y, Seta T, Nakagawa K, Lonergan P, Imai K. Optimizing production of in vivo-matured oocytes from superstimulated Holstein cows for in vitro production of embryos using X-sorted sperm. J Dairy Sci 2014; 97: 743–753. [DOI] [PubMed] [Google Scholar]
- 17.Kassens A, Held E, Salilew-Wondim D, Sieme H, Wrenzycki C, Tesfaye D, Schellander K, Hoelker M. Intrafollicular oocyte transfer (IFOT) of abattoir-derived and in vitro-matured oocytes results in viable blastocysts and birth of healthy calves. Biol Reprod 2015; 92: 150. [DOI] [PubMed] [Google Scholar]
- 18.Hoelker M, Kassens A, Salilew-Wondim D, Sieme H, Wrenzycki C, Tesfaye D, Neuhoff C, Schellander K, Held-Hoelker E. Birth of healthy calves after intra-follicular transfer (IFOT) of slaughterhouse derived immature bovine oocytes. Theriogenology 2017; 97: 41–49. [DOI] [PubMed] [Google Scholar]
- 19.Leibfried L, First NL. Characterization of bovine follicular oocytes and their ability to mature in vitro. J Anim Sci 1979; 48: 76–86. [DOI] [PubMed] [Google Scholar]
- 20.Hazeleger NL, Hill DJ, Stubbing RB, Walton JS. Relationship of morphology and follicular fluid environment of bovine oocytes to their developmental potential in vitro. Theriogenology 1995; 43: 509–522. [DOI] [PubMed] [Google Scholar]
- 21.Nagano M, Katagiri S, Takahashi Y. Relationship between bovine oocyte morphology and in vitro developmental potential. Zygote 2006; 14: 53–61. [DOI] [PubMed] [Google Scholar]
- 22.Calarco PG. Polarization of mitochondria in the unfertilized mouse oocyte. Dev Genet 1995; 16: 36–43. [DOI] [PubMed] [Google Scholar]
- 23.Hyttel P, Xu KP, Smith S, Greve T. Ultrastructure of in-vitro oocyte maturation in cattle. J Reprod Fertil 1986; 78: 615–625. [DOI] [PubMed] [Google Scholar]
- 24.Kruip TAM, Cran DG, Van Beneden TH, Dieleman SJ. Structural changes in bovine oocytes during final maturation in vivo. Gamete Res 1983; 8: 29–47. [Google Scholar]
- 25.Van Blerkom J, Runner MN. Mitochondrial reorganization during resumption of arrested meiosis in the mouse oocyte. Am J Anat 1984; 171: 335–355. [DOI] [PubMed] [Google Scholar]
- 26.Nagano M, Katagiri S, Takahashi Y. ATP content and maturational/developmental ability of bovine oocytes with various cytoplasmic morphologies. Zygote 2006; 14: 299–304. [DOI] [PubMed] [Google Scholar]
- 27.Wanka F, Van Zoelen EJJ. Force generation by cellular motors. Cell Mol Biol Lett 2003; 8: 1017–1033. [PubMed] [Google Scholar]
- 28.Koyama K, Kang SS, Huang W, Yanagawa Y, Takahashi Y, Nagano M. Aging-related changes in in vitro-matured bovine oocytes: oxidative stress, mitochondrial activity and ATP content after nuclear maturation. J Reprod Dev 2014; 60: 136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagano M, Hishinuma M, Katagiri S, Takahashi Y. The relationship between oocyte morphology and ovarian status in cattle. J Reprod Dev 2007; 53: 953–958. [DOI] [PubMed] [Google Scholar]
- 30.Nagai K, Yanagawa Y, Katagiri S, Nagano M. Fertilizability of oocytes derived from Holstein cows having different antral follicle counts in ovaries. Anim Reprod Sci 2015; 163: 172–178. [DOI] [PubMed] [Google Scholar]
- 31.Arlotto T, Schwartz JL, First NL, Leibfried-Rutledge ML. Aspects of follicle and oocyte stage that affect in vitro maturation and development of bovine oocytes. Theriogenology 1996; 45: 943–956. [DOI] [PubMed] [Google Scholar]
- 32.Otoi T, Yamamoto K, Koyama N, Tachikawa S, Suzuki T. Bovine oocyte diameter in relation to developmental competence. Theriogenology 1997; 48: 769–774. [DOI] [PubMed] [Google Scholar]
- 33.Fair T, Hyttel P, Greve T. Bovine oocyte diameter in relation to maturational competence and transcriptional activity. Mol Reprod Dev 1995; 42: 437–442. [DOI] [PubMed] [Google Scholar]
- 34.Merton JS, de Roos AP, Mullaart E, de Ruigh L, Kaal L, Vos PL, Dieleman SJ. Factors affecting oocyte quality and quantity in commercial application of embryo technologies in the cattle breeding industry. Theriogenology 2003; 59: 651–674. [DOI] [PubMed] [Google Scholar]
- 35.Abdel-Ghani MA, Sakaguchi K, Kanno C, Yanagawa Y, Katagiri S, Nagano M. Effects of pre-maturational culture duration on developmental competence of bovine small-sized oocytes. J Reprod Dev 2018; 64: 365–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang W, Kang SS, Nagai K, Yanagawa Y, Takahashi Y, Nagano M. Mitochondrial activity during pre-maturational culture in in vitro-grown bovine oocytes is related to maturational and developmental competences. Reprod Fertil Dev 2016; 28: 349–356. [DOI] [PubMed] [Google Scholar]
- 37.Huang W, Kang SS, Yanagawa Y, Yang Y, Takahashi Y, Nagano M. Effects of in vitro-growth culture duration on fertilizability of bovine growing oocytes and proliferation of cells surrounding oocytes. Jpn J Vet Res 2014; 62: 135–141. [PubMed] [Google Scholar]
- 38.Huang W, Nagano M, Kang SS, Yanagawa Y, Takahashi Y. Effects of in vitro growth culture duration and prematuration culture on maturational and developmental competences of bovine oocytes derived from early antral follicles. Theriogenology 2013; 80: 793–799. [DOI] [PubMed] [Google Scholar]
- 39.Huang W, Nagano M, Kang SS, Yanagawa Y, Takahashi Y. Prematurational culture with 3-isobutyl-1-methylxanthine synchronizes meiotic progression of the germinal vesicle stage and improves nuclear maturation and embryonic development in in vitro-grown bovine oocytes. J Reprod Dev 2014; 60: 9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagai K, Yanagawa Y, Katagiri S, Nagano M. The relationship between antral follicle count in a bovine ovary and developmental competence of in vitro-grown oocytes derived from early antral follicles. Biomed Res 2016; 37: 63–71. [DOI] [PubMed] [Google Scholar]
- 41.Sakaguchi K, Huang W, Yang Y, Yanagawa Y, Nagano M. Relationship between in vitro growth of bovine oocytes and steroidogenesis of granulosa cells cultured in medium supplemented with bone morphogenetic protein-4 and follicle stimulating hormone. Theriogenology 2017; 97: 113–123. [DOI] [PubMed] [Google Scholar]
- 42.Sakaguchi K, Tanida T, Abdel-Ghani MA, Kanno C, Yanagawa Y, Katagiri S, Nagano M. Relationship between the antral follicle count in bovine ovaries from a local abattoir and steroidogenesis of granulosa cells cultured as oocyte-cumulus-granulosa complexes. J Reprod Dev 2018; 64: 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang Y, Kanno C, Huang W, Kang SS, Yanagawa Y, Nagano M. Effect of bone morphogenetic protein-4 on in vitro growth, steroidogenesis and subsequent developmental competence of the oocyte-granulosa cell complex derived from bovine early antral follicles. Reprod Biol Endocrin2016; 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang Y, Kanno C, Sakaguchi K, Yanagawa Y, Katagiri S, Nagano M. Extension of the culture period for the in vitro growth of bovine oocytes in the presence of bone morphogenetic protein-4 increases oocyte diameter, but impairs subsequent developmental competence. Anim Sci J 2017; 88: 1686–1691. [DOI] [PubMed] [Google Scholar]
- 45.Abdel-Ghani MA, Yanagawa Y, Balboula AZ, Sakaguchi K, Kanno C, Katagiri S, Takahashi M, Nagano M. Astaxanthin improves the developmental competence of in vitro-grown oocytes and modifies the steroidogenesis of granulosa cells derived from bovine early antral follicles. Reprod Fertil Dev 2019; 31: 272–281. [DOI] [PubMed] [Google Scholar]
- 46.Naguib YMA. Antioxidant activities of astaxanthin and related carotenoids. J Agric Food Chem 2000; 48: 1150–1154. [DOI] [PubMed] [Google Scholar]
- 47.Jang HY, Ji SJ, Kim YH, Lee HY, Shin JS, Cheong HT, Kim JT, Park IC, Kong HS, Park CK, Yang BK. Antioxidative effects of astaxanthin against nitric oxide-induced oxidative stress on cell viability and gene expression in bovine oviduct epithelial cell and the developmental competence of bovine IVM/IVF embryos. Reprod Domest Anim 2010; 45: 967–974. [DOI] [PubMed] [Google Scholar]