Abstract
Importance
The need for improved methods of hemorrhage control and resuscitation has resulted in a reappraisal of resuscitative endovascular balloon occlusion of the aorta (REBOA). However, there is a paucity of data regarding the use of REBOA on a multi-institutional level in the United States.
Objective
To evaluate the outcomes in trauma patients after REBOA placement.
Design, Setting, and Participants
A case-control retrospective analysis was performed of the 2015-2016 American College of Surgeons Trauma Quality Improvement Program data set, a national multi-institutional database of trauma patients in the United States. A total of 593 818 adult trauma patients (aged ≥18 years) were analyzed and 420 patients were matched and included in the study; patients who were dead on arrival or were transferred from other facilities were excluded. Trauma patients who underwent REBOA placement in the ED were identified and matched with a similar cohort of patients (the no-REBOA group). Both groups were matched in a 1:2 ratio using propensity score matching for demographics, vital signs, mechanism of injury, injury severity score, head abbreviated injury scale score, each body region abbreviated injury scale score, pelvic fractures, lower extremity vascular injuries and fractures, and number and grades of intra-abdominal solid organ injuries.
Main Outcomes and Measures
Outcome measures were the rates of complications and mortality.
Results
Of 593 818 trauma patients, 420 patients (the REBOA group, 140 patients; 36 women and 104 men; mean [SD] age, 44 [20] years; the no-REBOA group, 280 patients; 77 women and 203 men; mean [SD] age, 43 [19] years) were matched and included in the analysis. Among the REBOA group, median injury severity score was 29 (interquartile range [IQR], 18-38) and 129 patients (92.1%) had a blunt mechanism of injury. There was no significant difference between groups in median 4-hour blood transfusion (REBOA: packed red blood cells, 6 U [IQR, 3-8 U]; platelets, 4 U [IQR, 3-9 U], and plasma, 3 U [IQR, 2-5 U]; and no-REBOA: packed red blood cells, 7 U [IQR, 3-9 U]; platelets, 4 U [IQR, 3-8 U], and plasma, 3 U [IQR, 2-6 U]) or 24-hour blood transfusion (REBOA: packed red blood cells, 9 U [IQR, 5-20 U]; platelets, 7 U [IQR, 3-13 U], and plasma, 9 U [IQR, 6-20 U]; and no-REBOA: packed red blood cells, 10 U [IQR, 4-21 U]; platelets, 8 U [IQR, 3-12 U], and plasma, 10 U [IQR, 7-20 U]), median hospital length of stay (REBOA, 8 days [IQR, 1-20 days]; and no-REBOA, 10 days [IQR, 5-22 days]), or median intensive care unit length of stay (REBOA, 5 days [IQR, 2-14 days]; and no-REBOA, 6 days [IQR, 3-15 days]). The mortality rate was higher in the REBOA group as compared with the no-REBOA group (50 [35.7%] vs 53 [18.9%]; P = .01). Patients who underwent REBOA placement were also more likely to develop acute kidney injury (15 [10.7%] vs 9 [3.2%]; P = .02) and more likely to undergo lower extremity amputation (5 [3.6%] vs 2 [0.7%]; P = .04).
Conclusions and Relevance
Placement of REBOA in severely injured trauma patients was associated with a higher mortality rate compared with a similar cohort of patients with no placement of REBOA. Patients in the REBOA group also had higher rates of acute kidney injury and lower leg amputations. There is a need for a concerted effort to clearly define when and in which patient population REBOA has benefit.
This case-control study uses the American College of Surgeons Trauma-Quality Improvement Program data set to evaluate the outcomes in trauma patients after placement of resuscitative endovascular balloon occlusion of the aorta.
Key Points
Question
Is there a benefit of placement of resuscitative endovascular balloon occlusion of the aorta for resuscitation of severely injured trauma patients?
Findings
In this case-control study that included 420 patients (resuscitative endovascular balloon occlusion of the aorta, 140; no resuscitative endovascular balloon occlusion of the aorta, 280), the patients who received resuscitative endovascular balloon occlusion of the aorta had significantly higher rates of acute kidney injury and lower-limb amputation and higher mortality compared with similarly injured patients who did not receive resuscitative endovascular balloon occlusion of the aorta.
Meaning
The use of resuscitative endovascular balloon occlusion of the aorta in severely injured trauma patients may increase the risk of complications and mortality.
Introduction
Trauma remains one of the leading causes of morbidity and mortality in the United States.1 More than 20% to 40% of trauma deaths occurring after hospital admission are caused by massive hemorrhage that is potentially preventable.2 Hemostatic resuscitation ensures normal hemostatic competence and resuscitation to improve prognosis in such patients.3 Temporary hemostatic measures such as aortic occlusion have been used for more than 50 years.4 Endoluminal occlusion of the aorta with a balloon has been described to occlude the blood flow distal to the diaphragm.5 Resuscitative endovascular balloon occlusion of the aorta (REBOA) in trauma was first used more than 50 years ago during resuscitative efforts for injured soldiers in the Korean War4; however, it was not mentioned in the emergency medicine literature until 1986.6 The use of REBOA declined in the 1990s and early 2000s. During the past decade, however, REBOA has gained the attention of trauma surgeons5,7,8,9 in both military and civilian settings.
Trauma with a noncompressible torso hemorrhage requires urgent hemorrhage control. Use of REBOA has been shown to provide circulatory support in such patients with hypovolemia. In animal model studies, the use of REBOA could temporize exsanguinating hemorrhage and was able to restore perfusion.9 Such therapy could be critical to definitive hemorrhage control. A national, multi-institutional study from Japan10 has shown that REBOA is associated with a higher mortality, while others7,8 have demonstrated its usefulness in clinical settings to avoid life-threating hemorrhage. However, in the United States, extensive use of REBOA is limited because of the lack of clinical and research evidence of its outcomes. Numerous small single-center studies have analyzed the use of REBOA in trauma patients. However, there is a paucity of multi-institutional data at a national level regarding the efficacy and safety of REBOA in the United States. Therefore, the aim of our study was to evaluate the outcomes in trauma patients after REBOA placement by using the national American College of Surgeons Trauma Quality Improvement Program data set (ACS-TQIP). We hypothesized that REBOA placement would be associated with improved survival.
Methods
Study Design and Population
We performed a retrospective analysis of the 2015-2016 ACS-TQIP database and identified all patients who received REBOA within 1 hour of admission. The ACS-TQIP is one of the largest databases of trauma patients in the United States: as of 2016, more than 740 hospitals were participating in the ACS-TQIP. Trained personnel abstract more than 100 patient and institutional variables. The University of Arizona Institutional Review Board granted this study exemption from approval because the ACS-TQIP contains only deidentified data.
Inclusion and Exclusion Criteria
We included all adult patients (≥18 years of age) who received REBOA within 1 hour of presentation to the emergency department (ED). We excluded patients who were dead on arrival, were transferred, had missing physiological parameters, or who underwent resuscitative thoracotomy. The following International Statistical Classification of Diseases and Related Health Problems, Tenth Revision procedure codes were used to identify patients who underwent REBOA placement: 04L03DZ, 04L03DJ, 04L04DZ, and 02LW3DJ.
Data Points
We abstracted the following data points: demographics (age, sex, race, and ethnicity), injury parameters (mechanism of injury, injury severity score [ISS], and each body region abbreviated injury scale score [AIS]), prehospital and ED vital signs (systolic blood pressure [SBP], heart rate [HR], temperature, and Glasgow Coma Scale [GCS] score), transfusion parameters (packed red blood cells [PRBCs], platelets, and fresh frozen plasma), hospital length of stay (LOS), in-hospital complications, and mortality.
Patient Stratification
Patients were stratified into 2 cohorts based on whether they received the intervention: those who received REBOA (the REBOA group) and those who did not receive REBOA (the no-REBOA group).
Outcomes
Our primary outcome measures were ED mortality, 24-hour mortality, and mortality after 24 hours in both groups. Secondary outcome measures were transfusion requirements at 4 hours and 24 hours after injury, in-hospital complications (deep venous thrombosis, pulmonary embolism, stroke, myocardial infarction, extremity compartment syndrome, unplanned return to the operating room, and lower limb amputation), hospital LOS, and intensive care unit LOS.
Missing Data Analysis
Missing data were treated as missing completely at random. We performed multiple imputations using a missing value analysis technique to account for the missing values. For multiple imputations, the original data set was analyzed for random missing data points using the Little missing completely at random test. The Markov-Chain Monte Carlo method was also used for multiple imputations. This method refers to a collection of methods for simulating random draws from nonstandard distributions.
Statistical Analysis
We performed propensity score matching. Patients who underwent REBOA placement were matched with a similar cohort of patients who did not undergo REBOA placement in a 1:2 ratio for demographics, vital signs (prehospital and ED SBP, HR, and GCS score), mechanism of injury, ISS, each body region AIS, pelvic fractures (intact, incompletely disrupted, and completely disrupted pelvic ring), lower extremity vascular injuries and fractures, and number and grades of intra-abdominal solid organ injuries (liver, spleen, and kidney injuries). A logistic regression model was used to generate a propensity score for each patient based on confounding factors. The patients in the 2 groups were then matched based on their propensity scores within 0.00001 of the estimated score. We also used multivariate regression analysis to perform multiple subanalyses.
Descriptive statistics were performed. Continuous parametric data are reported as a mean and SD, continuous nonparametric data as a median and interquartile range (IQR), and categorical data as a proportion. To analyze the differences between the 2 groups, a χ2 test was used for categorical variables, a Mann-Whitney test for continuous nonparametric data, and a t test for continuous parametric data. All P values were from 2-sided tests and results were deemed statistically significant at P < .05. All statistical analyses were performed using SPSS, version 24 (SPSS Inc).
Results
We analyzed 593 818 trauma patients, of which 140 received REBOA. The demographics and injury parameters of the prematched data are summarized in Table 1. Patients who received REBOA were more likely than those who did not receive REBOA to be younger (mean [SD] age, 44 [20] vs 53 [21] years) nonwhite (51 [36.4%] vs 157 325 of 593 678 [26.5%]; P = .003), and male (104 [74.3%] vs 379 954 of 593 678 [64.0%]; P = .01). Patients who received REBOA were more likely than those who did not receive REBOA to have a lower mean (SD) SBP (108.8 [32.7] vs 138.0 [27.0] mm Hg; P < .001), a higher mean (SD) HR (102.0 [30.0] vs 88.8 [20.0] beats per minute; P < .001), and a lower median GCS score (14 [IQR, 3-15] vs 15 [IQR, 15-15]; P < .001) on admission. Furthermore, patients who received REBOA had a higher median ISS (29 [IQR, 18-38] vs 15 [IQR, 9-17]; P < .001) and a median higher head-AIS (0 [IQR, 0-3] vs 0 [IQR, 0-2]; P < .001) than those who did not receive REBOA. Regarding injuries, patients who received REBOA were more likely than those who did not receive REBOA to have a liver injury (43 [30.7%] vs 27 309 of 593 678 [4.6%]; P < .001), splenic injury (47 [33.6%] vs 29 090 of 593 678 [4.6%]; P < .001), or kidney injury (22 [15.7%] vs 14 248 of 593 678 [2.4%]; P < .001); a lower limb fracture (41 [29.3%] vs 39 776 of 593 678 [6.7%]; P < .001); and vascular injuries (41 [29.3%] vs 6530 of 593 678 [1.1%]; P < .001).
Table 1. Prematch Demographics and Injury Parameters of the 2 Groups.
| Variables | Patients, No. (%) | P Value | |
|---|---|---|---|
| No-REBOA Group (n = 593 678) | REBOA Group (n = 140) | ||
| Age, mean (SD), y | 53 (21) | 44 (20) | <.001 |
| Male sex | 379 954 (64.0) | 104 (74.3) | .01 |
| White race | 436 353 (73.5) | 89 (63.6) | .003 |
| Vital signs in ED | |||
| SBP, mean (SD), mm Hg | 138.0 (27.0) | 108.8 (32.7) | <.001 |
| HR, mean (SD), bpm | 88.8 (20.0) | 102.0 (30.0) | <.001 |
| GCS score, median (IQR) | 15 (15-15) | 14 (3-15) | <.001 |
| Injury parameters | |||
| Blunt MOI | 565 181 (95.2) | 129 (92.1) | .11 |
| ISS, median (IQR) | 15 (9-17) | 29 (18-38) | <.001 |
| h-AIS score, median (IQR) | 0 (0-2) | 0 (0-3) | <.001 |
| Pelvic fractures, total | 46 307 (7.8) | 74 (52.9) | <.001 |
| With intact posterior arch | 29 684 (5.0) | 25 (17.9) | |
| Incompletely disrupted posterior arch | 13 061 (2.2) | 33 (23.6) | |
| Completely disrupted posterior arch | 3562 (0.6) | 16 (11.4) | |
| Liver injuries, total | 27 309 (4.6) | 43 (30.7) | <.001 |
| Grades I-III | 25 528 (4.3) | 37 (26.4) | |
| Grades IV-VI | 1187 (0.2) | 6 (4.3) | |
| Splenic injuries, total | 29 090 (4.9) | 47 (33.6) | <.001 |
| Grades I-III | 22 560 (3.8) | 36 (25.7) | |
| Grades IV-V | 6530 (1.1) | 11 (7.9) | |
| Kidney injuries, total | 14 248 (2.4) | 22 (15.7) | <.001 |
| Grades I-III | 11 280 (1.9) | 19 (13.6) | |
| Grades IV-V | 2968 (0.5) | 3 (2.1) | |
| Lower limb fractures, total | 39 776 (6.7) | 41 (29.3) | <.001 |
| Femur | 31 465 (5.3) | 27 (19.3) | |
| Tibia | 9499 (1.6) | 20 (14.3) | |
| Fibula | 20 185 (3.4) | 21 (15.0) | |
| Vascular injuries, total | 6530 (1.1) | 41 (29.3) | <.001 |
| Iliac | 2375 (0.4) | 29 (20.7) | |
| Lower extremity | 5937 (1.0) | 11 (7.9) | |
| Other | 2375 (0.4) | 38 (27.1) | |
Abbreviations: ED, emergency department; GCS, Glasgow Coma Scale; h-AIS, head Abbreviated Injury Scale; HR, heart rate; IQR, interquartile range; ISS, Injury Severity Score; MOI, mechanism of injury; REBOA, resuscitative endovascular balloon occlusion of the aorta; SBP, systolic blood pressure.
Of the 593 818 trauma patients, 420 patients (the no-REBOA group, 280 patients; the REBOA group, 140 patients) were matched. Among the REBOA group, there were 36 women and 104 men, the mean (SD) age was 44 (20) years, the median ISS was 29 (IQR, 18-38), and the mechanism of injury was blunt injury in 129 patients (92.1%). The demographics and injury parameters of the matched cohort of trauma patients are demonstrated in Table 2. There was no difference between the REBOA and no-REBOA groups regarding mean (SD) age (44 [20] vs 43 [19] years; P = .88), sex (104 men [74.3%] vs 203 men [72.5%]; P = .76), race (89 white patients [63.6%] vs 180 white patients [64.3%]; P = .37), mean (SD) SBP (108.8 [32.7] vs 106.5 [28.7] mm Hg; P = .65), mean (SD) HR (102 [30] vs 104 [27] beats per minute; P = .74), median GCS score (14 [IQR, 3-15] vs 13 [IQR, 3-15]; P = .88), mechanism of injury (blunt injury, 129 [92.1%] vs 257 [91.8%]; P = .87), median ISS score (29 [IQR, 18-38] vs 28 [IQR, 17-35]; P = .91), median head AIS (0 [IQR, 0-3] vs 0 [IQR, 0-3]; P = .98), pelvic fractures and type of pelvic fractures (total pelvic fractures, 74 [52.9%] vs 144 [51.4%]; P = .65), liver injury or severity of liver injury (total liver injuries, 43 [30.7%] vs 89 [31.8%]; P = .79), splenic injury or severity of splenic injury (total splenic injuries, 47 [33.6%] vs 90 [32.1%]; P = .81), kidney injury or severity of kidney injury (total kidney injuries, 22 [15.7%] vs 39 [13.9%]; P = .82), lower limb fractures (total fractures, 41 [29.3%] vs 78 [27.9%]; P = .69) (including femur, tibia or fibula fractures), or vascular injuries (total injuries, 41 [29.3%] vs 76 [27.1%]; P = .11).
Table 2. Postmatch Demographics and Injury Parameters of the 2 Groups.
| Variables | Patients, No. (%) | P Value | |
|---|---|---|---|
| No-REBOA Group (n = 280) | REBOA Group (n = 140) | ||
| Age, mean (SD), y | 43 (19) | 44 (20) | .88 |
| Male sex | 203 (72.5) | 104 (74.3) | .76 |
| White race | 180 (64.3) | 89 (63.6) | .37 |
| Vital signs in the ED | |||
| SBP, mean (SD), mm Hg | 106.5 (28.7) | 108.8 (32.7) | .65 |
| HR, mean (SD), bpm | 104 (27) | 102 (30) | .74 |
| GCS score, median (IQR) | 13 (3-15) | 14 (3-15) | .88 |
| Injury parameters | |||
| Blunt MOI | 257 (91.8) | 129 (92.1) | .87 |
| ISS, median (IQR) | 28 (17-35) | 29 (18-38) | .91 |
| h-AIS score, median (IQR) | 0 (0-3) | 0 (0-3) | .98 |
| Pelvic fractures, total | 144 (51.4) | 74 (52.9) | .65 |
| With intact posterior arch | 45 (16.1) | 25 (17.9) | |
| Incompletely disrupted posterior arch | 68 (24.3) | 33 (23.6) | |
| Completely disrupted posterior arch | 31 (11.1) | 16 (11.4) | |
| Liver injuries, total | 89 (31.8) | 43 (30.7) | .79 |
| Grades I-III | 76 (27.1) | 37 (26.4) | |
| Grades IV-VI | 13 (4.6) | 6 (4.3) | |
| Splenic injuries, total | 90 (32.1) | 47 (33.6) | .81 |
| Grades I-III | 67 (23.9) | 36 (25.7) | |
| Grades IV-V | 22 (7.9) | 11 (7.9) | |
| Kidney injuries, total | 39 (13.9) | 22 (15.7) | .82 |
| Grades I-III | 35 (12.5) | 19 (13.6) | |
| Grades IV-V | 5 (1.8) | 3 (2.1) | |
| Lower limb fractures, total | 78 (27.9) | 41 (29.3) | .69 |
| Femur | 48 (17.1) | 27 (19.3) | |
| Tibia | 45 (16.1) | 20 (14.3) | |
| Fibula | 32 (11.4) | 21 (15.0) | |
| Vascular injuries, total | 76 (27.1) | 41 (29.3) | .11 |
| Iliac | 53 (18.9) | 29 (20.7) | |
| Lower extremity | 20 (7.1) | 11 (7.9) | |
| Other | 11 (3.9) | 38 (27.1) | |
Abbreviations: ED, emergency department; GCS, Glasgow Coma Scale; h-AIS, head Abbreviated Injury Scale; HR, heart rate; IQR, interquartile range; ISS, Injury Severity Score; MOI, mechanism of injury; REBOA, resuscitative endovascular balloon occlusion of the aorta; SBP, systolic blood pressure.
The overall ED mortality rate among all 420 patients was 2.1% (n = 9) (Table 3). The 24-hour mortality rate was 16.7% (n = 70), and the in-hospital mortality rate was 24.5% (n = 103). The median 4-hour requirements among all 420 patients for PRBCs were 7 U (IQR, 3-8 U), for platelets were 4 U (IQR, 3-8 U), and for plasma was 3 U (IQR, 2-5 U), while the median 24-hour requirements among all 420 patients for PRBCs were 10 U (IQR, 4-20 U), for platelets were 8 U (IQR, 3-12 U), and for plasma was 10 U (IQR, 6-20 U). The median hospital LOS among all 420 patients was 9 days (IQR, 4-20 days), and the median intensive care unit LOS was 6 days (IQR, 3-14 days). The median time from ED presentation to the placement of REBOA was 19 minutes (IQR, 14-29 minutes).
Table 3. Outcomes of Patients.
| Variable | Patients, No. (%) | P Value | |
|---|---|---|---|
| No-REBOA Group (n = 280) | REBOA Group (n = 140) | ||
| 4-h Transfusion, median (IQR), U | |||
| PRBCs | 7 (3-9) | 6 (3-8) | .14 |
| Platelets | 4 (3-8) | 4 (3-9) | .13 |
| Plasma | 3 (2-6) | 3 (2-5) | .17 |
| 24-h Transfusion, median (IQR), U | |||
| PRBCs | 10 (4-21) | 9 (5-20) | .21 |
| Platelets | 8 (3-12) | 7 (3-13) | .12 |
| Plasma | 10 (7-20) | 9 (6-20) | .11 |
| Hemorrhage control intervention | |||
| Angioembolization | 85 (30.4) | 40 (28.6) | .18 |
| Time to angioembolization, median (IQR), min | 46 (31-69) | 59 (39-78) | .04 |
| Laparotomy | 190 (67.9) | 96 (68.6) | .33 |
| Time to laparotomy, median (IQR), min | 33 (26-62) | 45 (35-69) | .04 |
| LOS, median (IQR), d | |||
| Hospital | 10 (5-22) | 8 (1-20) | .21 |
| ICU | 6 (3-15) | 5 (2-14) | .19 |
| Complications | |||
| Acute kidney injury | 9 (3.2) | 15 (10.7) | .02 |
| Amputation of lower limb | 2 (0.7) | 5 (3.6) | .04 |
| Deep venous thrombosis | 14 (5.0) | 6 (4.3) | .42 |
| Pulmonary embolism | 5 (1.8) | 2 (1.4) | .28 |
| Stroke | 3 (1.1) | 2 (1.4) | .37 |
| Myocardial infarction | 1 (0.4) | 0 | .51 |
| Extremity compartment syndrome | 2 (0.7) | 1 (0.7) | .39 |
| Overall mortality | 53 (18.9) | 50 (35.7) | .01 |
| Mortality in the ED | 5 (1.8) | 4 (2.9) | .35 |
| 24-h Mortality | 33 (11.8) | 37 (26.4) | .01 |
| In-hospital mortality after 24 h | 15 (5.4) | 9 (6.4) | .21 |
Abbreviations: ED, emergency department; ICU, intensive care unit; IQR, interquartile range; LOS, length of stay; PRBCs, packed red blood cells; REBOA, resuscitative endovascular balloon occlusion of the aorta.
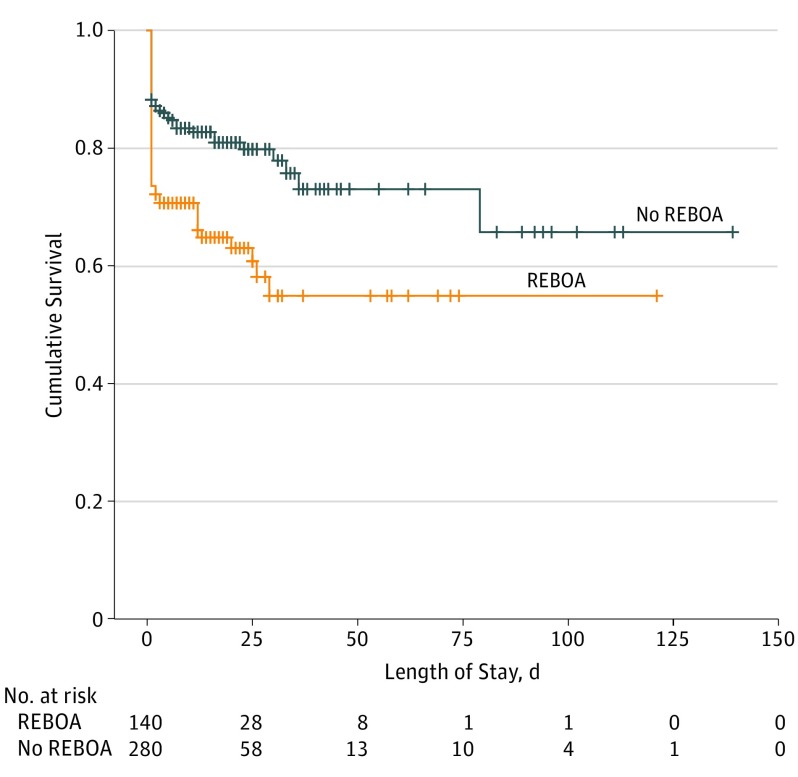
The primary and secondary outcome measures of the analysis are presented in Table 3. Compared with patients in the no-REBOA group, patients in the REBOA group had a higher 24-hour mortality rate (37 [26.4%] vs 33 [11.8%]; P = .01), as well as higher rates of acute kidney injury (15 [10.7%] vs 9 [3.2%]; P = .02) and amputation of a lower limb (5 [3.6%] vs 2 [0.7%]; P = .04). However, there was no significant difference in ED mortality (4 [2.9%] vs 5 [1.8%]; P = .35), mortality after 24 hours (9 [6.4%] vs 15 [5.4%]; P = .21), rate of deep venous thrombosis (6 [4.3%] vs 14 [5.0%]; P = .42), pulmonary embolism (2 [1.4%] vs 5 [1.8%]; P = .28), myocardial infarction (0 vs 1 [0.4%]; P = .51), stroke (2 [1.4%] vs 3 [1.1%]; P = .37), and extremity compartment syndrome (1 [0.7%] vs 2 [0.7%]; P = .39) between the REBOA and no-REBOA groups. Moreover, there was no difference between groups in 4-hour or 24-hour blood transfusion requirements for PRBCs, platelets, or plasma, and no difference in hospital or intensive care unit LOS. All the patients who survived the ED underwent hemorrhage control intervention with either angioembolization or exploratory laparotomy. However, there was no difference between the REBOA and no-REBOA groups regarding the rate of angioembolization (40 [28.6%] vs 85 [30.4%]; P = .18) or exploratory laparotomy (96 [68.6%] vs 190 [67.9%]; P = .33). The median time from ED presentation to angioembolization (59 minutes [IQR, 39-78 minutes] vs 46 minutes [31-69 minutes]; P = .04) or exploratory laparotomy (45 minutes [IQR, 35-69 minutes] vs 33 minutes [IQR, 26-62 minutes]; P = .04) was higher among patients who underwent REBOA placement than those who did not undergo REBOA placement. The Figure demonstrates the survival functions for patients who underwent REBOA placement vs those who did not undergo REBOA placement.
Figure. Survival Curve Analysis.
The probability of survival over time in the group that received resuscitative endovascular balloon occlusion of the aorta (REBOA) vs the no-REBOA group (P < .01).
The demographics and injury parameters of patients who underwent REBOA who survived (n = 90) vs those who died (n = 50) are demonstrated in Table 4. Patients who survived REBOA placement were more likely to have a higher mean (SD) SBP (114 [32] vs 98 [31] mm Hg; P = .006) and median GCS score (15 [IQR, 13-15] vs 3 [IQR, 3-13]; P = .04), but a lower mean (SD) HR (99.0 [27.0] vs 109.4 [25.0] beats per minute; P = .02), median ISS (27 [IQR, 17-34] vs 38 [26-50]; P = .043), and median head AIS (0 [IQR, 0-2] vs 2 [IQR, 0-4]; P = .002). Moreover, those who died were more likely to sustain liver injuries (21 [42.0%] vs 22 [24.4%]; P = .04). Regarding blood product requirements, patients who received REBOA and died were more likely to receive PRBCs, platelets, and plasma at 4 hours and 24 hours after injury.
Table 4. Subanalysis of Patients Who Received REBOA.
| Variable | Patients, No. (%) | P Value | |
|---|---|---|---|
| Survived (n = 90) | Died (n = 50) | ||
| Age, mean (SD), y | 42 (19) | 48.2 (19) | .12 |
| Male sex | 57 (63.3) | 32 (64.0) | .20 |
| Vital signs in the ED | |||
| SBP, mean (SD), mm Hg | 114 (32) | 98 (31) | .006 |
| HR, mean (SD), bpm | 99.0 (27.0) | 109.4 (25.0) | .02 |
| GCS score, median (IQR) | 15 (13-15) | 3 (3-13) | .04 |
| Injury parameters | |||
| Blunt MOI | 82 (91.1) | 47 (94.0) | .54 |
| ISS, median (IQR) | 27 (17-34) | 38 (26-50) | .043 |
| h-AIS, median (IQR) | 0 (0-2) | 2 (0-4) | .002 |
| Injuries | |||
| Pelvic fractures | 48 (53.3) | 26 (52.0) | .14 |
| Liver injuries | 22 (24.4) | 21 (42.0) | .04 |
| Splenic injuries | 32 (35.6) | 14 (28.0) | .39 |
| Kidney injuries | 16 (17.8) | 5 (10.0) | .23 |
| Lower limb fractures | 29 (32.2) | 11 (22.0) | .65 |
| Vascular injuries | 26 (28.9) | 14 (28.0) | .11 |
| Transfusion requirements, median (IQR), U | |||
| PRBCs | |||
| 4 h | 0 (0-5) | 12 (7-19) | <.001 |
| 24 h | 1 (1-6) | 14 (9-22) | <.001 |
| Platelets | |||
| 4 h | 0 (0-1) | 2 (1-3) | <.001 |
| 24 h | 1 (0-2) | 3 (2-6) | <.001 |
| Plasma | |||
| 4 h | 0 (0-3) | 9 (4-15) | <.001 |
| 24 h | 1 (1-5) | 13 (6-20) | <.001 |
Abbreviations: ED, emergency department; GCS, Glasgow Coma Scale; h-AIS, head Abbreviated Injury Scale; HR, heart rate; IQR, interquartile range; ISS, Injury Severity Score; MOI, mechanism of injury; PRBCs, packed red blood cells; REBOA, resuscitative endovascular balloon occlusion of the aorta; SBP, systolic blood pressure.
We performed a subanalysis of patients based on SBP. In a subset of patients with an SBP greater than 80 mm Hg, REBOA placement was associated higher odds of mortality (odds ratio, 4.67; 95% CI, 1.35-8.42; P = .03). Similarly, in the subset of patients with an SBP less than 80 mm Hg, REBOA placement was independently associated with higher odds of mortality (odds ratio, 2.51; 95% CI, 1.16-6.41; P = .03) on multivariate regression analysis. Another subanalysis was performed for patients who underwent exploratory laparotomy (n = 286: REBOA group, 96; no-REBOA group, 190); on regression analysis REBOA placement was associated with higher mortality (odds ratio, 2.12; 95% CI, 1.67-3.84; P = .01).
Discussion
In our propensity-matched analysis from the ACS-TQIP data bank, REBOA placement was associated with a higher mortality rate in severely injured trauma patients compared with those who did not receive REBOA. Moreover, patients who underwent REBOA placement had higher rates of acute kidney injury and lower limb amputation. On the contrary, there was no difference between groups in requirements for blood products at 4 hours and 24 hours after the injury.
Resuscitative endovascular balloon occlusion of the aorta has emerged as a promising technique for hemostasis in severely injured trauma patients.11 In animal models, REBOA has been demonstrated as a potential hemostatic measure in exsanguinating animals that improves survival, increases blood pressure, and improves brain oxygenation and carotid arterial blood flow.12,13 This finding was followed up by a case series by Brenner et al14 in which 6 trauma patients underwent REBOA placement. They concluded that REBOA is feasible and effective in preventing hemorrhage in patients with end-stage shock. These findings were then followed by a prospective observational study by DuBose et al,7 who analyzed 114 patients, of which 46 underwent REBOA placement. They concluded that REBOA has emerged as a viable option to open aortic occlusion in centers that have the capability of performing REBOA placement.
We found that REBOA placement was associated with higher mortality and poorer outcomes in trauma patients. Our results are contrary to 2 previously published prospective observational studies in the United States.7,15 These differences can be explained by several limitations in those 2 studies. First, both of those studies compared REBOA with open aortic occlusion or resuscitative thoracotomy and the indications for resuscitative thoracotomy are different than those for REBOA. Thoracotomy in the emergency department is performed in patients who are experiencing cardiac arrest, while REBOA is indicated for trauma patients who are hypotensive and have a pelvic fracture or abdominal fluid detected on initial ultrasonographic scan in the trauma bay. Moreover, those studies did not have a true control group (ie, patients who did not undergo REBOA placement and/or resuscitative thoracotomy). We have overcome this limitation by excluding the patients who underwent thoracotomy in the ED and comparing patients who underwent REBOA with similarly injured trauma patients who did not undergo REBOA. Second, both studies had small patient cohorts (Brenner et al,15 83 patients; and DuBose et al,7 46 patients), which may be an inadequate sample size with low power to draw a conclusion regarding the effectiveness of REBOA. On the other hand, we included 140 patients in the REBOA group matched with 280 patients in the no-REBOA group, which is the largest patient cohort to our knowledge for REBOA in the United States. Third, there were significant differences regarding vital signs in the ED and injury patterns between the 2 patient cohorts in the studies by Brenner et al15 and DuBose et al.7 To overcome this limitation, the patient cohorts in our analysis were matched in terms of patient demographics, prehospital vital signs, ED vitals, global injury severity, severity of injury in each body region, mechanism of injury, solid organ injuries, vascular injuries, pelvic fractures, and lower extremity fractures. The results of our study are consistent with those of a previously published study by Norii et al,10 who analyzed the safety and efficacy of REBOA in severely injured trauma patients from the Japanese National Trauma Registry and reported a higher mortality rate in the REBOA group after adjustment for the likelihood of REBOA treatment. The study by Norii et al10 had some limitations. They did not have data regarding the vital signs at presentation or blood product transfusion. In our analysis, we included these data points and there was no difference between the 2 patient cohorts regarding these variables. In addition, Norii et al10 did not analyze the complications of REBOA deployment such as acute kidney injury and lower limb amputation.
The patients in the REBOA group in our study had a longer time to angioembolization or laparotomy, which might be associated with a higher mortality rate. It is a well-established fact in the trauma literature that delay of every minute in establishing definitive surgical control increases mortality by 0.35%.16 In addition, although REBOA may improve cerebral and myocardial perfusion, it decreases blood flow distal to the point of occlusion and causes a state of ischemia in the lower torso and lower extremities. This ischemia may increase the overall inflammatory burden in the body and may cause a reperfusion injury when the occlusion of the aorta is released. Multiple studies in trauma have reported that an increase in inflammatory and reactive oxygen particles leads to higher mortality.
Duration of occlusion of the aorta is critical once the patient survives the ED phase. Extended occlusion of the aorta can cause ischemic damage to organs distal to the site of occlusion secondary to low blood flow. Moreover, mechanical damage to the aorta can cause aortic dissection or rupture, or embolization of a thrombus. In our analysis, patients who underwent REBOA placement had higher rates of acute kidney injury and amputation of a lower limb. Similar to our results, Brenner et al15 reported a need for amputation and distal embolism in patients who underwent REBOA. In addition, Wasicek et al17 analyzed lower-limb complications in 31 patients and found that, among the 20 patients who received REBOA at zone I, 15% developed lower extremity compartment syndrome; they also found that a longer duration of aortic occlusion at zone I is associated with higher rates of calf and thigh fasciotomies. Regarding zone of placement, Tibbits et al18 found in a swine model that REBOA at zone I was independently associated with a higher burden of ischemia and higher rates of reperfusion injury on deflation compared with control group. However, we could not obtain the duration of occlusion nor the zone of occlusion, which is a limitation of our study. Furthermore, Pieper et al19 published 20 years of experience with REBOA in France and demonstrated that REBOA placement was associated with high rates of vascular complications (including lower-limb ischemia and aortic dissection), acute renal failure, and rhabdomyolysis. Our study did not demonstrate any difference in the requirements for blood products at 4 hours and 24 hours after injury in either group of severely injured trauma patients. The median PRBC and plasma requirements in the first 24 hours in the REBOA group is similar to that seen in the previously published literature.15
The ACS Committee on Trauma in collaboration with the American College of Emergency Physicians has recently published clinical guidelines regarding the safe use of REBOA.20 The American Association for the Surgery of Trauma, Aortic Occlusion in Resuscitation for Trauma and Acute Care Surgery (AORTA) group has already published 2 studies from 11 centers in the United States. However, they lack a standard protocol for REBOA placement and an appropriate control group. Most REBOA placement is at the discretion of the attending trauma surgeon rather than in accordance with a specific protocol. Currently, a REBOA device is available in 149 level 1 trauma centers,21 but the protocol varies for each institution depending on resources as well as other factors.22 Because of many reasons, including lack of funding and complex regulations related to patient consent, much of trauma care is driven by the surgeon’s experience and retrospective studies. Resuscitative endovascular balloon occlusion of the aorta has potential application in the civilian and military settings. In a civilian setting, REBOA may be beneficial in hemorrhaging patients with noncompressible torso hemorrhage who do not have rapid access to a trauma center or the operating room for definitive control of bleeding. In the military setting, REBOA might be beneficial in the field, because REBOA can be placed in injured soldiers for a temporary control of lower torso or extremity hemorrhage while waiting for transportation to a trauma unit for definitive surgery. However, there is still a lack of clinical data that adequately address the appropriate use of REBOA and guide the absolute duration of full or partial aortic occlusion. There needs to be a concerted effort to clearly define when and in which patient population REBOA should be used. Thus, further randomized clinical trials are required to properly evaluate the indications of REBOA use in trauma patients in accordance with specific well-defined protocols. Currently, a randomized clinical trial is ongoing in the United Kingdom, which might identify the specific subset of trauma patients that might benefit from REBOA placement.23
Limitations
Our study has some limitations. Because of the retrospective nature of the database, we were not able to account for unmeasured confounders, including, but not limited to, the type and size of the catheter used, the zone of placement (ie, zone 1, zone 2, or zone 3), and the duration of aortic occlusion. Moreover, we could not determine the responsiveness of the patient to initial resuscitation before REBOA placement. This factor could have produced the greatest potential bias in the selection of patients for REBOA placement. However, in our propensity score matching, we tried to minimize selection bias by matching the demographics, injury parameters, physiological parameters, and intra-abdominal solid organ injuries, along with the grading. In addition, because only small number of trauma centers have used REBOA, the data may be skewed, which should be interpreted in similar contexts. Nonetheless, to our knowledge, this is the first national study to compare the outcomes of REBOA in severely injured trauma patients. Our study has the strengths of a large sample size derived from multiple institutions across the United States. In addition, the ACS-TQIP database is adequately representative of the trauma population in the United States and its use is well established in trauma research.
Conclusions
Placement of REBOA in severely injured trauma patients was associated with higher mortality compared with a similar cohort of patients who did not undergo REBOA placement. Resuscitative endovascular balloon occlusion of the aorta was also associated with higher rates of acute kidney injury and lower-leg amputations. There is a need for a concerted effort to clearly define when and in which patient population REBOA has benefit.
References
- 1.Rhee P, Joseph B, Pandit V, et al. . Increasing trauma deaths in the United States. Ann Surg. 2014;260(1):13-21. doi: 10.1097/SLA.0000000000000600 [DOI] [PubMed] [Google Scholar]
- 2.Acosta JA, Yang JC, Winchell RJ, et al. . Lethal injuries and time to death in a level I trauma center. J Am Coll Surg. 1998;186(5):528-533. doi: 10.1016/S1072-7515(98)00082-9 [DOI] [PubMed] [Google Scholar]
- 3.Stensballe J, Ostrowski SR, Johansson PI. Haemostatic resuscitation in trauma: the next generation. Curr Opin Crit Care. 2016;22(6):591-597. doi: 10.1097/MCC.0000000000000359 [DOI] [PubMed] [Google Scholar]
- 4.Hughes CW. Use of an intra-aortic balloon catheter tamponade for controlling intra-abdominal hemorrhage in man. Surgery. 1954;36(1):65-68. [PubMed] [Google Scholar]
- 5.Stannard A, Eliason JL, Rasmussen TE. Resuscitative endovascular balloon occlusion of the aorta (REBOA) as an adjunct for hemorrhagic shock. J Trauma. 2011;71(6):1869-1872. [DOI] [PubMed] [Google Scholar]
- 6.Low RB, Longmore W, Rubinstein R, Flores L, Wolvek S. Preliminary report on the use of the Percluder occluding aortic balloon in human beings. Ann Emerg Med. 1986;15(12):1466-1469. doi: 10.1016/S0196-0644(86)80945-3 [DOI] [PubMed] [Google Scholar]
- 7.DuBose JJ, Scalea TM, Brenner M, et al. ; AAST AORTA Study Group . The AAST prospective Aortic Occlusion for Resuscitation in Trauma and Acute Care Surgery (AORTA) registry: data on contemporary utilization and outcomes of aortic occlusion and resuscitative balloon occlusion of the aorta (REBOA). J Trauma Acute Care Surg. 2016;81(3):409-419. doi: 10.1097/TA.0000000000001079 [DOI] [PubMed] [Google Scholar]
- 8.Brenner M, Teeter W, Hoehn M, et al. . Use of resuscitative endovascular balloon occlusion of the aorta for proximal aortic control in patients with severe hemorrhage and arrest. JAMA Surg. 2018;153(2):130-135. doi: 10.1001/jamasurg.2017.3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrison JJ, Ross JD, Houston R IV, Watson JDB, Sokol KK, Rasmussen TE. Use of resuscitative endovascular balloon occlusion of the aorta in a highly lethal model of noncompressible torso hemorrhage. Shock. 2014;41(2):130-137. doi: 10.1097/SHK.0000000000000085 [DOI] [PubMed] [Google Scholar]
- 10.Norii T, Crandall C, Terasaka Y. Survival of severe blunt trauma patients treated with resuscitative endovascular balloon occlusion of the aorta compared with propensity score–adjusted untreated patients. J Trauma Acute Care Surg. 2015;78(4):721-728. doi: 10.1097/TA.0000000000000578 [DOI] [PubMed] [Google Scholar]
- 11.Harma M, Harma M, Kunt AS, Andac MH, Demir N. Balloon occlusion of the descending aorta in the treatment of severe post-partum haemorrhage. Aust N Z J Obstet Gynaecol. 2004;44(2):170-171. doi: 10.1111/j.1479-828X.2004.00181.x [DOI] [PubMed] [Google Scholar]
- 12.Avaro J-P, Mardelle V, Roch A, et al. . Forty-minute endovascular aortic occlusion increases survival in an experimental model of uncontrolled hemorrhagic shock caused by abdominal trauma. J Trauma. 2011;71(3):720-725. doi: 10.1097/TA.0b013e318221a94a [DOI] [PubMed] [Google Scholar]
- 13.Scott DJ, Eliason JL, Villamaria C, et al. . A novel fluoroscopy-free, resuscitative endovascular aortic balloon occlusion system in a model of hemorrhagic shock. J Trauma Acute Care Surg. 2013;75(1):122-128. doi: 10.1097/TA.0b013e3182946746 [DOI] [PubMed] [Google Scholar]
- 14.Brenner ML, Moore LJ, DuBose JJ, et al. . A clinical series of resuscitative endovascular balloon occlusion of the aorta for hemorrhage control and resuscitation. J Trauma Acute Care Surg. 2013;75(3):506-511. doi: 10.1097/TA.0b013e31829e5416 [DOI] [PubMed] [Google Scholar]
- 15.Brenner M, Inaba K, Aiolfi A, et al. ; AAST AORTA Study Group . Resuscitative endovascular balloon occlusion of the aorta and resuscitative thoracotomy in select patients with hemorrhagic shock: early results from the American Association for the Surgery of Trauma’s Aortic Occlusion in Resuscitation for Trauma and Acute Care Surgery Registry. J Am Coll Surg. 2018;226(5):730-740. doi: 10.1016/j.jamcollsurg.2018.01.044 [DOI] [PubMed] [Google Scholar]
- 16.Clarke JR, Trooskin SZ, Doshi PJ, Greenwald L, Mode CJ. Time to laparotomy for intra-abdominal bleeding from trauma does affect survival for delays up to 90 minutes. J Trauma. 2002;52(3):420-425. [DOI] [PubMed] [Google Scholar]
- 17.Wasicek PJ, Teeter WA, Yang S, et al. . Life over limb: lower extremity ischemia in the setting of resuscitative endovascular balloon occlusion of the aorta (REBOA). Am Surg. 2018;84(6):971-977. [PubMed] [Google Scholar]
- 18.Tibbits EM, Hoareau GL, Simon MA, et al. . Location is everything: the hemodynamic effects of REBOA in zone 1 versus zone 3 of the aorta. J Trauma Acute Care Surg. 2018;85(1):101-107. doi: 10.1097/TA.0000000000001858 [DOI] [PubMed] [Google Scholar]
- 19.Pieper A, Thony F, Brun J, et al. . Resuscitative endovascular balloon occlusion of the aorta for pelvic blunt trauma and life-threatening hemorrhage: a 20-year experience in a level I trauma center. J Trauma Acute Care Surg. 2018;84(3):449-453. doi: 10.1097/TA.0000000000001794 [DOI] [PubMed] [Google Scholar]
- 20.Brenner M, Bulger EM, Perina DG, et al. . Joint statement from the American College of Surgeons Committee on Trauma (ACS COT) and the American College of Emergency Physicians (ACEP) regarding the clinical use of resuscitative endovascular balloon occlusion of the aorta (REBOA). Trauma Surg Acute Care Open. 2018;3(1):e000154. doi: 10.1136/tsaco-2017-000154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prytime Medical. ER-REBOA catheter. https://prytimemedical.com/. Accessed June 25, 2018.
- 22.Rasmussen TE, Eliason JL. Military-civilian partnership in device innovation: development, commercialization and application of resuscitative endovascular balloon occlusion of the aorta. J Trauma Acute Care Surg. 2017;83(4):732-735. doi: 10.1097/TA.0000000000001661 [DOI] [PubMed] [Google Scholar]
- 23.UK REBOA Trial. Resuscitative endovascular balloon occlusion of the aorta for trauma: protocol. https://w3.abdn.ac.uk/hsru/REBOA/Public/download.aspx?ID=142. Accessed June 27, 2018.