ABSTRACT
Exosomes are a subset of extracellular vesicles and their size is approximately 100 nm in diameter. They are surrounded by a lipid bilayer membrane and secreted from almost all of cells. Exosomes are generated within the endocytic system as ILV (intraluminal membrane vesicle) and secreted during the fusion of MVB (multivesicular body) with the cell membrane. Recently it has been reported that exosomes modulate cell-cell communication contributing to the maintenance of tissue homeostasis by molecules including exosomes. Moreover, exosomes released from cancer cells are involved in cancer progression. Thus, data regarding the role of the exosomes in malignant cancer will lead to development of novel diagnostic and therapeutic methods.
Keywords: cancer progression, exosome, extracellular vesicle
Extracellular vesicles (EVs) are vesicles secreted by the cell and are classified into three groups, namely apoptotic bodies, microvesicles (MVs), and exosomes according to their size and mechanism of formation. Apoptotic bodies are vesicles of 1μm or more in diameter that are released from the apoptotic cells, and contain fragmented nuclei and intracellular organelle. While both MV and exosomes contain proteins and nucleic acids, their sizes differ, with MVs ranging from 100 to 1,000 nm while exosomes are approximately 100 nm in diameter. Based on the composition of RNA in each type of vesicle, apoptotic bodies primarily contain ribosomal RNA (rRNA), whereas MVs contain almost no RNA. Exosomes contain large amounts of low molecular weight RNA but not rRNA.1 While apoptotic bodies and MV are directly formed and released by the cell membrane, exosomes are secreted through the endocytosis of the multivesicular bodies (MVBs). Each vesicle also differs in the surface molecules expressed, such as phosphatidylserine (apoptotic bodies), integrin, secretin and CD40 (MVs), and tetraspanin (exosome). Although there have been studies regarding each type of vesicle, in recent years, research on exosomes has increased particularly, and there have been reports related to disease progression by exosome, in addition to understanding the biological significance of exosomes. In this review, we summarize an overview of the characteristics of the exosome and its involvement in cancer progression. Moreover, we discuss the issues in exosome research and future prospects for its clinical application.
OVERVIEW OF THE EXOSOME
History —From discovery to arriving on the front stage—
In 1983, Johnstone et al. discovered a 100 nm vesicle having a lipid bilayer structure secreted from the sheep reticulocytes2 and named the vesicle “exosome” in 1987. Exosomes were initially thought to play a garbage bag-like role to eliminate unnecessary substances from the cells. However, it was shown in 1996 that exosomes secreted by the B lymphocytes induced activation of the T lymphocytes, thereby revealing that exosomes affect other cells.3 In 2007, Valadi and colleagues reported that exosomes encapsulated RNA and transferred them to other cells, as well as the fact that proteins encoded by the mRNA contained in the exosomes are expressed in cells receiving the exosome.4 Furthermore, in 2010, Kosaka, Pegtel, and Zhang et al. used different cell lines to show that microRNA (miRNA) encapsulated in the exosomes inhibited expression of the target genes in the receiving cells.5,6,7 These reports revealed that exosomes are not merely garbage bags, but they function as tools of communication between cells, and this led to exosomes gathering more attention.
Biogenesis of exosomes
Exosomes originate from the endosome; thus they are differentiated from the previously mentioned apoptotic bodies and MV that are formed directly from the cell membrane.8 The initial endosome is formed by encapsulating the cell membrane proteins through endocytosis and intracellular proteins and nucleic acids. This initial endosome matures into a late endosome through a maturation process which is accompanied by decreasing pH, and multiple ILV (intraluminal membrane vesicle) are formed inside the endosome, as if to sprout. Vesicle containing multiple ILVs are called MVBs, and the ILVs are released into the extracellular space as MVB fuses with the cell membrane again. These secreted ILVs are exosomes (Fig. 1).
Fig. 1.
Biogenesis of Exosome.
The intracellular formation of ILV has been reported via two pathways; firstly, a pathway involving ESCRT (endosomal sorting complex required for transport) and, secondly a pathway independent of the ESCRT. In the former, proteins such as ESCRT-0, -I, -II, and -III form complexes and are involved in a series of ILV formation.9,10,11 In the latter, exosomes are secreted even when ESCRT is inhibited12 and instead involve ceramide and tetraspanin. Kosaka and colleagues have reported that exosome secretion is suppressed by inhibition of n-SMase2, an enzyme that promotes ceramide synthesis, and conversely, exosome secretion is promoted by overexpression of n-SMase2.5, 13 In addition, van Niel et al. reported that CD63, a type of tetraspanin, is involved in protein transfer to ILV independent of ESCRT and ceramide.14
As a protein involved in extracellular release of ILV, that is, secretion of exosomes, the Rab family, which is a small molecule GTP-binding protein involved in vesicular trafficking, is known. Among the RAB family, RAB 7,15 RAB 11,16 RAB 27 A,17 RAB 27 B,17 and RAB 3518 regulate exosome secretion. Moreover, their involvement has also been confirmed in various cancer cells.19,20,21 The RAB family proteins are thought to be involved in the binding of MVBs to the cell membrane, but the associated RAB family protein differ from cell to cell. In addition to the RAB family protein, it has also been clarified that the p53 gene, which is a tumor suppressor gene, controls the secretion through its target gene TSAP6,22, 23 showing a glimpse of complexity involving various kinds of molecules. As described above, many molecules are involved during the formation of the exosomes, and multiple pathways have been reported, but the complete mechanism still remains unknown.
Exosome structure and encapsulated substances
As mentioned above, exosomes are vesicles of approximately 100 nm in diameter consisting of a lipid bilayer and can be largely classified into membrane components and encapsulated molecules (Fig. 2). The membrane components consist of lipids and proteins, like a typical cell membrane. It has been reported that the lipid composition of the exosome membrane is dependent on the cell producing the exosome.24 Exosomal membranes contain more cholesterol, sphingolipids and phosphatidylserines, which are usually found on the inner side of cell membranes, are found on the outer side of exosome.25 As proteins existing in the exosome membrane, tetraspanins, such as CD9, CD63, CD81 etc., which are proteins with four transmembrane domains, MHC molecules that are proteins related to antigen presentation, and the cell adhesion molecule integrin were observed. Tetraspanin is used as a relatively specific exosome marker,26 and integrin is involved in exosome tropism.27
Fig. 2.
Main features of Exosome.
On the contrary, the encapsulated molecules in exosomes includes proteins and nucleic acids. The protein contains a large amount of heat shock and cytoskeletal proteins, as well as endosome-related proteins (SNARE, annexin, and flotillin), and Alix and TSG101 that are related to MVBs biosynthesis. Similar to the lipid composition, the protein composition is also known to change depending on the type of cell producing it and the surrounding microenvironment.28,29,30,31 Nucleic acids encapsulated in the cells include RNA and DNA. On the former, the presence of miRNA,32 IncRNA,33, 34 and circRNA35 etc., have been confirmed in addition to the mRNA.4, 36 Cells containing exosomes that have RNA molecules have been shown to translate mRNA into proteins,4 while the miRNA blocks translation.5,6,7 While there are many other non-coding RNAs in exosomes, they have been strongly suggested to be involved in transcription and translation processes in the exosome-received cells. On the latter, it has been reported that exosomes contain DNA, and Thakur et al. has demonstrated that using enzymes that specifically break down dsDNA and ssDNA respectively, most DNAs contained in the exosomes are dsDNAs.37 In recent years, Takahashi et al. showed that inhibition of exosome secretion by normal cells led to the accumulation of nuclear DNA fragments, thereby causing cellular aging and induction of apoptosis.38 This suggests that the accumulation of harmful DNA fragments in the cytoplasm is released into the external environment using exosomes and the exosomes contribute to the maintenance of cellular homeostasis. While there are reports suggesting that DNA is encapsulated in the exosomes, Kawamura et al. showed that DNA is present on the surface of exosomes,39 and the significance of its localization needs to be elucidated.
The exosome database ExoCarta (www.exocarta.org) contains a list of molecules present in the exosome. As of March 2019, 9,769 types of proteins, 3,408 types of mRNAs, 2,838 types of miRNAs, and 1,116 types of lipids have been registered. Moreover, the exosome constituents can be searched according to conditions like the animal species or cell type etc.
EXOSOME AND PROGRESSION OF CANCER
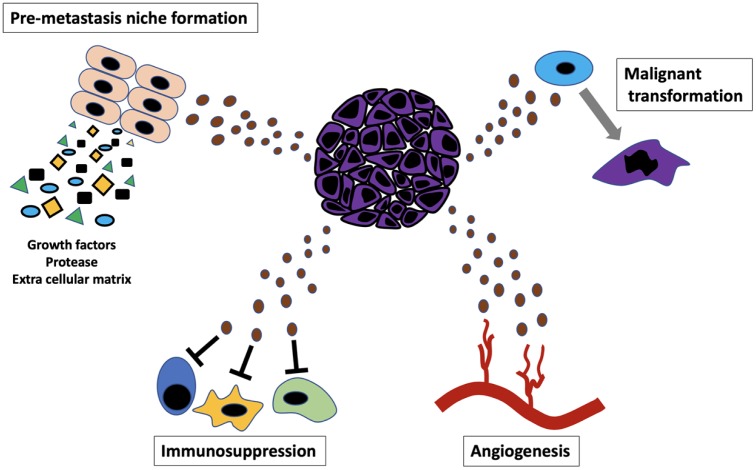
Proliferative cancer cells show (i) enlargement of cancer tissue with angiogenesis, (ii) acquisition of migratory and invasive capacity, and (iii) acquisition of capacity to avoid attack from immune cells and ultimately (iv) the formation of metastatic lesions. Based on the studies so far, it has become apparent that exosomes are involved in each of these processes (Fig. 3). Here, we describe the role of exosomes in each process.
Fig. 3.
Cancer progression regulated by “Exosome.”
Role of exosomes in the enlargement of cancer tissue with angiogenesis
During tumor growth, it has been reported that angiogenesis is induced when the tumor size exceeds 2 to 3 mm in diameter.40 Angiogenesis plays an important role in supplying oxygen and nutrition to the cancer cells, and it is an essential phenomenon for enlargement of solid tumors, i.e. a primary lesion or a metastatic lesion.
In 2008, Skog et al. reported that exosomes secreted by the glioblastoma promotes angiogenesis, as it is taken in by vascular endothelial cells,41 while in 2009, Hong et al. reported that cell cycle-related mRNA contained in the exosome from the colon cancer cells promotes the proliferation of vascular endothelial cells.42 In 2011, Grande et al. reported that the exosomes secreted by the renal cancer cell line, CD105-positive exosomes affected vascular endothelial cells and promoted growth, while CD105-negative exosomes had no such effect.43 Furthermore, Svensson et al. reported that exosome secreted by the glioblastoma under hypoxic environment had tissue factor and induced angiogenesis through the activation of PAR-2 in the vascular endothelial cells.44 These reports suggest that the surface molecules on the exosomes secreted by the cancer cells differ according to their function and change according to the environment. Furthermore, Kosaka et al. demonstrated that miR-210 contained in the exosomes secreted by breast cancer cells promotes angiogenesis and that suppression of exosome secretion from breast cancer cells by n-SMase2 knockdown can suppress metastasis through the inhibition of angiogenesis.13 In 2019, Li et al. reported that exosomes from the liver cancer cells are taken up by vascular endothelial cells and the LOXL4 in the exosomes acts to promote proliferation of the vascular endothelial cells.45 In the same year, Sun et al. reported that colon cancer-cell derived exosomes containing IRF-2 are taken up by the macrophages and induce VEGFC production, which then promotes lymph node metastasis through lymphatic endothelial cell proliferation and lymphatic vessel remodeling.46
Role of exosomes in cancer cell migration or invasion
As cancer cells proliferate, metastasis occurs by destroying the surrounding existing tissues. The migration and invasion of cancer cells is important in this process, and it is considered the first step to metastasis. The expression and activation of factors that degrade proteins are important in the invasion process.
In 2010, McCready et al. reported that HSP90α exists on the surface of the exosomes derived from cancer cells with high invasive capacity, acts in an autocrine manner, and activates plasminogen to enhance the invasive capacity of cancer cells.47 While plasmin formed by activation of plasminogen is known to degrade the extracellular matrix, it has also been reported to contribute to cell migration and invasion through the degradation of E-cadherin, which plays a role in epithelial cell adhesion,48 and therefore, it was believed that exosomes containing HSP90α are involved in the invasion of cancer cells through the mechanism mentioned above. In 2016, Sakha et al. reported that exosomes secreted by highly metastatic oral squamous cell carcinoma contained a large amount of miR-1246, and introduction of these exosomes to lowly metastatic cells negatively controlled DENND2D, the target molecule of miR-1246 and thereby promoted the migratory and invasive capacity of the cell.49 In 2018, Guo et al. showed that migration and invasion were suppressed in Rab27A-KO melanoma cells, but there was no difference in the size and expression level of the exosomes secreted, compared to cells that showed a high expression of Rab27A.50 Comparison of the contents of exosomes derived from the two cell lines showed that Rab27A-KO cells had a decreased amount of gene product that promotes cell migration and invasion, which suggested the possibility of Rab27A being involved in the production of exosomes that have the capacity to promote invasion.
It has been reported that the interaction between fibroblasts present in the cancer tissue and exosomes is important for cancer cell migration and invasion. In 2009, Castellana et al. showed that the chemokine receptor CXCR1 is present on the surface of cells derived from prostate cancer cells and that this increases the expression of the matrix metalloprotease (MMP) in fibroblasts through phosphorylation (=activation) of ERK.51 Although MMP is a representative of the enzymes that degrade the extracellular matrix and plays an important role in cancer cell invasion, thus, an environment convenient for cancer cells is produced by secretion of these enzymes not by the cancer cell itself but through by the surrounding fibroblasts. In 2012, Luga et al. reported that through interaction with exosomes secreted by fibroblasts around breast cancer cells, breast cancer cells secrete exosomes containing Wnt, which acts on other surrounding breast cancer cells and promotes migration and invasion.52 Fibroblasts present in cancer tissue are called cancer-associated fibroblasts (CAFs). These fibroblasts are known to contribute to forming a favorable environment for cancer cells, and exosomes are used for interaction between cancer cells and CAFs.
Exosomes and their role in avoiding attack from the immune cells
For cancer cells to continue surviving and proliferating, it is necessary for them to avoid interaction with the immune cells. Starting with the proliferation in primary lesions, it is necessary for cancer cells to avoid being attacked by immune cells even while moving around in vessels.
In 2005, Huber et al. reported that the Fas ligand expressed on the surface of the exosomes derived from colon cancer cells thereby induced apoptosis of the T cells.53 Moreover, in 2006, Liu et al. reported that exosomes derived from breast cancer cells contribute to growth of tumor by blocking IL-2-mediated proliferation and activation of NK cell.54 Similarly, Clayton et al. reported that exosomes derived from the cancer cells suppress the expression of NKG2D that is necessary for activation of NK cells.55 In 2007, Yu et al. revealed that exosomes derived from breast cancer cells act on the CD11-positive cells in the bone marrow, promoting IL-6 secretion and ultimately suppressing the differentiation of the dendritic cells.56 In 2014, Zhou et al. reported that miR-203 encapsulated in the exosomes derived from the pancreatic cancer cells inhibits the expression of TLR4 (toll-like receptor 4), which is a foreign body recognition receptor in dendritic cells.57 Moreover, de Vrij et al. reported in 2015 that exosomes derived from glioma induce differentiation of monocytes into immunosuppressive macrophages.58
Thus, exosomes derived from the cancer cells induce death of the immune cells or inhibit their activation or differentiation in the body to play an important role in helping cancer cells avoid attacks by such cells.
Exosomes and their role in formation of metastatic niche
The metastasis of cancer is the hallmark of malignant cancer, and it is an important prognostic factor clinically. Exosomes are actively involved in the formation of metastatic lesions, and under certain conditions exosomes promote metastasis when they are secreted by cancer cells with the purpose of metastasis, or when exosomes, secreted from the primary lesion, reach a distant organ through the blood.
As mentioned previously, cells that migrate from the primary lesion and reach a distant organ through the blood vessels, while avoiding attacks from immune cells, migrate from the blood vessel to the tissue. In 2014, Zhou et al. reported that exosomes derived from the metastatic breast cancer tissue contain miR-105, and by acting on the vascular endothelial cells, these exosomes downregulate the expression of the adhesion molecule ZO-1, ultimately promoting migration of cancer cells out of the blood vessels.59 In addition, a positive correlation was shown between the miR-105 content in the blood exosomes and distant metastasis in human breast cancer patients, which strongly suggested the importance of miR-105. In 2015, Tominaga et al. identified the exosome related to disruption of the blood-brain-barrier (BBB) that controls the movement of substances in and out of the brain.60 miR-181c contained in the exosomes derived from the breast cancer cells that metastasize to the brain inhibits PDPK1 and inhibits polymerization of actin in the cytoskeleton, which results in morphological changes. This changes the structure of the vascular endothelial cells that compose the BBB, producing gaps between cells and promotes the migration of the breast cancer cells into the brain parenchyma. This report was epochal. Furthermore, in 2017, an exosome related to the peritoneal dissemination of ovarian cancer was identified. Yokoi et al. reported that as exosomes derived from the metastatic ovarian cancer cells were taken up by the mesothelial cells composing the peritoneum, they induced apoptosis and led to formation of a hole in the peritoneum, leading to peritoneal dissemination of the cancer.61 This exosome contained MMP-1 mRNA, and it has been shown that this mRNA is translated to its protein in the mesothelial cells.
While the mechanism by which exosome secreted by cancer cells acts on the target tissue has been elucidated, it has also been shown that exosomes secreted by the primary lesion lead to the formation of the metastatic lesion in distant tissues (i.e., pre-metastatic niche formation) before the cancer cells themselves migrate to the particular tissue. In 2012, Peinado et al. revealed that when exosomes derived from melanoma cells, with highly metastatic potential to the lungs, were administered to mice, they promoted vascular permeability through introduction of bone marrow cells to the lungs and thereby contributed to the formation of a pre-metastatic niche.20 In fact, it has been reported that administration of the exosome prior to transplantation of melanoma cells to mice promoted metastasis in those mice, compared to mice that were devoid of the exosomes. In 2015, Costa-Silva et al. reported that exosomes derived from the pancreatic cancer cells formed the pre-metastatic niche for liver metastasis.62 Pancreatic cancer cell-derived exosomes contain the macrophage migration inhibitory factor, and the hepatic Kuppfer cells that took in these exosomes induced fibronectin production by hepatic stellate cells through production of TGF-beta and ultimately formed a pre-metastatic niche through introduction of bone marrow cells to the liver. As normal pancreatic tissue-derived exosomes do not show such phenomena, the pre-metastatic niche formation was thought to be due to pancreatic cancer cell-derived exosomes. As such, it was understood that exosomes secreted by cancer cells were involved in the formation of pre-metastatic niche, and there are questions regarding the function of the exosomes in specific organs. Hoshino et al. have reported that this is dependent on the pattern of integrins on the surface of the exosomes secreted by the cancer cells.27 Hoshino et al. showed that exosomes, showing tropism to the lungs, have integrin α6β4 and α6β1, while exosomes showing tropism to the liver have αvβ5. In addition to the formation of a pre-metastatic niche in the lungs and liver by the respective exosomes, downregulation of the specific integrin expressions in the exosomes weakened their respective tropism and subsequently suppressed the metastasis of cancer cells secreting these exosomes. Thus, these reports partially, demonstrated the “Seed and soil hypothesis” proposed by Paget in 1889 at the molecular level.
PROSPECT OF THE CLINICAL APPLICATION OF EXOSOME RESEARCH AND FUTURE CHALLENGES
As the role of exosomes in cancer progression become clearer, there have been increasing efforts towards their clinical application, and in recent years, cancer diagnosis by liquid biopsy has gained attention. Liquid biopsy is a method of diagnosis that analyzes the disease information contained in body fluids like blood, and cancer cell-derived exosomes are certainly one such type of information. As a novel diagnostic technology for colon cancer, Yoshioka et al. developed the “ExoScreen method.”63 This is a quick and easy method for detecting colon cancer cell-derived exosomes contained in the blood. This method works by trapping the exosome, using two types of monoclonal antibodies, one against the general exosome marker CD9 and the other against the colon cancer cell-derived exosome-specific marker CD147. When the two antibodies are in close proximity i.e. within 200 nm, a fluorescence signal is generated. As such, if we are able to identify a molecule that is specifically present in the exosomes derived from cancer cells, either for all cancers in general or individual cancer types, this can provide an epochal diagnostic marker for cancer(s). Furthermore, if exosomes contain information related to pathology or drug resistance, it would prove to be a useful marker for understanding patient condition or for choosing a treatment. In addition to diagnosis using exosomes, development of treatments targeting exosomes is underway at the research level. Nishida-Aoki et al. produced a mouse model with breast cancer by orthotopic transplantation of human breast cancer cell line and administered antibodies against human CD9 or CD63 to these mice. Whereas mice without antibody administration showed lymph node and lung metastasis of the cancer, use of antibodies against cancer cell-derived exosomes have been shown to suppress cancer metastasis in the models.64 Although the antibodies used in the study showed a reaction specific to breast cancer cell-derived exosomes because the cancer cell and the host species are different from one another, the report strongly suggested that identification of cancer cell-specific exosomal surface marker(s) will be able to lead to a novel treatment against cancers. Furthermore, by using molecules that specify the tropism of an exosome as described in 2.4, exosomes will enable the use as a drug delivery system (DDS). Therefore, exosomes can be expected to develop as a diagnostic marker, a therapeutic target, and for drug discovery and has the potential to change the future of medicine.
CONCLUSION
This review provides an overview of exosomes and its role in cancer progression. Although exosomes were discovered 40 years ago, in the 1980s, there has been a dramatic progression in research intended for understanding their biological significance and application to medicine. However, there are many details that still remain unknown, and new questions arise based on recent research. For instance, while the content of exosomes varies among cells and changes even for the same cell depending on the environment, the mechanism for this variety is yet to be understood. Elucidating the role of the molecules on the exosomal surface and those encapsulated within would help reveal the pathological condition caused by the exosome and develop methods to counter them. With the exosome as a key word, basic researchers and clinical researchers need to take a challenge to overcome “cancer,” and we believe there will be a significant breakthrough.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Crescitelli R,Lässer C,Szabó TG,Kittel A,Eldh M,Dianzani I,et al. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles. 2013;2:20677. 10.3402/jev.v2i0.20677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan BT,Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967-78. 10.1016/0092-8674(83)90040-5 [DOI] [PubMed] [Google Scholar]
- 3.Raposo G,Nijman HW,Stoorvogel W,Liejendekker R,Harding CV,Melief CJ,et al. B lymphocytes secrete antigen-presenting vesicles. J Cell Biol. J Exp Med.-1995;131:1403-19.1996;183:1161-72. PubMed -https://doi.org/. 10.1084/jem.183.3.1161 [DOI] [PMC free article] [PubMed]
- 4.Valadi H,Ekström K,Bossios A,Sjöstrand M,Lee JJ,Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654-9. 10.1038/ncb1596 [DOI] [PubMed] [Google Scholar]
- 5.Kosaka N,Iguchi H,Yoshioka Y,Takeshita F,Matsuki Y,Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285:17442-52. 10.1074/jbc.M110.107821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pegtel DM,Cosmopoulos K,Thorley-Lawson DA,van Eijndhoven MAJ,Hopmans ES,Lindenberg JL,et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci USA. 2010;107:6328-33. 10.1073/pnas.0914843107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y,Liu D,Chen X,Li J,Li L,Bian Z,et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell. 2010;39:133-44. 10.1016/j.molcel.2010.06.010 [DOI] [PubMed] [Google Scholar]
- 8.van Niel G,D’Angelo G,Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213-28. 10.1038/nrm.2017.125 [DOI] [PubMed] [Google Scholar]
- 9.Henne WM,Stenmark H,Emr SD. Molecular mechanisms of the membrane sculpting ESCRT pathway. Cold Spring Harb Perspect Biol. 2013;5:a016766. 10.1101/cshperspect.a016766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katzmann DJ,Babst M,Emr SD. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001;106:145-55. 10.1016/S0092-8674(01)00434-2 [DOI] [PubMed] [Google Scholar]
- 11.Hanson PI,Cashikar A. Multivesicular body morphogenesis. Annu Rev Cell Dev Biol. 2012;28:337-62. 10.1146/annurev-cellbio-092910-154152 [DOI] [PubMed] [Google Scholar]
- 12.Trajkovic K,Hsu C,Chiantia S,Rajendran L,Wenzel D,Wieland F,et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244-7. 10.1126/science.1153124 [DOI] [PubMed] [Google Scholar]
- 13.Kosaka N,Iguchi H,Hagiwara K,Yoshioka Y,Takeshita F,Ochiya T. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. J Biol Chem. 2013;288:10849-59. 10.1074/jbc.M112.446831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Niel G,Charrin S,Simoes S,Romao M,Rochin L,Saftig P,et al. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell. 2011;21:708-21. 10.1016/j.devcel.2011.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoshino D,Kirkbride KC,Costello K,Clark ES,Sinha S,Grega-Larson N,et al. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Reports. 2013;5:1159-68. 10.1016/j.celrep.2013.10.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savina A,Fader CM,Damiani MT,Colombo MI. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic. 2005;6:131-43. 10.1111/j.1600-0854.2004.00257.x [DOI] [PubMed] [Google Scholar]
- 17.Ostrowski M,Carmo NB,Krumeich S,Fanget I,Raposo G,Savina A,et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19-30, 1-13. 10.1038/ncb2000 [DOI] [PubMed]
- 18.Hsu C,Morohashi Y,Yoshimura S,Manrique-Hoyos N,Jung S,Lauterbach MA,et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A–C. J Cell Biol. 2010;189:223-32. 10.1083/jcb.200911018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamai K,Tanaka N,Nakano T,Kakazu E,Kondo Y,Inoue J,et al. Exosome secretion of dendritic cells is regulated by Hrs, an ESCRT-0 protein. Biochem Biophys Res Commun. 2010;399:384-90. 10.1016/j.bbrc.2010.07.083 [DOI] [PubMed] [Google Scholar]
- 20.Peinado H,Alečković M,Lavotshkin S,Matei I,Costa-Silva B,Moreno-Bueno G,et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Erratum in: Nat Med. 2012;18:883-91. . [DOI] [PMC free article] [PubMed]
- 21.Bobrie A,Krumeich S,Reyal F,Recchi C,Moita LF,Seabra MC,et al. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res. 2012;72:4920-30. 10.1158/0008-5472.CAN-12-0925 [DOI] [PubMed] [Google Scholar]
- 22.Yamini B,Yu X,Dolan ME,Wu MH,Kufe DW,Weichselbaum RR. Inhibition of nuclear factor-kappaB activity by temozolomide involves O6-methylguanine-induced inhibition of p65 DNA binding. Cancer Res. 2007;67:6889-98. 10.1158/0008-5472.CAN-06-4496 [DOI] [PubMed] [Google Scholar]
- 23.Lespagnol A,Duflaut D,Beekman C,Blanc L,Fiucci G,Marine J-C,et al. Exosome secretion, including the DNA damage-induced p53-dependent secretory pathway, is severely compromised in TSAP6/Steap3-null mice. Cell Death Differ. 2008;15:1723-33. 10.1038/cdd.2008.104 [DOI] [PubMed] [Google Scholar]
- 24.Laulagnier K,Motta C,Hamdi S,Roy S,Fauvelle F,Pageaux JF,et al. Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem J. 2004;380:161-71. 10.1042/bj20031594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Llorente A,Skotland T,Sylvänne T,Kauhanen D,Róg T,Orłowski A,et al. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim Biophys Acta BBAMol Cell Biol Lipids. 2013;1831:1302-9. 10.1016/j.bbalip.2013.04.011 [DOI] [PubMed] [Google Scholar]
- 26.Yoshioka Y,Konishi Y,Kosaka N,Katsuda T,Kato T,Ochiya T. Comparative marker analysis of extracellular vesicles in different human cancer types. J Extracell Vesicles. 2013;2:20424. 10.3402/jev.v2i0.20424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoshino A,Costa-Silva B,Shen TL,Rodrigues G,Hashimoto A,Tesic Mark M,et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329-35. 10.1038/nature15756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.André F,Chaput N,Schartz NEC,Flament C,Aubert N,Bernard J,et al. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J Immunol. 2004;172:2126-36. 10.4049/jimmunol.172.4.2126 [DOI] [PubMed] [Google Scholar]
- 29.Webber J,Steadman R,Mason MD,Tabi Z,Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010;70:9621-30. 10.1158/0008-5472.CAN-10-1722 [DOI] [PubMed] [Google Scholar]
- 30.Segura E,Nicco C,Lombard B,Véron P,Raposo G,Batteux F,et al. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood. 2005;106:216-23. 10.1182/blood-2005-01-0220 [DOI] [PubMed] [Google Scholar]
- 31.King HW,Michael MZ,Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421. 10.1186/1471-2407-12-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mittelbrunn M,Gutiérrez-Vázquez C,Villarroya-Beltri C,González S,Sánchez-Cabo F,González MÁ,et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. 10.1038/ncomms1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang X,Yuan T,Tschannen M,Sun Z,Jacob H,Du M,et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics. 2013;14:319. 10.1186/1471-2164-14-319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kogure T,Yan IK,Lin WL,Patel T. Extracellular vesicle-mediated transfer of a novel long noncoding RNA TUC339: A mechanism of intercellular signaling in human hepatocellular cancer. Genes Cancer. 2013;4:261-72. 10.1177/1947601913499020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu Z,Yan Y,Zeng S,Dai S,Chen X,Wei J,et al. Circular RNAs: clinical relevance in cancer. Oncotarget. 2017;9:1444-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen TS,Lai RC,Lee MM,Choo ABH,Lee CN,Lim SK. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010;38:215-24. 10.1093/nar/gkp857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thakur BK,Zhang H,Becker A,Matei I,Huang Y,Costa-Silva B,et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 2014;24:766-9. 10.1038/cr.2014.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi A,Okada R,Nagao K,Kawamata Y,Hanyu A,Yoshimoto S,et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat Commun. 2017;8:15287. 10.1038/ncomms15287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawamura Y,Yamamoto Y,Sato TA,Ochiya T. Extracellular vesicles as trans-genomic agents: emerging roles in disease and evolution. Cancer Sci. 2017;108:824-30. 10.1111/cas.13222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Folkman J. Anti-Angiogenesis. Ann Surg. 1972;175:409-16. 10.1097/00000658-197203000-00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skog J,Würdinger T,van Rijn S,Meijer DH,Gainche L,Curry WT,et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470-6. 10.1038/ncb1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hong B,Cho JH,Kim H,Choi EJ,Rho S,Kim J,et al. Colorectal cancer cell-derived microvesicles are enriched in cell cycle-related mRNAs that promote proliferation of endothelial cells. BMC Genomics. 2009;10:556. 10.1186/1471-2164-10-556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grange C,Tapparo M,Collino F,Vitillo L,Damasco C,Deregibus MC,et al. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011;71:5346-56. 10.1158/0008-5472.CAN-11-0241 [DOI] [PubMed] [Google Scholar]
- 44.Svensson KJ,Kucharzewska P,Christianson HC,Sköld S,Löfstedt T,Johansson MC,et al. Hypoxia triggers a proangiogenic pathway involving cancer cell microvesicles and PAR-2–mediated heparin-binding EGF signaling in endothelial cells. Proc Natl Acad Sci USA. 2011;108:13147-52. 10.1073/pnas.1104261108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li R,Wang Y,Zhang X,Feng M,Ma J,Li J,et al. Exosome-mediated secretion of LOXL4 promotes hepatocellular carcinoma cell invasion and metastasis. Mol Cancer. 2019;18:18. 10.1186/s12943-019-0948-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun B,Zhou Y,Fang Y,Li Z,Gu X,Xiang J Colorectal cancer exosomes induce lymphatic network remodeling in lymph nodes. Int J Cancer. 2019. . [DOI] [PubMed]
- 47.McCready J,Sims JD,Chan D,Jay DG. Secretion of extracellular hsp90α via exosomes increases cancer cell motility: a role for plasminogen activation. BMC Cancer. 2010;10:294. 10.1186/1471-2407-10-294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayashido Y,Hamana T,Yoshioka Y,Kitano H,Koizumi K,Okamoto T. Plasminogen activator/plasmin system suppresses cell-cell adhesion of oral squamous cell carcinoma cells via proteolysis of E-cadherin. Int J Oncol. 2005;27:693-8. [PubMed] [Google Scholar]
- 49.Sakha S,Muramatsu T,Ueda K,Inazawa J. Exosomal microRNA miR-1246 induces cell motility and invasion through the regulation of DENND2D in oral squamous cell carcinoma. Sci Rep. 2016;6:38750. 10.1038/srep38750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo D,Lui GYL,Lai SL,Wilmott JS,Tikoo S,Jackett LA,et al. RAB27A promotes melanoma cell invasion and metastasis via regulation of pro‐invasive exosomes. Int J Cancer. 2019;144:3070-85. 10.1002/ijc.32064 [DOI] [PubMed] [Google Scholar]
- 51.Castellana D,Zobairi F,Martinez MC,Panaro MA,Mitolo V,Freyssinet JM,et al. Membrane microvesicles as actors in the establishment of a favorable prostatic tumoral niche: a role for activated fibroblasts and CX3CL1-CX3CR1 axis. Cancer Res. 2009;69:785-93. 10.1158/0008-5472.CAN-08-1946 [DOI] [PubMed] [Google Scholar]
- 52.Luga V,Zhang L,Viloria-Petit AM,Ogunjimi AA,Inanlou MR,Chiu E,et al. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151:1542-56. 10.1016/j.cell.2012.11.024 [DOI] [PubMed] [Google Scholar]
- 53.Huber V,Fais S,Iero M,Lugini L,Canese P,Squarcina P,et al. Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: role in immune escape. Gastroenterology. 2005;128:1796-804. 10.1053/j.gastro.2005.03.045 [DOI] [PubMed] [Google Scholar]
- 54.Liu C,Yu S,Zinn K,Wang J,Zhang L,Jia Y,et al. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J Immunol. 2006;176:1375-85. 10.4049/jimmunol.176.3.1375 [DOI] [PubMed] [Google Scholar]
- 55.Clayton A,Mitchell JP,Court J,Linnane S,Mason MD,Tabi Z. Human tumor-derived exosomes down-modulate NKG2D expression. J Immunol. 2008;180:7249-58. 10.4049/jimmunol.180.11.7249 [DOI] [PubMed] [Google Scholar]
- 56.Yu S,Liu C,Su K,Wang J,Liu Y,Zhang L,et al. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J Immunol. 2007;178:6867-75. 10.4049/jimmunol.178.11.6867 [DOI] [PubMed] [Google Scholar]
- 57.Zhou M,Chen J,Zhou L,Chen W,Ding G,Cao L. Pancreatic cancer derived exosomes regulate the expression of TLR4 in dendritic cells via miR-203. Cell Immunol. 2014;292:65-9. 10.1016/j.cellimm.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 58.de Vrij J,Maas SLN,Kwappenberg KMC,Schnoor R,Kleijn A,Dekker L,et al. Glioblastoma-derived extracellular vesicles modify the phenotype of monocytic cells. Int J Cancer. 2015;137:1630-42. 10.1002/ijc.29521 [DOI] [PubMed] [Google Scholar]
- 59.Zhou W,Fong MY,Min Y,Somlo G,Liu L,Palomares MR,et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25:501-15. 10.1016/j.ccr.2014.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tominaga N,Kosaka N,Ono M,Katsuda T,Yoshioka Y,Tamura K,et al. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood–brain barrier. Nat Commun. 2015;6:6716. 10.1038/ncomms7716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yokoi A,Yoshioka Y,Yamamoto Y,Ishikawa M,Ikeda S,Kato T,et al. Malignant extracellular vesicles carrying MMP1 mRNA facilitate peritoneal dissemination in ovarian cancer. Nat Commun. 2017;8:14470. 10.1038/ncomms14470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Costa-Silva B,Aiello NM,Ocean AJ,Singh S,Zhang H,Thakur BK,et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17:816-26. 10.1038/ncb3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoshioka Y,Kosaka N,Konishi Y,Ohta H,Okamoto H,Sonoda H,et al. Ultra-sensitive liquid biopsy of circulating extracellular vesicles using ExoScreen. Nat Commun. 2014;5:3591. 10.1038/ncomms4591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nishida-Aoki N,Tominaga N,Takeshita F,Sonoda H,Yoshioka Y,Ochiya T. Disruption of circulating extracellular vesicles as a novel therapeutic strategy against cancer metastasis. Mol Ther. 2017;25:181-91. 10.1016/j.ymthe.2016.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]