Key Points
Question
What is the independent impact of quantitative metastatic nodal burden in hypopharyngeal and laryngeal malignancies?
Findings
This study of 8351 cases identified continuously escalating mortality risk with increasing number of metastatic lymph nodes, eclipsing conventional nodal staging factors such as node size and contralaterality. A simplified nodal staging system based on metastatic node number exhibited improved prognostic value and discrimination compared with the TNM staging system outlined in the American Joint Committee on Cancer’s AJCC Staging Manual, 8th edition.
Meaning
Greater incorporation of numerical metastatic nodal burden into nodal classification for hypopharyngeal and laryngeal cancers may streamline staging, refine patient prognosis, and triage patients who may benefit from adjuvant treatment.
This analysis examines the association between metastatic lymph node burden and overall survival in patients with laryngohypopharyngeal cancers.
Abstract
Importance
Nodal staging for laryngohypopharyngeal cancers is based primarily on size and laterality, with less value placed on absolute number of metastatic lymph nodes (LNs). We are aware of no studies to date that have specifically addressed the prognostic effect of quantitative nodal burden in larynx or hypopharynx malignancies.
Objective
To assess the independent impact of quantitative metastatic LN burden on mortality risk.
Design, Setting, and Participants
Univariate and multivariable models were constructed to evaluate the association between patients’ number of metastatic LNs and their survival, adjusting for factors such as nodal size, laterality, extranodal extension, margin status, and adjuvant treatment. Participants were patients with squamous cell carcinoma of the larynx or hypopharynx undergoing upfront surgical resection for curative intent at a US hospital between 2004 and 2013, as identified in the National Cancer Database. A neck dissection of a minimum of 10 LNs was required.
Main Outcomes and Measures
Overall survival.
Results
Overall, 8351 cases were included (mean [SD] age, 61 [10.1] years; 6499 men [77.8%]; 4710 patients with metastatic LNs and 3641 with no metastatic LNs). Mortality risk escalated continuously without plateau as number of metastatic nodes increased, with the hazard per node (hazard ratio [HR], 1.19; 95% CI, 1.16-1.23; P < .001) most pronounced up to 5 positive LNs. Extranodal extension was also associated with increased mortality (HR, 1.34; 95% CI, 1.13-1.59; P < .001). Increasing number of nodes examined was associated with improved survival, albeit to a lesser degree (per 10 LNs: HR, 0.97; 95% CI, 0.96-0.98; P < .001) and without a detectable change point. Other nodal factors, including nodal size, contralateral LN involvement (TNM stage N2c), and lower LN involvement (levels 4-5), were not associated with mortality in multivariable models when accounting for number of positive LNs. A novel, parsimonious nodal staging system derived by recursive partitioning analysis exhibited greater concordance with survival than the TNM staging system outlined in the American Joint Committee on Cancer’s AJCC Staging Manual, 8th edition.
Conclusions and Relevance
The number of metastatic nodes is a predominant independent factor associated with mortality in hypopharyngeal and laryngeal cancers. Moreover, standard nodal staging factors like LN size and contralaterality have no independent prognostic value when accounting for positive LN number. Deeper integration of quantitative metastatic nodal disease may simplify staging and better triage the need for adjuvant therapy.
Introduction
The management of laryngohypopharyngeal cancers remains daunting, owing to functional morbidity and poor prognosis. Though organ preservation trials have shown efficacy with chemoradiation,1,2,3,4,5,6 surgery remains the standard of care for resectable disease, especially with extralaryngeal extension or pretreatment laryngeal dysfunction.7
The American Joint Committee on Cancer’s AJCC Staging Manual, 8th edition8 (AJCC 8E) TNM staging system considers a combination of lymph node (LN) factors, including number, size, laterality, and extranodal extension. However, the independent impact of each factor remains poorly defined. Current staging may underestimate the cumulative effect of escalating metastatic nodal burden; for example, patients with 2 positive LNs may be staged the same as those with 10, despite the likelihood that the latter will do much worse clinically. We therefore investigated quantitative metastatic nodal burden in a large hypopharyngeal and laryngeal cancer cohort.
Methods
Patients
This study was deemed exempt by the Cedars-Sinai institutional review board, and the requirement for patient consent was waived. Data from the National Cancer Database between 2004 and 2013 were evaluated. All patients 18 years or older undergoing surgery and neck dissection for hypopharyngeal or laryngeal squamous cell carcinoma for curative intent were assessed. Cases with fewer than 10 LNs examined were eliminated to remove excisional biopsies and substandard neck dissections.
Statistical Analysis
Detailed methods are listed in the Supplement. Missing values were imputed using the multivariate imputation by chained equations algorithm.9,10 Univariate and multivariable survival analyses were performed with Cox proportional hazards models. Restricted cubic spline functions were used to model relationships between positive LN number and overall survival (OS). Optimal numbers of knots were chosen based on the lowest Akaike information criterion. Three knots were placed at 1, 3, and 9 positive LNs corresponding to 55th, 75th, and 95th percentiles, respectively. Change points were estimated with piecewise linear regression modeling.11 Recursive partitioning analysis was used to create a nodal classification system, with performance assessed via the C statistic using the bootstrap method.
Results
Number of Positive Metastatic LNs
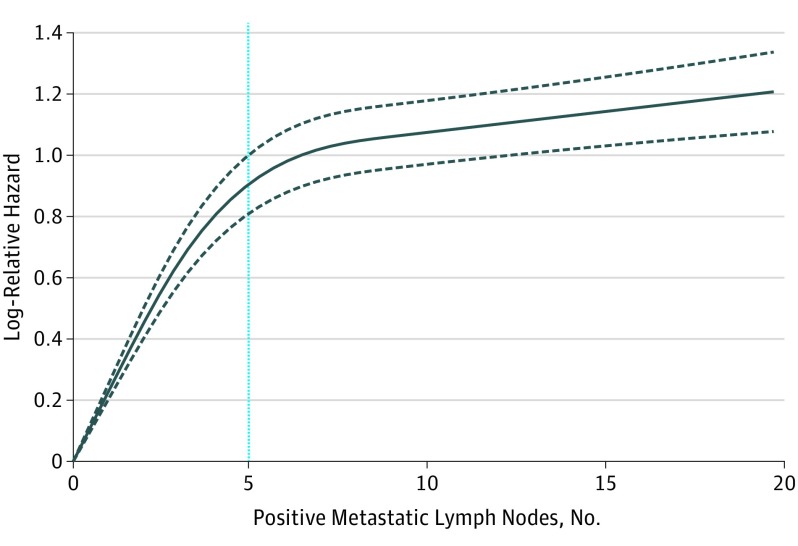
Overall, 8351 patients (mean [SD] age, 61 [10.1] years; 6499 men [77.8%]) met inclusion criteria (eFigure 1 and eTable 1 in the Supplement). Increased number of metastatic LNs correlated with worse OS (eTable 2 and eFigure 2 in the Supplement). Mortality risk continually increased with increasing number of positive LNs without plateau (Figure 1) even after adjustment for nodal and nonnodal covariates. The relationship between OS and the number of positive LNs was nonlinear: the hazard of death per positive LN increased to a change point of 5 metastatic LNs (hazard ratio [HR], 1.19; 95% CI, 1.16-1.23; P < .001). Beyond this, the risk of death increased modestly (HR, 1.01; 95% CI, 1.01-1.02; P = .001) (eTable 3 in the Supplement). Because significant interactions between anatomic site and number of positive LNs on survival were detected, patients with larynx and hypopharynx cancers were analyzed separately (eTable 4 in the Supplement). The results were similar for both larynx and hypopharynx cancers, although the hazard per LN was greater for larynx cancers vs hypopharynx cancers for each positive LN up to 5. The reverse was true for more than 5 positive LNs.
Figure 1. Escalating Mortality Risk With Increase in Metastatic Lymph Node Number.
Escalating adjusted hazard ratio (HR) with increasing number of positive metastatic lymph nodes (LNs) in hypopharyngeal and laryngeal cancers. Gray dashed lines represent estimated 95% CIs of the predicted HRs. Black solid line represents multivariable smoothed restricted cubic spline plot of the natural logarithm of adjusted HR vs the number of positive metastatic LNs. Blue vertical line represents the estimated change point at 5 positive LNs.
Number of LNs Examined
Number of LNs examined exhibited a linear association with mortality, with no change point observed. The risk of death decreased continuously with each additional node harvested (per 10 LNs examined: HR, 0.97; 95% CI, 0.96-0.98; P < .001) (eFigure 3 and eTable 3 in the Supplement). An interaction between margin status and number of LNs examined was identified (eTable 5 in the Supplement).
Metastatic LN Features
Extranodal extension remained independently associated with decreased OS in multivariable models (HR, 1.34; 95% CI, 1.13-1.59; P = .001). Node size, lower LN involvement (level 4-5), and contralateral LN involvement (TNM stage N2c) had no independent impact on survival (eTable 2 in the Supplement).
Proposed Nodal Staging System
Recursive partitioning analysis was used to generate a novel nodal staging system (eFigure 4 in the Supplement). One positive LN with extranodal extension and 2 to 3 positive LNs clustered separately but were grouped as N2 owing to similar survival rates (Table). The proposed system showed improvement in predictive ability (optimism-corrected C statistic, 0.674; 95% CI, 0.661-0.687) over the AJCC 8E TNM system (C statistic, 0.671; 95% CI, 0.658-0.684) (eTable 6 in the Supplement).
Table. Overall Survival for Proposed and AJCC 8th Edition TNM Nodal Staging Systems for Hypopharyngeal and Laryngeal Cancers.
| N Category | Criteria | 3-Year OS, % |
|---|---|---|
| Proposed Nodal Staging System | ||
| N0 | 0 LN+ | 73.2 |
| N1 | 1 LN+ without ENE | 62.3 |
| N2 | 2-3 LN+ or 1 LN+ with ENE | 51.7 |
| N3a | 4-6 LN+ | 43.2 |
| N3b | ≥7 LN+ | 27.9 |
| AJCC 8th Edition TNM Nodal Staging System | ||
| N0 | 0 LN+ | 73.2 |
| N1 | 1 Ipsilateral LN+, ≤3 cm, without ENE | 61.6 |
| N2a | 1 Ipsilateral or contralateral LN+, ≤3 cm, with ENE; or 1 ipsilateral LN+ 3-6 cm, without ENE | 52.8 |
| N2b | >1 Ipsilateral LN+, ≤6 cm, without ENE | 55.1 |
| N2c | >1 Bilateral or contralateral LN+, ≤6 cm, without ENE | 48.7 |
| N3a | ≥1 LN+, >6 cm, without ENE | NA |
| N3b | 1 Ipsilateral LN+, >3 cm, with ENE; or >1 ipsilateral, contralateral, or bilateral LN+, with ENE | 38.8 |
Abbreviations: AJCC, American Joint Committee on Cancer; ENE, extranodal extension; LN+, metastatic lymph node; NA, not applicable; OS, overall survival.
Discussion
This study systematically addressed quantitative metastatic nodal disease burden in larynx and hypopharynx cancers. In continuous multivariable regression models, we observed that successive positive LNs were associated with increased risk of death without plateau. Notably, each metastatic node up to 5 was associated with an additional 19% mortality risk, while each positive node beyond 5 was associated with continually escalating cumulative hazard. The results were similar for larynx and hypopharynx cancers individually, although risk of death per LN increased more rapidly for larynx cancers than hypopharynx cancers with each positive LN up to 5, while the opposite trend was observed with each positive LN beyond 5. It is intuitive that increasing number of metastatic nodes might increase risk of death; it is moreover provocative that factors used by TNM staging, including size and laterality, had no prognostic significance. As these covariates influenced survival in univariate analysis, they likely act as surrogates for metastatic nodal number. Consistent with this theme, divergent outcomes were observed within TNM N2b and N2c subgroups (eFigure 2B and C in the Supplement).
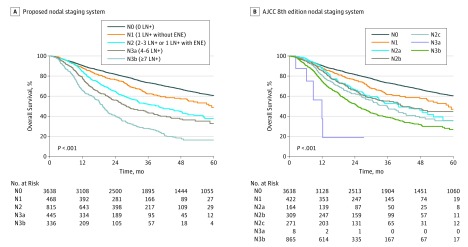
The predictive capability of this system was mildly improved in comparison to AJCC 8E. This may be because contralaterality and LN size associate tightly with number of positive LNs. Nonetheless, the proposed nodal staging system derived by recursive partitioning analysis confers several advantages considered qualitatively desirable by AJCC, beyond what can be measured by the C statistic.12 It is concise, consisting of fewer substratifications and based largely on a single variable. The distribution of patients across stages is relatively even (Figure 2). In contrast, AJCC 8E TNM staging for N2a through N2c classifications exhibits overlapping survival curves, with very few patients (n = 8) classified as N3a. The proposed system also partitions patients across a wider spectrum of outcomes. The patients at highest risk in the proposed system (those with 7 or more positive LNs) have 4.7 times the risk of death as patients with no LN metastases (eTable 6 in the Supplement). In comparison, the patients at highest risk in the AJCC 8E TNM system have 2.9 times higher risk of death than patients with no LN metastases. Altogether, the proposed system is simpler, is more discriminating, eliminates nonprognostic factors, and captures a greater range of mortality risk (Table).
Figure 2. Overall Survival for Proposed and AJCC 8th Edition TNM Nodal Staging Systems.
A, Kaplan-Meier estimate for proposed nodal staging system. B, Kaplan-Meier estimate for AJCC 8th edition TNM staging system. ENE indicates extranodal extension; LN+, metastatic lymph node.
These results are remarkably consistent with what we have observed in oral cavity cancer,13 suggesting unifying relationships among human papillomavirus–negative head and neck cancers that differ from virally driven oropharyngeal or nasopharyngeal carcinomas.14 As such, the influential role of positive LN number warrants additional investigation for adjuvant therapy. For example, treatment intensification with adjuvant chemoradiation may augment survival in those with 7 or more positive LN, given their poor prognosis.
Limitations
Several caveats deserve mention, including the study’s retrospective analysis and the focus on laryngeal and hypopharyngeal cancers undergoing surgery. Certain factors correlating with outcome, including smoking status, chemotherapy type, and perineural invasion, were not available. Our analysis is specific to pathologic staging and may not fully translate to clinical staging, given that imaging and physical examinations are less precise for determining metastatic LN number. Our proposed system may thus be more helpful in determining adjuvant, rather than definitive, treatment decision making. This distinction is relevant for laryngohypopharyngeal cancers, for which definitive chemoradiation can be equally considered for treatment. Nevertheless, this study provides strong empirical evidence to guide pathologic nodal staging.
Conclusions
Our data underscore the principal importance of metastatic LN number in delineating larynx and hypopharynx cancer prognosis. Each additional positive LN is associated with an escalation in mortality risk without plateau, with conventional nodal factors like size and contralaterality eclipsed in prognostic value. We advocate metastatic LN number as a prime component in nodal classification for larynx and hypopharynx cancers to refine staging and drive adjuvant treatment recommendations.
eFigure 1. Consolidated standards of reporting trials (CONSORT) diagram.
eFigure 2. Kaplan-Meier estimates of overall survival in hypopharyngeal and laryngeal cancer, stratified by number of positive metastatic lymph nodes in (a). all cases, (b). N2b cases, and (c). N2c cases.
eFigure 3. Adjusted hazard ratio (HR) with increasing number of lymph nodes (LN) examined in hypopharyngeal and laryngeal cancers.
eFigure 4. Novel proposed nodal staging system developed by recursive partitioning analysis in hypopharyngeal and laryngeal cancer patients with determinable AJCC 8th Edition stage.
eTable 1. Baseline patient demographics stratified by nodal status.
eTable 2. Multivariable analyses with proposed N-classification system and AJCC 8th edition N-classification system in hypopharyngeal and laryngeal cancer.
eTable 3. Summary of hazard ratios for positive metastatic lymph nodes and number of lymph nodes examined in hypopharyngeal and laryngeal cancer, stratified by change point. LN, lymph nodes.
eTable 4. Summary of hazard ratios for positive metastatic lymph nodes, stratified by hypopharyngeal or laryngeal cancer site and change point. LN, lymph nodes.
eTable 5. Summary of hazard ratios for number of lymph nodes examined stratified by margin status, and for gender stratified by hypopharynx or larynx cancer site. LN, lymph nodes.
eTable 6. Multivariable analyses with proposed N-Classification system and AJCC 8th Edition N-Classification system in hypopharyngeal and laryngeal cancer.
References
- 1.Lefebvre JL, Chevalier D, Luboinski B, Kirkpatrick A, Collette L, Sahmoud T; EORTC Head and Neck Cancer Cooperative Group . Larynx preservation in pyriform sinus cancer: preliminary results of a European Organization for Research and Treatment of Cancer phase III trial. J Natl Cancer Inst. 1996;88(13):890-899. [DOI] [PubMed] [Google Scholar]
- 2.Lefebvre JL, Andry G, Chevalier D, et al. ; EORTC Head and Neck Cancer Group . Laryngeal preservation with induction chemotherapy for hypopharyngeal squamous cell carcinoma: 10-year results of EORTC trial 24891. Ann Oncol. 2012;23(10):2708-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pointreau Y, Garaud P, Chapet S, et al. Randomized trial of induction chemotherapy with cisplatin and 5-fluorouracil with or without docetaxel for larynx preservation. J Natl Cancer Inst. 2009;101(7):498-506. [DOI] [PubMed] [Google Scholar]
- 4.Janoray G, Pointreau Y, Garaud P, et al. Long-term results of a multicenter randomized phase III trial of induction chemotherapy with cisplatin, 5-fluorouracil, ± docetaxel for larynx preservation. J Natl Cancer Inst. 2015;108(4):djv368. [DOI] [PubMed] [Google Scholar]
- 5.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349(22):2091-2098. [DOI] [PubMed] [Google Scholar]
- 6.Forastiere AA, Zhang Q, Weber RS, et al. Long-term results of RTOG 91-11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol. 2013;31(7):845-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adelstein D, Gillison ML, Pfister DG, et al. NCCN guidelines insights: head and neck cancers, version 2.2017. J Natl Compr Canc Netw. 2017;15(6):761-770. [DOI] [PubMed] [Google Scholar]
- 8.American Joint Committee on Cancer AJCC Cancer Staging Manual. 8th ed New York, NY: Springer International Publishing; 2017. [Google Scholar]
- 9.van Buuren S, Groothuis-Oudshoorn K. MICE: multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1-67. [Google Scholar]
- 10.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16(3):219-242. [DOI] [PubMed] [Google Scholar]
- 11.Toms JD, Lesperance ML. Piecewise regression: a tool for identifying ecological thresholds. Ecology. 2003;84:2034-2041. [Google Scholar]
- 12.Lydiatt WM, Patel SG, O’Sullivan B, et al. Head and neck cancers—major changes in the American Joint Committee on Cancer eighth edition Cancer Staging Manual. CA Cancer J Clin. 2017;67(2):122-137. [DOI] [PubMed] [Google Scholar]
- 13.Ho AS, Kim S, Tighiouart M, et al. Metastatic lymph node burden and survival in oral cavity cancer. J Clin Oncol. 2017;JCO2016711176. doi: 10.1200/JCO.2016.71.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Sullivan B, Huang SH, Su J, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol. 2016;17(4):440-451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Consolidated standards of reporting trials (CONSORT) diagram.
eFigure 2. Kaplan-Meier estimates of overall survival in hypopharyngeal and laryngeal cancer, stratified by number of positive metastatic lymph nodes in (a). all cases, (b). N2b cases, and (c). N2c cases.
eFigure 3. Adjusted hazard ratio (HR) with increasing number of lymph nodes (LN) examined in hypopharyngeal and laryngeal cancers.
eFigure 4. Novel proposed nodal staging system developed by recursive partitioning analysis in hypopharyngeal and laryngeal cancer patients with determinable AJCC 8th Edition stage.
eTable 1. Baseline patient demographics stratified by nodal status.
eTable 2. Multivariable analyses with proposed N-classification system and AJCC 8th edition N-classification system in hypopharyngeal and laryngeal cancer.
eTable 3. Summary of hazard ratios for positive metastatic lymph nodes and number of lymph nodes examined in hypopharyngeal and laryngeal cancer, stratified by change point. LN, lymph nodes.
eTable 4. Summary of hazard ratios for positive metastatic lymph nodes, stratified by hypopharyngeal or laryngeal cancer site and change point. LN, lymph nodes.
eTable 5. Summary of hazard ratios for number of lymph nodes examined stratified by margin status, and for gender stratified by hypopharynx or larynx cancer site. LN, lymph nodes.
eTable 6. Multivariable analyses with proposed N-Classification system and AJCC 8th Edition N-Classification system in hypopharyngeal and laryngeal cancer.