Key Points
Question
What percentage of adults with impairment in sexual functioning report durable improvements in the 5 years after bariatric surgery?
Findings
In this cohort study of adults with impairment in sexual functioning before bariatric surgery, more than half of women experienced meaningful postsurgical improvements in the degree to which physical health limited sexual activity (74%) and satisfaction with sexual life (52%) 5 years after surgery, and more than one-third of women had improvements in frequency of sexual desire (41%) and sexual activity (35%) 5 years after surgery. At least half of men experienced improvements in all 4 domains in year 5.
Meaning
In this study, a considerable portion of adults experienced durable improvements in several domains of sexual functioning after bariatric surgery.
This cohort study assesses sexual desire, sexual activity, satisfaction with sexual life, and the degree to which physical health limits sexual activity in a cohort of men and women in the 5 years after bariatric surgery.
Abstract
Importance
Short-term improvements in sexual functioning are reported after bariatric surgery, but to our knowledge, little is known about the durability of these improvements.
Objective
To determine the percentage of adults with impairment in sexual functioning who experience durable improvements in sexual functioning after bariatric surgery and to identify factors associated with improvements.
Design, Setting, and Participants
The Longitudinal Assessment of Bariatric Surgery-2 is an observational cohort study conducted at 10 hospitals in 6 US clinical centers. Adults undergoing their first bariatric procedure were recruited from 2005 through 2009, data were collected through August 2014. Data analysis was conducted from 2016 to April 2018.
Interventions
Participants completed assessments before the procedure and annually thereafter for 5 years.
Main Outcomes and Measures
A self-administered questionnaire was used to assess clinically meaningful differences before and after surgery in past-month sexual satisfaction, desire, and activity and physical health limitations to sexual activity among subgroups who reported sexual functioning at less than domain-specific thresholds before surgery.
Results
Of 2215 participants eligible for sexual function follow-up, 2036 (91.9%) completed 1 or more follow-up assessment (1431 [64.6%] at year 5), of whom 1607 (78.9%) were women. At the presurgery assessment, median (interquartile range) age was 47 (37-55) years, and the median (interquartile range) body mass index was 45.8 (41.7-51.3). Among those who were not satisfied with their sexual life before surgery (1015 of 1456 women [69.7%]; 304 of 409 men [74.3%]), 56.0% of women (95% CI, 52.5%-59.5%) and 49.2% of men (95% CI, 42.4%-55.9%) experienced clinically meaningful improvements at year 1; these percentages did not significantly differ during further follow-up. Among those who reported physical limitations to sexual activity at baseline (892 of 1490 women [59.9%] and 267 of 406 men [65.8%]), the percentage experiencing improvement in this domain decreased during follow-up, but 73.6% (95% CI, 69.3%-78.0%) of women and 67.6% (95% CI, 59.6%-75.6%) of men continued to report improvements at year 5. Greater postsurgical reduction in depressive symptoms was independently associated with improvement in 4 domains of sexual life among women (frequency of sexual desire: adjusted relative risk [aRR] per 5-point decrease in Beck Depression Inventory score, 1.12 [95% CI, 1.07-1.18]; P < .001; frequency of sexual activity: aRR, 1.13 [95% CI, 1.08-1.18]; P < .001; the degree to which physical health limited sexual activity: aRR, 1.19 [95% CI, 1.14-1.23]; P < .001; and satisfaction with sexual life: aRR, 1.25 [95% CI, 1.19-1.31]; P < .001) and 2 domains among men (physical health limitations: aRR, 1.14 [95% CI, 1.04-1.26]; P = .008 and satisfaction with sexual life: aRR, 1.55 [95% CI, 1.33-1.81]; P < .001). Surgical procedure was not associated with improvement.
Conclusions and Relevance
Per this study, approximately half of women and men who were not satisfied with their sexual life prior to bariatric surgery experienced improvements in satisfaction in 5 years of follow-up.
Trial Registration
ClinicalTrials.gov Identifier: NCT00465829
Introduction
Sexual functioning is an important contributor to health-related quality of life, and it may be diminished by obesity.1 Numerous studies have documented associations between higher body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) or weight status and problems with various aspects of sexual functioning or sexual quality of life.2,3,4,5,6,7,8 In particular, we have previously reported9 that adults with severe obesity seeking surgical treatment have impairments in several domains of sexual functioning, and approximately half are dissatisfied with their sexual life.9
Several prospective studies have evaluated changes in sexual functioning after bariatric surgery in men10,11,12,13,14,15,16 and women.7,14,15,17,18,19,20 Collectively, these data suggest that surgery leads to improvements in sexual functioning. However, the literature is limited by small sample sizes and short follow-up, such that the durability of improvement is unknown. There are also, to our knowledge, few studies that address factors, apart from weight loss that may be associated with postsurgical changes in sexual functioning. Several obesity–associated comorbid medical and psychological conditions, as well as some of the medications used to treat them, have been associated with sexual dysfunction in prior literature.21,22,23,24,25,26,27,28 Therefore, reductions in the presence or severity of comorbid conditions after bariatric surgery may contribute to improvements in postsurgery sexual functioning.
Data collected within the multicenter Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) study provided the unique opportunity to prospectively characterize sexual functioning in a large cohort of men and women. The primary aims of this investigation were to describe various domains of sexual functioning by time relative to surgery and evaluate change over 5 years, with an emphasis on clinically meaningful improvements after surgery among those with impairment at time of surgery. A secondary aim was to identify factors associated with clinically meaningful improvements after surgery in sexual functioning.
Methods
Participants
Between February 2006 and February 2009, patients 18 years and older who were preparing to undergo their first bariatric surgical procedure from participating surgeons at 10 centers throughout the United States were recruited to participate in LABS-2. Prior to data collection, the institutional review boards at each center approved the protocol, and all participants gave written informed consent to participate.
A recruitment flowchart is shown in the eFigure in the Supplement. Research assessments were conducted by personnel trained and certified by LABS-2 within 30 days prior to scheduled surgery dates and annually postsurgery, independent of surgical care. Assessments of women who were pregnant, were 6 months postpregnancy, had at least 2 pregnancies in the past 12 months, or self-reported using fertility medication in the past 12 months were excluded from analysis. This report uses follow-up data through the 5-year follow-up (through August 2014). Participants who completed the presurgery assessment and at least 1 follow-up assessment were included in the analysis sample (eFigure in the Supplement).
Measures
Sexual functioning and weight-associated sexual quality of life were assessed using a self-administered, structured-item questionnaire that participants were given regardless of history of sexual activity. Select questionnaire items were drawn from 3 existing measures: the Sexual Function Questionnaire (SFQ),29 the Program to Reduce Incontinence by Diet and Exercise sexual function questionnaire,30 and the Female Sexual Function Index.31 The assessment was standardized to evaluate sexual functioning in the past month. Questions and response options for frequency of sexual desire, frequency of sexual activity, degree to which physical health limited sexual activity, and degree of satisfaction with sexual life are available in eTable 1 in the Supplement, as are the definitions of dichotomous postsurgical improvement variables for each of these domains, which represent clinically meaningful improvement. The questionnaire also included a series of yes/no items to indicate whether various aspects of physical health (eg, fatigue, embarrassment, pain, difficulty with function) limited sexual activity, and if applicable, reasons for no sexual activity (eg, no partner, partner not interested).
As part of the Impact of Weight on Quality of Life–lite (IWQOL-lite) questionnaire,32 participants responded to 4 Likert-scale items composing the sexual life subscale, which assessed the degree to which weight affected their sexual activity and function. The score ranges from 0 to 100, with 100 representing the lowest influence of weight on sexual life.
Sociodemographics and Health Status
Sex, age, race, ethnicity, education, marital status, and smoking status were assessed using self-administered questionnaires.33 Race was set to missing for participants who did not self-report 1 or more of the following: white, black/African American, Asian, American Indian/Alaska Native, or Native Hawaiian/other Pacific Islander. In women, a reproductive questionnaire also assessed pregnancies, parity (ie, prior live births or stillbirths), use of hormone therapy, number of periods in the past 12 months, and if applicable, reasons for no periods (eg, hysterectomy, natural menopause), which were used in conjunction with age to determine menopausal status.34 Height and weight were measured by standardized protocols and used for BMI calculations. Symptoms of depression over the past week were assessed using the Beck Depression Inventory, version 1 (BDI-135,36), which was scaled from 0 to 63, with higher scores indicating greater severity of depression. Alcohol use was measured with the Alcohol Use Disorders Identification Test (AUDIT37). Regular alcohol consumption was defined as consuming alcohol at least twice a week. Alcohol use disorder symptoms were defined at an Alcohol Use Disorders Identification Test score of 8 or more or indication of harm or dependence.38 Comorbid conditions, including sleep apnea, hypertension, dyslipidemia, diabetes with or without insulin use, history of cardiovascular disease, and urinary incontinence, were assessed through self-report questionnaires, medical record reviews, patient interviews, physical examinations, and laboratory assays. Owing to lack of objective testing of sleep apnea at follow-up, only presurgical sleep apnea status was included. Detailed comorbidity definitions and the technical details pertaining to assays have been previously described.39
Medication Use
Using a study-specific form,33 participants recorded the names and frequency of use of all prescribed medications taken within the past 90 days. Among women and men,25 antidepressant medications (except bupropion, trazodone, mirtazapine, or nefazodone) taken at least daily were categorized as antidepressant medications that may impair sexual functioning. Among men only, β-adrenergic blockers and diuretics taken at least daily were categorized as antihypertensive medications that may impair sexual functioning.23 Among women and men, bupropion taken at least daily was categorized as a medication that may improve sexual functioning. Among men only, phosphodiesterase type-5 inhibitors and androgens taken at any frequency were also categorized as medications that may improve sexual function. Among women only, estrogens, progestins, and androgens, either alone or in combination, not including typical combined oral contraceptives, taken at any frequency, were categorized as hormonal medications that may influence sexual functioning (direction unknown).
Analysis
Analyses were conducted using SAS version 9.4 (SAS Institute). All reported P values are 2-sided; P values less than or equal to .05 are considered to be statistically significant. Analysis took place from 2016 to April 2018.
Analyses were stratified by sex. Descriptive statistics used to summarize baseline characteristics include frequencies and percentages for categorical data and medians and interquartile ranges (25th and 75th percentiles) for continuous data. Preoperative characteristics of LABS-2 participants in the analysis sample were compared with those excluded using Pearson χ2 test for categorical variables, the Cochran-Armitage test for ordinal variables, and the Wilcoxon rank sum test for continuous variables.
Longitudinal analyses were performed with mixed models assuming the unstructured covariance matrix using all available data, with controls for site and presurgical age, smoking status, and marital status (which were independently associated with missing follow-up data40), and time entered as a discrete fixed effect. Sensitivity analyses were performed to examine the robustness of results with respect to the missing-at-random assumption.
Among the full sample, mixed-effects ordinal logistic regression models were used to test the odds of ordinal sexual functioning variables changing over time (presurgery through year 5). The proportional odds assumption was assessed and met. Poisson mixed models with robust error variance were used to test change over time in dichotomous sexual functioning variables41; linear mixed models were used to test change over time in the continuous Sexual Life IWQOL-lite score. Pairwise comparisons were made between presurgical data and year 1 data (to assess short-term effects) and presurgical data and year 5 data (to assess long-term effects). Additionally, a linear trend between years 1 and 5, constructed with a linear contrast of regression coefficients, was used to evaluate changes across the follow-up period. The 3 comparisons were tested using t statistics with P values adjusted to control for overall type I error.42 Modeled percentages or means and 95% CIs and adjusted P values are reported. The P values were not adjusted for multiple response variables.
To address the primary aim of this study, Poisson mixed models with robust error variance were used to model the percentage of participants with meaningful postsurgical improvements in frequency of sexual desire, frequency of sexual activity, the degree to which physical health limits sexual activity, and satisfaction with sexual life at each year of follow-up and test for linear trends between years 1 and 5. This analysis excluded participants reporting relatively high functioning and satisfaction before surgery (ie, sexual desire a few times a week or at least once per day, sexual activity a few times a week or at least once per day, no physical health limitations to sexual activity, and being moderately or very satisfied with sexual life; eTable 1 in the Supplement), who therefore had little opportunity to demonstrate improvement. Poisson mixed models with robust error variance were also used to identify factors independently associated with meaningful improvements among participants with impairment before surgery in each of the 4 domains. Based on previous literature,17,21,23,25,43,44,45,46 the static independent variables included were race, ethnicity, age at surgery, presurgical BMI, presurgical BDI score, and surgical procedure, with controls for site and the presurgical value of the corresponding sexual functioning variable. In addition, the time-dependent independent variables included in presurgical and postsurgical assessments were marital status, menopausal status (in women), cardiovascular disease history (in men), medications that may impair sexual functioning, medications that may improve sexual functioning, change in BDI score (ie, postsurgical score minus presurgical score), and weight percentage change. In addition, variables that were of interest but had less support from the literature were entered and retained through backward elimination if they reached statistical significance (P ≤ .05): presurgical education level and presurgical sleep apnea status (both static) and presurgical and postsurgical status of smoking, regular alcohol use, alcohol use disorder symptoms, cardiovascular disease history (in women), hypertension, diabetes requiring insulin use, dyslipidemia, urinary incontinence, and hormonal medications that may influence sexual functioning (in women), and postsurgical status of prior live or still birth (in women). All of these were time dependent. Additionally, to specifically evaluate the association between surgical procedure and outcomes, a second set of models controlled for presurgical variables only. Adjusted relative risks, 95% CIs, and P values are reported.
Results
Presurgery Characteristics and Surgical Procedure
A total of 1931 women and 527 men were recruited, and 2215 participants completed the presurgical assessment and did not die prior to the assessment at 1 year. Of these, 2036 completed at least 1 follow-up assessment and were included in the analysis sample (eFigure in the Supplement). Of these participants, more than three-fourths (n = 1607; 78.6%) were women. Presurgery median (interquartile range [IQR]) age was 47 (37-55) years, and median (IQR) body mass index was 45.8 (41.7-51.3). Presurgical characteristics, stratified by sex, are shown in eTable 4 in the Supplement. Most participants underwent Roux-en-Y gastric bypass (1154 of 1607 women [71.8%] and 288 of 429 men [67.1%]).
A comparison of presurgical clinical and demographic characteristics of those included vs excluded from the analysis samples is presented in eTable 5 in the Supplement. Women who were excluded had higher BMIs (median [IQR], 46.5 [42.0-52.2] vs 45.5 [41.6-50.8]; P = .03) but were younger (median [IQR] age, 42 [35-51] years vs 46 [37-54] years; P < .001) and less likely to be menopausal (25 of 324 [7.7%] vs 494 of 1607 [30.7%]; P < .001), have sleep apnea (134 of 323 [41.5%] vs 770 of 1606 [47.9%]; P = .03), or have at least slight physical limitations to sexual activity (114 of 137 [83.2%] vs 1351 of 1516 [89.1%]; P = .03). Men who were excluded were also younger (median [IQR] age, 44 [38-51] years vs 50 [39-58] years; P = .001), more likely to smoke (22 of 98 [22.4%] vs 37 of 428 [8.6%]; P < .001), and less likely to have sleep apnea (55 of 97 [56.7%] vs 329 of 429 [76.7%]; P < .001) or cardiovascular disease (1 of 23 [4.4%] vs 80 of 429 [18.7%]; P = .03) and had higher frequency of sexual activity (9 of 45 [20.0%] vs 30 of 424 [7.1%] had sexual activity at least once a day; P = .01).
Retention
All participants who completed the baseline sexual function assessment and did not die prior to the assessment at 1 year were considered eligible for follow-up. Sexual functioning–associated data were obtained from 1356 of the 1733 women eligible for follow-up (78.2%) at year 1, 1204 of 1709 women (70.5%) at year 2, 1154 of 1708 women (68.6%) at year 3, 1131 of 1706 women (66.3%) at year 4, and 1152 of 1697 women (67.9%) at year 5. Similarly, data were received from 365 of 470 men eligible for follow-up (77.7%) in year 1, 314 of 469 men (67.0%) in year 2, 293 of 468 men (62.6%) in year 3, 282 of 468 men (60.3%) in year 4, and 279 of 466 men (59.9%) in year 5 (eFigure in the Supplement).
Of the 1607 women included in the analysis sample, 745 (46.4%) completed 5 follow-up assessments, with 301 (18.7%) completing 4, 219 (13.6%) completing 3, 167 (10.4%) completing 2, and 175 (10.9%) providing data at 1 follow-up assessment. Of the 429 men included in the sample, 180 (42.0%) provided data at 5 follow-up assessment 5 times, 75 (17.5%) did so 4 times, 48 (11.2%) did so 3 times, 63 (14.7%) completed 2 assessments, and 63 (14.7%) completed 1. Among those missing vs not missing an assessment of sexual function at a particular follow-up, sexual functioning data at other points were similar (eTables 2 and 3 in the Supplement).
Changes in Sexual Functioning After Surgery in the Full Sample
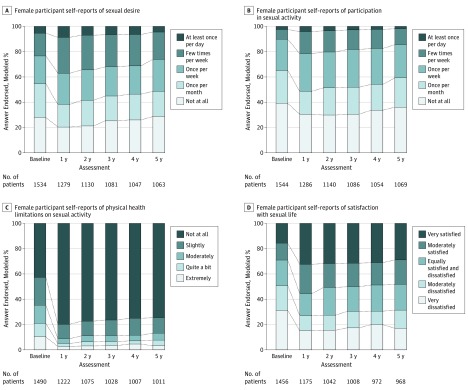
Modeled frequency of sexual desire and sexual activity, the degree to which physical health limits sexual activity, and satisfaction with sexual life are shown by time among all women and men in Figures 1 and 2, respectively. Among women, the adjusted odds ratio (aOR) of an improved value at year 1 compared with the presurgical assessment was 1.50 (95% CI, 1.23-1.82; P < .001) for frequency of sexual desire, 1.53 (95% CI, 1.25-1.86; P < .001) for frequency of sexual activity, 3.70 (95% CI, 2.83-4.82; P < .001) for degree of physical health limitations, and 2.11 (95% CI, 1.72-2.60; P < .001) for satisfaction with sexual life. There was a significant linear trend in frequency of sexual desire and activity from postoperative years 1 to 5, indicating a decrease in frequency across the 4-year postoperative follow-up (sexual desire: aOR, 0.55 [95% CI, 0.34-0.90]; P = .01; activity: aOR, 0.59 [95% CI, 0.36-0.97]; P = .03), such that the frequency of sexual desire and activity were no longer significantly different than the presurgical values by year 5 (sexual desire: aOR, 1.17 [95% CI, 0.95-1.44]; P = .17; sexual activity: aOR, 1.23 [95% CI, 0.99-1.51]; P = .053). Trends across the 4-year postoperative follow-up period (ie, years 1 to 5) did not reach statistical significance for the degree of physical limitations (aOR, 0.53 [95% CI, 0.27-1.04]; P = .06) or satisfaction with sexual life (aOR, 0.63 [95% CI, 0.38-1.05]; P = .08), and both remained significantly better than presurgical levels through year 5 (degree of physical limitations: aOR, 2.81 [95% CI, 2.14-3.68]; P < .001; satisfaction with sexual life: aOR, 1.72 [95% CI, 1.38-2.14]; P < .001).
Figure 1. Domains of Sexual Function Before and After Bariatric Surgery in Women.
Discrepancies between Table and Figure data are owing to the number of responses to specific questions differing from the number of participants who completed a given assessment (answered any of the questions) and the adjustment of these models for covariates that may have missing data (ie, smoking and martial status).
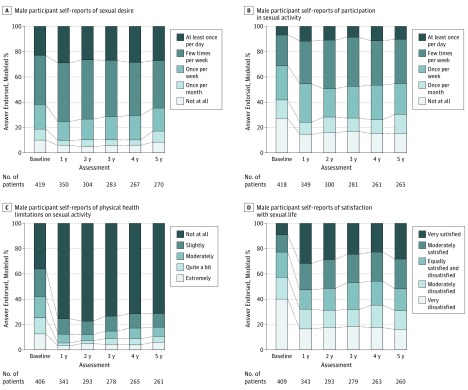
Figure 2. Domains of Sexual Function Before and After Bariatric Surgery in Men.
Discrepancies between Table and Figure data are owing to the number of responses to specific questions differing from the number of participants who completed a given assessment (answered any of the questions) and the adjustment of these models for covariates that may have missing data (ie, smoking and martial status).
Among men, aORs of an improved value at year 1 compared with baseline were 1.57 (95% CI, 1.06-2.34; P = .02) for frequency of sexual desire, 1.53 (95% CI, 1.04-2.23; P = .02) for frequency of sexual activity, 3.97 (95% CI, 2.47-6.37; P < .001) for the degree of physical health limitations, and 2.37 (95% CI, 1.61-3.49; P < .001) for satisfaction with sexual life. None of the trend tests across the 4-year postoperative period (ie, years 1 to 5) were significant (frequency of sexual desire: aOR, 0.55 [95% CI, 0.22-1.50]; P = .33; frequency of sexual activity: aOR, 0.88 [95% CI, 0.33-2.33]; P = .97; degree of physical health limitations: aOR, 0.42 [95% CI, 0.12-1.44]; P = .22; satisfaction with sexual life: aOR, 0.78 [95% CI, 0.30-2.05]; P = .85). However, only the degree of physical health limitations (aOR, 2.98 [95% CI, 1.80-4.93]; P < .001) and satisfaction with sexual life (aOR, 2.24 [95% CI, 1.47-3.40]; P < .001) were significantly better at year 5 compared with presurgical values (frequency of sexual desire: aOR, 1.18 [95% CI, 0.77-1.82]; P = .67; frequency of sexual activity: aOR, 1.45 [95% CI, 0.96-2.20]; P = .09).
Observed data and modeled percentages or means with 95% CI and adjusted P values for all sexual functioning measures by sex and time are provided in eTables 6 and 7 in the Supplement, respectively. Among women and men, reasons for no sexual activity (eg, fatigue, lack of a partner, lack of interest from a partner) were not significantly different at year 5 vs baseline, except that physical problems were reported by fewer women (6.2% [95% CI, 5.0%-7.5%] at baseline; 3.6% [95% CI, 2.6%-4.7%] at year 5; P = .001) and men (11.5% [95% CI, 6.3%-16.7%] at baseline; 5.7% [2.7%-8.6%] at year 5; P = .005; eTable 7 in the Supplement). All types of physical health limitations to sexual activity (eg, pain, functional difficulty) were significantly less common at year 1 and year 5 vs before surgery in women (fatigue or low energy or not interested: baseline, 42.8% [95% CI, 40.3%-45.4%]; year 1, 15.0% [95% CI, 13.2%-16.8%]; year 5, 21.0% [95% CI, 18.8%-23.3%]; pain or fear of damaging health: baseline, 15.3% [95% CI, 13.6%-17.0%]; year 1, 8.3% [95% CI, 7.0%-9.6%]; year 5, 11.4% [95% CI, 9.8%-13.0%]; embarrassment or fear of hurting partner: baseline, 28.5% [95% CI, 26.1%-30.8%]; year 1, 6.4% [95% CI, 5.0%-7.7%]; year 5, 7.5% [95% CI, 5.9%-9.1%]; difficulty become aroused, becoming lubricated, or having orgasm: baseline, 28.7% [95% CI, 26.5%-31.0%]; year 1, 11.0% [95% CI, 9.5%-12.5%]; year 5, 14.3% [95% CI, 12.5%-16.2%]; year 1 vs presurgery P < .001 for all; year 5 vs presurgery P < .001 for all). Likewise, among men, almost all types of physical health limitations to sexual activity were significantly less common at year 1 and year 5 vs before surgery (fatigue, low energy, or disinterest: baseline, 46.5% [95% CI, 38.9%-54.2%]; year 1, 16.4% [95% CI, 12.2%-20.7%]; year 5, 21.3% [95% CI, 15.8%-26.9%]; year 1 vs baseline P < .001; year 5 vs baseline P < .001; pain or fear of damaging health: baseline, 12.5% [95% CI, 8.5%-16.4%]; year 1, 6.8% [95% CI, 4.1%-9.5%]; year 5, 9.0% [95% CI, 5.5%-12.6%]; year 1 vs baseline P = .001; year 5 vs baseline P = .20; embarrassment or fear of hurting partner: baseline, 32.4% [95% CI, 25.2%-39.6%]; year 1, 8.2% [95% CI, 5.1%-11.4%]; year 5, 9.7% [95% CI, 5.8%-13.7%]; year 1 vs baseline P < .001; year 5 vs baseline P < .001; difficulty with arousal or orgasm or other functional difficulty: baseline, 49.5% [95% CI, 41.2%-57.7%]; year 1, 22.9% [95% CI, 17.7%-28.1%]; year 5, 23.5% [95% CI, 17.6%-29.5%]; year 1 vs baseline P < .001; year 5 vs baseline P < .001). An exception was that among men, pain or fear of damaging health was no longer significantly different at year 5 (eTable 7 in the Supplement).
Mean scores on the IWQOL-lite sexual life scale improved significantly from 50.3 (95% CI, 48.6-52.0) before surgery to 80.2 (95% CI, 78.6-81.7; P < .001) at year 1 among all women and 53.4 (95% CI, 49.8-57.0) before surgery to 80.1 (95% CI, 77.0-83.1; P < .001) at year 1 among all men. There was a significant decrease in scores between years 1 and 5, but scores were still higher at year 5 (mean: women, 76.9 [95% CI, 75.1-78.7]; men, 76.1 [95% CI, 72.6-79.6]; P < .001) than presurgery (eTable 7 in the Supplement).
Clinically Meaningful Improvement Among Adults With Impairment Prior to Surgery
Table 1 shows the modeled percentage of participants with meaningful postsurgical improvements among the applicable subgroups (ie, participants with impairments before surgery). There were significantly fewer women with improvements by year 5 vs year 1 in most domains. Still, by year 5, more than one-third of women had significant improvements from presurgical assessment in frequency of sexual desire (40.6% [95% CI, 37.2%-44.0%]; vs year 1, 45.8% [95 % CI, 42.4%-49.1%]; P < .001) and frequency of sexual activity (34.5% [95% CI, 31.5%-37.5%]; vs year 1, 37.8% [95% CI, 35.0%-40.6%]; P = .01), while 892 of the 1490 women (59.9%) who reported physical limitations to sexual function before surgery had improvements in the degree to which physical health limits sexual activity (73.6% [95% CI, 69.3%-78.0%]; vs year 1, 81.6 [95% CI, 77.5%-85.8%]; P = .01). Among women who were not satisfied with their sexual life before surgery (1015 of 1456 women [69.7%]), 56.0% of women (95% CI, 52.5%-59.5%) experienced clinically meaningful improvements at year 1. Satisfaction with sexual life improved in approximately half of female participants by year 5 (51.9% [95% CI, 48.1%-55.7%]); there was not a significant trend across follow-up. Among men, half or more experienced improvements in all 4 domains at year 1 and year 5; improvement rates did not significantly change from year 1 to year 5 for all but 1 domain (the degree to which physical health limits sexual activity, for which 267 of 406 men (65.8%) reported such limitations prior to surgery; year 1, 75.1% [95% CI, 68.4%-81.7%]; year 5, 67.6% [95% CI, 59.6%-75.6%]; P = .05). Corresponding observed data are shown in eTable 8 in the Supplement.
Table 1. Percentages of Patients With Clinically Meaningful Improvements in Domains of Sexual Function Following Bariatric Surgery, by Sex.
| Variable | Model-Based Estimates, % (95% CI)a | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants, No. | 1 y | 2 y | 3 y | 4 y | 5 y | P Value for Linear Trend | ||||||
| Participants, No. | Estimate | Participants, No. | Estimate | Participants, No. | Estimate | Participants, No. | Estimate | Participants, No. | Estimate | |||
| Women | ||||||||||||
| Frequency of sexual desire | 1069b | 896 | 45.8 (42.4-49.1) | 796 | 46.4 (42.9-49.9) | 781 | 42.5 (39.1-45.9) | 735 | 41.4 (38.0-44.9) | 763 | 40.6 (37.2-44.0) | .001 |
| Frequency of sexual activity | 1249c | 1038 | 37.8 (35.0-40.6) | 926 | 39.1 (36.1-42.1) | 882 | 37.6 (34.6-40.5) | 863 | 35.0 (32.1-38.0) | 877 | 34.5 (31.5-37.5) | .01 |
| Degree physical health limits sexual activity | 892d | 720 | 81.6 (77.5-85.8) | 641 | 76.5 (72.3-80.7) | 613 | 77.7 (73.3-82.1) | 603 | 77.4 (73.2-81.7) | 607 | 73.6 (69.3-78.0) | .01 |
| Satisfaction with sexual life | 1015e | 815 | 56.0 (52.5-59.5) | 725 | 52.5 (48.8-56.2) | 688 | 51.7 (47.8-55.5) | 685 | 52.2 (48.4-56.1) | 675 | 51.9 (48.1-55.7) | .08 |
| Men | ||||||||||||
| Frequency of sexual desire | 165b | 139 | 58.9 (50.8-67.0) | 125 | 60.9 (52.5-69.2) | 113 | 59.0 (50.3-67.8) | 101 | 62.1 (52.5-71.6) | 107 | 57.9 (49.1-66.7) | .93 |
| Frequency of sexual activity | 265c | 224 | 53.9 (45.9-61.8) | 196 | 53.6 (45.4-61.8) | 181 | 50.9 (42.4-59.3) | 169 | 52.7 (44.0-61.5) | 173 | 55.6 (47.1-64.1) | .80 |
| Degree physical health limits sexual activity | 267d | 225 | 75.1 (68.4-81.7) | 196 | 77.2 (70.1-84.3) | 181 | 76.0 (68.2-83.7) | 172 | 71.8 (63.8-79.7) | 177 | 67.6 (59.6-75.6) | .05 |
| Satisfaction with sexual life | 304e | 254 | 49.2 (42.4-55.9) | 222 | 50.7 (43.5-57.9) | 212 | 50.2 (42.7-57.6) | 203 | 45.0 (37.6-52.4) | 204 | 50.3 (43.0-57.7) | .69 |
All models were adjusted for factors associated with missing follow-up data (site, baseline age, smoking status, and marital status). Observed data are reported in eTable 8 in the Supplement.
Excludes 465 women (30.3%) and 254 men (60.6%) who reported a few times a week or at least once per day at the baseline assessment.
Excludes 300 women (19.4%) and 153 men (36.6%) who reported a few times a week or at least once per day at the baseline assessment.
Excludes 598 women (40.1%) and 139 men (34.2%) who reported not at all at the baseline assessment.
Excludes 441 women (30.3%) and 105 men (25.7%) who reported they were moderately satisfied or very satisfied at the baseline assessment.
Factors Associated With Clinically Meaningful Improvement
After controlling for presurgical factors only, surgical procedure (eg, laparoscopic adjustable gastric banding vs Roux-en-Y gastric bypass) was not significantly associated with postsurgical improvement in sexual functioning among women and men (eTables 9 and 10 in the Supplement). Independent associations between demographic and clinical characteristics and improvement in sexual functioning among women and men are shown in Tables 2 and 3, respectively, and described in the following sections.
Table 2. Associations of Clinically Meaningful Postsurgical Improvements With Domains of Sexual Function in Years 1 Through 5 After Bariatric Surgery in Womena.
| Variable | Frequency of Sexual Desire (n = 919) | Frequency of Sexual Activity (n = 1068) | Degree to Which Physical Health Limits Sexual Activity (n = 773) | Satisfaction With Sexual Life (n = 868) | ||||
|---|---|---|---|---|---|---|---|---|
| Adjusted Relative Risk (95% CI) | P Value | Adjusted Relative Risk (95% CI) | P Value | Adjusted Relative Risk (95% CI) | P Value | Adjusted Relative Risk (95% CI) | P Value | |
| Presurgery Status | ||||||||
| Age, per 10 y younger | 1.30 (1.20-1.40) | <.001 | 1.28 (1.18-1.39) | <.001 | 1.03 (0.98-1.07) | .20 | 1.03 (0.97-1.10) | .35 |
| Nonwhite race (vs white)b | 1.13 (0.95-1.36) | .17 | 1.17 (0.98-1.40) | .09 | 1.11 (1.01-1.23) | .04 | 1.03 (0.89-1.21) | .68 |
| Hispanic ethnicity (vs non-Hispanic ethnicity) | 0.88 (0.61-1.29) | .52 | 1.21 (0.95-1.54) | .13 | 1.10 (0.96-1.26) | .23 | 1.45 (1.19-1.77) | <.001 |
| Fewer depressive symptoms, per 5 BDI points | 1.14 (1.07-1.21) | <.001 | 1.14 (1.08-1.21) | <.001 | 1.19 (1.14-1.24) | <.001 | 1.25 (1.19-1.32) | <.001 |
| Postsurgical improvement | ||||||||
| Weight loss, per 5% | 1.03 (0.995-1.06) | .10 | 1.04 (1.01-1.07) | .01 | 1.03 (1.01-1.05) | .004 | 1.03 (1.01-1.05) | .01 |
| Decrease in depressive symptoms, per −5 BDI points | 1.12 (1.07-1.18) | <.001 | 1.13 (1.08-1.18) | <.001 | 1.19 (1.14-1.23) | <.001 | 1.25 (1.19-1.31) | <.001 |
| Postsurgical Status Changes | ||||||||
| Marital status | ||||||||
| Got married vs stayed single | 1.35 (1.11-1.63) | <.001 | 1.59 (1.28-1.96) | <.001 | 0.89 (0.73-1.07) | <.001 | 1.32 (1.10-1.60) | .003 |
| Got divorced vs stayed married | 1.41 (1.15-1.72) | 1.18 (0.92-1.52) | 1.12 (0.98-1.28) | 1.00 (0.80-1.24) | ||||
| Stayed married vs stayed single | 1.10 (0.93-1.29) | 1.42 (1.21-1.68) | 0.86 (0.78-0.94) | 1.07 (0.93-1.22) | ||||
| Regular alcohol use | ||||||||
| Started vs no history | 1.17 (0.98-1.40) | .045 | 1.28 (1.09-1.52) | .002 | NAc | NA | 1.21 (1.05-1.38) | .01 |
| Stopped vs continued | 0.93 (0.57-1.54) | 0.90 (0.53-1.51) | NAc | 1.07 (0.76-1.51) | ||||
| Continued vs no history | 1.24 (0.95-1.62) | 1.24 (0.90-1.69) | NAc | 0.97 (0.75-1.26) | ||||
| Menopause | ||||||||
| Developed vs no history | 0.83 (0.72-0.97) | .049 | 0.93 (0.79-1.09) | .39 | 0.97 (0.88-1.07) | .27 | 1.01 (0.89-1.15) | .96 |
| Continued vs no history | 0.88 (0.66-1.17) | 0.87 (0.67-1.15) | 1.09 (0.96-1.22) | 1.01 (0.84-1.22) | ||||
| Urinary incontinenced | ||||||||
| Started/continued vs no history | NAc | NA | NAc | NA | NAc | NA | 0.85 (0.74-0.97) | .009 |
| Remitted vs started/continued | NAc | NAc | NAc | 1.05 (0.92-1.21) | ||||
| Antidepressant medication that may impair sexual functiond | ||||||||
| Started/continued vs no history | 0.84 (0.73-0.97) | .02 | 0.86 (0.75-0.99) | .06 | 0.99 (0.92-1.07) | .61 | 1.00 (0.90-1.12) | .76 |
| Stopped vs started/continued | 1.11 (0.92-1.33) | 0.89 (0.74-1.09) | 0.96 (0.87-1.00) | 0.95 (0.82-1.11) | ||||
| Hormonal medication that may influence sexual functiond | ||||||||
| Started/continued vs no history | NAc | NA | NAc | NA | 0.92 (0.78-1.07) | .04 | NAc | NA |
| Stopped vs started/continued | NAc | NAc | 0.88 (0.69-1.13) | NAc | ||||
In addition to the variables shown in this Table, all 4 models also controlled for site and presurgery smoking, which were associated with missing follow-up data and baseline values of the corresponding outcome. Presurgery body mass index, surgical procedure, and presurgical and postsurgical use of medications that may improve sexual function were also forced in the models but were not significantly associated with any outcome. The models did not adjust for presurgical marital status because changes in marital status were a factor of interest. Presurgical education level, presurgical sleep apnea, presurgical and postsurgical smoking status, regular alcohol use, alcohol use disorder symptoms, prior live birth, prior still birth, hypertension, diabetes with insulin, dyslipidemia, cardiovascular disease history, urinary incontinence, and hormonal medications that may influence sexual functioning were also entered and retained through backward elimination if they reached significance.
The race categories black and other were collapsed owing to low representation. For the model of improvement in frequency of sexual desire, this included 74 black participants or 23 of other races; for the model of improvement in frequency of sexual activity, 94 black participants or 34 of other races; for the model of improvement in the degree to which physical health limits sexual activity, 63 black participants or 23 of other races black; for the model of improvement in satisfaction with sexual life, 73 black participants or 25 of other races.
The adjusted relative risks are not reported for variables that were not retained in each model owing to lack of significance.
Because so few participants developed urinary incontinence or started antidepressant medication after surgery, starting and continuing groups were collapsed for analysis. For the model of improvement in frequency of sexual desire, antidepressant medication use at year 1, the analysis included 30 who started and 216 who continued; at year 2, 30 who started and 190 who continued; at year 3, 43 who started and 169 who continued; at year 4, 42 who started and 166 who continued; at year 5, 62 who started and 166 who continued. For the model of improvement in frequency of sexual activity, antidepressant medication use year 1, the model included 36 who started and 237 who continued; at year 2, 43 who started and 206 who continued; at year 3, 54 who started and 183 who continued; at year 4, 56 who started and 186 who continued; at year 5, 72 who started and 180 who continued. For the model of improvement in degree physical health limits sexual activity, with respect to antidepressant medication use at year 1, the model included 26 who started and 172 who continued; at year 2, 32 who started and 144 who continued; at year 3, 40 who started and 129 who continued; at year 4, 41 who started and 136 who continued; at year 5, 54 who started and 138 who continued. For the model of improvement in satisfaction with sexual life, with respect to antidepressant medication use at year 1, the model included 28 who started and 187 who continued; at year 2, 35 who started and 161 who continued; at year 3, 43 who started and 137 who continued; at year 4, 45 who started and 142 who continued; at year 5, 59 who started and 138 who continued. For the model of improvement in satisfaction with sexual life, with respect to urinary incontinence at year 1, the model included 11 who started and 93 who continued; at year 2, 18 who started and 110 who continued; at year 3, 13 who started and 106 who continued; at year 4, 24 who started and 119 who continued; at year 5, 29 who started and 129 who continued.
Table 3. Associations of Clinically Meaningful Postsurgical Improvements With Domains of Sexual Function in Years 1 Through 5 After Bariatric Surgery in Mena.
| Variable | Frequency of Sexual Desire (n = 110) | Frequency of Sexual Activity (n = 181) | Degree to Which Physical Health Limits Sexual Activity (n = 178) | Satisfaction With Sexual Life (n = 200) | ||||
|---|---|---|---|---|---|---|---|---|
| Adjusted Relative Risk (95% CI) | P Value | Adjusted Relative Risk (95% CI) | P Value | Adjusted Relative Risk (95% CI) | P Value | Adjusted Relative Risk (95% CI) | P Value | |
| Presurgery status | ||||||||
| Nonwhite race (vs white)b | 1.60 (0.93-2.74) | .09 | 1.33 (0.82-2.17) | .24 | 1.17 (0.87-1.56) | .30 | 1.58 (1.04-2.41) | .03 |
| Smoking (vs not smoking) | 1.43 (0.97-2.12) | .07 | 1.77 (1.14-2.72) | .01 | 1.04 (0.82-1.32) | .74 | 1.05 (0.73-1.51) | .81 |
| Fewer depressive symptoms, per 5 BDI points | 0.96 (0.85-1.08) | .49 | 1.16 (0.97-1.38) | .10 | 1.15 (1.04-1.27) | .007 | 1.55 (1.30-1.77) | <.001 |
| Postsurgical improvement | ||||||||
| Weight loss, per 5% | 0.99 (0.93-1.06) | .79 | 1.04 (0.97-1.11) | .23 | 1.06 (1.02-1.11) | .001 | 1.04 (0.98-1.10) | .17 |
| Decrease in depressive symptoms, per −5 BDI points | 1.09 (0.97-1.22) | .11 | 1.08 (0.93-1.25) | .31 | 1.14 (1.04-1.26) | .008 | 1.55 (1.33-1.81) | <.001 |
| Use of antidepressant medication that may impair sexual functionc | ||||||||
| Started/continued vs no history | 0.79 (0.54-1.17) | .02 | 0.77 (0.51-1.15) | .03 | 0.88 (0.72-1.08) | .37 | 0.96 (0.70-1.31) | .01 |
| Stopped vs started/continued | 1.66 (1.10-2.51) | 1.72 (1.09-2.71) | 1.13 (0.84-1.50) | 1.80 (1.16-2.79) | ||||
In addition to the variables shown in this table, all 4 models also controlled for site and age, which were associated with missing follow-up data and baseline values of the corresponding outcome. Hispanic ethnicity, presurgical body mass index, surgical procedure, cardiovascular disease history, presurgical and postsurgical marital status, use of hypertensive medications that may impair sexual function, and use of medications that may improve sexual function were also forced in the models but were not significantly associated with any outcome. The models did not adjust for presurgical marital status because change in marital status was a factor of interest. Presurgical education level, presurgical sleep apnea, and presurgical and postsurgical smoking status, regular alcohol use, alcohol use disorder symptoms, hypertension, diabetes with insulin, dyslipidemia, and urinary incontinence were also entered and retained through backward elimination if they reached significance.
The race categories of black and other were collapsed owing to low representation. For the model of improvement in frequency of sexual desire and model of improvement in frequency of sexual activity, 5 black participants 0 of other races were included; for the model of improvement in degree physical health limits sexual activity, 10 black participants and 3 of other races were included; for the model of improvement in satisfaction with sexual life, 9 black participants and 3 of other races were included.
Because so few participants started antidepressant medication following surgery, the starting and continuing groups were collapsed for analysis. For model of improvement in frequency of sexual desire, with respect to antidepressant medication use at year 1, the model included 5 who started and 29 who continued; at year 2, 6 who started and 20 who continued; at year 3, 6 who started and 16 who continued; at year 4, 7 who started and 12 who continued; and at year 5, 8 who started and 13 who continued. For the model of improvement in frequency of sexual activity, with respect to antidepressant medication use at year 1, the model included 9 who started and 42 who continued; at year 2, 9 who started and 26 who continued; at year 3, 7 who started and 25 who continued; at year 4, 10 who started and 22 who continued; and at year 5, 11 who started and 21 who continued. For the model of improvement in degree physical health limits sexual activity, with respect to antidepressant medication use at year 1, the model included 9 who started and 41 who continued; at year 2, 8 who started and 22 who continued; at year 3, 6 who started and 21 who continued; at year 4, 7 who started and 18 who continued; and at year 5, 8 who started and 16 who continued. For the model of improvement in satisfaction with sexual life, with respect to antidepressant medication use at year 1, the model included 13 who started and 37 who continued; at year 2, 12 who started and 21 who continued, at year 3, 7 who started and 23 who continued; at year 4, 10 who started and 20 who continued; and at year 5, 13 who started and 17 who continued.
Women
Fewer presurgical depressive symptoms and a postsurgical decrease in depressive symptoms were independently associated with higher chance of improvement in all 4 domains of sexual functioning in women. Specifically, the adjusted relative risk (aRR) for a presurgical score 5 BDI points lower was 1.14 (95% CI, 1.07-1.21; P < .001) for frequency of sexual desire; 1.14 (95% CI, 1.08-1.21; P < .001) for frequency of sexual activity; 1.19 (95% CI, 1.14-1.24; P < .001) for the degree to which physical health limits sexual activity; and 1.25 (95% CI, 1.19-1.32; P < .001) for satisfaction with sexual life. The aRR per a 5-point decrease in BDI scores from presurgery to postsurgery was 1.12 (95% CI, 1.07-1.18; P < .001) for frequency of sexual desire; 1.13 (95% CI, 1.08-1.18; P < .001) for frequency of sexual activity; 1.19 (95% CI, 1.14-1.23; P < .001) for degree physical health limits sexual activity; and 1.25 (95% CI, 1.19-1.31; P < .001) for satisfaction with sexual life.
Greater weight loss was associated with a higher chance of improvement in 3 domains (increase in frequency of sexual activity: aRR, 1.04 [95% CI, 1.01-1.07] per 5% greater weight loss; P = .01; improvement in the degree that physical health limits sexual activity: aRR, 1.03 [95% CI, 1.01-1.05] per 5% greater weight loss; P = .01; improvement in satisfaction with sexual life: aRR, 1.03 [95% CI, 1.01-1.05] per 5% greater weight loss; P = .01), as was getting married vs staying single (increase in frequency of sexual desire: aRR, 1.35 [95% CI, 1.11-1.63]; P < .001; increase in frequency of sexual activity: aRR, 1.59 [95% CI, 1.28-1.96]; P < .001; improvement in satisfaction with sexual life: aRR, 1.32 [95% CI, 1.10-1.60]; P = .003).
Younger age was associated with a higher chance of improvement in 2 domains (increase in frequency of sexual desire: aRR, 1.30 [95% CI, 1.20-1.40] per 10 years younger; P < .001; increase in frequency of sexual activity: aRR, 1.28 [95% CI, 1.18-1.39] per 10 years younger; P < .001), as was initiating regular alcohol use (increase in frequency of sexual activity: aRR, 1.28 [95% CI, 1.09-1.52]; P = .002; improvement in satisfaction with sexual life: aRR, 1.21 [95% CI, 1.05-1.38]; P = .01).
Several additional characteristics were associated with 1 of the 4 domains (Table 2): nonwhite race with the degree to which physical health limits physical activity (aRR, 1.11 [95% CI, 1.01-1.23]; P = .04); Hispanic ethnicity with satisfaction with sexual life (aRR, 1.45 [95% CI, 1.19-1.77]; P < .001); menopause with frequency of sexual desire (developed postsurgery vs no history: aRR, 0.83 [95% CI, 0.72-0.97]; P = .049); urinary incontinence with satisfaction with sexual life (started or continued postsurgery vs no history: aRR, 0.85 [95% CI, 0.74-0.97]; P = .009); and antidepressant use (started or continued postsurgery vs no history: 0.84 [95% CI, 0.73-0.97]; P = .02).
Men
Fewer presurgery depressive symptoms and a postsurgical decrease in depressive symptoms were associated with a higher chance of improvement in the degree physical health limits sexual activity and satisfaction with sexual life. Specifically, the adjusted relative risk (aRR) for a presurgical BDI score 5 points lower was 1.15 (95% CI, 1.04-1.27; P = .007) for the degree to which physical health limits sexual activity and 1.55 (95% CI, 1.30-1.77; P < .001) for satisfaction with sexual life, with lower scores associated with better sexual functioning. The aRR per 5–BDI point postsurgical decrease was 1.14 (95% CI, 1.04-1.26; P = .008) for the degree to which physical health limits sexual activity and 1.55 (95% CI, 1.33-1.81; P = .001) for satisfaction with sexual life. Stopping antidepressant use was associated with a higher chance of improvement in 3 domains: satisfaction with sexual life (aRR, 1.80 [95% CI, 1.16-2.79]; P = .01), frequency of sexual desire (aRR, 1.66 [95% CI, 1.10-2.51]; P = .02), and activity (aRR, 1.72 [95% CI, 1.09-2.71]; P = .03). Other factors were associated with 1 domain (Table 3): weight loss with the degree to which physical health limits sexual activity (aRR, 1.06 [95% CI, 1.02-1.11]; P = .001); smoking with frequency of sexual activity (aRR, 1.77 [95% CI, 1.14-2.72]; P = .01); and nonwhite race with satisfaction with sexual life (aRR, 1.58 [95% CI, 1.04-2.41]; P = .03).
Discussion
The primary findings of this study are that early (1-year) and persistent (5-year) clinically meaningful postsurgical improvements in all assessed domains of sexual functioning were observed in a substantial percentage of adults who had impairment prior to bariatric surgery. Importantly, about half of women and men who reported some level of dissatisfaction with their sexual life prior to surgery experienced clinically meaningful improvement in satisfaction in each of the 5 years of follow-up. Additionally, this study identified several presurgical factors, as well as postsurgical changes, that were associated with improvements in sexual function. These findings provide new evidence to reinforce and extend findings of previous, smaller studies examining sexual functioning in women and men after bariatric surgery which have collectively, although not uniformly, shown improvement in sexual functioning following bariatric surgery.7,10,11,12,13,14,15,16,17,18,19,20
Factors associated with improvement in sexual functioning varied somewhat between women and men and also differed according to which sexual functioning domain was considered. However, lower depressive symptom scores before surgery and postsurgical improvement in depression symptoms were independently associated with improvement in all aspects of sexual functioning among women, as well as improvements in the degree to which physical health limits sexual functioning and sexual satisfaction among men. Among women and men, antidepressant use was also associated with a decreased likelihood of improvement in 1 or more domains of sexual functioning. In contrast, most other medical comorbidities considered (eg, dyslipidemia, sleep apnea, diabetes) were not independently associated with meaningful improvement in sexual function after weight loss and other factors were taken into account. Also, while change in weight after surgery was associated with 3 of 4 domains of improvement in sexual functioning in women, in men it was only independently associated with improvement in the degree to which physical health limits sexual activity. Strengths of this study include the large sample size, 5-year duration of follow-up, and examination of comorbid factors associated with changes in sexual functioning.
Limitations
Several limitations of these data should also be considered. The study did not have a nonsurgical control group, nor did it randomize participants to surgery. The psychometric properties of the sexual functioning questionnaire used in this study have not been characterized. Although LABS-2 participants who were excluded from the analysis sample owing to missing follow-up data did not differ from those included on most factors, women who were excluded were less likely to have physical limitations to sexual activity, and men who were excluded had higher frequency of sexual activity. These differences, likely explained by a difference in age, affected the percentage of participants excluded from evaluation of clinically meaningful improvement but not necessarily from estimates of the percentage of participants who improved or associations of improvement. Although missing follow-up data are a concern, analyses controlled for presurgical factors associated with missing follow-up data, and the sensitivity analysis indicated missing data were not associated with sexual functioning.
This report evaluates change in sexual functioning and satisfaction in the overall sample and specifically among participants with limitations prior to surgery. Future research should more fully explore how bariatric surgery affects sexual functioning and satisfaction among patients who report doing relatively well in these domains prior to surgery. Of note, Sarwer et al have published data on the change in sexual functioning after bariatric surgery in smaller samples of women20 and men16 through an ancillary grant associated with LABS-2. Some individuals may be represented in the articles by Sarwer et al16,20 and this article. Finally, additional factors beyond those analyzed may influence sexual functioning, such as history of sexual abuse, body image, surplus skin, and body-contouring surgery.
Conclusions
Among a cohort of patients undergoing bariatric surgery, approximately half of women and men who reported presurgical dissatisfaction with sexual life experienced postsurgical improvements in satisfaction over 5 years. Additionally, a large percentage experienced improvements in the frequency of sexual desire and activity and in physical limitations to sexual activity. However, there was deterioration in the frequency of sexual desire and activity (ie, the percentage of participants with improvement decreased) between years 1 and 5.
Depressive symptoms and antidepressant medication were associated with sexual functioning among adults who underwent bariatric surgery. Thus, future research should further explore the association between depression symptoms, treatment for depression, and sexual functioning after bariatric surgery.
Clinicians should assess patient satisfaction with sexual functioning before and after bariatric surgery. They should also consider interventions targeted to modifiable factors that may influence the likelihood of improvement.
eFigure. Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) Study Flow from Approached Patients to Analysis Sample
eTable 1. Items on Sexual Function Questionnaire and Definitions of Meaningful Improvement from Pre-surgery to Post-surgery
eTable 2. Sensitivity Analysis of Missing at Random Assumption
eTable 3. Mean IWQOL-lite Sexual Life Score by Time Point by Whether that Outcome was Observed or Missing at Each Follow-up Assessment
eTable 4. Demographic and Clinical Characteristics of Adults Prior to Bariatric Surgery, by Sex.
eTable 5. Baseline Demographic and Clinical Characteristics of Participants Included in and Excluded from Analysis Samples
eTable 6. Observed Parameters of Sexual Function Before and After Bariatric Surgery, by Sex
eTable 7. Modeled Parameters of Sexual Function Before and After Bariatric Surgery, by Sex
eTable 8. Observed Proportion of Patients with Clinically Important Improvements in Parameters of Sexual Function Following Bariatric Surgery, by Sex
eTable 9. Associations between Baseline Characteristics and Surgical Procedure with Clinically Meaningful Presurgery-to-Postsurgery Improvements in Parameters of Sexual Function in Years 1-5 Following Bariatric Surgery in Women.a
eTable 10. Associations between Baseline Characteristics and Surgical Procedure with Clinically Meaningful Presurgery-to-Postsurgery Improvements in Parameters of Sexual Function in Years 1-5 Following Bariatric Surgery in Men.a
References
- 1.Sarwer DB, Lavery M, Spitzer JC. A review of the relationships between extreme obesity, quality of life, and sexual function. Obes Surg. 2012;22(4):668-676. doi: 10.1007/s11695-012-0588-1 [DOI] [PubMed] [Google Scholar]
- 2.Assimakopoulos K, Panayiotopoulos S, Iconomou G, et al. Assessing sexual function in obese women preparing for bariatric surgery. Obes Surg. 2006;16(8):1087-1091. doi: 10.1381/096089206778026442 [DOI] [PubMed] [Google Scholar]
- 3.Kolotkin RL, Crosby RD, Williams GR. Health-related quality of life varies among obese subgroups. Obes Res. 2002;10(8):748-756. doi: 10.1038/oby.2002.102 [DOI] [PubMed] [Google Scholar]
- 4.Kolotkin RL, Binks M, Crosby RD, Østbye T, Gress RE, Adams TD. Obesity and sexual quality of life. Obesity (Silver Spring). 2006;14(3):472-479. doi: 10.1038/oby.2006.62 [DOI] [PubMed] [Google Scholar]
- 5.Kolotkin RL, Zunker C, Østbye T. Sexual functioning and obesity: a review. Obesity (Silver Spring). 2012;20(12):2325-2333. doi: 10.1038/oby.2012.104 [DOI] [PubMed] [Google Scholar]
- 6.Veronelli A, Mauri C, Zecchini B, et al. Sexual dysfunction is frequent in premenopausal women with diabetes, obesity, and hypothyroidism, and correlates with markers of increased cardiovascular risk: a preliminary report. J Sex Med. 2009;6(6):1561-1568. doi: 10.1111/j.1743-6109.2009.01242.x [DOI] [PubMed] [Google Scholar]
- 7.Sarwer DB, Spitzer JC, Wadden TA, et al. Sexual functioning and sex hormones in persons with extreme obesity and seeking surgical and nonsurgical weight loss. Surg Obes Relat Dis. 2013;9(6):997-1007. doi: 10.1016/j.soard.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore RH, Sarwer DB, Lavenberg JA, et al. Relationship between sexual function and quality of life in obese persons seeking weight reduction. Obesity (Silver Spring). 2013;21(10):1966-1974. doi: 10.1002/oby.20398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steffen KJ, King WC, White GE, et al. Sexual functioning of men and women with severe obesity before bariatric surgery. Surg Obes Relat Dis. 2017;13(2):334-343. doi: 10.1016/j.soard.2016.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammoud A, Gibson M, Hunt SC, et al. Effect of Roux-en-Y gastric bypass surgery on the sex steroids and quality of life in obese men. J Clin Endocrinol Metab. 2009;94(4):1329-1332. doi: 10.1210/jc.2008-1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dallal RM, Chernoff A, O’Leary MP, Smith JA, Braverman JD, Quebbemann BB. Sexual dysfunction is common in the morbidly obese male and improves after gastric bypass surgery. J Am Coll Surg. 2008;207(6):859-864. doi: 10.1016/j.jamcollsurg.2008.08.006 [DOI] [PubMed] [Google Scholar]
- 12.Reis LO, Favaro WJ, Barreiro GC, et al. Erectile dysfunction and hormonal imbalance in morbidly obese male is reversed after gastric bypass surgery: a prospective randomized controlled trial. Int J Androl. 2010;33(5):736-744. doi: 10.1111/j.1365-2605.2009.01017.x [DOI] [PubMed] [Google Scholar]
- 13.Mora M, Aranda GB, de Hollanda A, Flores L, Puig-Domingo M, Vidal J. Weight loss is a major contributor to improved sexual function after bariatric surgery. Surg Endosc. 2013;27(9):3197-3204. doi: 10.1007/s00464-013-2890-y [DOI] [PubMed] [Google Scholar]
- 14.Efthymiou V, Hyphantis T, Karaivazoglou K, et al. The effect of bariatric surgery on patient HRQOL and sexual health during a 1-year postoperative period. Obes Surg. 2015;25(2):310-318. doi: 10.1007/s11695-014-1384-x [DOI] [PubMed] [Google Scholar]
- 15.Strain GW, Kolotkin RL, Dakin GF, et al. The effects of weight loss after bariatric surgery on health-related quality of life and depression. Nutr Diabetes. 2014;4:e132. doi: 10.1038/nutd.2014.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarwer DB, Spitzer JC, Wadden TA, et al. Sexual functioning and sex hormones in men who underwent bariatric surgery. Surg Obes Relat Dis. 2015;11(3):643-651. doi: 10.1016/j.soard.2014.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Legro RS, Dodson WC, Gnatuk CL, et al. Effects of gastric bypass surgery on female reproductive function. J Clin Endocrinol Metab. 2012;97(12):4540-4548. doi: 10.1210/jc.2012-2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bond DS, Wing RR, Vithiananthan S, et al. Significant resolution of female sexual dysfunction after bariatric surgery. Surg Obes Relat Dis. 2011;7(1):1-7. doi: 10.1016/j.soard.2010.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Assimakopoulos K, Karaivazoglou K, Hyphantis T, et al. P02-115: bariatric surgery is associated with reduced psychological distress and improved sexual function in obese female patients. Eur Psychiatry. 2011;26:711-711. doi: 10.1016/S0924-9338(11)72416-6 [DOI] [PubMed] [Google Scholar]
- 20.Sarwer DB, Spitzer JC, Wadden TA, et al. Changes in sexual functioning and sex hormone levels in women following bariatric surgery. JAMA Surg. 2014;149(1):26-33. doi: 10.1001/jamasurg.2013.5022 [DOI] [PubMed] [Google Scholar]
- 21.Coyne KS, Wein A, Nicholson S, Kvasz M, Chen CI, Milsom I. Comorbidities and personal burden of urgency urinary incontinence: a systematic review. Int J Clin Pract. 2013;67(10):1015-1033. doi: 10.1111/ijcp.12164 [DOI] [PubMed] [Google Scholar]
- 22.Coyne KS, Kvasz M, Ireland AM, Milsom I, Kopp ZS, Chapple CR. Urinary incontinence and its relationship to mental health and health-related quality of life in men and women in Sweden, the United Kingdom, and the United States. Eur Urol. 2012;61(1):88-95. doi: 10.1016/j.eururo.2011.07.049 [DOI] [PubMed] [Google Scholar]
- 23.Manolis A, Doumas M. Antihypertensive treatment and sexual dysfunction. Curr Hypertens Rep. 2012;14(4):285-292. doi: 10.1007/s11906-012-0276-5 [DOI] [PubMed] [Google Scholar]
- 24.Manolis A, Doumas M. Sexual dysfunction: the ‘prima ballerina’ of hypertension-related quality-of-life complications. J Hypertens. 2008;26(11):2074-2084. doi: 10.1097/HJH.0b013e32830dd0c6 [DOI] [PubMed] [Google Scholar]
- 25.Montgomery SA, Baldwin DS, Riley A. Antidepressant medications: a review of the evidence for drug-induced sexual dysfunction. J Affect Disord. 2002;69(1-3):119-140. doi: 10.1016/S0165-0327(01)00313-5 [DOI] [PubMed] [Google Scholar]
- 26.Maiorino MI, Bellastella G, Esposito K. Diabetes and sexual dysfunction: current perspectives. Diabetes Metab Syndr Obes. 2014;7:95-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosman L, Cahill JM, McCammon SL, Sears SF. Sexual health concerns in patients with cardiovascular disease. Circulation. 2014;129(5):e313-e316. doi: 10.1161/CIRCULATIONAHA.113.004846 [DOI] [PubMed] [Google Scholar]
- 28.Steffen KJ, King WC, White GE, et al. Sexual functioning of men and women with severe obesity before bariatric surgery. Surg Obes Relat Dis. 2017;13(2):334-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Syrjala KL, Schroeder TC, Abrams JR, et al. Sexual function measurement and outcomes in cancer survivors and matched controls. J Sex Res. 2000;37(3):213-225. doi: 10.1080/00224490009552042 [DOI] [Google Scholar]
- 30.Huang AJ, Stewart AL, Hernandez AL, Shen H, Subak LL; Program to Reduce Incontinence by Diet and Exercise . Sexual function among overweight and obese women with urinary incontinence in a randomized controlled trial of an intensive behavioral weight loss intervention. J Urol. 2009;181(5):2235-2242. doi: 10.1016/j.juro.2009.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26(2):191-208. doi: 10.1080/009262300278597 [DOI] [PubMed] [Google Scholar]
- 32.Kolotkin RL, Crosby RD, Kosloski KD, Williams GR. Development of a brief measure to assess quality of life in obesity. Obes Res. 2001;9(2):102-111. doi: 10.1038/oby.2001.13 [DOI] [PubMed] [Google Scholar]
- 33.Belle SH, Berk PD, Courcoulas AP, et al. ; Longitudinal Assessment of Bariatric Surgery Consortium Writing Group . Safety and efficacy of bariatric surgery: longitudinal assessment of bariatric surgery. Surg Obes Relat Dis. 2007;3(2):116-126. doi: 10.1016/j.soard.2007.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gosman GG, King WC, Schrope B, et al. Reproductive health of women electing bariatric surgery. Fertil Steril. 2010;94(4):1426-1431. doi: 10.1016/j.fertnstert.2009.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beck AT, Steer RA, Garbin MG. psychometric properties of the Beck Depression Inventory: 25 years of evaluation. Clin Psychol Rev. 1988;8(1):77-100. doi: 10.1016/0272-7358(88)90050-5 [DOI] [Google Scholar]
- 36.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561-571. doi: 10.1001/archpsyc.1961.01710120031004 [DOI] [PubMed] [Google Scholar]
- 37.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption—II. Addiction. 1993;88(6):791-804. doi: 10.1111/j.1360-0443.1993.tb02093.x [DOI] [PubMed] [Google Scholar]
- 38.King WC, Chen JY, Mitchell JE, et al. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA. 2012;307(23):2516-2525. doi: 10.1001/jama.2012.6147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belle SH, Berk PD, Chapman WH, et al. ; LABS Consortium . Baseline characteristics of participants in the Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) study. Surg Obes Relat Dis. 2013;9(6):926-935. doi: 10.1016/j.soard.2013.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.King WC, Chen JY, Belle SH, et al. Change in pain and physical function following bariatric surgery for severe obesity. JAMA. 2016;315(13):1362-1371. doi: 10.1001/jama.2016.3010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702-706. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 42.Edwards D, Berry JJ. The efficiency of simulation-based multiple comparisons. Biometrics. 1987;43(4):913-928. doi: 10.2307/2531545 [DOI] [PubMed] [Google Scholar]
- 43.Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281(6):537-544. doi: 10.1001/jama.281.6.537 [DOI] [PubMed] [Google Scholar]
- 44.Janik MR, Bielecka I, Paśnik K, Kwiatkowski A, Podgórska L. Female sexual function before and after bariatric surgery: a cross-sectional study and review of literature. Obes Surg. 2015;25(8):1511-1517. doi: 10.1007/s11695-015-1721-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yee LM, Kaimal AJ, Nakagawa S, Houston K, Kuppermann M. Predictors of postpartum sexual activity and function in a diverse population of women. J Midwifery Womens Health. 2013;58(6):654-661. doi: 10.1111/jmwh.12068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Avis NE, Green R. The perimenopause and sexual functioning. Obstet Gynecol Clin North Am. 2011;38(3):587-594. doi: 10.1016/j.ogc.2011.05.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) Study Flow from Approached Patients to Analysis Sample
eTable 1. Items on Sexual Function Questionnaire and Definitions of Meaningful Improvement from Pre-surgery to Post-surgery
eTable 2. Sensitivity Analysis of Missing at Random Assumption
eTable 3. Mean IWQOL-lite Sexual Life Score by Time Point by Whether that Outcome was Observed or Missing at Each Follow-up Assessment
eTable 4. Demographic and Clinical Characteristics of Adults Prior to Bariatric Surgery, by Sex.
eTable 5. Baseline Demographic and Clinical Characteristics of Participants Included in and Excluded from Analysis Samples
eTable 6. Observed Parameters of Sexual Function Before and After Bariatric Surgery, by Sex
eTable 7. Modeled Parameters of Sexual Function Before and After Bariatric Surgery, by Sex
eTable 8. Observed Proportion of Patients with Clinically Important Improvements in Parameters of Sexual Function Following Bariatric Surgery, by Sex
eTable 9. Associations between Baseline Characteristics and Surgical Procedure with Clinically Meaningful Presurgery-to-Postsurgery Improvements in Parameters of Sexual Function in Years 1-5 Following Bariatric Surgery in Women.a
eTable 10. Associations between Baseline Characteristics and Surgical Procedure with Clinically Meaningful Presurgery-to-Postsurgery Improvements in Parameters of Sexual Function in Years 1-5 Following Bariatric Surgery in Men.a