Abstract
Objective
To investigate delay in diagnosis by both patients and doctors, and to evaluate its effect on outcomes of high‐grade sarcoma of bone in a single‐referral oncological center.
Methods
Fifty‐four patients with osteosarcoma, 29 with Ewing sarcoma and 19 with chondrosarcoma were enrolled in this retrospective study. Delay in diagnosis was defined as the period between initial clinical symptoms and histopathological diagnosis at our center. The delays were categorized as patient‐ or doctor‐related. Short total delays were defined as <4 months; prolonged delays >4 months were assumed to have prognostic relevance.
Results
Total delay in diagnosis was 688.0 days in patients with chondrosarcoma, which is significantly longer than the 163.3 days for osteosarcoma (P < 0.01) and 160.2 days for Ewing sarcoma (P < 0.01). Most doctor‐related delays were at the pre‐hospital stage, occurring at the general practitioner (GP)'s office. However, prolonged total delays (≥4 months) did not result in lower survival rates. Five‐year‐overall survival rates were 67.0% for osteosarcoma, 49.0% for Ewing sarcoma and 60.9% for chondrosarcoma. Survival was significantly lower for patients with metastatic disease for all three types of sarcoma.
Conclusion
Prolonged delay in diagnosis does not result in lower survival. Metastatic disease has a pronounced effect on survival. Aggressive tumor behavior results in shorter delays. Minimizing GP‐related delays could be achieved by adopting a lower threshold for obtaining plain radiographs at the pre‐hospital stage.
Keywords: Doctor‐related delay, Patient‐related delay, Sarcoma, Survival
Introduction
High‐grade primary bone sarcomas are rare and aggressively invade soft tissue from bone. The most common high‐grade bone sarcomas are osteosarcoma, Ewing sarcoma and chondrosarcoma. Because of their malignant nature and ability to metastasize, aggressive treatment is required1. The introduction of chemotherapy has dramatically improved survival of individuals with osteosarcoma and Ewing sarcoma2, 3, 4, 5, 6. While surgical and medical treatment options have also evolved since then, there has been no further remarkable improvement in survival rates7, 8, 9. Early diagnosis and treatment are still vital because local control is easier and may help prevent metastasis. Metastasis of high‐grade sarcomas of bone greatly impacts survival10, 11. A cooperative group of oncologists in the Netherlands, Stichting Oncologische Samenwerking (SONCOS), has issued a general guideline on timely diagnosis of cancer. Diagnosing bone tumors is notoriously difficult and sometimes time‐consuming, as in most cases multidisciplinary diagnosis by local or nationwide musculoskeletal tumor committees like the Dutch Committee on Bone Tumors is necessary. Hence, in some cases it is impossible to meet these guidelines.
Only a few series regarding delay in diagnosis have been published12, 13, 14, 15, 16. Prolonged duration of symptoms is associated with larger tumor size and increased rate of metastasis, but not with inferior outcomes12, 17. Reducing patients’ associated delay seems difficult because of the low incidence of these diseases. Most general practitioner s (GPs) will only encounter a primary bone sarcoma a few times during their entire career, even though they deal with musculoskeletal complaints daily. Kim et al. demonstrated that doctor‐related delays followed by inappropriate primary procedures significantly influence survival18. A detailed analysis of diagnostic delays may reveal new insights on how to improve awareness among patients and physicians.
The University Medical Center Groningen (UMCG), one of four accredited bone tumor centers in the Netherlands, provides regional coverage for the treatment of bone sarcomas, as shown in Fig. 1. In this study, we quantified and analyzed patient‐ and doctor‐related delays and their effects on clinical outcomes in a large series of high‐grade bone sarcomas with the aim of identifying new strategies for shortening delays.
Figure 1.
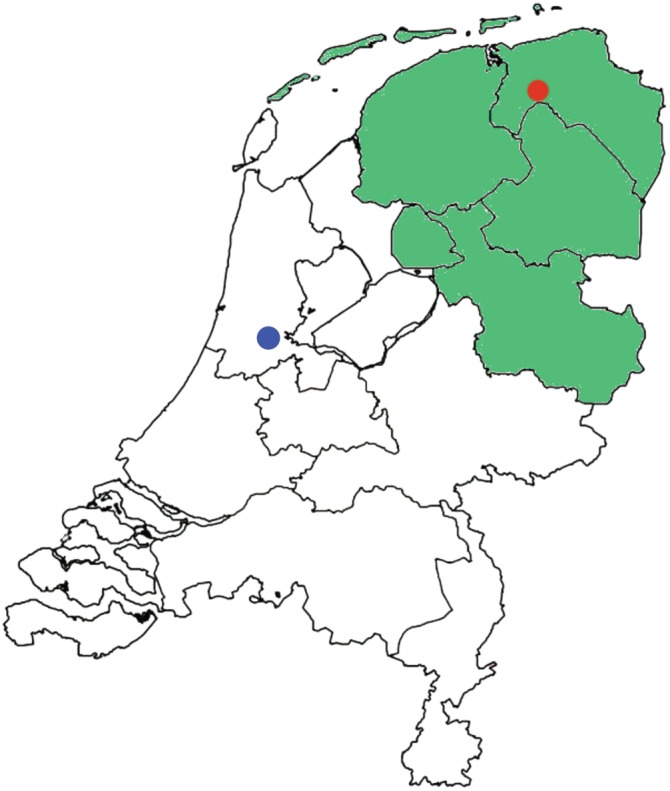
Regional coverage for the treatment of bone sarcomas in the Netherlands. The capital of the Netherlands, Amsterdam, is marked in blue and the city of Groningen in red. The University Medical Center Groningen is located in Groningen and provides regional coverage, being the oncology center for the treatment of bone sarcomas in the northern provinces of the Netherlands. These northern provinces are marked in green.
Material and Methods
All cases were selected from a prospectively maintained bone tumor registry at UMCG. A minimum follow‐up of 12 months was an inclusion criterion. All 102 consecutive patients with high‐grade bone sarcoma diagnosed between October 2000 and October 2012 were included. They comprised 54 patients with osteosarcoma, 29 with Ewing sarcoma and 19 with intermediate or high‐grade chondrosarcoma.
Delays in diagnosis were calculated in days and categorized as patient‐related or doctor‐related. Patient‐related delays were defined as the period between the initial symptom and first consultation with a GP, which is required for all Dutch patients prior to referral to a specialized service. GPs were asked to provide the date of the first entry in their medical records concerning tumor‐related symptoms (swelling, daytime/night‐time pain, loss of function, etc.). Doctor‐related delays were further subdivided as follows: (i) between presentation to the GP's office and presentation to a primary hospital, defined as pre‐hospital doctor‐related delay; (ii) between presentation at a primary hospital and an oncology center, defined as primary clinic doctor‐related delay; and (iii) between presentation to an oncology center and definitive histopathological diagnosis, defined as referral clinic doctor‐related delay. The third group was further subdivided into two subgroups, ≤42 days and >42 days, according to the SONCOS guidelines. The date of presentation at each general primary hospital was obtained from the referral letters. When these were not available, the hospital was contacted with a request for the date of first presentation. Not every patient had been referred to a general secondary hospital; some had been referred directly to an oncology (referral) center or had presented to an emergency room. Short total delays were defined as <4 months; total delays longer than 4 months were assumed to have prognostic relevance based on expert opinion.
Local presenting symptoms comprised pain, swelling, pathological fracture and/or loss of function. The presence of systemic symptoms (fatigue, loss of appetite, weight loss or fever) was recorded separately.
The primary outcome measure was the effect of delay (patient‐ or doctor‐related) on oncological outcomes. The secondary aims were to assess patients’ symptoms in and determine whether outcomes were is affected by transgression of the 6‐week rule. Statistical analysis was performed using SPSS statistics 20 for Windows. Normality was tested using the Shapiro–Wilk test. Survival analysis was performed separately for each pathological type of sarcoma using Kaplan–Meier survival curves. The impact of delay in diagnosis on joint salvage rate and local recurrence was also investigated using binary logistic regression analysis.
Results
In all, 102 patients, 57 of whom were male and 45 female, were enrolled in this study. The mean age at presentation was 30.0 years (range, 5–89 years). The subjects were categorized according to pathological diagnosis: 54 had osteosarcomas, 29 Ewing sarcoma and 19 chondrosarcoma. Clinicopathological characteristics according to pathological diagnosis are shown in Table 1.
Table 1.
Clinicopathological characteristics according to sarcoma type
| Characteristic | Osteosarcoma (n = 54) | Ewing sarcoma (n = 29) | Chondrosarcoma (n = 19) |
|---|---|---|---|
| Age at primary therapy (years, mean [range]) | 28.9 (8–86) | 17.4 (5–56) | 52.4 (21–89) |
| Sex (cases [%]) | |||
| Male | 30 (55.6) | 19 (65.5) | 8 (42.1) |
| Female | 24 (44.4) | 10 (34.5) | 11 (57.9) |
| Location (cases [%]) | |||
| Long bones | 50 (92.6) | 10 (34.5) | 8 (42.1) |
| Axial skeleton | 4 (7.4) | 18 (62.1) | 7 (36.8) |
| Other | — | 1 (3.4) | 4 (21.1) |
| Primary therapy (cases [%]) | |||
| Surgical excision | 50 (92.6) | 16 (55.2) | 19 (100) |
| Chemotherapy/radiotherapy | 4 (7.4) | 13 (44.8) | — |
| Wide excision (cases [%]) | 46 (92.0) | 9 (56.3) | 16 (84.2) |
| Additional therapy (cases [%]) | |||
| (Neo‐) adjuvant chemotherapy | 44 (81.5) | 27 (93.1) | — |
| Radiotherapy | 10 (18.5) | 22 (75.9) | 3 (15.8) |
| Status after primary therapy (cases [%]) | |||
| Disease‐free | 37 (68.5) | 15 (51.7) | 15 (78.9) |
| Non‐radical resection | 3 (5.6) | 3 (10.3) | 2 (10.5) |
| Unresectable lesion | 1 (1.9) | — | — |
| Metastatic disease | 13 (24.1) | 11 (37.9) | 2 (10.5) |
| Follow‐up (months, mean [SD]) | 54.9 (42.8) | 39.2 (31.2) | 54.6 (35.8) |
| Current status (cases [%]) | |||
| Continuously disease‐free | 24 (44.4) | 11 (37.9) | 10 (52.6) |
| Alive with disease | 2 (3.7) | 1 (3.4) | 1 (5.3) |
| No evidence of disease | 8 (14.8) | 4 (13.8) | 1 (5.3) |
| Dead of disease | 18 (33.3) | 13 (44.8) | 6 (31.6) |
| Dead of other disease | 2 (3.7) | — | 1 (5.3) |
SD, standard deviation.
Clinical Characteristics
Osteosarcomas were located in the long bones in 50 patients (92.6%), the femur being the predominant site (31 patients, 57.4%). Pain was the most frequent symptom, being present in 44 patients (81.4%). Most Ewing sarcomas were located in the axial skeleton (18 patients, 62.1%) with the spine or ribs as the predominant site in nine patients (31.0%), followed by the pelvis in eight patients (27.6%). Ten patients (34.5%) presented with Ewing sarcoma in their long bones. Pain was the commonest symptom, being present in 20 patients (68.9%). Chondrosarcomas were most in the long bones (eight patients, 42.1%); the most frequent symptom was pain, which was present in 13 patients (68.4%). Six patients (11%) with osteosarcoma and 10 (34.5%) with Ewing sarcoma presented with systemic symptoms, whereas were none of the patients with chondrosarcoma had systemic symptoms.
Delays in Diagnosis
The mean delay in diagnosis is shown in Table 2 and Fig. 2. The mean total delay was 163.3 days (standard deviation [SD], 176.5 days) in patients with osteosarcoma, 160.2 days (SD, 193.7 days) in patients with Ewing sarcoma, and 688.0 days (SD, 678.4 days) in patients with chondrosarcoma, this mean total delay being significantly longer than that for osteosarcoma and Ewing sarcoma (P < 0.01). The mean patient‐related delay for all types of sarcoma was 83.2 days (SD, 208.1 days), being 244.1 days for chondrosarcoma, which is somewhat longer than that for osteosarcoma (44.8 days, P = 0.058) and significantly longer than that for Ewing sarcoma (41.0 days, P = 0.034).
Table 2.
Delay in diagnosis (days, mean [standard deviation]) according to sarcoma type
| Mean delay in days | Osteosarcoma (n = 54) | Ewing sarcoma (n = 29) | Chondrosarcoma (n = 19) |
|---|---|---|---|
| Total delay | 163.3 (176.5) | 160.2 (193.7) | 688.0 (678.4)* |
| Patient‐related delay | 44.8 (41.8) | 41.0 (40.5) | 244.1 (433.5)* |
| Mean doctor‐related delay | 100.0 (94.6) | 130.6 (217.6) | 332.3 (312.7)* |
| Pre‐hospital doctor‐related delay | 57.8 (56.1) | 103.6 (223.8) | 197.2 (291.9) |
| Primary clinic doctor‐related delay | 16.5 (21.3) | 16.3 (21.3) | 49.7 (43.6)* |
| Referral clinic doctor‐related delay | 26.6 (28.2) | 24.5 (28.0) | 34.9 (36.0) |
Significant difference (P < 0.05).
Figure 2.
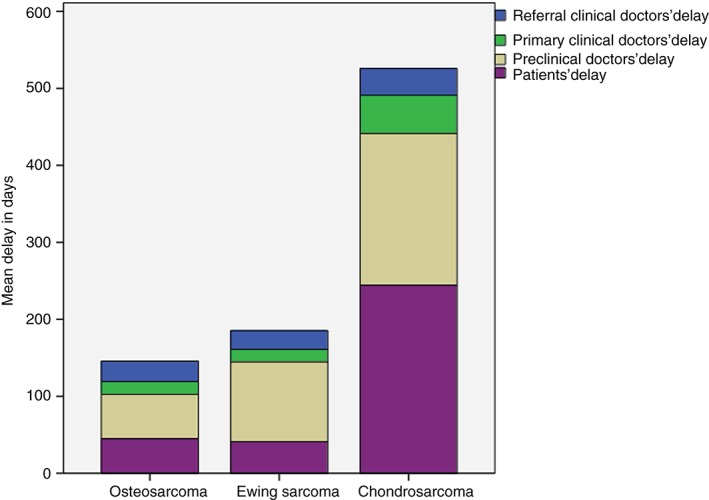
Mean diagnostic delay in days.
The mean overall doctor‐related delay was 156.2 days (SD, 210.9 days). Mean doctor‐related delay for chondrosarcoma was 332.3 days, which is significantly longer than for osteosarcoma (100.0 days, P ≤ 0.01) and Ewing sarcoma (130.6 days, P < 0.01). Mean overall pre‐hospital doctor‐related delay was 101.5 days (SD, 189.7 days); data on this were unavailable for 14 cases (13.5%). The mean overall primary clinic doctor‐related delay was 23.5 days (SD, 30.4 days), data being unavailable for 3.9% of cases. This delay was significantly longer for patients with chondrosarcoma (49.7 days) than for those with osteosarcoma (16.5 days; P < 0.01) or Ewing sarcoma (16.3 days; P < 0.01). The mean overall referral clinic doctor‐related delay was 27.6 days (SD, 29.6 days), all data being available.
Outcomes
Metastatic disease was present at diagnosis in 24.1% of patients with osteosarcoma, 37.9% with Ewing sarcoma and 10.5% with chondrosarcoma. Surgical resection of the primary tumor was performed in 92.4% of patients with osteosarcoma and 55.2% with Ewing sarcoma; all patients with chondrosarcoma underwent surgical resection. There was no association between length of delay and rate of limb salvage procedures. Overall, 16 patients with tumors in the axial skeleton underwent surgical excision, resulting in intralesional resections in 43.8% of them, compared with 9.4% of 60 patients with tumors in the long bones (P < 0.01). Adjuvant chemotherapy was given to 93.7% of patients with Ewing sarcoma. Local recurrence was diagnosed in 16 patients (15.7%), six of whom had osteosarcomas (11.1%), five Ewing sarcomas (17.2%) and five chondrosarcomas (26.3%). There was no association between length of delay and local recurrence rate for any single pathological type or overall. At the end of follow‐up, 44.4% of patients with osteosarcoma, 37.9% with Ewing sarcoma and 52.6% with chondrosarcoma were continuously disease‐free. After primary treatment, no evidence of disease was seen in 14.8% of subjects with osteosarcoma, 13.8% of those with Ewing sarcoma and 5.3% of those with chondrosarcoma. Patient mortality was highest for Ewing sarcoma; 44.8% of cases dying of disease.
Survival
Thirty‐eight patients (37.3%) had a minimum follow‐up of 5 years. The mean duration of follow‐up in patients with osteosarcoma was 54.9 months (SD, 42.8 months) with a 5‐year overall survival rate (60 months) of 67.0% (SD, 6.6 months). The mean duration of follow‐up in patients with Ewing sarcoma was 39.2 months (SD, 31.2 months) with a 5‐year overall survival rate (62 months) of 49.0% (SD, 11.1 months). The mean duration of follow‐up in patients with chondrosarcoma was 54.6 months (SD, 35.8 months) with a 5‐year overall survival rate (61 months) of 60.9% (SD, 13.0 months). Five‐year overall survival curves are displayed in Fig. 3.
Figure 3.
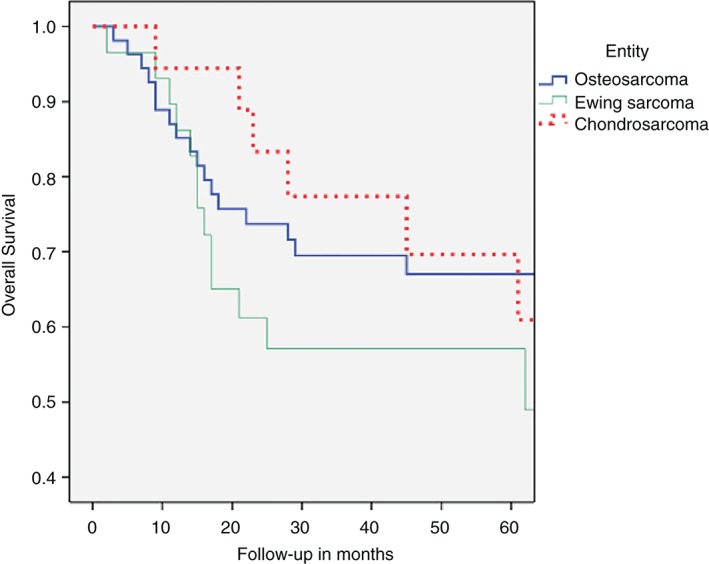
Five‐year‐overall survival for high‐grade sarcoma of bone according to sarcoma type.
Overall (all sarcoma types), 5‐year‐overall survival rates were significantly lower for patients with tumors in the axial skeleton (46.0%) than for those with long bone tumors (72.3%; P = 0.016). In patients with osteosarcoma, the 5‐year‐overall survival was significantly lower for tumors in the axial skeleton (25.0%) than for those in long bones (71.0%; P = 0.026). For Ewing sarcoma and chondrosarcoma, tumor location did not significantly impact 5‐year‐overall survival.
The 5‐year‐overall survival of patients with osteosarcoma was significantly lower after intralesional resection (25.0%) than after wide resection (77.3%; P = 0.01). Similarly, for Ewing sarcoma, the 5‐year‐overall survival was significantly lower after intralesional resection (42.9%) than after wide resection (71.1%; P = 0.048). The excision margin did not significantly impact 5‐year‐overall survival in subjects with chondrosarcoma.
Five‐year‐overall survival rates were significantly lower in patients with metastatic osteosarcoma (26.9%) than in patients who remained disease‐free after resection (79.3%; P < 0.01). Survival of subjects with metastatic Ewing sarcoma was also significantly lower (36.4%) than for disease‐free patients (72.7% after 62 months; P < 0.01). The 5‐year overall survival rates of patients with chondrosarcoma and metastases was 50% compared 69.5% in patients who remained disease‐free (P < 0.01).
The mean overall delay from presentation at UMCG to histological diagnosis for each sarcoma type was compared based on the SONCOS guidelines and it was found that the 5‐year overall survival rate for osteosarcoma diagnosed in <42 days was 58.2%, as against 76.9% in patients diagnosed ≥42 days (not significant [NS]). The overall survival rate after 62 months for patients with Ewing sarcoma diagnosed in <42 days was 39.4%, compared to 80.0% of patients diagnosed ≥42 days (NS). The overall survival after 61 months for chondrosarcoma was 46.2% in patients diagnosed in <42 days and 83.3% in patients diagnosed ≥42 days (NS).
There were no significant differences in 5‐year overall survival rates between total delay <4 months and a longer total delay for patients with osteosarcoma and Ewing sarcoma. There was no significant difference in overall survival rates between a total delay <4 months (two patients, 50% overall survival after 40 months) and a delay ≥4 months (17 patients, 63.6% overall survival after 61 months) for patients with chondrosarcoma. No significant differences were identified in metastatic disease rates after a total delay <4 months compared to a longer total delay for any of the three pathological types or for all subjects combined.
Discussion
Delay in diagnosis may have an adverse effect on oncologic outcomes. SONCOS guidelines state that diagnosis at an oncology center within 42 days minimizes negative effects on outcome. The purpose of this study was to investigate patient‐ and doctor‐related delays and evaluate their effects on outcomes. High‐grade bone sarcomas are rare neoplasms; 102 lesions were seen in our referral oncology center over 12 years. The present study provides a valuable addition to data of other published Dutch series on outcomes of high‐grade bone sarcomas19, 20, 21, 22. Furthermore, this report includes 8.3% of all patients with high‐grade bone sarcomas who underwent orthopaedic oncological treatment in the Netherlands during that 12 years23. Because our study focused on the effect of delay in diagnosis, one of its limitations is that we did not include tumor size as a prognostic factor24.
There are few relevant published reports, as shown in Table 3. Bacci et al. reported a shorter delay in patients with metastatic osteosarcomas than in those with localized disease12. They concluded that aggressive tumor behavior results in shorter delays. This conclusion is in accordance with our results, since patients with chondrosarcoma had significantly longer delays in diagnosis than those with osteosarcoma and Ewing sarcoma. However, the long delay in diagnosis of intermediate and high‐grade chondrosarcoma did not result in lower survival rates. We believe that this can be explained by less aggressive tumor behavior, absence of systemic symptoms and the inclusion of intermediate‐grade chondrosarcomas. However, it is important to recognize that chondrosarcoma can dedifferentiate and that this is associated with poor survival.
Table 3.
Relevant published reports on delay in diagnosis of high‐grade bone sarcomas
| Authors | Years | Sarcoma type | Conclusion |
|---|---|---|---|
| Sneppen and Hansen14 | 1984 |
84 osteosarcomas 40 Ewing sarcomas |
No association between delay and survival |
| Wurtz et al. 25 | 1999 | 68 pelvic chondrosarcomas | No association between delay and survival |
| Widhe and Widhe15 | 2000 |
102 osteosarcomas 47 Ewing sarcomas |
Doctor‐related delay significantly longer for Ewing sarcoma |
| Bacci et al. 26 | 2000 | 965 high‐grade osteosarcomas | Aggressive tumor behavior results in shorter delay |
| Kim et al. 18 | 2009 | 26 osteosarcomas | Doctor‐related delay superimposed on an inappropriate primary procedure has a detrimental effect on survival |
| Pan et al. 13 | 2010 | 30 knee‐region osteosarcomas | Total delay in diagnosis 17 weeks |
| Goedhart et al. (current study) | 2016 |
54 osteosarcomas 29 Ewing sarcomas 19 chondrosarcomas |
Longer delay in patients with chondrosarcoma, no effect on outcome |
Sneppen and Hansen defined treatment delay as the time from the first symptom until presentation at an oncology center14. Their mean treatment delay was 6.4 months for osteosarcoma and 9.6 months for Ewing sarcoma, which is longer than our series, in which there was a treatment delay of 3.9 months for osteosarcoma and 5.3 months for Ewing sarcoma. Comparison between the Bacci study and our own is difficult because referral patterns and accuracy have evolved over the past 30 years.
Primary orthopaedic hospitals in the Netherlands follow the guidelines on bone tumors, which specify that biopsy and treatment should be performed in an oncology referral center. Although diagnostic delay at an oncology center makes up only a small slice of the total delay, it is the most visible type of delay. According to SONCOS, the acceptable referral clinic doctor‐related delay for a Dutch oncological center to diagnose a neoplasm and start treatment is 42 days27. In this study, the referral clinic doctor‐related delay from presentation to diagnosis was 27.6 days and thus within our national standards. For high‐grade chondrosarcoma it was 34.9 days. Because these tumors often present in the pelvis and may therefore be difficult to access for biopsy, multiple biopsies may be performed before a definitive diagnosis is made. Delay in diagnosis is also associated with other clinical variables; Kim et al . found that misdiagnosis and inappropriate treatment resulted in inferior outcomes in subjects with osteosarcoma18. This is in accordance with our study, in which we found significantly lower survival rates in patients with osteosarcoma and Ewing sarcoma after intralesional surgical resection. Furthermore, intralesional surgical resection occurred more often with tumors located in the axial skeleton. Location of a tumor in the axial skeleton is also associated with lower survival rates.
According to our data, diagnosis after 42 days of high‐grade bone sarcomas does not result in lower survival; neither do total delays exceeding 4 months. And yet, paradoxically, metastatic disease after primary resection is associated with significantly lower survival rates. This discrepancy may be explained by the fact that aggressive tumor behavior results in early clinical symptoms, thereby facilitating a timely diagnosis. Our findings imply that tumor location and resectability have more influence on survival than delay in diagnosis in patients with osteosarcoma and Ewing sarcoma.
Tumor location and resectability, metastatic disease at diagnosis, response to chemotherapy and local recurrence are known prognostic factors for osteosarcoma and Ewing sarcoma according to published reports8, 26, 28, 29, 30, 31, 32, 33, 34.
Most of the diagnostic delay occurred in the pre‐hospital setting at the GP's office. We realize that is very difficult for GPs to recognize a bone malignancy because they generally only encounter one or two primary bone sarcomas in their entire careers. Pain was the most common symptom in our study and in other reports14, 15. Therefore GPs should have a low‐threshold for requesting plain radiographs in patients with pain and no history of trauma; such a policy would likely decrease mean doctor‐related delay by accelerating referral to an oncology center. Persistent pain for more than 6 weeks is a red flag and an indication for a radiograph.
In conclusion, this study provides valuable insight into diagnostic delay patterns for high‐grade bone sarcomas in the Netherlands. Prolonged delay in diagnosis of high‐grade sarcomas of the bone does not result in lower survival. The SONCOS guidelines for diagnosing neoplasms are easily met, but do not seem clinically relevant to high‐grade bone sarcomas. Persistent pain for more than 6 weeks in the pre‐hospital (GP) setting is an indication for a radiograph.
Disclosure: The authors have no conflicts of interest to declare.
References
- 1. Bleyer A, O'Leary M, Barr R, Ries L. Cancer Epidemiology in Older Adolescents and Young Adults 15 to 29 Years of Age, Including SEER Incidence and Survival: 1975–2000. NIH Publication. Bethesda: National Cancer Institute, 2006. [Google Scholar]
- 2. Ferrari S, Palmerini E, Staals EL, et al The treatment of nonmetastatic high grade osteosarcoma of the extremity: review of the Italian Rizzoli experience. Impact on the future. Cancer Treat Res, 2009, 152: 275–287. [DOI] [PubMed] [Google Scholar]
- 3. Bruland ØS, Bauer H, Alvegaard T, Smeland S. Treatment of osteosarcoma. The Scandinavian Sarcoma Group experience. Cancer Treat Res, 2009, 152: 309–318. [DOI] [PubMed] [Google Scholar]
- 4. Jaffe N. Osteosarcoma: review of the past, impact on the future. The American experience. Cancer Treat Res, 2009, 152: 239–262. [DOI] [PubMed] [Google Scholar]
- 5. Whelan J, Seddon B, Perisoglou M. Management of osteosarcoma. Curr Treat Options Oncol, 2006, 7: 444–455. [DOI] [PubMed] [Google Scholar]
- 6. Sciubba DM, Okuno SH, Dekutoski MB, Gokaslan ZL. Ewing and osteogenic sarcoma: evidence for multidisciplinary management. Spine (Phila Pa 1976), 2009, 34: S58–S68. [DOI] [PubMed] [Google Scholar]
- 7. Damron TA, Ward WG, Stewart A. Osteosarcoma, chondrosarcoma, and Ewing's sarcoma: national cancer data base report. Clin Orthop Relat Res, 2007, 459: 40–47. [DOI] [PubMed] [Google Scholar]
- 8. Anninga JK, Gelderblom H, Fiocco M, et al Chemotherapeutic adjuvant treatment for osteosarcoma: where do we stand? Eur J Cancer, 2011, 47: 2431–2445. [DOI] [PubMed] [Google Scholar]
- 9. Luetke A, Meyers PA, Lewis I, Juergens H. Osteosarcoma treatment – where do we stand? A state of the art review. Cancer Treat Rev, 2014, 40: 523–532. [DOI] [PubMed] [Google Scholar]
- 10. Cangir A, Vietti TJ, Gehan EA, et al Ewing's sarcoma metastatic at diagnosis. Results and comparisons of two intergroup Ewing's sarcoma studies. Cancer, 1990, 66: 887–893. [DOI] [PubMed] [Google Scholar]
- 11. Cotterill SJ, Ahrens S, Paulussen M, et al Prognostic factors in Ewing's tumor of bone: analysis of 975 patients from the European intergroup cooperative Ewing's sarcoma study group. J Clin Oncol, 2000, 18: 3108–3114. [DOI] [PubMed] [Google Scholar]
- 12. Bacci G, Ferrari S, Longhi A, Mellano D, Giacomini S, Forni C. Delay in diagnosis of high‐grade osteosarcoma of the extremities. Has it any effect on the stage of disease? Tumori, 2000, 86: 204–206. [DOI] [PubMed] [Google Scholar]
- 13. Pan KL, Chan WH, YY C. Initial symptoms and delayed diagnosis of osteosarcoma around the knee joint. J Orthop Surg (Hong Kong), 2010, 18: 55–57. [DOI] [PubMed] [Google Scholar]
- 14. Sneppen O, Hansen LM. Presenting symptoms and treatment delay in osteosarcoma and Ewing's sarcoma. Acta Radiol Oncol, 1984, 23: 159–162. [DOI] [PubMed] [Google Scholar]
- 15. Widhe B, Widhe T. Initial symptoms and clinical features in osteosarcoma and Ewing sarcoma. J Bone Joint Surg Am, 2000, 82: 667–674. [DOI] [PubMed] [Google Scholar]
- 16. Yang JY, Cheng FW, Wong KC, et al Initial presentation and management of osteosarcoma, and its impact on disease outcome. Hong Kong Med J, 2009, 15: 434–439. [PubMed] [Google Scholar]
- 17. Bielack SS, Kempf‐Bielack B, Delling G, et al Prognostic factors in high‐grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol, 2002, 20: 776–790. [DOI] [PubMed] [Google Scholar]
- 18. Kim MS, Lee SY, Cho WH, et al Prognostic effects of doctor‐associated diagnostic delays in osteosarcoma. Arch Orthop Trauma Surg, 2009, 129: 1421–1425. [DOI] [PubMed] [Google Scholar]
- 19. Ham SJ, Kroon HM, Koops HS, Hoekstra HJ. Osteosarcoma of the pelvis–oncological results of 40 patients registered by the Netherlands committee on bone Tumours. Eur J Surg Oncol, 2000, 26: 53–60. [DOI] [PubMed] [Google Scholar]
- 20. Renard AJ, Veth RP, Pruszczynksi M, et al Ewing's sarcoma of bone: oncologic and functional results. J Surg Oncol, 1995, 60: 250–256. [DOI] [PubMed] [Google Scholar]
- 21. Renard AJ, Veth RP, Schreuder HW, et al Osteosarcoma: oncologic and functional results. A single institutional report covering 22 years. J Surg Oncol, 1999, 72: 124–129. [DOI] [PubMed] [Google Scholar]
- 22. Smorenburg CH, van Groeningen CJ, Meijer OW, Visser M, Boven E. Ewing's sarcoma and primitive neuroectodermal tumour in adults: single‐centre experience in the Netherlands. Neth J Med, 2007, 65: 132–136. [PubMed] [Google Scholar]
- 23. Integraal Kankercentrum Nederland . Dutch cancer registration 2015.
- 24. Grimer RJ. Size matters for sarcomas! Ann R Coll Surg Engl, 2006, 88: 519–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wurtz LD, Peabody TD, Simon MA. Delay in the diagnosis and treatment of primary bone sarcoma of the pelvis. J Bone Joint Surg Am, 1999, 81: 317–325. [DOI] [PubMed] [Google Scholar]
- 26. Bacci G, Ferrari S, Bertoni F, et al Prognostic factors in nonmetastatic Ewing's sarcoma of bone treated with adjuvant chemotherapy: analysis of 359 patients at the Istituto Ortopedico Rizzoli. J Clin Oncol, 2000, 18: 4–11. [DOI] [PubMed] [Google Scholar]
- 27. Stichting Oncologische Samenwerking . Multidisciplinaire normering oncologische zorg in Nederland. SONCOS Normeringsrapport, 2014, 3: 4 (in Dutch). [Google Scholar]
- 28. Pakos EE, Nearchou AD, Grimer RJ, et al Prognostic factors and outcomes for osteosarcoma: an international collaboration. Eur J Cancer, 2009, 45: 2367–2375. [DOI] [PubMed] [Google Scholar]
- 29. Bramer JA, van Linge JH, Grimer RJ, Scholten RJ. Prognostic factors in localized extremity osteosarcoma: a systematic review. Eur J Surg Oncol, 2009, 35: 1030–1036. [DOI] [PubMed] [Google Scholar]
- 30. Duchman KR, Gao Y, Miller BJ. Prognostic factors for survival in patients with Ewing's sarcoma using the surveillance, epidemiology, and end results (SEER) program database. Cancer Epidemiol, 2015, 39: 189–195. [DOI] [PubMed] [Google Scholar]
- 31. Oksüz DC, Tural D, Dincbas FÖ, et al Non‐metastatic Ewing's sarcoma family of tumors of bone in adolescents and adults: prognostic factors and clinical outcome‐single institution results. Tumori, 2014, 100: 452–458. [DOI] [PubMed] [Google Scholar]
- 32. Whelan JS, Jinks RC, McTiernan A, et al Survival from high‐grade localised extremity osteosarcoma: combined results and prognostic factors from three European osteosarcoma intergroup randomised controlled trials. Ann Oncol, 2012, 23: 1607–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Berner K, Hall KS, Monge OR, Weedon‐Fekjær H, Zaikova O, Bruland ØS. Prognostic factors and treatment results of high‐grade osteosarcoma in Norway: a scope beyond the "classical" patient. Sarcoidosis, 2015, 2015: 516843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bacci G, Longhi A, Ferrari S, Mercuri M, Versari M, Bertoni F. Prognostic factors in non‐metastatic Ewing's sarcoma tumor of bone: an analysis of 579 patients treated at a single institution with adjuvant or neoadjuvant chemotherapy between 1972 and 1998. Acta Oncol, 2006, 45: 469–475. [DOI] [PubMed] [Google Scholar]


