Abstract
Anterior lumbar interbody fusion (ALIF) is one of the surgical procedures for the relief of chronic back pain, radiculopathy and neurogenic claudication in patients with degenerative lumbar spine disease that is refractory to conservative therapy, low‐grade spondylolisthesis and pseudo arthrosis. Over the past half century, both the surgical techniques and instrumentation required for ALIF have changed significantly. In particular, the designs of ALIF cage and the materials used have evolved dramatically, the common goal being to improve fusion rates and optimize clinical outcomes. The increasing popularity of ALIF is reflected by the increasing abundance of published studies reporting clinical outcomes, surgical techniques and grafting options for ALIF. Developments in cage designs include cylindrical Bagby and Kuslich, cylindrical ray, cylindrical mesh, lumbar‐tapered, polyethyl‐etherketone cage and integral fixation cages. Biologic implants include bone dowels and femoral ring allografts. Methods for optimization of cage design have included cage dimensions, use of novel composite cage materials and integral fixation technologies. However, the historical development and evolution of cages used for ALIF has not been extensively documented. This article therefore aims to provide an overview of the historical basis for the anterior approach, evolution in design of ALIF cage implants and potential future research directions.
Keywords: ALIF, Anterior lumbar interbody fusion, Cage, Design, Review of published reports
Introduction
For patients with degenerative lumbar spine disease refractory to conservative treatment, anterior lumbar interbody fusion (ALIF) is one of the potential surgical procedures for the relief of chronic back pain, radiculopathy and neurogenic claudication1, 2. There is a limited but increasing amount of evidence for the safety and efficacy of the anterior interbody fusion approach compared with posterior approaches3. In 1932, Capener was the first to describe the use of an anterior approach for treatment of spondylolisthesis4. Since this initial study, ALIF has evolved to become an effective surgical option for various lumbar degenerative pathologies, including degenerative disc disease (Fig. 1), low‐grade spondylolisthesis and pseudoarthrosis5.
Figure 1.
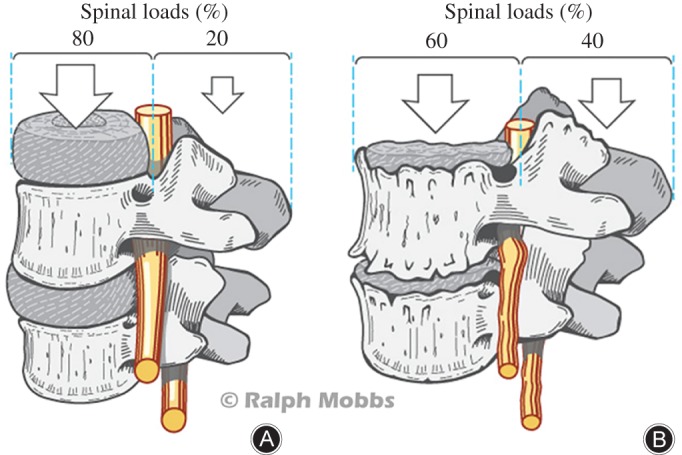
Degenerative disc disease as a rationale for ALIF. (A) Distribution of spinal loads on the anterior and posterior weight‐bearing columns in a normal lumbar spine. (B) Shifting of spinal loads to the posterior column as a result of degenerative pathology in the lumbar spine.
The ALIF procedure involves retraction of the great vessels in the retroperitoneal region to allow ventral access to the spinal structures, followed by discectomy and cage implantation. ALIF offers the potential advantages of facilitating normal lumbar lordosis, indirect enlargement of neural foramina space and increased intervertebral height, whilst reducing the risk of damaging posterior paraspinal muscles and neural structures6, 7, 8, 9. Furthermore, the anterior approach also allows implantation of larger bone cages and grafts, facilitating improved initial stability and compression of the fusion construct10, 11, 12, 13, 14. Some studies have reported that ALIF may be associated with reduced blood loss, shorter operative duration and reduced blood transfusion requirements than other approaches15. However, the ALIF procedure is also associated with risks of injury to major vasculature, intestinal and urethral damage, and injury to the hypogastric nerve plexus leading to retrograde ejaculation16, 17, 18.
Over the past half century, both the surgical techniques and instrumentation required for ALIF have changed significantly. In particular, the designs and materials of ALIF cages have evolved dramatically, the common goal being to improve fusion rates and optimize clinical outcomes. The increasing popularity of ALIF is reflected by the increasing abundance of published studies reporting clinical outcomes, surgical techniques and grafting options for ALIF3, 6, 10, 12, 15, 19, 20, 21, 22, 23, 24. In recent years, development of lumbar fusion surgery has focused on reduction of surgical trauma by using minimally invasive approaches, new graft materials and cage designs25. However, the historical development and evolution of cages used for ALIF has not been extensively documented. This article therefore aims to overview the historical basis for the anterior approach, evolution in the design of ALIF cages and potential future research directions.
Methods
A review of published reports concerning the evolution of cage designs for ALIF was performed. In accordance with international guidelines and recommendations, an electronic search the Medline/Pubmed database from inception to February 2015 was performed using the following key words and MeSH terms: “ALIF”, “anterior lumbar interbody”, “fusion”, “cage”, “implant” and “design26, 27, 28”. Inclusion criteria included studies focusing on the design, methodology or outcomes of the ALIF approach. Studies that focused on other fusion approaches, non‐English language studies and non‐human studies were excluded. Related articles were also assessed; original articles are cited where possible. The present review includes assessment and overview of 109 articles (Fig. 2).
Figure 2.
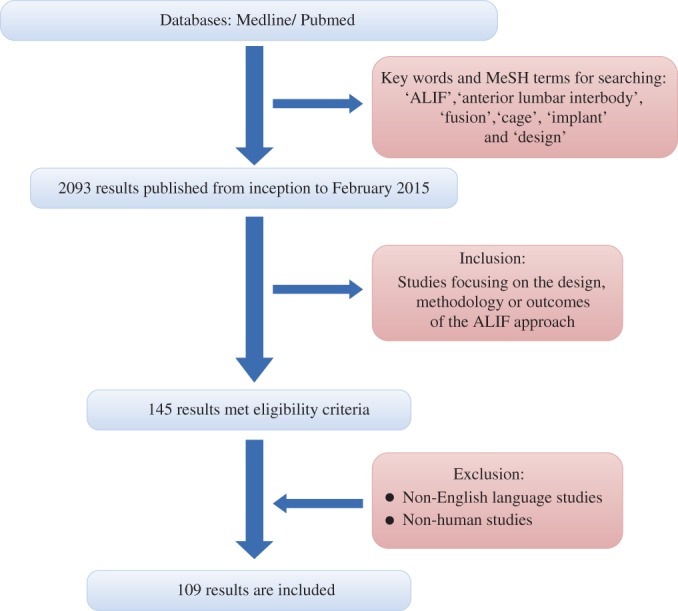
Flow‐chart of search of published reports showing the process of inclusion and exclusion.
Development of Anterior Fusion Surgery
Capener first introduced the anterior approach for insertion of a bone graft spacer for treatment of 32 patients with spondylolisthesis in 19324. Although he concluded that the anterior approach was theoretically biomechanically ideal, this view was met with resistance from his peers, Stauffer and Coventry making the criticism that “too much surgical trauma to the patient” was involved29. However, shortly thereafter several surgeons reported successful utilization of the anterior approach for spondylolisthesis, including Mercer, Friberg, d'Aubigne and Cauchoix30, 31, 32. In 1944, Iwahara proposed a retroperitoneal technique and soon after, in 1948, Lane and Moore reported the first use of ALIF for the treatment of lumbar degenerative disc disease33, 34. They used allogenic bone graft in 97 patients and achieved an excellent clinical success rate of 94% and fusion rate of 54%.
Considered by many as the initial foundation for modern era ALIF surgery, Hodgson and Stock employed Iwahara's retroperitoneal technique and different types of graft material to treat Pott disease35, 36. Their approach involved debridement of necrotic tissue, followed by decompression of the spinal canal and insertion of corticocancellous blocks of autogenous bone. Around the same time, Cloward utilized a similar technique, the difference being use of a cylindrical‐shaped corticocancellous dowel37. Whilst Cloward used a posterior approach, his use of dowels and his techniques for disc removal and endplate preparation were extensively adopted by surgeons opting for the anterior fusion approach, including Harmon in 1963 and Sacks in 196538, 39. O'Brien et al. later proposed the use of trapezoid blocks as a modification to prior grafts for treatment of discogenic pain by ALIF40. O'Brien et al. subsequently developed this into a hybrid approach involving a biologic fusion cage comprising femoral cortical allograft rings packed with autogenous cancellous bone graft (Fig. 3).
Figure 3.
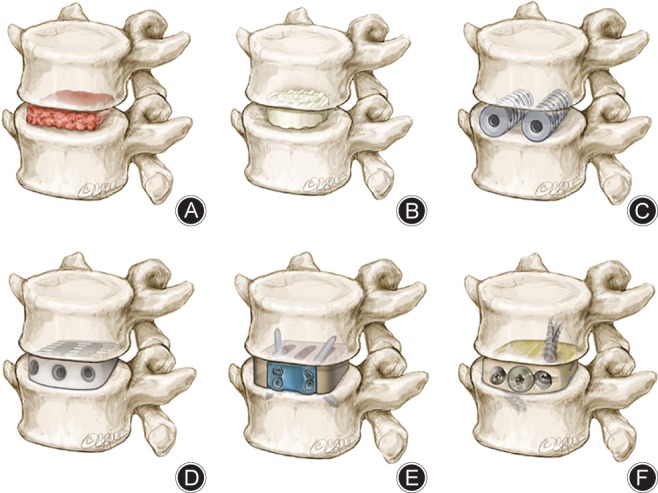
Evolution of anterior lumbar interbody fusion (ALIF) cage designs. (A) Autograft block; (B) femoral ring allograft; (C) BAK cage; (D) PEEK cage; (E) SynFix implant; (F) Ti‐PEEK cage.
Following the above pioneering innovations, there was an increasing use of stand‐alone ALIF in the 1970s and 1980s characterized by great variation in surgical techniques and discrepancies between groups concerning ALIF fusion success rates. For example, Lane and Moore reported fusion rates of 54%, Adkins fusion rates of 1% and Harmon fusion rates of up to 95%34, 38, 41. As a consequence, ALIF procedures supplemented by posterior fusion became increasingly popular. Even in current clinical practice, there is still debate as to whether stand‐alone ALIF or posterior supplemented ALIF is the optimal approach for lumbar degenerative spinal disease. However, during this time, there have also been advances in ALIF instrumentation and access techniques, including the introduction of implant cages or devices to improve stabilization and restore disc height.
Cage Design Evolution
Threaded Titanium Cages
Cylindrical BAK Cage
The history of cages used for ALIF stems back to the original cage implant developed by Bagby, an orthopaedic surgeon in Washington, for the treatment of cervical instability and myelopathy due to Wobbler's syndrome in thoroughbred horses42. Known as the “Bagby basket”, it was the first cage to consist of a stainless steel cylinder and was packed with horse autograft, facilitating successful fusion with good early stability and improved arthrodesis42, 43, 44.
To adapt the Bagby basket for human use, in the late 1980s Kuslich et al. introduced several changes to the cage design, including the use of a threaded hollow titanium cylinder with thick perforated walls. This allowed the cage to be screwed onto the endplates of the adjacent vertebrae, thus promoting stabilization and fusion. Furthermore, the hollow cage could be packed with cancellous bone chips, which eliminated the need for autografts42, 43, 44, 45. At the time, the use of autografts as in the Cloward technique had produced poor results with high mortality and morbidity. This new cage for human use was named the Bagby and Kuslich (BAK) titanium cage (BAK; Spine‐Tech, Minneapolis, MI, USA) and was first successfully implanted in humans in 1992 using a posterior approach46. Soon after, the use of interbody cages was quickly adapted for an anterior approach for fusion, these being approved by the Food and Drug Administration in 1996.
Cylindrical Ray Cage
Ray further modified the BAK cage, using a design with deeper threads47. This promoted “self‐tapping” and facilitated stabilization. Furthermore, the Ray cage is reportedly associated with fewer artefacts on imaging studies than BAK cages and can be implanted via a posterior or anterior approach48.
Cylindrical Mesh Cage
Titanium mesh cages was first developed and introduced in 1986 by Harms and Biederman. Although there have been few reports concerning the use of titanium mesh cages for anterior lumbar fusion, the results published thus far have cited optimistic results49, 50, 51. The design of these cages involves titanium mesh that has been rolled into a cylindrical shape and reinforced with rings at each end. Titanium mesh cages are traditionally filled with autograft and achieve a high rate of arthrodesis; however, there may be up to 25% complication rates associated with obtaining bone from the iliac crest52, 53, 54. Recent studies have shown that alternatives to grafts, including coralline hydroxyapatite and demineralized bone matrix, are effective55.
Lumbar‐tapered Cage
Several studies have demonstrated that wider implants are associated with improved segmental stability20, 56, 57. These have higher axial strength to resist subsidence than the narrower area of cylindrical implants. This finding has led to the introduction of lumbar‐tapered or trapezoid cages. Specifically, lumbar‐tapered cages are wedge‐shaped, allowing restoration of the spine to more physiologically correct alignments and angles. These provide similar benefits to the cylindrical cages, however, lumbar‐tapered cages allows symmetric reaming of endplates, improving lordosis. These cages can be packed with bone morphogenic protein or autograft.
The use of ALIF for patients with severe discogenic pain for stabilization of vertebral segments has accelerated since the development of these titanium cages46, 47, 48, 58, 59, 60, 61, 62. Limitations of threaded cylindrical titanium cages include the use of solid titanium, which prevents accurate radiological assessment of the fusion mass. The stiffness of the titanium cage may also promote its subsidence into adjacent vertebrae, a long‐term complication associated with this cage.
Polyetheretherketone (PEEK) Cage Devices
The use of titanium and titanium alloys has proliferated in the spine surgery realm since the 1940s because of its biocompatibility, low density of approximately 4700 kg/m3, and robust passivation due to TiO2 formation, which provides impressive resistance to corrosion63. However, the use of titanium and its alloys also poses several issues for anterior lumbar fusion implants. Firstly, there is a mismatch between the elastic modulus of titanium (110 GPa) and that of vertebrae trabecular bone (2.1 GPa) and cortical bone (2.4 GPa64). This elastic modulus mismatch results in reduced stress shielding around the implant which, together with local inflammation, can precipitate graft subsidence and bone–graft interface fractures65, 66, 67, 68. A second major issue with the use of titanium cages and elastic modulus mismatch is that it causes imaging artefacts because of its high radiodensity, and thus hinders accurate assessment of fusion status69.
To address the above‐mentioned disadvantages of titanium and titanium alloys, alternative materials, including PEEK and carbon fiber, were developed. PEEK fusion cages were introduced by AcroMed (Raynham, MA, USA) in the 1990s, and pioneered by polymer engineer McMillin; these implants were known as Brantigan cages70. The initial anterior interbody cage devices constructed of PEEK comprised either a hexagonal or round device designed as a spacer with a central cavity for bone graft placement. PEEK offers several advantages: PEEK cages have a modulus of elasticity similar to that of cortical bone, which may promote even load sharing and stress distribution71, 72. This may translate into lower subsidence rates and potentially higher fusion rates. Furthermore, the use of carbon fiber reinforcement may further reduce any differences in elastic modulus between PEEK and bone, PEEK anterior fusion cages are biocompatible and the radiolucency of PEEK implants permits improved assessment of fusion on imaging73, 74. Some studies have suggested that PEEK materials are relatively resistant to microbial adhesion and hence associated with lower infection rates than their titanium counterparts75, 76.
Several studies have evaluated anterior lumbar fusion using PEEK cages. In one of the earliest biochemical studies, Schleicher et al. demonstrated acceptable flexion and extension loading in a test PEEK cage compared with an established anterior lumbar fusion cage77. In a prospective 2‐year follow‐up study, Hoff et al. demonstrated significant improvements in Oswestry Disability Index and Visual Analog Scale scores in 32 patients undergoing ALIF with PEEK cages. Fusion rates were reportedly 93% postoperatively and 70% at final 24‐month follow‐up. More recently, a study of 40 patients demonstrated solid interbody fusion in 96.4% of them after ALIF using PEEK cages with posterior instrumentation78.
Integral Fixation Cages
Stand‐alone ALIF cages have been developed over the last 15 years and rapidly grown in popularity because of ease of instrumentation and improved biomechanics that eliminate the need for further posterior fixation. In a study by Cain et al., the first stand‐alone ALIF implant “Test‐device” demonstrated significantly more stability than a traditional anterior cage with translaminar facet screws in flexion and rotation and similar stability to pedicle screw fixation79. Stand‐alone integral fixation ALIF cages have the potential to reduce complications associated with a combined anterior and posterior instrumentation77, 80, 81.
The first integral fixation device on the market that was constructed of PEEK with an integral fixation screw construct was the SynFix (Synthes Bettlach, Solothurn, Switzerland) and gained widespread popularity worldwide because of its excellent biomechanics and ease of use. The SynFix system is a PEEK implant with an integrated anterior plate that additionally stabilizes the motion segment using four angle‐locked screws. An early study investigating this cage in stand‐alone ALIF demonstrated a 70.6% fusion rate, and 68.7% fusion when posterior instrumentation was added. More recently, an investigation of 32 ALIF procedures performed using the SynFix system with recombinant human bone morphogenetic protein‐2 demonstrated solid fusion in 29 patients (90.6%) at 6‐month follow‐up, similar to values reported for ALIF with posterior instrumentation and bone morphogenetic protein‐282. In another recent study of stand‐alone ALIF with PEEK, a fusion rate of 96.3% was demonstrated at 12‐month follow‐up in 65 patients with degenerative lumbar disc disease83. These preliminary studies provide promising results for integral fixation cages for stand‐alone ALIF; these findings require further confirmation by additional long‐term follow‐up studies.
Biologic Implants
Bone Dowels
Threaded bone dowels derived from the diaphysis of freeze‐dried femurs or tibias have been used as an alternative to titanium cage constructs. Bone dowels are deployed in a similar fashion to traditional titanium cages and involve a dowel holder for impacting the dowel into the appropriate position. Bone dowels offer several advantages, including the transmission of more physiological forces than titanium. Similar construct stiffness and biomechanical stability has been demonstrated for dowel and interbody fusion cage constructs84, 85. Bone dowels also have a modulus of elasticity similar to that of native bone and would therefore provide better fusion rates than titanium and carbon fiber alternatives. Furthermore, natural bone material is more radiolucent than titanium, thus making fusion with bone dowels easier to assess by imaging intraoperatively. Bone dowels can be used in the presence of infections because they are made of a biological substrate.
Femoral Ring Allografts
Allograft biological cages were developed with the aim of providing mechanical support and stability whilst using biocompatible materials with physiological properties. Femoral ring allografts are biological cages machined from allograft into wedge‐shaped rings with “teeth” that can grip onto adjacent vertebrae and improve the stability of the spacer. Given that these spacers have hollow centers, they can be filled with further allograft or bone morphogenic protein to further promote fusion of the biologic cage86, 87, 88, 89.
Cage Design Optimisation
Cage Dimensions
To optimize the design of anterior lumbar fusion implants, pathological changes to spinal anatomy must be considered. The primary consideration of a fusion technique and implant cage is its ability to restore normal anatomy, including foraminal area and volume, disc height, lumbar lordosis and sagittal balance6, 7, 8, 9. Degenerative changes in the lumbar spine may affect these variables; a commonly quoted change is foraminal narrowing leading to radicular pain and loss of disc height90, 91. Recently, the pedicle‐to‐pedicle technique was developed as a standardized radiographic approach for indirectly demonstrating significant improvements in foraminal dimensions and disc height with ALIF using a stand‐alone PEEK interbody implant92. Foraminal height and area are important variables to consider when designing new ALIF implant and determining whether one design is superior to another92, 93.
Modern ALIF cage designs have a variety of different features and dimensions to ensure maximal clinical and fusion outcomes3, 6, 10, 12, 15, 19, 20, 21, 22, 23, 24. Because of differences in initial stability and disc space height between lumbar levels and individuals, ALIF cages are manufactured in a variety of sizes. ALIF cages relieve radicular pain by restoring disc space height, which thus indirectly restores foraminal height and area92, 93. The architectural design of an implanted ALIF fusion cage has been recognized as a key factor in modulating mechanical dynamics and biological functions in ALIF. However, it must also be recognized that over‐distraction can lead to the complications of non‐union, postoperative neck pain and poor clinical outcomes resulting from high pressures between graft and lumbar vertebra end plates.
Cage width and length are also important variables for ensuring maximal surface contact and stability of ALIF. Greater implant areas improve transmission of loads to adjacent segments. A further advantage of this is the potential reduction in adjacent level pain94. Greater implant area may also facilitate restoration of a lordotic curve, thus creating better physiological stress95. Biomechanical studies have demonstrated that covering more than 30% of the endplate area with bone grafts facilitates improved load carrying capacity. However, it must be noted that the optimal dimensions are dictated by lumbar anatomy: an implant that is too small will provide inadequate stability whereas an implant that is too large can damage surrounding structures.
Composite Cage Materials
Although PEEK has favorable mechanical properties, its chemical inertness limits its ability to osseointegrate into the surrounding bone environment. Thus, a myriad of options for cage materials have been developed with the aim of improving PEEK bioactivity, including hydroxyapatite (HA)‐PEEK and Ti‐PEEK composite cages (Fig. 4).
Figure 4.
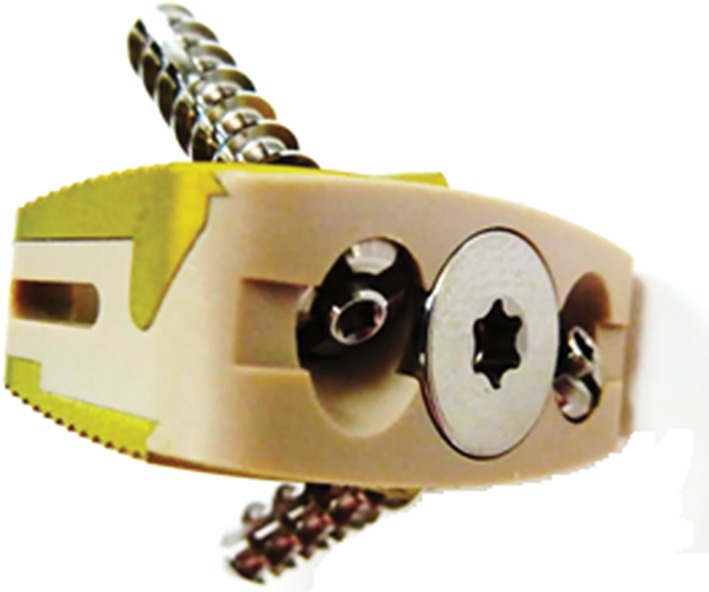
TI/PEEK composite device for improved osseointegration. Recent developments have seen increasing use of PEEK in vertebral body fusion. More novel approaches to improving PEEK have included the introduction of Ti‐PEEK composites and coatings.
Given that natural bone consists of fine HA, the natural response would be to develop a composite cage with HA, which would improve bio‐integration with its environment. There have been several approaches to integrating HA with PEEK cages. In 1988, HA was added to PEEK in an attempt to create a composite material that would more closely mimic natural bone substance96. This concept was recently reintroduced by Wong et al., who developed a strontium‐containing HA‐PEEK composite with similar bending modulus to cortical bone that enhanced in vitro bioactivity97. Other recent studies by Khor's group have demonstrated that PEEK with 30% volume HA has an elastic modulus similar to that of human cortical bone98, 99. Another way to create a HA‐PEEK composite is to coat PEEK cages in nanocrystalline HA, which has been shown to be superior to uncoated implants in terms of osseointegration100.
Another type of composite material currently under investigation is Ti‐PEEK. Using a PEEK cage composite with TiO2 particles manufactured by mixing compression and model, Wu et al. demonstrated significantly better osseointegration than with PEEK alone101. They reported better cell attachment and spreading than with pure PEEK and better bone regeneration around the composite implant in vivo. Similar conclusions have been reported by Han et al., who have also demonstrated that Ti‐PEEK composite materials have the promise of improved bioactivity102.
Other implant materials, including silicon nitride and tantalum, are also under investigation. Nitinol, an alloy consisting of 50% nickel and 50% Ti with shape memory and superelastic properties, is also a potential option for implant material103, 104, 105. Recent studies have also tested bio‐absorbable cages manufactured using poly‐l‐lactide‐co‐D, l‐lactide. This material absorbs over time without leaving any foreign material in the spinal segment106. These materials exhibit the necessary rigidity at the time of implantation, then gradually degrade, improving radiological assessment. However, there has been limited experience with this implant material with contradictory results; thus, further studies are warranted107, 108, 109, 110, 111.
Integral Fixation Technologies
Most integral fixation devices use screw technologies to secure the implant to the endplate above and below the device. There are a variety of devices with two, three or four screw designs for assisting initial implant fixation. The design of the fixation method in an integral fixation device may affect the biomechanics of the ALIF cage and must therefore be taken into consideration. Buttermann et al. compared three cage types: a PEEK spacer with small ridges, a modular interbody device with end‐plate spikes and a dual tapered threaded interbody cage112. Cages with end‐plate spikes provided better motion segment rigidity in bending modes and particularly in torsion, which may have implications in terms of the design of future ALIF implants112.
A variety of other devices use additional fixation methods such as an implantable fin (ROI‐A Oblique; Zimmer‐Biomet, Brognard, France), rotatable teeth and expanding screws (A‐Spine ASIA, Taipei, Taiwan) for fixation (Figs 5, 6).
Figure 5.

Redmond lumbar cage (A‐SPINE Asia). (A) Superior view showing central beveled keel. (B) Lateral view shwoing angulated ridges and composite design and Ti end‐plate inlay, fixed in a PEEK body. (C) Anterior view showing two integrated screw fixation.
Figure 6.

Cage with Fin device for improving initial stability. Current developments in cage fixation technology include an implantable fin (ROI‐A Oblique; LDR), rotatable teeth and expanding screws (A‐Spine ASIA) for fixation.
Conclusions
Whilst great strides have been made in the development of ALIF cages over the past decade, from bone grafts to composite cages, the ALIF cage continues to evolve into the future. Current efforts are focused on improving bioactivity and osseointegration of ALIF cages and on streamlining anterior fixation with integrated screw cages. Multiple promising new designs are currently in experimentation and testing, however, the inadequate available clinical evidence and lack of comparisons between different models have prevented definitive conclusions regarding the advantages and disadvantages of one implant over another. Future designs will benefit from continued collaborative biomechanical studies, experimentation and clinical studies.
Disclosure: No funds were received in support of this work.
References
- 1. Mobbs RJ, Loganathan A, Yeung V, Rao PJ. Indications for anterior lumbar interbody fusion. Orthop Surg, 2013, 5: 153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rao PJ, Loganathan A, Yeung V, Mobbs RJ. Outcomes of anterior lumbar interbody fusion surgery based on indication: a prospective study. Neurosurgery, 2015, 76: 7–23. [DOI] [PubMed] [Google Scholar]
- 3. Mobbs RJ, Phan K, Malham G, Seex K, Rao PJ. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI‐TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg, 2015, 1: 2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Capener N. Spondylolisthesis. Br J Surg, 1932, 19: 374–386. [Google Scholar]
- 5. Czerwein JK Jr, Thakur N, Migliori SJ, Lucas P, Palumbo M. Complications of anterior lumbar surgery. J Am Acad Orthop Surg, 2011, 19: 251–258. [DOI] [PubMed] [Google Scholar]
- 6. Dennis S, Watkins R, Landaker S, Dillin W, Springer D. Comparison of disc space heights after anterior lumbar interbody fusion. Spine, 1989, 14: 876–878. [DOI] [PubMed] [Google Scholar]
- 7. Mobbs RJ, Phan K, Thayaparan GK, Rao PJ. Anterior lumbar interbody fusion as a salvage technique for pseudarthrosis following posterior lumbar fusion surgery. Global Spine J, 2016, 6: 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mobbs RJ, Phan K, Daly D, Rao PJ, Lennox A. Approach‐related complications of anterior lumbar interbody fusion: results of a combined spine and vascular surgical team. Global Spine J, 2016, 6: 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hsieh PC, Koski TR, O'Shaughnessy BA, et al. Anterior lumbar interbody fusion in comparison with transforaminal lumbar interbody fusion: implications for the restoration of foraminal height, local disc angle, lumbar lordosis, and sagittal balance. J Neurosurg Spine, 2007, 7: 379–386. [DOI] [PubMed] [Google Scholar]
- 10. Lee CS, Hwang CJ, Lee DH, Kim YT, Lee HS. Fusion rates of instrumented lumbar spinal arthrodesis according to surgical approach: a systematic review of randomized trials. Clin Orthop Surg, 2011, 3: 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hueng DY, Chung TT, Chuang WH, Hsu CP, Chou KN, Lin SC. Biomechanical effects of cage positions and facet fixation on initial stability of the anterior lumbar interbody fusion motion segment. Spine (Phila Pa 1976), 2014, 39: E770–E776. [DOI] [PubMed] [Google Scholar]
- 12. Shim JH, Kim WS, Kim JH, Kim DH, Hwang JH, Park CK. Comparison of instrumented posterolateral fusion versus percutaneous pedicle screw fixation combined with anterior lumbar interbody fusion in elderly patients with L5‐S1 isthmic spondylolisthesis and foraminal stenosis. J Neurosurg Spine, 2011, 15: 311–319. [DOI] [PubMed] [Google Scholar]
- 13. Sørensen KH. Anterior interbody lumbar spine fusion for incapacitating disc degeneration and spondylolisthesis. Acta Orthop Scand, 1978, 49: 269–277. [DOI] [PubMed] [Google Scholar]
- 14. Sasso RC, Kitchel SH, Dawson EG. A prospective, randomized controlled clinical trial of anterior lumbar interbody fusion using a titanium cylindrical threaded fusion device. Spine Spine (Phila Pa 1976), 2004, 29: 113–122. [DOI] [PubMed] [Google Scholar]
- 15. Phan K, Thayaparan GK, Mobbs RJ. Anterior lumbar interbody fusion versus transforaminal lumbar interbody fusion–systematic review and meta‐analysis. Br J Neurosurg, 2015, 29: 705–711. [DOI] [PubMed] [Google Scholar]
- 16. Wood KB, Devine J, Fischer D, Dettori JR, Janssen M. Vascular injury in elective anterior lumbosacral surgery. Spine (Phila Pa 1976), 2010, 35: S66–S75. [DOI] [PubMed] [Google Scholar]
- 17. Than KD, Wang AC, Rahman SU, et al. Complication avoidance and management in anterior lumbar interbody fusion. Neurosurg Focus, 2011, 31: E6. [DOI] [PubMed] [Google Scholar]
- 18. Jarrett CD, Heller JG, Tsai L. Anterior exposure of the lumbar spine with and without an "access surgeon": morbidity analysis of 265 consecutive cases. J Spinal Disord Tech, 2009, 22: 559–564. [DOI] [PubMed] [Google Scholar]
- 19. Burke PJ. Anterior lumbar interbody fusion. Radiol Technol, 2001, 72: 423–430. [PubMed] [Google Scholar]
- 20. Pavlov PW, Meijers H, van Limbeek J, et al. Good outcome and restoration of lordosis after anterior lumbar interbody fusion with additional posterior fixation. Spine (Phila Pa 1976), 2004, 29: 1893–1899. [DOI] [PubMed] [Google Scholar]
- 21. Strube P, Hoff E, Hartwig T, Perka CF, Gross C, Putzier M. Stand‐alone anterior versus anteroposterior lumbar interbody single‐level fusion after a mean follow‐up of 41 months. J Spinal Disord Tech, 2012, 25: 362–369. [DOI] [PubMed] [Google Scholar]
- 22. Matgé G, Leclercq TA. Rationale for interbody fusion with threaded titanium cages at cervical and lumbar levels. Results on 357 cases. Acta Neurochir (Wien), 2000, 142: 425–433. [DOI] [PubMed] [Google Scholar]
- 23. Kim Y. Finite element analysis of anterior lumbar interbody fusion: threaded cylindrical cage and pedicle screw fixation. Spine (Phila Pa 1976), 2007, 32: 2558–2568. [DOI] [PubMed] [Google Scholar]
- 24. Gumbs AA, Bloom ND, Bitan FD, Hanan SH. Open anterior approaches for lumbar spine procedures. Am J Surg, 2007, 194: 98–102. [DOI] [PubMed] [Google Scholar]
- 25. Pannell WC, Savin DD, Scott TP, Wang JC, Daubs MD. Trends in the surgical treatment of lumbar spine disease in the United States. Spine J, 2015, 15: 1719–1727. [DOI] [PubMed] [Google Scholar]
- 26. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Int J Surg, 2010, 8: 336–341. [DOI] [PubMed] [Google Scholar]
- 27. Phan K, Tian DH, Cao C, Black D, Yan TD. Systematic review and meta‐analysis: techniques and a guide for the academic surgeon. Ann Cardiothorac Surg, 2015, 4: 112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Phan K, Mobbs RJ. Systematic reviews and meta‐analyses in spine surgery, neurosurgery and orthopedics: guidelines for the surgeon scientist. J Spine Surg, 2015, doi: 10.3978/jss.2015.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stauffer RN, Coventry MB. Anterior interbody lumbar spine fusion. Analysis of Mayo Clinic series. J Bone Joint Surg Am, 1972, 54: 756–768. [PubMed] [Google Scholar]
- 30. Mercer W. Spondylolisthesis with a description of a new method of operative treatment and notes of ten cases. Edinb Med J, 1936, 43: 545–572. [PMC free article] [PubMed] [Google Scholar]
- 31. Friberg S. Low back and sciatic pain caused by intervertebral disc herniation: anatomic and clinical investigation. Acta Chir Scand, 1941, 64: 85. [Google Scholar]
- 32. Merle d'Aubigne R, Cauchoix J, Faulong M. Anterior transperitoneal arthrodesis in the therapy of spondylolisthesis. Rev Orthop Chir Appar Mot, 1950, 36: 490–494. [PubMed] [Google Scholar]
- 33. Iwahara T. A new method of vertebral body fusion. Surgery, 1944, 8: 271–287. [Google Scholar]
- 34. Lane JD Jr, Moore ES Jr. Transperitoneal approach to the intervertebral disc in the lumbar area. Ann Surg, 1948, 127: 537–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hodgson A, Stock F. Anterior spinal fusion. Br J Surg, 1956, 44: 226–275. [DOI] [PubMed] [Google Scholar]
- 36. Hodgson AR, Stock FE. Anterior spine fusion for the treatment of tuberculosis of the spine. J Bone Joint Surg Br, 1960, 42: 295–310. [Google Scholar]
- 37. Cloward RB. Lesions of the intervertebral disks and their treatment by interbody fusion methods. Clin Orthop, 1963, 27: 51–77. [PubMed] [Google Scholar]
- 38. Harmon PH. Anterior excision and vertebral body fusion operation for intervertebral disc syndromes of the lower lumbar spine. Clin Orthop Relat Res, 1963, 26: 107–127. [PubMed] [Google Scholar]
- 39. Sacks S. Anterior interbody fusion of the lumbar spine. J Bone Joint Surg Br, 1965, 47: 211–223. [PubMed] [Google Scholar]
- 40. O'Brien JP, Dawson MH, Heard CW, Momberger G, Speck G, Weatherly CR. Simultaneous combined anterior and posterior fusion. A surgical solution for failed spinal surgery with a brief review of the first 150 patients. Clin Orthop Relat Res, 1986, 203: 191–195. [PubMed] [Google Scholar]
- 41. Adkins EW. Lumbo‐sacral arthrodesis after laminectomy. J Bone Joint Surg Br, 1955, 37: 208–223. [DOI] [PubMed] [Google Scholar]
- 42. DeBowes RM, Grant BD, Bagby GW, Gallina AM, Sande RD, Ratzlaff MH. Cervical vertebral interbody fusion in the horse: a comparative study of bovine xenografts and autografts supported by stainless steel baskets. Am J Vet Res, 1984, 45: 191–199. [PubMed] [Google Scholar]
- 43. Bagby GW. Arthrodesis by the distraction‐compression method using a stainless steel implant. Orthopedics, 1988, 11: 931–934. [DOI] [PubMed] [Google Scholar]
- 44. Crawley GR, Grant BD, White KK, Barbee DD, Gallina AM, Ratzlaff MH. A modified Cloward's technique for arthrodesis of the normal metacarpophalangeal joint in the horse. Vet Surg, 1988, 17: 117–127. [DOI] [PubMed] [Google Scholar]
- 45. Wagner PC, Grant BD, Bagby GW, Gallina AM, Sande RD, Ratzlaff M. Evaluation of cervical spinal fusion as a treatment in the equine “wobbler” syndrome. Vet Surg, 1979, 8: 84–88. [Google Scholar]
- 46. Kuslich SD, Ulstrom CL, Griffith SL, Ahern JW, Dowdle JD. The Bagby and Kuslich method of lumbar interbody fusion. History, techniques, and 2‐year follow‐up results of a United States prospective, multicenter trial. Spine (Phila Pa 1976), 1998, 23: 1267–1278. [DOI] [PubMed] [Google Scholar]
- 47. Ray CD. Threaded fusion cages for lumbar interbody fusions. An economic comparison with 360 degrees fusions. Spine (Phila Pa 1976), 1997, 22: 681–685. [DOI] [PubMed] [Google Scholar]
- 48. Ray CD. Threaded titanium cages for lumbar interbody fusions. Spine (Phila Pa 1976), 1997, 22: 667–679. [DOI] [PubMed] [Google Scholar]
- 49. Bohm H, Harms J, Donk R, Zielke K. Correction and stabilization of angular kyphosis. Clin Orthop Relat Res, 1990, 258: 56–61. [PubMed] [Google Scholar]
- 50. Harms J, Stoltze D. The indications and principles of correction of post‐traumatic deformities. Eur Spine J, 1992, 1: 142–151. [DOI] [PubMed] [Google Scholar]
- 51. Lowery GL, Harms J. Titanium Surgical Mesh for Vertebral Defect Replacement and Intervertebral Spacers: Manual of Internal Fixation of the Spine. Philadelphia: Lippincott‐Raven Publishers, 1996; 127–146. [Google Scholar]
- 52. Hu RW, Bohlman HH. Fracture at the iliac bone graft harvest site after fusion of the spine. Clin Orthop Relat Res, 1994, 309: 208–213. [PubMed] [Google Scholar]
- 53. Fernyhough JC, Schimandle JJ, Weigel MC, Edwards CC, Levine AM. Chronic donor site pain complicating bone graft harvesting from the posterior iliac crest for spinal fusion. Spine (Phila Pa 1976), 1992, 17: 1474–1480. [DOI] [PubMed] [Google Scholar]
- 54. Laurie SW, Kaban LB, Mulliken JB, Murray JE. Donor‐site morbidity after harvesting rib and iliac bone. Plast Reconstr Surg, 1984, 73: 933–938. [DOI] [PubMed] [Google Scholar]
- 55. Thalgott JS, Giuffre JM, Klezl Z, Timlin M. Anterior lumbar interbody fusion with titanium mesh cages, coralline hydroxyapatite, and demineralized bone matrix as part of a circumferential fusion. Spine J, 2002, 2: 63–69. [DOI] [PubMed] [Google Scholar]
- 56. Steffen T, Tsantrizos A, Aebi M. Effect of implant design and endplate preparation on the compressive strength of interbody fusion constructs. Spine (Phila Pa 1976), 2000, 25: 1077–1084. [DOI] [PubMed] [Google Scholar]
- 57. Spruit M, Pavlov PW, Leitao J, De Kleuver M, Anderson PG, Den Boer F. Posterior reduction and anterior lumbar interbody fusion in symptomatic low‐grade adult isthmic spondylolisthesis: short‐term radiological and functional outcome. Eur Spine J, 2002, 11: 428–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. McAfee PC, Regan JJ, Geis WP, Fedder IL. Minimally invasive anterior retroperitoneal approach to the lumbar spine. Emphasis on the lateral BAK. Spine, 1998, 23: 1476–1484. [DOI] [PubMed] [Google Scholar]
- 59. Nesheiwat F, Brown WM, Healey KM. Post‐traumatic first metatarsal reconstruction using coralline hydroxyapatite. J Am Podiatr Med Assoc, 1998, 88: 130–134. [DOI] [PubMed] [Google Scholar]
- 60. Nibu K, Panjabi MM, Oxland T, Cholewicki J. Multidirectional stabilizing potential of BAK interbody spinal fusion system for anterior surgery. J Spinal Disord, 1997, 10: 357–362. [PubMed] [Google Scholar]
- 61. Rahimi F, Maurer BT, Enzweiler MG. Coralline hydroxyapatite: a bone graft alternative in foot and ankle surgery. J Foot Ankle Surg, 1997, 36: 192–203. [DOI] [PubMed] [Google Scholar]
- 62. Olinger A, Hildebrandt U, Pistorius G, Lindemann W, Menger MD. Laparoscopic 2‐level fusion of the lumbar spine with Bagby and Kuslich implants. Chirurg, 1996, 67: 348–350. [PubMed] [Google Scholar]
- 63. Ramakrishna S, Mayer J, Wintermantel E, Leong KW. Biomedical applications of polymer‐composite materials: a review. Compos Sci Technol, 2001, 61: 1189–1224. [Google Scholar]
- 64. Kurtz SM, Devine JN. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials, 2007, 28: 4845–4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bal BS, Rahaman MN. Orthopedic applications of silicon nitride ceramics. Acta Biomater, 2012, 8: 2889–2898. [DOI] [PubMed] [Google Scholar]
- 66. Niu CC, Liao JC, Chen WJ, Chen LH. Outcomes of interbody fusion cages used in 1 and 2‐levels anterior cervical discectomy and fusion: titanium cages versus polyetheretherketone (PEEK) cages. J Spinal Disord Tech, 2010, 23: 310–316. [DOI] [PubMed] [Google Scholar]
- 67. Liao JC, Niu CC, Chen WJ, Chen LH. Polyetheretherketone (PEEK) cage filled with cancellous allograft in anterior cervical discectomy and fusion. Int Orthop, 2008, 32: 643–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rosa AL, Beloti MM. Effect of cpTi surface roughness on human bone marrow cell attachment, proliferation, and differentiation. Braz Dent J, 2003, 14: 16–21. [DOI] [PubMed] [Google Scholar]
- 69. Rao PJ, Pelletier MH, Walsh WR, Mobbs RJ. Spine interbody implants: material selection and modification, functionalization and bioactivation of surfaces to improve osseointegration. Orthop Surg, 2014, 6: 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Brantigan JW, Steffee AD. A carbon fiber implant to aid interbody lumbar fusion. Two‐year clinical results in the first 26 patients. Spine (Phila Pa 1976), 1993, 18: 2106–2107. [DOI] [PubMed] [Google Scholar]
- 71. Schimmel JJ, Poeschmann MS, Horsting PP, Schonfeld DH, van Limbeek J, Pavlov PW. PEEK cages in lumbar fusion: mid‐term clinical outcome and radiological fusion. J Spinal Disord Tech, 2012. [Epub ahead of print]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22907069 (accessed 2 February 2016). [DOI] [PubMed] [Google Scholar]
- 72. Galbusera F, Schmidt H, Wilke HJ. Lumbar interbody fusion: a parametric investigation of a novel cage design with and without posterior instrumentation. Eur Spine J, 2012, 21: 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Blumenthal SL, Gill K. Can lumbar spine radiographs accurately determine fusion in postoperative patients? Correlation of routine radiographs with a second surgical look at lumbar fusions. Spine (Phila Pa 1976), 1993, 18: 1186–1189. [DOI] [PubMed] [Google Scholar]
- 74. McAfee PC, Boden SD, Brantigan JW, et al. Symposium: a critical discrepancy‐a criteria of successful arthrodesis following interbody spinal fusions. Spine (Phila Pa 1976), 2001, 26: 320–334. [DOI] [PubMed] [Google Scholar]
- 75. Kakinuma H, Ishii K, Ishihama H, et al. Antibacterial polyetheretherketone implants immobilized with silver ions based on chelate‐bonding ability of inositol phosphate: processing, material characterization, cytotoxicity, and antibacterial properties. Biomed Mater Res A, 2015, 103: 57–64. [DOI] [PubMed] [Google Scholar]
- 76. Gallo J, Holinka M, Moucha CS. Antibacterial surface treatment for orthopaedic implants. Int J Mol Sci, 2014, 15: 13849–13880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Schleicher P, Gerlach R, Schar B, et al. Biomechanical comparison of two different concepts for stand alone anterior lumbar interbody fusion. Eur Spine J, 2008, 17: 1757–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hoff E, Strube P, Gross C, Hartwig T, Putzier M. Monosegmental anterior lumbar interbody fusion with the SynFix‐LR™ device. A prospective 2-year follow-up study. Orthopade, 2010, 39: 1044–1050. [Article in German]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20821188 [DOI] [PubMed] [Google Scholar]
- 79. Cain CM, Schleicher P, Gerlach R, Pflugmacher R, Scholz M, Kandziora F. A new stand‐alone anterior lumbar interbody fusion device: biomechanical comparison with established fixation techniques. Spine (Phila Pa 1976), 2005, 30: 2631–2636. [DOI] [PubMed] [Google Scholar]
- 80. Rao PJ, Ghent F, Phan K, Lee K, Reddy R, Mobbs RJ. Stand‐alone anterior lumbar interbody fusion for treatment of degenerative spondylolisthesis. J Clin Neurosci, 2015, 22: 1619–1624. [DOI] [PubMed] [Google Scholar]
- 81. Phan K, Mobbs RJ. Sacrum fracture following L5‐S1 stand‐alone interbody fusion for isthmic spondylolisthesis. J Clin Neurosci, 2015, 22: 1837–1839. [DOI] [PubMed] [Google Scholar]
- 82. Burkus JK, Gornet MF, Schuler TC, Kleeman TJ, Zdeblick TA. Six‐year outcomes of anterior lumbar interbody arthrodesis with use of interbody fusion cages and recombinant human bone morphogenetic protein‐2. J Bone Joint Surg Am, 2009, 91: 1181–1189. [DOI] [PubMed] [Google Scholar]
- 83. Allain J, Delecrin J, Beaurain J, Poignard A, Vila T, Flouzat‐Lachaniette CH. Stand‐alone ALIF with integrated intracorporeal anchoring plates in the treatment of degenerative lumbar disc disease: a prospective study on 65 cases. Eur Spine J, 2014, 23: 2136–2143. [DOI] [PubMed] [Google Scholar]
- 84. Kanayama M, Cunningham BW, Haggerty CJ, Abumi K, Kaneda K, McAfee PC. In vitro biomechanical investigation of the stability and stress‐shielding effect of lumbar interbody fusion devices. J Neurosurg, 2000, 93 (Suppl. 2): 259–265. [DOI] [PubMed] [Google Scholar]
- 85. Cagli S, Crawford NR, Sonntag VK, Dickman CA. Biomechanics of grade I degenerative lumbar spondylolisthesis. Part 2: treatment with threaded interbody cages/dowels and pedicle screws. J Neurosurg, 2001, 94 (Suppl. 1): 51–60. [DOI] [PubMed] [Google Scholar]
- 86. Holte DC, O'Brien JP, Renton P. Anterior lumbar fusion using a hybrid interbody graft. A preliminary radiographic report. Eur Spine J, 1994, 3: 32–38. [DOI] [PubMed] [Google Scholar]
- 87. Kozak JA, O'Brien JP. Simultaneous combined anterior and posterior fusion. An independent analysis of a treatment for the disabled low‐back pain patient. Spine (Phila Pa 1976), 1990, 15: 322–328. [DOI] [PubMed] [Google Scholar]
- 88. Liljenqvist U, O'Brien JP, Renton P. Simultaneous combined anterior and posterior lumbar fusion with femoral cortical allograft. Eur Spine J, 1998, 7: 125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sarwat AM, O'Brien JP, Renton P, Sutcliffe JC. The use of allograft (and avoidance of autograft) in anterior lumbar interbody fusion: a critical analysis. Eur Spine J, 2001, 10: 237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sengupta DK, Herkowitz HN. Degenerative spondylolisthesis: review of current trends and controversies. Spine (Phila Pa 1976), 2005, 30 (Suppl. 6): S71–S81. [DOI] [PubMed] [Google Scholar]
- 91. Mulholland RC. Degenerative lumbar spondylolisthesis: a meta‐analysis of literature 1970‐1993. Spine (Phila Pa 1976), 1995, 20: 1957–1958. [PubMed] [Google Scholar]
- 92. Rao PJ, Maharaj MM, Phan K, Lakshan Abeygunasekara M, Mobbs RJ. Indirect foraminal decompression after anterior lumbar interbody fusion: a prospective radiographic study using a new pedicle‐to‐pedicle technique. Spine J, 2015, 15: 817–824. [DOI] [PubMed] [Google Scholar]
- 93. Phan K, Mobbs RJ, Rao PJ. Foraminal height measurement techniques. J Spine Surg, 2015, 1: 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kumar N, Judith MR, Kumar A, Mishra V, Robert MC. Analysis of stress distribution in lumbar interbody fusion. Spine (Phila Pa 1976), 2005, 30: 1731–1735. [DOI] [PubMed] [Google Scholar]
- 95. Closkey RF, Parsons JR, Lee CK, Blacksin MF, Zimmerman MC. Mechanics of interbody spinal fusion. Analysis of critical bone graft area. Spine (Phila Pa 1976), 1993, 18: 1011–1015. [DOI] [PubMed] [Google Scholar]
- 96. Bonfield W. Hydroxyapatite‐reinforced polyethylene as an analogous material for bone replacement. Ann N Y Acad Sci, 1988, 523: 173–177. [DOI] [PubMed] [Google Scholar]
- 97. Wong KL, Wong CT, Liu WC, et al. Mechanical properties and in vitro response of strontium‐containing hydroxyapatite/polyetheretherketone composites. Biomaterials, 2009, 30: 3810–3817. [DOI] [PubMed] [Google Scholar]
- 98. Abu Bakar MS, Cheang P, Khor KA. Tensile properties and microstructural analysis of spheroidized hydroxyapatite–poly (etheretherketone) biocomposites. Mater Sci Eng A, 2003, 345: 55–63. [Google Scholar]
- 99. Tang SM, Cheang P, AbuBakar MS, Khor KA, Liao K. Tension–tension fatigue behavior of hydroxyapatite reinforced polyetheretherketone composites. Int J Fatigue, 2004, 26: 49–57. [Google Scholar]
- 100. Barkarmo S, Wennerberg A, Hoffman M, et al. Nano‐hydroxyapatite‐coated PEEK implants: a pilot study in rabbit bone. J Biomed Mater Res a, 2013, 101: 465–471. [DOI] [PubMed] [Google Scholar]
- 101. Wu X, Liu X, Wei J, Ma J, Deng F, Wei S. Nano‐TiO2/PEEK bioactive composite as a bone substitute material: in vitro and in vivo studies. Int J Nanomedicine, 2012, 7: 1215–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Han CM, Lee EJ, Kim HE, et al. The electron beam deposition of titanium on polyetheretherketone (PEEK) and the resulting enhanced biological properties. Biomaterials, 2010, 31: 3465–3470. [DOI] [PubMed] [Google Scholar]
- 103. Fernandez‐Fairen M, Sala P, Dufoo M Jr, Ballester J, Murcia A, Merzthal L. Anterior cervical fusion with tantalum implant: a prospective randomized controlled study. Spine(Phila Pa 1976), 2008, 33: 465–472. [DOI] [PubMed] [Google Scholar]
- 104. Kato H, Nakamura T, Nishiguchi S, et al. Bonding of alkali‐ and heat‐treated tantalum implants to bone. J Biomed Mater Res, 2000, 53: 28–35. [DOI] [PubMed] [Google Scholar]
- 105. Tarnita D, Tarnita DN, Bizdoaca N, Mindrila I, Vasilescu M. Properties and medical applications of shape memory alloys. Rom J Morphol Embryol, 2009, 50: 15–21. [PubMed] [Google Scholar]
- 106. Pflugmacher R, Schleicher P, Gumnior S, et al. Biomechanical comparison of bioabsorbable cervical spine interbody fusion cages. Spine(Phila Pa 1976), 2004, 29: 1717–1722. [DOI] [PubMed] [Google Scholar]
- 107. Jiya T, Smit T, Deddens J, Mullender M. Posterior lumbar interbody fusion using nonresorbable poly‐ether‐ether‐ketone versus resorbable poly‐L‐lactide‐co‐D, L‐lactide fusion devices: a prospective, randomized study to assess fusion and clinical outcome. Spine(Phila Pa 1976), 2009, 34: 233–237. [DOI] [PubMed] [Google Scholar]
- 108. van Dijk M, Smit TH, Arnoe MF, Burger EH, Wuisman PI. The use of poly‐L‐lactic acid in lumbar interbody cages: design and biomechanical evaluation in vitro . Eur Spine J, 2003, 12: 34–40. [DOI] [PubMed] [Google Scholar]
- 109. Coe JD. Instrumented transforaminal lumbar interbody fusion with bioabsorbable polymer implants and iliac crest autograft. Neurosurg Focus, 2004, 16: E11. [DOI] [PubMed] [Google Scholar]
- 110. van Dijk M, Smit TH, Sugihara S, Burger EH, Wuisman PI. The effect of cage stiffness on the rate of lumbar interbody fusion: an in vivo model using poly(l‐lactic Acid) and titanium cages. Spine(Phila Pa 1976), 2002, 27: 682–688. [DOI] [PubMed] [Google Scholar]
- 111. Smit TH, Muller R, van Dijk M, Wuisman PI. Changes in bone architecture during spinal fusion: three years follow‐up and the role of cage stiffness. Spine(Phila Pa 1976), 2003, 28: 1802–1808. [DOI] [PubMed] [Google Scholar]
- 112. Buttermann GR, Beaubien BP, Freeman AL, Stoll JE, Chappuis JL. Interbody device endplate engagement effects on motion segment biomechanics. Spine J, 2009, 9: 564–573. [DOI] [PubMed] [Google Scholar]


