Abstract
Purpose: To date, increasing evidences have demonstrated that the aberrant expression of miR-371–3 cluster has been verified in various cancers and could be potentially used as a biomarker for tumor diagnosis and prognosis. To explore the role of miR-371–3 cluster in tumor diagnosis and prognosis, we conducted this study based on the published data.
Methods: We searched electronic databases (PubMed, EMBASE and Web of Science databases) (Jan 1, 2007 to Jun 1, 2018). The pooled sensitivity, specificity and area under the curve (AUC) of summary receiver operator characteristic (SROC) curve were used for diagnostic values, meanwhile the pooled hazard ration (HR) and 95% CI were used to explore the prognosis capacity of miR-372 and miR-373. In addition, the publication bias of the enrolled studies was tested and a sensitivity analysis of each study was performed to evaluate the stability of the pooled result.
Results: A total of eleven eligible studies containing six eligible studies containing 870 participants for diagnosis and 1218 cancer cases for prognosis were selected for this study. For diagnosis, the pooled results revealed that the miR-371 (sensitivity: 0.85, specificity: 0.92, AUC: 0.92) and miR-373 (sensitivity: 0.81, specificity: 0.93, AUC: 0.93) could be used as diagnostic biomarkers. For prognosis, we observed that elevated miR-372 indicated poor prognosis (HR=2.31, 95% CI: 1.04–5.14), especially in the cutoff value subgroup of median (HR=2.62, 95% CI: 1.54–4.46). In addition, pooled results showed that expression of miR-373 was not related to prognosis because of the significant heterogeneity, and the high miR-373 expression presented favorable prognosis in Asians (HR=0.34, 95% CI: 0.23–0.50) after omitting the study of heterogeneity origin.
Conclusion: The current studies demonstrated that miR-371 and miR-373 could be predictive tumor diagnostic biomarkers and the expression of miR-372 and miR-373 may indicate prognosis of cancer patients.
Keywords: cancer, miR-371, miR-372, miR-373, diagnosis, prognosis, biomarkers
Introduction
MicroRNAs (miRNAs), a primary class of endogenous, small, and noncoding RNA containing 18–24 nucleotides, have been found to have vital functions in post-transcriptional regulation of genes.1 MiRNAs degrade transcription and repress translation via implicating in target messenger RNA (mRNA) to form RNA-induced silencing complexes (RISCs), and consequently lead to mRNA decapping and deadenylation.2 There have been overwhelming evidence suggesting that miRNAs dysregulation in cancers could affect the biological behaviors of cells, such as abnormal proliferation, invasion, metastasis and epithelial-mesenchyme transition (EMT) of cancer cells, by influencing oncogenes and tumor-suppressor genes.3 Therefore, abnormal miRNAs expression could be potentially used as diagnosis and prognosis tumor biomarkers, and miRNAs also could be a treatment target.
MicroRNA-371–3 (miR-371–3) cluster locates on chromosome 19 and has three members, miR-371, MiR-372 and miR-373. MiR-371–3 cluster has been discussed to be involved in several diseases, such as canine visceral leishmaniasis,4 stroke,5 Kawasaki diseases,6 colon cancer,7 and moreover, it’s role in the pathology of cancers is currently being focused on in several studies.8–11 MiR-372 and miR-373 were reported to have the capacity to activate wild-type p53, counteract p53-mediated CDK inhibition and nourish oncogenic RAS to promote testicular germ cell cancerous process,12 and up regulated miR-371–3p could reverse the acquired drug resistance and improve overall survival of cancer patients.8 For miR-372, over-expression represses insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1) to suppress cancer proliferation and metastasis.13 For miR-373, it has been identified as being significantly elevated in lymph node-positive breast tumor samples, indicating that it plays a role in invasion and metastasis of tumors.14 However, there are some reports holding different attitudes that down regulation of miR-373 in non-small-cell-lung-cancer (NSCLC) cells was observed to be associated with suppression of the cell EMT, proliferation, migration, and invasion.15 Therefore, the role of miR-371–3 in development and progression of cancers remains obscure. In addition, the cluster was also reported as a diagnostic or prognostic biomarker, but the conclusions have remained elusive. MiR-371 and miR-372 in serum was reported to have a diagnostic value for germ cell tumors,16 testicular germ cell cancer,17 gastric cancer,18 pancreatic cancer,19 and breast cancer,20,21 respectively. However, for miR-371, the sensitivity and specificity ranged from 75% to 98.60%, and 63.41% to 99.00% with AUC from 0.715 to 0.929, respectively and for miR-372, the sensitivity and specificity ranged from 68.00% to 96.80%, and 84.3% to 100% with AUC from 0.84 to 0.879, respectively. Such a varied result was arose from the different sample size, cut-off value, or cancer types. On the other hand, studies have evaluated the prognostic value of miR-372 and miR-373 for cancers, and reported they could serve as favorable survival indicators,15,19,22–24 as well as adverse survival indicators.25–30 Therefore, the diagnostic and prognostic values of miR-371–3 cluster are unclear currently. Hence, considering the disputable evaluation of the miR-371–3 cluster, this study were performed to integrate related published studies to evaluate the potential role of these miRNAs as diagnostic and prognostic biomarkers in various cancers.
Materials and methods
Search strategy
To identify the related published articles, we searched three databases including PubMed, Web of Science, and Embase between Jan 1, 2007, and Jun 1, 2018, by the following keywords restricting, namely (‘microRNA-371ʹ OR ‘miR-371ʹ OR ‘microRNA-372ʹ OR ‘miR-372ʹ OR ‘microRNA-373ʹ OR ‘miR-373ʹ OR ‘microRNA-371–3ʹ OR ‘miR-371–3ʹ) and (“carcinoma” OR “cancer” OR “neoplasm” OR “tumor”). To get additional articles, some potential related articles were included for full-text review based on the headline and abstract, and the references of full-text articles were also traced.
Inclusion and exclusion criteria
Inclusion criteria for articles identification were as following: 1) the patients reported were definitively diagnosed by the gold standard; 2) the expression of miR-371 or miR-372 or miR-373 were detected in body fluids, such as plasma, serum, tissue or sputum; 3) the diagnostic or prognostic value of one or more of the miR-371–3 cluster were connected; and 4) the sufficient data were provided to extract or calculate the diagnostic value for true positives (TP), false positives (FP), false negatives (FN), and true negatives (TN) and the prognostic value for overall survival (OS), progression-free survival (PFS), recurrence-free survival (RFS), or disease-free survival (DFS).
We deemed the studies which had one of the following features as ineligible: 1) non-English publications; 2) lacking indispensable data for meta-analysis; and 3) containing duplicate data.
The NOS scale was applied to evaluate the quantity of articles before we excluded the inappropriate ones. We strictly followed the principles of the scale to award scores for each item and the articles scoring 6 or more were selected out of full marks of 9 points.
Data extraction and checking
Two authors reviewed and extracted information from the studies using a uniform criteria list independently. The extracted information below were concerned with outcome indicator: date of publication, type of microRNA, nationality of case, dominant ethnicity of case, mean or median age of participants, kinds of malignant disease, source of data, size of research cohort, sampling method, detection method, follow-up time, HR and 95%CI, cut-off value, grade of cancer, and diagnostic power (including TP, FP, TN and FN).
Statistical analysis
To explore the performance of the cancer process and cancer outcomes impacted by dysfunction of miR-371–3, we pooled the HR of miR-372 and miR-373 expression for OS/RFS/PFS/DFS (high expression vs low expression). The data of two studies which didn’t present directly were extracted from the Kaplan-Meier survival curves via the way Tierney reported.31 In addition, Engauge Digitizer version 4.1 was used to recognize the graphical survival plots. The effects of heterogeneity were investigated by Chi-square test and I2 statistic. If I2 was >50% or P<0.05, a random-effects model was applied; or else a fixed-effects model was selected.32 Sources of heterogeneity across contained articles were investigated with stratified analyses. We executed funnel plots, Begg’s and Egger’s tests to identify publication bias. With respect to diagnostic power, the bivariate regression model was used to combine sensitivity, specificity, positive likelihood ratios (PLRs), negative likelihood ratios (NLRs), and diagnostic odds ratio (DOR) with 95% CIs, based on the original statistics from the diagnostic four-fold table. We established summary receiver operator characteristic (SROC) curve and figured out the corresponding area under the SROC curve (AUC) based on the sensitivity and specificity of each study. Potential sources of heterogeneity were tested by the subgroup of participant characteristics and meta-regression. In addition, we adopted Deeks’ funnel plot to describe the publication bias.
The data for prognosis was calculated by the STATA (11.0) software. We deemed it was not statistically significant if P>0.05, on the contrary, it would be elaborated.
Results
Eligible studies
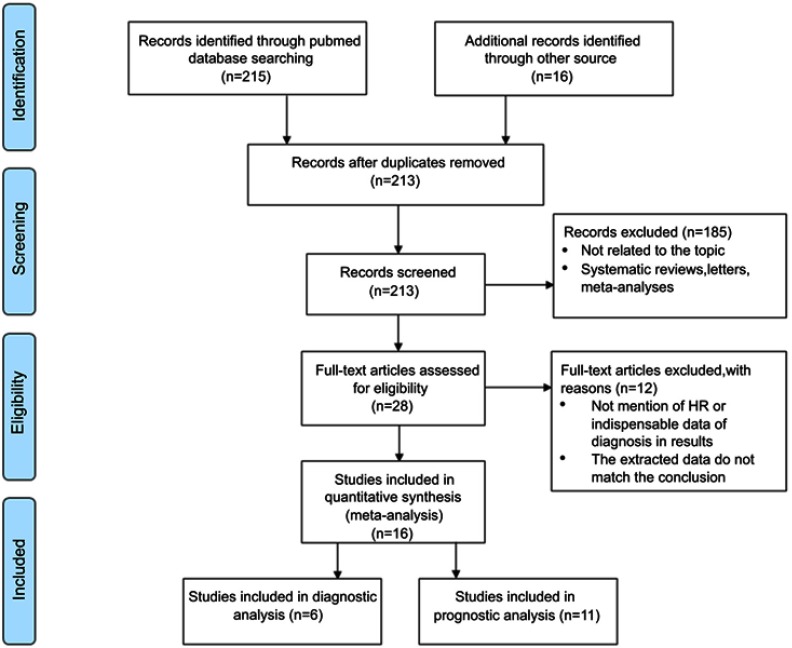
We searched the three databases and 231 (PubMed, Web of science and Embase) articles matched the keywords. After screening headline, abstract, authors and their institutions, we identified that 18 studies were duplicates, and 185 articles were systematic reviews, letters, meta-analyses, or not related to the topic. And then, 28 eligible studies with available date were enrolled for the data extraction. Due to lack of crucial statistic data, a total of 16 eligible studies were included finally, with a combination of eleven studies for prognostic and six studies for diagnostic meta-analysis (Figure 1).
Figure 1.
Flow diagram of the study selection process.
The enrolled 16 studies recruited 1,318 participants totally. As for prognosis, five studies (n=538) were prospective studies of the impact of miR-373 on the survival rate of cancer patients.15,19,23,24,30 The data related to miR-372 was extracted from five studies (n=580).22,25,27–29 Moreover, one study (n=100) contained both miR-372 and miR-373 for prognostic value.26 Regarding diagnosis, miR-371 was presented by three studies (n=497),16–18 and three studies (n=373) were linked to miR-373.19–21
Diagnostic meta-analysis
Study characteristics
Six articles which discussed the role of miR-371 and miR-373 in diagnosis of cancers were selected and the details of participants are presented in Table 1. For miR-371, two studies explored germ cell tumors and the other one was concerned with gastric cancer; and for miR-373, one study was focused on pancreatic cancer and two studies were about breast cancer. In all enrolled studies, quantitative real-time polymerase chain reaction (qRT-PCR) was applied to detect expression of miRNAs and all the samples were collected from serum except one which was taken from plasma.
Table 1.
Characteristics of the studies included for diagnosis in the meta-analysis
| Author | Year | microRNA | Ethnicity | Cancer type | Cancer grade |
Sample type |
Mean/Median age (years) | Sample size | Diagnostic power | Sensitivity | Specificity | AUC | Cut-off value |
Method | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca | Co | Ca | Co | TP | FP | TN | FN | ||||||||||||
| Dieckmann | 2017 | miR-371–3p | Caucasian | Germ cell tumours | CS1 | Serum | 38.5 | 38 | 107 | 106 | 87 | 8 | 98 | 20 | 81.40% | 92.50% | / | Median | qRT-PCR |
| CS2-CS3 | 43 | 42 | 8 | 98 | 1 | 98.60% | 92.50% | / | |||||||||||
| Syring | 2014 | miR-371-3p | Caucasian | Testicular germ cell cancer |
Serum | NM | NM | 59 | 101 | 50 | 1 | 100 | 9 | 84.70% | 99.00% | 0.929 | 0.004 | qRT-PCR | |
| Liu | 2012 | miR-371-5p | Asian | Gastric cancer | I–IV | Serum | 56 | 58 | 40 | 41 | 30 | 15 | 26 | 10 | 75.00% | 63.41% | 0.715 | 6.66 | qRT-PCR |
| Hua | 2017 | miR-373 | Asian | Pancreatic cancer | I–IV | Serum | NM | NM | 103 | 50 | 83 | 8 | 42 | 20 | 80.60% | 84.30% | 0.852 | Median | qRT-PCR |
| Eichelser | 2013 | miR-373 | Caucasian | Breast cancer | M0 | Serum | 65 | 65 | 120 | 40 | 92 | 0 | 40 | 28 | 76.60% | 100.00% | 0.879 | / | qRT-PCR |
| M1 | 32 | 31 | 2 | 38 | 1 | 96.80% | 94.10% | / | / | ||||||||||
| Chen | 2013 | miR-373 | Caucasian | Breast ductal carcinoma |
I–IV | Plasma | 49 | 47 | 35 | 25 | 24 | 3 | 22 | 11 | 68.00% | 89.00% | 0.84 | 6.97 | qRT-PCR |
Notes: M0, patients with primary breast cancer after surgery and before chemotherapy; M1, patients with distant metastases at diagnosis (We roughly incorporated M0 into the early stage, and correspondingly incorporate M1 into the late stage.); qRT-PCR, quantitative real-time PCR.
Abbreviations: NM, not mentioned; CS, clinical stage.
Expression of miR-371/3 and diagnosis
To discern whether the miR-371–3 level in serum of patients correlated with cancer development, we pooled the diagnostic parameters shown as forest plots. For miR-371, the pooled sensitivity, specificity and AUC of SROC were 0.85 (95% CI: 0.75–0.92, PHeterogeneity=0.02, I2=69.64), 0.92 (95% CI: 0.76–0.98, PHeterogeneity<0.001, I2=93.38) and 0.92 (Figure 2A, 2B). For miR-373, the pooled sensitivity, specificity and AUC of SROC were 0.81 (95% CI: 0.69–0.89, PHeterogeneity=0.02, I2=69.09), 0.93 (95% CI: 0.82–0.98, PHeterogeneity=0.03, I2=65.42) and 0.93 (Figure 3A, B).
Figure 2.

Diagnostic value of miR-371. (A) Forest plots. (B) SROC curve.
Figure 3.

Diagnostic value of miR-373. (A) Forest plots. (B) SROC curve.
Prognostic meta-analysis
Study characteristics and quality assessment
The dominant characteristics of the included eleven studies are summarized in Tables 2 and 3. For miR-372, the expression of miRNA was detected by qRT-PCR except one which was detected by in situ hybridization (ISH) and all six studies performed target miRNA expression screening in tumor tissues and corresponding para-carcinoma tissues. There were two articles evaluating HR about hepatocellular carcinoma (HCC) and the other four studies were about urothelial carcinoma (UC), oral squamous cell carcinoma (OSCC), colorectal cancer (CRC), and gliomas, respectively. For miR-373, the included six studies detected miR-373 expression with qRT-PCR, two of the six presented prognostic value in the serum samples and four studies in tissue samples. Bladder cancer (BCa), pancreatic cancer, non-small cell lung cancer (NSCLC), gliomas, oral squamous cell carcinoma (OSCC) and epithelial ovarian cancer (EOC) were explored. To assess the quality of enrolled studies, we adopted the Newcastle-Ottawa Scale (NOS)33 to score the studies based on nine criteria (Tables 2 and 3).
Table 2.
Characteristics of the studies included for miR-372 prognosis in tissue
| First author | Year | Ethnicity | Age(median/mean) | Malignant disease |
Survival indicator |
Follow up months | Method | Cut-off value |
Case number | OS | RFS/PFS/DFS | NOS sore | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | L | HR(95%CI)(U/M) | P-value | HR (95%CI) (U/M) | P-Value | ||||||||||
| Bellmunt | 2016 | Caucasian | NM | UC | PFS | 24 | qRT-PCR | Median | 40 | 40 | 1.73 (1.04, 2.89) U/ 1.70 (1.00, 2.89) M |
0.05 U | 5 | ||
| Tu | 2015 | Asian | 52.6 | OSCC | DFS | 160 | qRT-PCR | Median | 50 | 50 | 2.57 (1.20–5.48) U | 0.002 U | 6 | ||
| Wu | 2015 | Asian | NM | HCC | OS | 70 | ISH | Mean | 33 | 87 | 0.57 (0.38–0.85) U/ 0.53 (0.35–0.83) M |
0.006 U/0.005 M | 6 | ||
| Li | 2013 | Asian | 42 | Gliomas | RR | 148 | qRT-PCR | Mean | 78 | 50 | 4.37 (2.11–8.93) M | 0.008 M | 7 | ||
| Yamashita | 2012 | Asian | 66.8 | CRC | OS | 70 | qRT-PCR | Median | 72 | 72 | 2.03 (1.006–4.351) U/ 2.76 (1.322–6.110) M |
0.048 U/0.006 M | 6 | ||
| Gu | 2012 | Asian | NM | HCC | OS/RFS | 38 | qRT-PCR | Median | 65 | 43 | 20.36 (2.90–42.32) U/ 9.53 (1.74–28.67) M |
0.001 U/0.008 M | 12.73 (2.31–30.87) U/ 6.83 (1.51–19.05) M |
0.0006 U/ 0.01 M |
6 |
Note: qRT-PCR, quantitative real-time PCR; ISH, in situ hybridization; H, high expression; L, low expression; U, univariate analysis, M, multivariate analysis.
Abbreviations: NM, not mentioned; UC, urothelial carcinoma; OSCC, oral squamous cell carcinoma; HCC, hepatocellular carcinoma; CRC, colorectal cancer.
Table 3.
Characteristics of the studies included for miR-373 prognosis in the meta-analysis
| First author | Year | Dominant ethnicity | Age(median/mean) | Malignant disease | Detected sample |
Survival analysis |
Source of HR |
Follow-up months | Male/ Female |
Cut-off value |
Case number | OS | RFS/PFS/DFS | NOS score | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | L | HR (95%CI)(U/M) | P-value | HR(95%CI) (U/M) | P-value | ||||||||||||
| Zhang | 2017 | Asian | NM | BCa | Tissues | OS | Reported | 24 | 27/13 | NM | 40 | 40 | 0.441 (0.210–0.699)U | 0.17 U | 7 | ||
| Hua | 2017 | Asian | NM | Pancreatic cancer | Serum | OS | Reported | 70 | 62/41 | Median | 50 | 53 | 0.192 (0.119–0.472) M | 0.014 M | 5 | ||
| Wang | 2017 | Asian | 58.67 | NSCLC | Tissues | OS | SC | 70 | 56/36 | Median | 46 | 46 | 0.37 (0.15,0.92) | <0.001 | 6 | ||
| Jing | 2017 | Asian | NM | Gliomas | Tissues | OS/RFS | SC | 60 | 113/57 | NM | 83 | 87 | 0.40 (0.145–0.549) M | 0.0007 | 0.3 (0.157,0.613)M | <0.001 | 7 |
| Tu | 2015 | Asian | 52.6 | OSCC | Tissues | DFS | Reported | 160 | 47/3 | Median | 50 | 50 | 2.62 (1.47–4.64) U | 0.001 U | 6 | ||
| Meng | 2015 | 60 | EOC | Serum | OS/PFS | Reported | 136 | / | NM | 47 | 46 | 2.1 (1.0–4.3)U/ 2.9 (1.3–6.8)M |
0.033 U/ 0.012 M |
1.6 (0.9–2.9) U | 0.099 U | 8 | |
Abbreviations: NM, not mentioned; SC, survival curve; R, retrospective; qRT-PCR, quantitative real-time PCR; H, high expression; L, low expression; U, univariate analysis, M, multivariate analysis; BCa, bladder cancer; NSCLC, non-small cell lung cancer; OSCC, oral squamous cell carcinoma; EOC, epithelial ovarian cancer.
Expression of miR-372 and prognosis
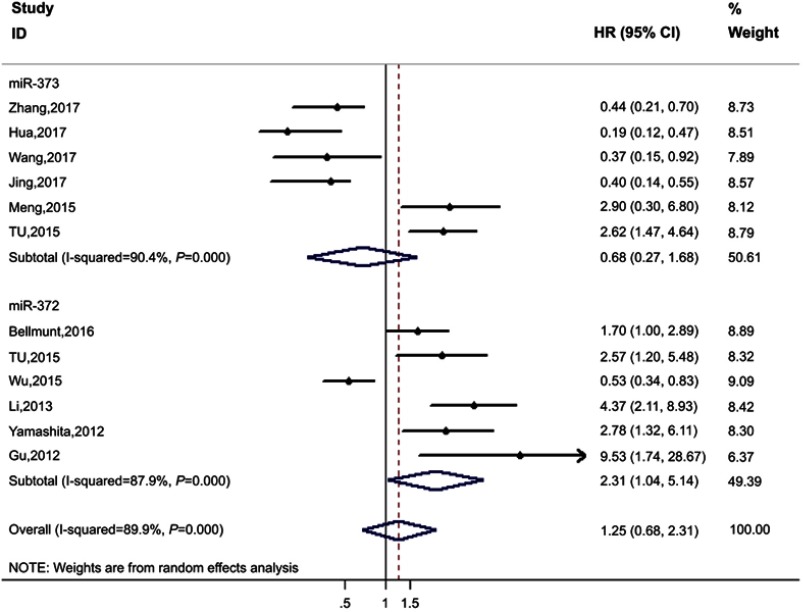
The association of miR-372 cluster relative expression (low or high) to prognosis was evaluated by seven studies, and the critical value of miRNA cluster was defined separately by each author. Pooled results showed that elevated miR-372 was associated with poor prognosis (high expression vs low expression: HR=2.31, 95% CI: 1.04–5.14, PHeterogeneity<0.001, I2=87.9%) (Figure 4).
Figure 4.
Forest plot of extracted HR for the association of miR-372 and miR-373 expression with OS/RFS/PFS/DFS.
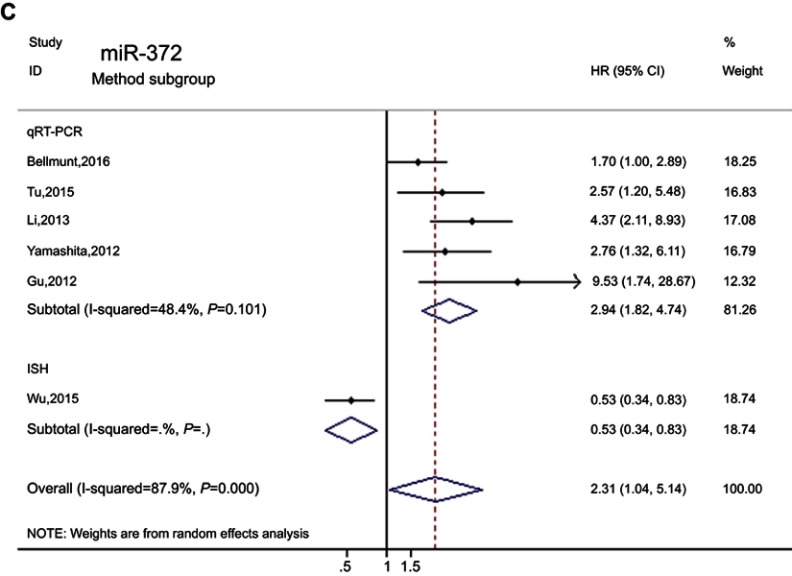
To identify the origin of the heterogeneity among studies, we performed subgroup analyses for race, main assay method, and cutoff value. There were no associations between miR-372 and the race subgroup, but a significant connection of up-regulated miR-372 with poor prognosis was found in the median of the cutoff subgroup (HR=2.62, 95% CI: 1.54–4.46, PHeterogeneity=0.139, I2=45.3%). The pooled results of the subgroup with qRT-RCR method showed that high expression of miR-372 indicated poor prognosis exceptfor one study with ISH (HR=2.94, 95% CI: 1.82–4.74, PHeterogeneity=0.101, I2=48.4%) (Figure 5, Table 4).
Figure 5.
(Continued).
Figure 5.

Forest plots of the miR-372 according to subgroup. (A) Cutoff value subgroup. (B) Detection method subgroup. (C) Race subgroup.
Table 4.
The pooled associations between characteristic subgroups of miR-371––3 expression and prognosis of patients
| microRNA | Subgroup | Number of studies | Number of cases (High/low) |
HR (95% CI) | Heterogeneity (I2) | P-values |
|---|---|---|---|---|---|---|
| miR-372 | Total | 6 | 338/342 | 2.31 (1.04,5.14) | 87.9% | <0.001 |
| Race | ||||||
| Asian | 5 | 298/302 | 2.55 (0.90,7.25) | 90.2% | <0.001 | |
| Caucasian | 1 | 40/40 | 1.70 (1.00,2.89) | / | / | |
| Method | ||||||
| qRT-PCR | 5 | 305/255 | 2.94 (1.82,4.74) | 48.4% | 0.101 | |
| ISH | 1 | 33/87 | 0.53 (0.35,0.83) | / | / | |
| Cutoff | ||||||
| Median | 4 | 227/208 | 2.62 (1.54,4.46) | 45.3% | 0.139 | |
| Mean | 2 | 111/137 | 1.50 (0.19,11.74) | 95.8% | <0.001 | |
| miR-373 | Total | 6 | 316/322 | 0.68 (0.27,1.68) | 90.04% | <0.001 |
| Race | ||||||
| Asian | 5 | 269/276 | 0.51 (0.20, 1.31) | 89.8% | <0.001 | |
| Caucasian | 1 | 47/46 | 2.90 (1.27,6.63) | / | / | |
| Sample source | ||||||
| Tissue | 4 | 172/177 | 0.66 (0.24,1.79) | 88.9% | <0.001 | |
| Serum | 2 | 97/99 | 0.74 (0.05,10.57) | 95.9% | <0.001 | |
| Cutoff | ||||||
| NM | 3 | 170/173 | 0.78 (0.25,2.42) | 87.7% | <0.001 | |
| Median | 3 | 146/149 | 0.58 (0.27,1.69) | 94.4% | <0.001 |
Abbreviations: NM, not mentioned; qRT-PCR, quantitative real-time PCR; ISH, in situ hybridization.
Expression of miR-373 and prognosis
Six studies involving miR-373 were included in this study, and there was one study reporting on miR-372 and miR-373 simultaneously.26 The pooled results showed that there was no significant association between expression of miR-373 and prognosis (HR=0.68, 95% CI: 0.27–1.69, PHeterogeneity<0.001, I2=90.4%) (Figure 4).
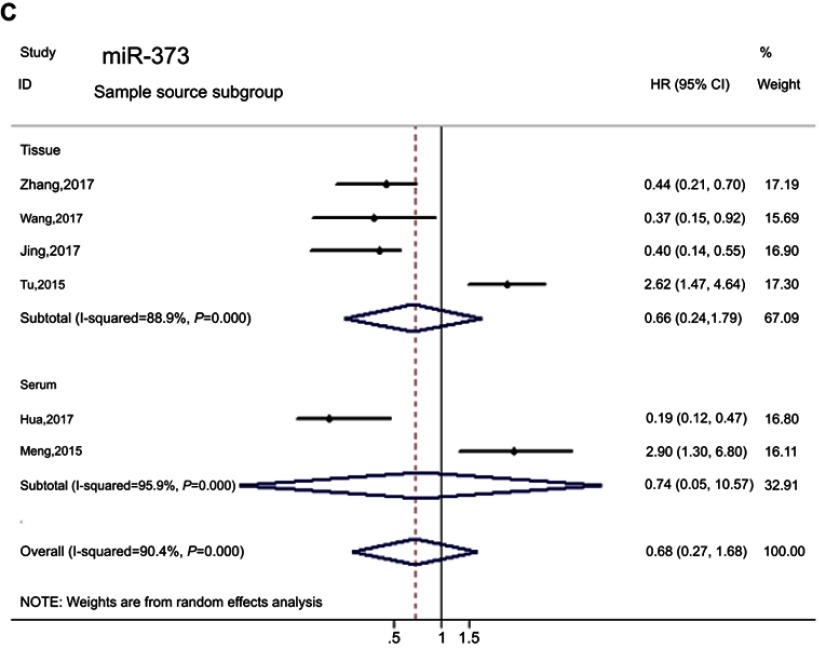
Due to the significant heterogeneity among studies, subgroup analyses for race, cutoff value and sample source were conducted. In the results of the race subgroup, we found miR-373 was not associated with prognosis (HR=0.51, 95 CI%: 0.20–1.31, PHeterogeneity<0.01, I2=89.8%), but elevated miR-373 predicted favorable prognosis in the Asian population (HR=0.338, 95% CI: 0.23–0.50, PHeterogeneity=0.305, I2=17.2%) after omitting one study. We failed to find any significant association between miR-373 expression and prognosis by high expression compared with low expression in the subgroups of sample source and cutoff value (Figure 6, Table 4).
Figure 6.
(Continued).
Figure 6.

Forest plots of the miR-373 according to subgroup. (A) Cutoff value subgroup. (B) Race subgroup. (C) Sample source subgroup.
Publication bias and sensitivity analyses
To evaluate the stability of the results, we conducted sensitivity analyses to calculate the HR and 95% CI by omitted studies one by one. For miR-373, results of pooled HR was stable; however, for miR-372, the HR was changed from 2.31 (95% CI: 1.04–5.14) to 2.94 (95% CI: 1.82–4.74) when one of the studies was discarded24 (Figure 7).
Figure 7.
Sensitivity analyses. (A) miR-372. (B) miR-373.
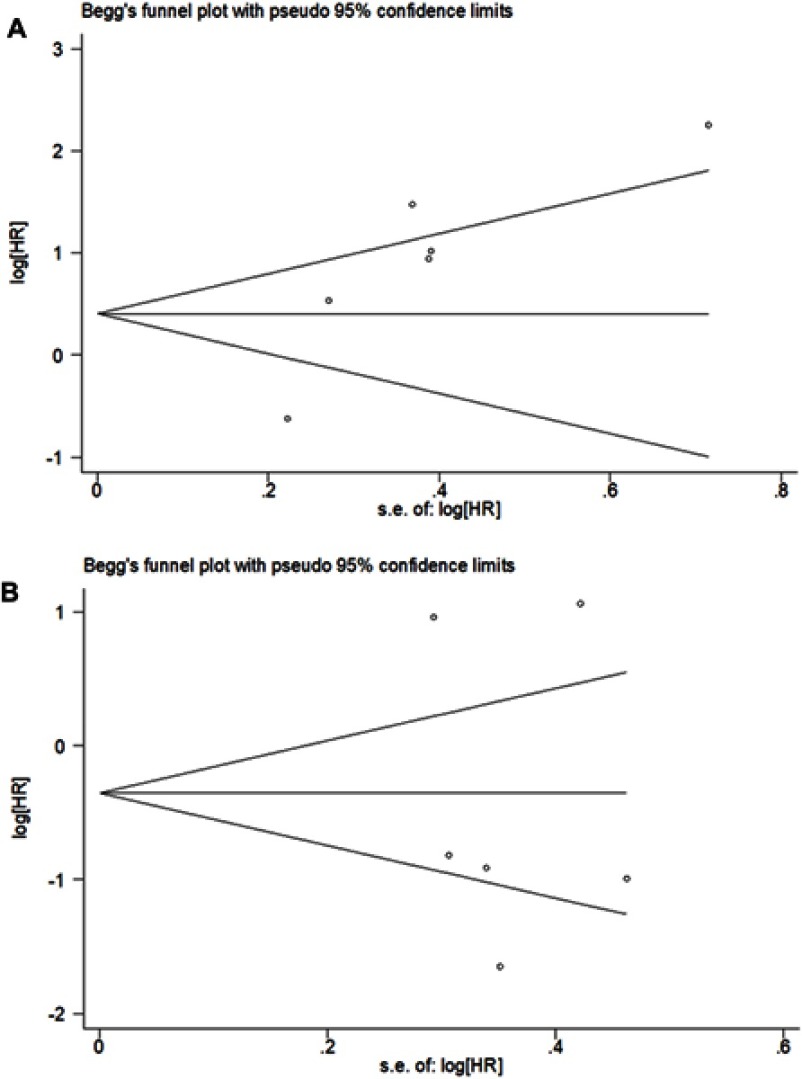
Egger’s and Begg’s tests were utilized to evaluate publication bias of studies. As the figure shows, the symmetric funnel plots indicated that there were no significant publication biases for the miR-373 (t=−0.28, P=0.792), but for miR-372, publication biases were existent (t=3.33, P=0.029) (Figure 8).
Figure 8.
Publication bias analysis. (A) miR-372. (B) miR-373.
Discussion
This study included 16 studies devoted to exploring the diagnostic and prognostic value of miR-371–3 cluster for cancers, and the pooled result showed that the members of miR-371–3 cluster could serve as cancer diagnosis biomarkers and prognostic indicators.
We observed miR-371 and miR-373 have diagnostic value for cancer based on pooled results of seven studies. In fact, studies have verified that miR-371–3 cluster could be used as a biomarker for cancer screening when combined with other miRNAs.Razzak et al34 and Kim et al35 reported that a panel of three miRNAs (mir-21, mir-210, mir-372) have sensitivity and specificity of 67% and 90%, respectively; and that five miRNAs (miR-21, miR-143, miR-155, miR-210, and miR-372) yielded a diagnostic sensitivity of 85.7% and specificity of 100% for early NSCLC detecting. Similarly, in breast cancer, it has been reported that, compared with miR-373, the combination of miR-373 and miR-10b could elevate the sensitivity and specificity from 68% and 89% to 72% and 94.3%,21 and, in germ cell tumors detection, a panel of miR-372-3p, miR-373-3p and miR-367-3p can increase sensitivity of miR-371a-3p from 88.7% to 92%.16 The result of these studies which are inconsistent with published data suggest that the combination of miR-371–3 cluster could be a favorable diagnostic biomarker for cancers. Recently, some reliable evidence has suggested that miR-371 and miR-373 could affect tumor initiation, cell proliferation and stemness of cancer cells via several complicated pathways. In metastasis-derived tumor initiated cells (TICs), TGFβ receptor 2 (TGFBR2), an identified target of miR-371–3, were repressed and the miR-371–3/TGFBR2/inhibitor of DNA binding 1 signaling pathway showed credible connection to self-renewal of TIC.7 MiR-373 regulated tumor cell proliferation and growth by prohibiting or enhancing multiple target genes expression, including large tumor suppressor homolog 2 (LATS2),12,36 CD44,14 nuclear factor I/B (NFIB),37 and estrogen receptor (ER).38 Moreover, the stemness of CRC cells was enhanced by miR-372/373 via repressing differentiation-related pathways, such as NFκB, MAPK/Erk, and VDR.39
For the predictive value of miR-372 and miR-373, up-regulated miR-372 was associated with poor prognosis and the results were more stable after omitting one study with ISH method. The pooled results indicated that there was no significant association between miR-373 and cancer patient survival (OS/RFS/PFS/DFS). However, increased miR-373 was associated with favorable OS based on the Asian population from four studies; the opposite results of the excepted study may be due to the unbalanced proportion of sex ratio (male/female: 47/3). We speculated that the prognostic trend about race may be due to the lack of studies reported in Caucasians, and still no study has verified the relationship between the role of miR-372 in cancer prognosis and race differences. MiR-371–3 cluster could influence cancer progress and recurrence rate mainly via the following three points. Firstly, lymph node metastasis is the most common result for tumor recurrence. In invasive cancer cells, overexpression of miR-373 induced by the zinc-dependent factor CREB could not only further down regulate TP53INP1 (nuclear protein), LATS2 or CD44 for breast and pancreatic cancer,14,40 but suppress the large tumor suppressor homolog 2 (LATS2), leading to p53 pathway restrained in TGCTs.41 Secondly, the emergence of drug resistance is a growing problem. Silenced miR-373 which induced by DNA methylation was reported to target RelA and PIK3CA mRNA, contributed to cisplatin sensitivity and tumor growth suppressed in lung cancer.42 Lastly, the tumor microenvironment is an indispensable factor to affect tumor development. Under hypoxia, the expression of miR-373 was increased in breast cancer cell line (MCF-7) and cervical cancer cell line (HeLa), which could lead to disordering of glucose metabolism and apoptotic pathway.43
There was obvious heterogeneity among the published data we included. These opposite results may be due to the difference of detection methods and cutoff value. For example, decreased miR-372 in HCC tissues reported by Wu et al22 was detected by in situ hybridization, while Gu et al29 showed the opposite outcomes by qRT-PCR; meanwhile, miR-372 was down regulated in HepG2 and SMMC7721 cell lines by Wu et al but up-regulated in the same HCC cell lines by Gu et al, who compared with different normal human hepatocyte. The different reference object and study method could result in the different experimental results. As for cutoff value, four of six studies for miR-372 divided the patients into high and low expression group by the median expression of target miRNA and the other two studies selected mean expression of miR-372. For miR-373, half of the six studies chose median expression and the others didn’t mention it. This subjective method of group division may also be one of the reasons for the heterogeneity.
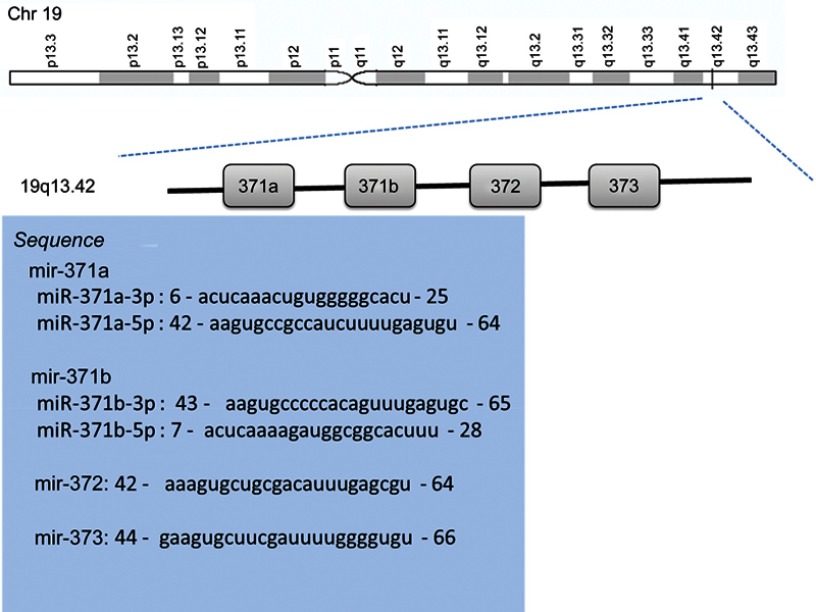
There are several limitations in this article we must to point out. Firstly, the HR and corresponding 95% CIs of two articles for OS which were extracted from survival curves may be inaccurate and have a certain impact on the final results. Secondly, the documents we screened are all in English, and deviations could be caused by the language restrictions. Thirdly, the number of articles included may be too small to summarize the diagnostic and prognostic value of miR-371-3 cluster. Lastly, although human miR-371, miR-372 and miR-373 are clustered within 1.1kb on chromosome 19, several types of microRNA are derived from this cluster, such as miR-371a-3p (previous ID: miR-371-3p), miR-371a-5p (previous ID: miR-371-5p), miR-371b-3p and miR-371b-5p (Figure S1). We combined the results and ignored the differences between miR-371-3p or miR-371-5p because up to date, there have been no studies concerned with the difference of miR-371-3p and miR-371-5p for the diagnostic or prognostic value. But it should be noted whether there are differences between mature miRNA of miR-371–3 cluster members when used as biomarkers, which should be considered by further studies.
Figure S1.
The genomic localization chart and sequences of miR-371-3 cluster.
In short, this study based on the published data suggested that the miR-3713 cluster member (miR-371/miR-373) in serum could be used as diagnostic biomarkers and that miR-372/miR-373 has a potential prognostic value for cancer survival.
Acknowledgments
This project was supported by grants from Key Project of Science and Technology Development of Nanjing Medicine (ZDX16001) to SW; The National Natural Science Foundation of China (No. 81802093) to HS; Innovation Team of Jiangsu Provincial Health-Strengthening Engineering by Science and Education (CXTDB2017008); Jiangsu Youth Medical Talents Training Project to BH (QNRC2016066) and YP (QNRC2016074); grants from Key Project of Science and Technology Development of Nanjing Medicine (ZKX18030) and Jiangsu 333 High-Level Talents Cultivating Project to BH (No. BRA201702).
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Supplementary materials
Table S1.
Characteristics of studies included in the meta-analysis
| First author | Year | miRNA | Ethnicity | Cancer type | Sample type | Method | Cancer grade |
Case (H) |
Control (L) |
|---|---|---|---|---|---|---|---|---|---|
| Liu | 2012 | miR-371-5p | Asian | Gastric cancer | Serum | qRT-PCR | I–IV | 40 | 41 |
| Yamashita | 2012 | miR-372 | Asian | CRC | Tissue | qRT-PCR | \ | 72 | 72 |
| Gu | 2012 | miR-372 | Asian | HCC | Tissue | qRT-PCR | \ | 65 | 43 |
| Li | 2013 | miR-372 | Asian | Gliomas | Tissue | qRT-PCR | \ | 78 | 50 |
| Eichelser | 2013 | miR-373 | Caucasian | Breast cancer | Serum | qRT-PCR | M0 | 120 | 40 |
| M1 | 32 | ||||||||
| Chen | 2013 | miR-373 | Caucasian | Breast ductal carcinoma |
Plasma | qRT-PCR | I–IV | 35 | 25 |
| Syring | 2014 | miR-371-3p | Caucasian | Testicular germ cell cancer |
Serum | qRT-PCR | \ | 59 | 101 |
| Tu | 2015 | miR-372 | Asian | OSCC | Tissue | qRT-PCR | \ | 50 | 50 |
| Tu | 2015 | miR-373 | Asian | OSCC | Tissues | qRT-PCR | \ | 50 | 50 |
| Meng | 2015 | miR-373 | Caucasian | EOC | Serum | qRT-PCR | \ | 47 | 46 |
| Wu | 2015 | miR-372 | Asian | HCC | Tissue | ISH | \ | 33 | 87 |
| Bellmunt | 2016 | miR-372 | Caucasian | UC | Tissue | qRT-PCR | \ | 40 | 40 |
| Dieckmann | 2017 | miR-371-3p | Caucasian | Germ cell tumours | Serum | qRT-PCR | CS1 | 107 | 106 |
| CS2-CS3 | 43 | ||||||||
| Hua | 2017 | miR-373 | Asian | Pancreatic cancer | Serum | qRT-PCR | I–IV | 103 | 50 |
| Zhang | 2017 | miR-373 | Asian | BCa | Tissues | qRT-PCR | \ | 40 | 40 |
| Hua | 2017 | miR-373 | Asian | Pancreatic cancer | Serum | qRT-PCR | \ | 50 | 53 |
| Jing | 2017 | miR-373 | Asian | Gliomas | Tissues | qRT-PCR | \ | 83 | 87 |
| Wang | 2017 | miR-373 | Asian | NSCLC | Tissues | qRT-PCR | \ | 46 | 46 |
Note: M0, patients with primary breast cancer after surgery and before chemotherapy; M1, patients with distant metastases at diagnosis (We roughly incorporated M0 into the early stage, and correspondingly incorporate M1 into the late stage).
Abbreviations: qRT-PCR, quantitative real-time PCR; H, high expression; L, low expression; CS, clinical stage; UC, urothelial carcinoma; OSCC, oral squamous cell carcinoma; HCC, hepatocellular carcinoma; CRC, colorectal cancer; BCa, bladder cancer; NSCLC, non-small cell lung cancer; EOC, epithelial ovarian cancer.
References
- 1.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small rnas with antisense complementarity to lin-14. Cell. 1993;75:843–854. [DOI] [PubMed] [Google Scholar]
- 2.Carthew RW, Sontheimer EJ. Origins and mechanisms of mirnas and sirnas. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bracken CP, Scott HS, Goodall GJ. A network-biology perspective of microrna function and dysfunction in cancer. Nat Rev Genet. 2016;17:719–732. doi: 10.1038/nrg.2016.134 [DOI] [PubMed] [Google Scholar]
- 4.Bragato JP, Melo LM, Venturin GL, Rebech GT, Garcia LE, Lopes FL. Relationship of peripheral blood mononuclear cells mirna expression and parasitic load in canine visceral leishmaniasis. PloS One 2018;13:e0206876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwardson MA. Plasma microrna markers of upper limb recovery following human stroke. PLoS One. 2018;8:12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang W, Wang Y. Serum mir-200c and mir-371-5p as the useful diagnostic biomarkers and therapeutic targets in Kawasaki disease. BioMed Res Int. 2017;2017:8257862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ullmann P, Rodriguez F, Schmitz M, et al. The mir-371 approximately 373 cluster represses colon cancer initiation and metastatic colonization by inhibiting the tgfbr2/id1 signaling axis. Cancer Res 2018;78:3793–3808. doi: 10.1158/0008-5472.CAN-17-3003 [DOI] [PubMed] [Google Scholar]
- 8.Sahu N, Stephan JP, Cruz DD, et al. Functional screening implicates mir-371-3p and peroxiredoxin 6 in reversible tolerance to cancer drugs. Nat Commun. 2016;7:12351. doi: 10.1038/ncomms12351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haan S, Letellier E, Guo H, et al. Microrna-371a-3p promotes progression of gastric cancer by targeting tob1. Cancer Res. 2019;443:179–188. [DOI] [PubMed] [Google Scholar]
- 10.Classon M, Settleman J, Ullmann P, et al. The mir-371 approximately 373 cluster represses colon cancer initiation and metastatic colonization by inhibiting the tgfbr2/id1 signaling axis. Nat Commun. 2018;78:3793–3808. [DOI] [PubMed] [Google Scholar]
- 11.Murray MJ, Huddart RA, Coleman N. The present and future of serum diagnostic tests for testicular germ cell tumours. Nat Rev Urol. 2016;13:715–725. doi: 10.1038/nrurol.2016.170 [DOI] [PubMed] [Google Scholar]
- 12.Voorhoeve PM, le Sage C, Schrier M, et al. A genetic screen implicates mirna-372 and mirna-373 as oncogenes in testicular germ cell tumors. Cell. 2006;124:1169–1181. doi: 10.1016/j.cell.2006.02.037 [DOI] [PubMed] [Google Scholar]
- 13.Huang X, Huang M, Kong L, Li Y. Mir-372 suppresses tumour proliferation and invasion by targeting igf2bp1 in renal cell carcinoma. Cell Prolif. 2015;48:593–599. doi: 10.1111/cpr.12207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Q, Gumireddy K, Schrier M, et al. The micrornas mir-373 and mir-520c promote tumour invasion and metastasis. Nat Cell Biol. 2008;10:202–210. doi: 10.1038/ncb1681 [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Qu J, Zhou L, Liao F, Wang J. Microrna-373 inhibits cell proliferation and invasion via targeting brf2 in human non-small cell lung cancer a549 cell line. Cancer Res Treat. 2018;50(3):936–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dieckmann KP, Radtke A, Spiekermann M, et al. Serum levels of microrna mir-371a-3p: A sensitive and specific new biomarker for germ cell tumours. Eur Urol. 2017;71:213–220. doi: 10.1016/j.eururo.2016.07.029 [DOI] [PubMed] [Google Scholar]
- 17.Syring I, Bartels J, Holdenrieder S, Kristiansen G, Muller SC, Ellinger J. Circulating serum mirna (mir-367-3p, mir-371a-3p, mir-372-3p and mir-373-3p) as biomarkers in patients with testicular germ cell cancer. J Urol. 2015;193:331–337. doi: 10.1016/j.juro.2014.07.010 [DOI] [PubMed] [Google Scholar]
- 18.Liu H, Zhu L, Liu B, et al. Genome-wide microrna profiles identify mir-378 as a serum biomarker for early detection of gastric cancer. Cancer Lett. 2012;316:196–203. doi: 10.1016/j.canlet.2011.10.034 [DOI] [PubMed] [Google Scholar]
- 19.Hua Y, Chen H, Wang L, et al. Low serum mir-373 predicts poor prognosis in patients with pancreatic cancer. Cancer Biomarkers. 2017;20:95–100. doi: 10.3233/CBM-170231 [DOI] [PubMed] [Google Scholar]
- 20.Eichelser C, Flesch-Janys D, Chang-Claude J, Pantel K, Schwarzenbach H. Deregulated serum concentrations of circulating cell-free micrornas mir-17, mir-34a, mir-155, and mir-373 in human breast cancer development and progression. Clin Chem. 2013;59:1489–1496. doi: 10.1373/clinchem.2013.205161 [DOI] [PubMed] [Google Scholar]
- 21.Chen W, Cai F, Zhang B, Barekati Z, Zhong XY. The level of circulating mirna-10b and mirna-373 in detecting lymph node metastasis of breast cancer: potential biomarkers. Tumour Biol. 2013;34:455–462. doi: 10.1007/s13277-012-0570-5 [DOI] [PubMed] [Google Scholar]
- 22.Wu G, Wang Y, Lu X, et al. Low mir-372 expression correlates with poor prognosis and tumor metastasis in hepatocellular carcinoma. BMC Cancer. 2015;15:182. doi: 10.1186/s12885-015-1584-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Q, Wang C, Miao S, Li C, Chen Z, Li F. Enhancing e-cadherin expression via promoter-targeted mir-373 suppresses bladder cancer cells growth and metastasis. Oncotarget. 2017;8:93969–93983. doi: 10.18632/oncotarget.21400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jing SY, Jing SQ, Liu LL, Xu LF, Zhang F, Gao JL. Down-expression of mir-373 predicts poor prognosis of glioma and could be a potential therapeutic target. Eur Rev Med Pharmacol Sci. 2017;21:2421–2425. [PubMed] [Google Scholar]
- 25.Bellmunt J, Zhou CW, Mullane SA, et al. Association of tumour microrna profiling with outcomes in patients with advanced urothelial carcinoma receiving first-line platinum-based chemotherapy. Br J Cancer. 2016;115:12–19. doi: 10.1038/bjc.2016.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tu HF, Chang KW, Cheng HW, Liu CJ. Upregulation of mir-372 and −373 associates with lymph node metastasis and poor prognosis of oral carcinomas. Laryngoscope. 2015;125:E365–370. doi: 10.1002/lary.25464 [DOI] [PubMed] [Google Scholar]
- 27.Li G, Zhang Z, Tu Y, et al. Correlation of microrna-372 upregulation with poor prognosis in human glioma. Diagn Pathol. 2013;8:1. doi: 10.1186/1746-1596-8-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamashita S, Yamamoto H, Mimori K, et al. Microrna-372 is associated with poor prognosis in colorectal cancer. Oncology. 2012;82:205–212. doi: 10.1159/000336809 [DOI] [PubMed] [Google Scholar]
- 29.Gu H, Guo X, Zou L, Zhu H, Zhang J. Upregulation of microrna-372 associates with tumor progression and prognosis in hepatocellular carcinoma. Mol Cell Biochem. 2013;375:23–30. doi: 10.1007/s11010-012-1521-6 [DOI] [PubMed] [Google Scholar]
- 30.Meng X, Pantel MV, Schwarzenbach H. Diagnostic and prognostic relevance of circulating exosomal mir-373, mir-200a, mir-200b and mir-200c in patients with epithelial ovarian cancer. Oncotarget. 2016. doi: 10.18632/oncotarget.7850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahid SS, Hornung CA, Minor KS, Turina M, Galandiuk S. Systematic reviews and meta-analysis for the surgeon scientist. Br J Surg. 2006;93:1315–1324. doi: 10.1002/bjs.5596 [DOI] [PubMed] [Google Scholar]
- 33.Stang A. Critical evaluation of the newcastle-ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 34.Razzak R, Bedard EL, Kim JO, et al. Microrna expression profiling of sputum for the detection of early and locally advanced non-small-cell lung cancer: A prospective case-control study. Current Oncol. 2016;23:e86–94. doi: 10.3747/co.23.2830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim JO, Gazala S, Razzak R, et al. Non-small cell lung cancer detection using microrna expression profiling of bronchoalveolar lavage fluid and sputum. Anticancer Res. 2015;35:1873–1880. [PubMed] [Google Scholar]
- 36.Lee KH, Goan YG, Hsiao M, et al. Microrna-373 (mir-373) post-transcriptionally regulates large tumor suppressor, homolog 2 (lats2) and stimulates proliferation in human esophageal cancer. Exp Cell Res. 2009;315:2529–2538. doi: 10.1016/j.yexcr.2009.06.001 [DOI] [PubMed] [Google Scholar]
- 37.Guo H, Liu H, Mitchelson K, et al. Micrornas-372/373 promote the expression of hepatitis b virus through the targeting of nuclear factor i/b. Hepatology. 2011;54:808–819. doi: 10.1002/hep.24441 [DOI] [PubMed] [Google Scholar]
- 38.Eichelser C, Stuckrath I, Muller V, et al. Increased serum levels of circulating exosomal microrna-373 in receptor-negative breast cancer patients. Oncotarget. 2014;5:9650–9663. doi: 10.18632/oncotarget.2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang LQ, Yu P, Li B, et al. Mir-372 and mir-373 enhance the stemness of colorectal cancer cells by repressing differentiation signaling pathways. Mol Oncol. 2018;12:1949–1964. doi: 10.1002/1878-0261.12376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Yang J, Cui X, et al. A novel epigenetic creb-mir-373 axis mediates zip4-induced pancreatic cancer growth. EMBO Mol Med. 2013;5:1322–1334. doi: 10.1002/emmm.201302507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei F, Cao C, Xu X, Wang J. Diverse functions of mir-373 in cancer. J Transl Med. 2015;13:162. doi: 10.1186/s12967-015-0541-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adi Harel S, Bossel Ben-Moshe N, Aylon Y, et al. Reactivation of epigenetically silenced mir-512 and mir-373 sensitizes lung cancer cells to cisplatin and restricts tumor growth. Cell Death Differ. 2015;22:1328–1340. doi: 10.1038/cdd.2014.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crosby ME, Kulshreshtha R, Ivan M, Glazer PM. Microrna regulation of DNA repair gene expression in hypoxic stress. Cancer Res. 2009;69:1221–1229. doi: 10.1158/0008-5472.CAN-08-2516 [DOI] [PMC free article] [PubMed] [Google Scholar]