Key Points
Question
What are the survival outcomes and costs associated with both radical cystectomy and trimodal therapy for older adults with muscle-invasive bladder cancer?
Findings
In this population-based cohort study of Surveillance, Epidemiology, and End Results–Medicare data from 3200 older adults with a clinical stage T2 to T4a bladder cancer diagnosis, patients who underwent trimodal therapy had significantly decreased overall and cancer-specific survival. The median total costs were substantially higher for trimodal therapy than for radical cystectomy ($827 million vs $492 million) for patients diagnosed in 2011.
Meaning
Compared with radical cystectomy, trimodal therapy was associated with significantly lower overall and cancer-specific survival rates at significantly higher costs.
Abstract
Importance
Radical cystectomy is the guidelines-recommended treatment of muscle-invasive bladder cancer, but a resurgence of trimodal therapy has occurred. Limited comparative data are available on outcomes and costs attributable to these 2 treatments.
Objective
To compare the survival outcomes and costs between trimodal therapy and radical cystectomy in older adults with muscle-invasive bladder cancer.
Design, Setting, and Participants
This population-based cohort study used data from the Surveillance, Epidemiology, and End Results–Medicare linked database. A total of 3200 older adults (aged ≥66 years) with clinical stage T2 to T4a bladder cancer diagnosed from January 1, 2002, to December 31, 2011, and with claims data available through December 31, 2013, were included in the analysis. Patients who received radical cystectomy underwent either only surgery or surgery in combination with radiotherapy or chemotherapy. Patients who received trimodal therapy underwent transurethral resection of the bladder followed by radiotherapy and chemotherapy. Propensity score matching by sociodemographic and clinical characteristics was used. Data analysis was performed from August 1, 2017, to March 11, 2018.
Main Outcomes and Measures
Overall survival and cancer-specific survival were evaluated using the Cox proportional hazards regression model and the Fine and Gray competing risk model. All Medicare health care costs for inpatient, outpatient, and physician services within 30, 90, and 180 days of treatment were compared. The total amount spent nationwide was estimated, using 180-day medical costs between treatments, by the total number of new cases of muscle-invasive bladder cancer in the United States in 2011.
Results
Of the 3200 patients who met the inclusion criteria, 2048 (64.0%) were men and 1152 (36.0%) were women, with a mean (SD) age of 75.8 (6.0) years. After propensity score matching, 687 patients (21.5%) underwent trimodal therapy and 687 patients (21.5%) underwent radical cystectomy. Patients who underwent trimodal therapy had significantly decreased overall survival (hazard ratio [HR], 1.49; 95% CI, 1.31-1.69) and cancer-specific survival (HR, 1.55; 95% CI, 1.32-1.83). No differences in costs at 30 days were observed between trimodal therapy ($15 233 in 2002 vs $18 743 in 2011) and radical cystectomy ($17 990 in 2002 vs $21 738 in 2011). However, median total costs were significantly higher with trimodal therapy than with radical cystectomy at 90 days ($80 174 vs $69 181; median difference, $8964; Hodges-Lehmann 95% CI, $3848-$14 079) and at 180 days ($179 891 vs $107 017; median difference, $63 771; Hodges-Lehmann 95% CI, $55 512-$72 029). Extrapolating these figures to the total US population revealed $335 million in excess spending for trimodal therapy compared with the less costly radical cystectomy ($492 million) for patients who received a muscle-invasive bladder cancer diagnosis in 2011.
Conclusions and Relevance
Trimodal therapy was associated with significantly decreased overall survival and cancer-specific survival as well as $335 million in excess spending in 2011. These findings have important health policy implications regarding the appropriate use of high value–based care among older adults with invasive bladder cancer who are candidates for either radical cystectomy or trimodal therapy.
This population-based study uses data from the Surveillance, Epidemiology, and End Results (SEER)–Medicare linked database to identify and compare the survival rates and costs attributed to muscle-invasive bladder cancer in older US adults.
Introduction
An estimated 81 190 new cases and 17 240 deaths from bladder cancer will occur in the United States in 2018.1 Neoadjuvant chemotherapy followed by radical cystectomy with extended pelvic lymphadenectomy is the guidelines-recommended treatment of muscle-invasive bladder cancer.2,3,4 Given the concerns regarding the nonnegligible morbidity and mortality associated with radical cystectomy along with patients often being older and having increased comorbidities, clinicians (eg, urologists, radiation and medical oncologists) and patients have sought alternative treatments.
The use of less-invasive trimodal “bladder-sparing” approaches that combine maximal transurethral resection, chemotherapy, and radiotherapy to treat muscle-invasive bladder cancer has increased.5 Several organizations, including the American Urological Association and the European Association of Urology, have updated their guidelines to support the use of radiotherapy combined with chemotherapy in select patients with muscle-invasive disease.3,4,6,7 No randomized data exist comparing trimodal therapy with radical cystectomy, but 2 single-center studies to date have noted comparable survival outcomes.8,9 These studies were limited by small numbers of patients and/or were derived from nonadjusted case-control series. Comparative effectiveness research using cancer registry data has reported conflicting overall survival outcomes between these 2 treatments.10 Against this backdrop, recent large population-based studies using the National Cancer Database have reported trimodal therapy to have inferior overall survival outcomes when compared with radical cystectomy.5,11 Furthermore, the costs associated with these treatments remain to be elucidated.12 Given this gap in the literature, we examined a nationally representative cohort to compare the survival outcomes and costs of radical cystectomy with those of trimodal therapy.
Methods
Data Source
We extracted data from the Surveillance, Epidemiology, and End Results (SEER)–Medicare linked database. This data set includes information regarding newly diagnosed cancers with 98% case ascertainment from 18 US regions.13 The institutional review board at The University of Texas Medical Branch, Galveston, deemed this study to be exempt from review because it used an administrative deidentified database. Patient informed consent was waived by this institutional review board. We performed data analysis from August 1, 2017, to March 11, 2018.
Ascertainment of Study Cohort
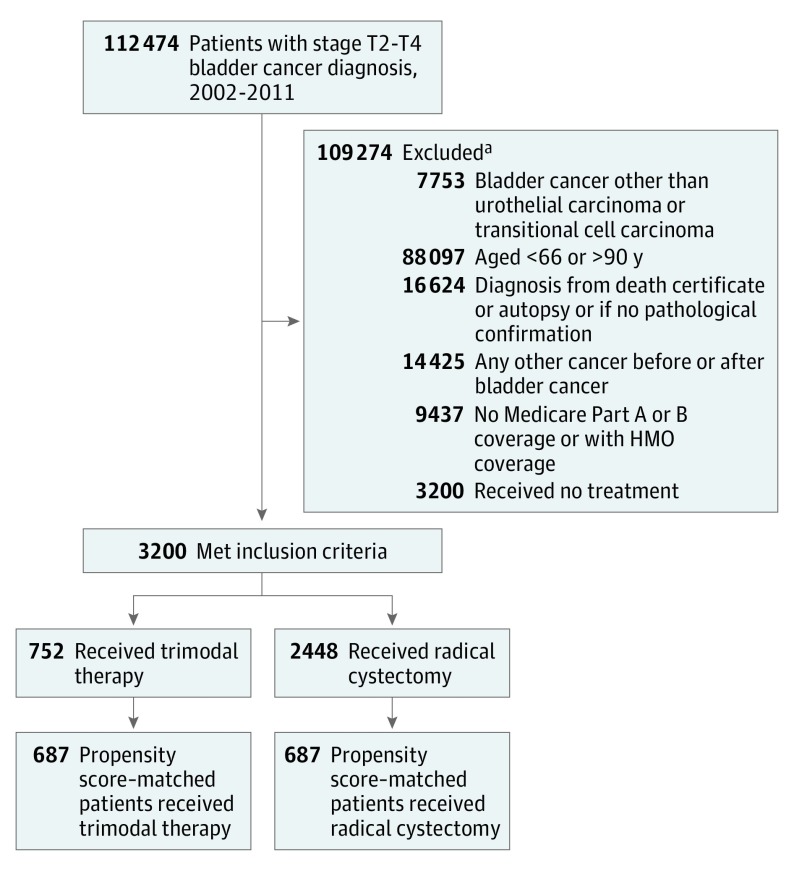
We restricted our analysis to patients with stage T2 to T4a bladder cancer that was diagnosed as either transitional cell or urothelial carcinoma between January 1, 2002, and December 31, 2011, and with claims data available through December 31, 2013. The study was restricted to Medicare fee-for-service beneficiaries with Medicare Part A and Part B claims data available. The final cohort consisted of 3200 patients (Figure 1).
Figure 1. Patient Selection Process.
HMO indicates health maintenance organization.
aSome patients met more than 1 exclusion criterion.
Identification of Bladder Cancer Treatments
Radical cystectomy was identified by procedure codes in Medicare claims, including for both open and robot-assisted laparoscopic surgical procedures with or without pelvic lymph node dissection. The radical cystectomy group comprised patients who underwent only surgery or surgery in combination with radiotherapy or chemotherapy. The trimodal therapy group consisted of patients who underwent transurethral resection of the bladder followed by radiotherapy and chemotherapy. Trimodal therapy was identified by diagnosis and procedure codes in Medicare claims for both radiotherapy and chemotherapy in the absence of a concomitant code for radical cystectomy.14 Radiotherapy dose typically consists of 60 to 66 Gy (39.6-50.4 Gy delivered to the bladder and pelvic lymph nodes with a sequential tumor boost) given in daily fractions of 1.8 to 2.0 Gy.3 Guidelines-recommended trimodal chemotherapy regimens include cisplatin or fluorouracil and mitomycin C.4 We counted the number of fractions within 90 days after the first radiation treatment and defined the number of fractions according to the number of radiation treatments delivered, as billed in Medicare claims, during the initial outpatient course of radiotherapy.15
Study Covariates
From the SEER database, we determined patient age, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, or non-Hispanic other race/ethnicity), marital status (single, married, or unknown), and US census region (Northeast, South, Midwest, or West). We obtained the socioeconomic characteristics of the patient’s neighborhood from the SEER database. Educational level was specified according to the percentage of residents who had at least 4 years of college education. County-level median annual household income was acquired through a link to the Area Health Resource Files data and then divided into quartiles. Comorbidity was assessed using the Klabunde modification for the year before cancer diagnosis.16
To determine treatment costs, we summed all Medicare health care expenditures from inpatient, outpatient, and physician services within 30, 90, and 180 days of treatment. We estimated the total amount spent nationwide on more expensive trimodal therapy by multiplying the mean differences of 180-day medical costs between trimodal therapy and radical cystectomy by the total number of new cases of muscle-invasive bladder cancer in the United States in 2011.17 All costs were inflated to 2017 dollars using previously established methods summing Medicare reimbursements, coinsurance reimbursements, and patient liability costs.12,18
Statistical Analysis
Patients who receive radical cystectomy may systematically differ from patients who receive trimodal therapy. For example, patients who are older, have greater comorbidities, and have a lower stage of cancer may be more likely to receive trimodal therapy. Propensity score matching acts as a pseudorandomization and makes 2 treatment groups comparable. Therefore, we used propensity score matching to control selection bias and confounding while comparing 2 treatments. The propensity score was derived from a multivariable logistic regression model that estimated the use of radical cystectomy. Reflecting previous research and clinical knowledge, we included patients’ sociodemographic (age, sex, race/ethnicity, annual household income, educational level, marital status, and region) and clinical characteristics (comorbidities, stage, and grade) in the propensity score model.14,19 All independent variables were parameterized, as illustrated in Table 1. We checked collinearity between educational level and income and the interaction among comorbidities, stage, and grade while building the propensity score model. Collinearity was important only for median annual household income and educational level. No notable interactions were observed. We performed 1:1 matching of patients undergoing radical cystectomy with patients undergoing trimodal therapy, without replacement, based on a 5-digit greedy matching algorithm.20 Standardized difference was used to assess the covariate balance; an absolute standardized difference less than 0.10 indicates a balance of covariates across the 2 groups.
Table 1. Patient Demographics and Clinical Characteristics Before and After Propensity Score Matching.
| Variable | Total | Treatment Before Matching | Standardized Difference | Treatment After Matching | Standardized Difference | ||
|---|---|---|---|---|---|---|---|
| Radical Cystectomy, No. (%) | Trimodal Therapy, No. (%) | Radical Cystectomy, No. (%) | Trimodal Therapy, No. (%) | ||||
| All patients | 3200 | 2448 (76.5) | 752 (23.5) | 687 (50.0) | 687 (50.0) | ||
| Age group, y | |||||||
| 66-69 | 588 | 498 (20.3) | 90 (12.0) | −0.229 | 84 (12.2) | 90 (13.1) | 0.026 |
| 70-74 | 802 | 674 (27.5) | 128 (17.0) | −0.255 | 139 (20.2) | 127 (18.5) | −0.044 |
| 75-79 | 864 | 675 (27.6) | 189 (25.1) | −0.055 | 186 (27.1) | 185 (26.9) | −0.003 |
| ≥80 | 946 | 601 (24.6) | 345 (45.9) | 0.458 | 278 (40.5) | 285 (41.5) | 0.021 |
| Sex | |||||||
| Male | 2048 | 1516 (61.9) | 532 (70.7) | 0.187 | 462 (67.3) | 475 (69.1) | 0.041 |
| Female | 1152 | 932 (38.1) | 220 (29.3) | −0.187 | 225 (32.8) | 212 (30.9) | −0.041 |
| Race/ethnicity | |||||||
| White | 2802 | 2136 (87.3) | 666 (88.6) | 0.040 | 600 (87.3) | 606 (88.2) | 0.027 |
| Black | 138 | 103 (4.2) | 35 (4.7) | 0.022 | 31 (4.5) | 32 (4.7) | 0.007 |
| Hispanic | 109 | 88 (3.6) | 21 (2.8) | −0.046 | 30 (4.4) | 21 (3.1) | −0.069 |
| Other | 151 | 121 (4.9) | 30 (4.0) | −0.046 | 26 (3.8) | 28 (4.1) | 0.015 |
| Marital status | |||||||
| Single | 441 | 341 (13.9) | 100 (13.3) | −0.018 | 93 (13.5) | 94 (13.7) | 0.004 |
| Married | 1920 | 1501 (61.3) | 419 (55.7) | −0.114 | 384 (55.9) | 396 (57.6) | 0.035 |
| Unknown | 839 | 606 (24.8) | 233 (31.0) | 0.139 | 210 (30.6) | 197 (28.7) | −0.042 |
| Census region | |||||||
| West | 1317 | 1009 (41.2) | 308 (41.0) | −0.005 | 271 (39.5) | 280 (40.8) | 0.027 |
| Northeast | 727 | 558 (22.8) | 169 (22.5) | −0.008 | 165 (24.0) | 154 (22.4) | −0.038 |
| Midwest | 373 | 276 (11.3) | 97 (12.9) | 0.050 | 79 (11.5) | 85 (12.4) | 0.027 |
| South | 783 | 605 (24.7) | 178 (23.7) | −0.024 | 172 (25.0) | 168 (24.5) | −0.014 |
| Median annual household income, USD | |||||||
| ≤42 992 | 706 | 530 (21.7) | 176 (23.4) | 0.042 | 147 (21.4) | 157 (22.9) | 0.035 |
| 42 993-56 188 | 802 | 626 (25.6) | 176 (23.4) | −0.050 | 161 (23.4) | 164 (23.9) | 0.010 |
| 56 189-73 827 | 825 | 608 (24.8) | 217 (28.9) | 0.091 | 201 (29.3) | 194 (28.2) | −0.023 |
| ≥73 828 | 867 | 684 (27.9) | 183 (24.3) | −0.082 | 178 (25.9) | 172 (25.0) | −0.020 |
| Educational level, %a | |||||||
| ≤20.58 | 880 | 681 (27.8) | 199 (26.5) | −0.031 | 188 (27.4) | 181 (26.4) | −0.023 |
| 20.59-27.36 | 796 | 608 (24.8) | 188 (25.0) | 0.004 | 173 (25.2) | 174 (25.3) | 0.003 |
| 27.37-34.83 | 742 | 546 (22.3) | 196 (26.1) | 0.088 | 179 (26.1) | 174 (25.3) | −0.017 |
| ≥34.84 | 782 | 613 (25.0) | 169 (22.5) | −0.060 | 147 (21.4) | 158 (23.0) | 0.039 |
| Comorbidity, No. | |||||||
| 0 | 1739 | 1385 (56.6) | 354 (47.1) | −0.191 | 328 (47.7) | 339 (49.3) | 0.032 |
| 1 | 852 | 646 (26.4) | 206 (27.4) | 0.023 | 184 (26.8) | 188 (27.4) | 0.013 |
| 2 | 344 | 240 (9.8) | 104 (13.8) | 0.125 | 87 (12.7) | 85 (12.4) | −0.009 |
| ≥3 | 265 | 177 (7.2) | 88 (11.7) | 0.153 | 88 (12.8 | 75 (10.9) | −0.059 |
| Cancer stage | |||||||
| II | 1498 | 966 (39.5) | 532 (70.7) | 0.663 | 470 (68.4) | 468 (68.1) | −0.006 |
| III | 857 | 761 (31.1) | 96 (12.8) | −0.454 | 98 (14.3) | 96 (14.0) | −0.008 |
| IV | 845 | 721 (29.5) | 124 (16.5) | −0.312 | 119 (17.3) | 123 (17.9) | 0.015 |
| Cancer grade | |||||||
| Low | 129 | 99 (4.0) | 30 (4.0) | −0.003 | 26 (3.8) | 28 (4.1) | 0.015 |
| High | 2967 | 2288 (93.5) | 679 (90.3) | −0.116 | 626 (91.1) | 624 (90.8) | −0.010 |
| Unknown | 104 | 61 (2.5) | 43 (5.7) | 0.163 | 35 (5.1) | 35 (5.1) | 0 |
| Year of diagnosis | |||||||
| 2002 | 320 | 256 (10.5) | 64 (8.5) | −0.067 | 63 (9.2) | 62 (9.0) | −0.005 |
| 2003 | 321 | 262 (10.7) | 59 (7.8) | −0.099 | 52 (7.6) | 58 (8.4) | 0.032 |
| 2004 | 352 | 274 (11.2) | 78 (10.4) | −0.027 | 65 (9.5) | 70 (10.2) | 0.025 |
| 2005 | 367 | 282 (11.5) | 85 (11.3) | −0.007 | 84 (12.2) | 79 (11.5) | −0.023 |
| 2006 | 336 | 243 (9.9) | 93 (12.4) | 0.078 | 78 (11.4) | 81 (11.8) | 0.014 |
| 2007 | 304 | 236 (9.6) | 68 (9.0) | −0.021 | 55 (8.0) | 65 (9.5) | 0.052 |
| 2008 | 317 | 245 (10.0) | 72 (9.6) | −0.015 | 77 (11.2) | 64 (9.3) | −0.062 |
| 2009 | 286 | 216 (8.8) | 70 (9.3) | 0.017 | 67 (9.8) | 64 (9.3) | −0.015 |
| 2010 | 308 | 225 (9.2) | 83 (11.0) | 0.061 | 74 (10.8) | 71 (10.3) | −0.014 |
| 2011 | 289 | 209 (8.5) | 80 (10.6) | 0.071 | 72 (10.5) | 73 (10.6) | 0.005 |
Abbreviation: USD, US dollar.
Educational level was specified according to the percentage of residents who had at least 4 years of college education.
All outcomes were evaluated in the matched sample. Unadjusted Kaplan-Meier curves were generated to estimate the overall survival and bladder cancer–specific survival for trimodal therapy and radical cystectomy. A Cox proportional hazards regression model was used to assess the association of treatment with overall survival. In the Cox model, we used a robust variance estimator to account for the clustering within matched sets. We conducted competing risk analyses to compare cancer-specific survival between trimodal therapy and radical cystectomy. The Fine and Gray21 competing risk model was used to obtain hazard ratios (HRs). The proportional hazards model assumption was tested to confirm the adequacy of the model. Because of the skewed nature of cost data, we reported median costs. The differences between overall and yearly median costs were evaluated with the Hodges-Lehmann estimator in the propensity score–matched sample.
Propensity score matching effectively reduces the sample size because unmatched patients are removed from the analyses. Therefore, we performed sensitivity analyses by including all patients. The association of bladder cancer treatment with overall survival and cancer-specific survival was modeled using the Cox proportional hazards regression model and competing risk model while accounting for all confounders.
All statistical tests were 2-sided, and all analyses were performed using SAS, version 9.4 (SAS Institute Inc). Statistical significance was defined as a 2-sided P < .05 using an unpaired, 2-tailed t test.
Results
Patient demographics according to treatment type before and after propensity score adjustments are summarized in Table 1. Of the 3200 patients who met the inclusion criteria, 2048 (64.0%) were men and 1152 (36.0%) were women, with a mean (SD) age of 75.8 (6.0) years. Overall, 752 patients (23.5%) underwent trimodal therapy and 2448 (76.5%) underwent radical cystectomy for muscle-invasive bladder cancer. Before matching, age, sex, and marital status as well as Charlson comorbidity index, stage, and grade according to treatments all had an SD greater than 0.10. Patients with high-grade disease more often underwent radical cystectomy, whereas patients with clinical stage T2 cancer more often underwent trimodal therapy (SD, >0.10).
Propensity score matching resulted in the matching of 687 patients (21.5%) receiving trimodal therapy with 687 patients (21.5%) receiving radical cystectomy (Figure 1). All baseline covariates were well balanced in the propensity score–matched sample. Multicollinearity was assessed for variables introduced into the model. The only substantial association observed was between annual household income and educational level. We removed educational level because of its high association with income. Tests for effect modification were performed for the following interaction terms: comorbidity with stage, comorbidity with grade, and stage with grade. No notable interactions were observed. The concordance index for the model was 0.74.
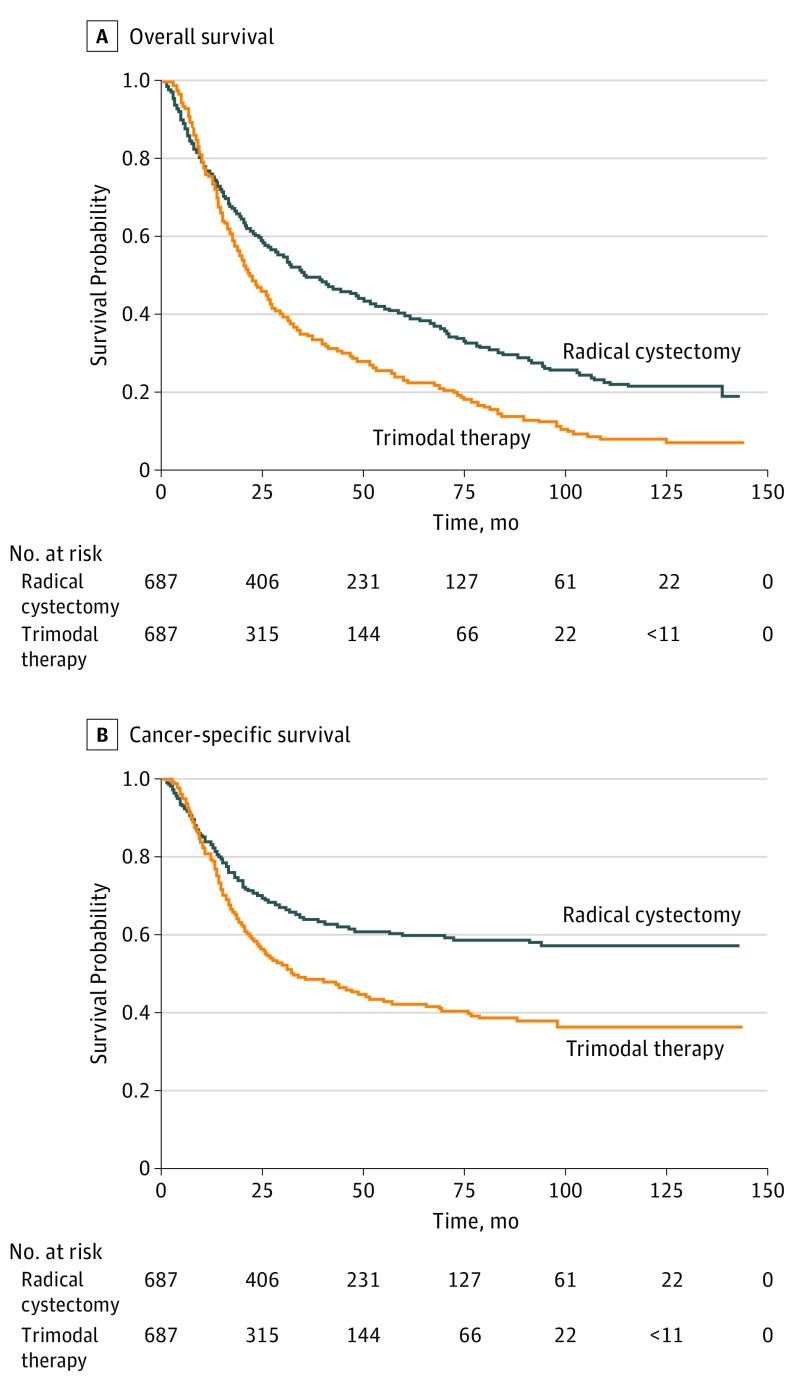
Patients who underwent trimodal therapy had significantly decreased overall survival (HR, 1.49; 95% CI, 1.31-1.69) and cancer-specific survival (HR, 1.55; 95% CI, 1.32-1.83) (Table 2). These findings persisted across all stages except in patients with stage IV disease, for whom no considerable differences between treatments were noted (eTable 1 in the Supplement). In sensitivity analyses, the effect estimates of trimodal therapy with overall survival (HR, 1.38; 95% CI, 1.25-1.53) and cancer-specific survival (HR, 1.50; 95% CI, 1.32-1.70) differed slightly compared with the effect estimates in propensity score analyses. However, the association was in the same direction, indicating improved outcomes associated with radical cystectomy. A total of 353 of 687 patients (51.4%) who underwent trimodal therapy received cisplatin or fluorouracil and mitomycin C. Although trimodal therapy resulted in worse survival when compared with radical cystectomy, this treatment with cisplatin or fluorouracil and mitomycin C vs with other chemotherapy resulted in improved overall survival (HR, 1.27; 95% CI, 1.10-1.47 vs HR, 1.69; 95% CI, 1.46-1.96) and cancer-specific survival (HR, 1.50; 95% CI, 1.23-1.83 vs HR, 1.97; 95% CI, 1.62-2.39) (eTable 2 in the Supplement). Among 326 patients who underwent trimodal therapy and had radiotherapy fraction data available, the total median number of fractions delivered was 18 (interquartile range, 9-27). When stratified by fractions delivered, trimodal therapy with 18 or more total fractions delivered compared with fewer than 18 showed improved overall survival (HR, 1.23; 95% CI, 1.02-1.48 vs HR, 1.39; 95% CI, 1.16-1.68) and cancer-specific survival (HR, 1.37; 95% CI, 1.05-1.78 vs HR, 1.48; 95% CI, 1.14-1.93) (eTable 3 in the Supplement). In addition, we found that, among the radical cystectomy cohort, patients who received neoadjuvant chemotherapy compared with those who received no chemotherapy improved overall survival (HR, 0.62; 95% CI, 0.48-0.79 vs HR, 0.70; 95% CI, 0.61-0.79) and cancer-specific survival (HR, 0.53; 95% CI, 0.37-0.76 vs HR, 0.59; 95% CI, 0.49-0.71) (eTable 4 in the Supplement). Figure 2 illustrates the unadjusted overall survival and cancer-specific survival rates for trimodal therapy and radical cystectomy.
Table 2. Proportional Hazards Regression Model of Treatment for Overall Survival and Cancer-Specific Survivala.
| Covariate | Survival, HR (95% CI) |
|---|---|
| Cox Proportional Hazards Regression Overall Survival | |
| Treatment | |
| Radical cystectomy | 1 [Reference] |
| Trimodal therapy | 1.49 (1.31-1.69) |
| Competing Risk Regression Cancer-Specific Survival | |
| Treatment | |
| Radical cystectomy | 1 [Reference] |
| Trimodal therapy | 1.55 (1.32-1.83) |
Abbreviation: HR, hazard ratio.
All baseline covariates were well balanced in the propensity score–matched sample. Therefore, no covariates were controlled for in the outcome model.
Figure 2. Unadjusted Kaplan-Meier Curves of Overall Survival and Cancer-Specific Survival According to Treatment After Propensity Score Matching.
P < .001 by the log-rank test for both overall and cancer-specific survival when radical cystectomy is compared with trimodal therapy.
Table 3 displays the median cost of each treatment in 2012 US dollars stratified by year of treatment. Cost-of-care analysis demonstrated a trend in increased costs from 2002 to 2011 for both treatments at 30 days and 90 days. However, the trend for increased costs remained significant for radical cystectomy ($80 431 in 2002 vs $140 122 in 2011; P < .001) but not for trimodal therapy at 180 days. For example, the 180-day median costs for radical cystectomy increased by 43% from $80 431 in 2002 to $140 122 in 2011, whereas the median costs for trimodal therapy increased by 8% from $159 693 in 2002 to $173 999. No differences were observed in annual median 30-day health care costs between trimodal therapy ($15 233 in 2002 vs $18 743 in 2011) and radical cystectomy ($17 990 in 2002 vs $21 738 in 2011). However, median total costs were significantly higher with trimodal therapy than with radical cystectomy at 90 days ($80 174 vs $69 181; median difference, $8964; Hodges-Lehmann 95% CI, $3848-$14 079) and at 180 days ($179 891 vs $107 017; median difference, $63 771; Hodges-Lehmann 95% CI, $55 512-$72 029). Extrapolating these figures to the total US population revealed $335 million in excess spending for trimodal therapy compared with the less costly radical cystectomy ($492 million) for patients who received a muscle-invasive bladder cancer diagnosis in 2011.
Table 3. Annual Median Medicare Costs for Radical Cystectomy and Trimodal Therapy.
| Year | Median Cost, USD | H-L Estimate (95% CI), USDa | |
|---|---|---|---|
| Radical Cystectomy | Trimodal Therapy | ||
| 30 d After Treatment | |||
| Total | 20 298 | 19 459 | −1282 (−2936 to 373) |
| 2002 | 17 990 | 15 233 | −2216 (−6824 to 2392) |
| 2003 | 14 388 | 18 779 | 235 (−4747 to 5217) |
| 2004 | 19 437 | 14 303 | −4367 (−8044 to −690) |
| 2005 | 20 534 | 20 246 | −797 (−5935 to 4340) |
| 2006 | 19 629 | 18 645 | −1616 (−6037 to 2805) |
| 2007 | 20 587 | 21 151 | −1139 (−7081 to 4803) |
| 2008 | 24 840 | 24 398 | −1197 (−7634 to 5240) |
| 2009 | 21 075 | 22 585 | 2797 (−3946 to 9539) |
| 2010 | 26 433 | 25 581 | −576 (−7204 to 6053) |
| 2011 | 21 738 | 18 743 | −2362 (−7928 to 3203) |
| 90 d After Treatment | |||
| Total | 69 181 | 80 174 | 8964 (3848 to 14 079) |
| 2002 | 60 498 | 80 150 | 14 071 (−768 to 28 911) |
| 2003 | 58 939 | 64 751 | 6232 (−11 357 to 23 821) |
| 2004 | 60 096 | 76 146 | 12 664 (−2138 to 27 465) |
| 2005 | 63 754 | 82 031 | 17 040 (1111 to 32 968) |
| 2006 | 62 286 | 74 943 | 8137 (5589 to 21 864) |
| 2007 | 69 650 | 88 312 | 10 134 (−9772 to 30 040) |
| 2008 | 81 492 | 85 683 | 8750 (−8612 to 26 112) |
| 2009 | 76 790 | 79 005 | 3239 (−15 189 to 21 667) |
| 2010 | 73 390 | 84 322 | 10 411 (−5973 to 26 795) |
| 2011 | 80 550 | 75 432 | −626 (−17 153 to 15 902) |
| 180 d After Treatment | |||
| Total | 107 017 | 179 891 | 63 771 (55 512 to 72 029) |
| 2002 | 80 431 | 159 693 | 69 180 (47 450 to 90 910) |
| 2003 | 99 079 | 179 445 | 73 249 (41 401 to 105 098) |
| 2004 | 78 932 | 168 208 | 81 206 (57 577 to 104 835) |
| 2005 | 93 323 | 181 577 | 76 597 (52 671 to 100 523) |
| 2006 | 99 232 | 163 651 | 65 040 (42 857 to 87 223) |
| 2007 | 124 072 | 186 129 | 55 873 (26 732 to 85 014) |
| 2008 | 134 600 | 181 036 | 44 271 (17 814 to 70 729) |
| 2009 | 124 707 | 186 535 | 55 558 (25 245 to 85 872) |
| 2010 | 131 653 | 213 161 | 74 307 (46 223 to 102 392) |
| 2011 | 140 122 | 173 999 | 39 559 (13 682 to 65 437) |
Abbreviations: H-L, Hodges-Lehmann; USD, US dollar.
Hodges-Lehmann median difference in costs (trimodal therapy minus radical cystectomy).
Discussion
Muscle-invasive bladder cancer is a lethal disease, which warrants definitive treatment. Previous research has shown that approximately half of these patients undergo definitive treatment, with only 19% receiving surgery.14,22 In recent years, the use of trimodal therapy has increased.5 Despite the lack of data from a randomized clinical trial comparing trimodal therapy with radical cystectomy and the conflicting survival outcomes shown in observational studies, trimodal therapy is recommended in select patients with muscle-invasive disease.3,6,7 In the present propensity score–matched study, we found that patients who underwent trimodal therapy had significantly worse overall or cancer-specific survival compared with those who received radical cystectomy. Furthermore, costs at 30 days of treatment were comparable between either intervention, but we observed significant increased costs associated with trimodal therapy at 90 days and 180 days of treatment.
Our study has several important findings. First, patients who underwent trimodal therapy instead of radical cystectomy had a significantly lower overall survival rate. This finding is consistent with that of recent population-based studies, which identified decreased overall survival among patients who underwent trimodal therapy compared with those who underwent radical cystectomy.5,11 A small single-center study using similar analytical methods found that the adverse association of trimodal therapy with age decreased considerably in older adults, suggesting that this treatment may be more appropriate for the elderly.23 In addition, decreased overall survival persisted across clinical stages except for stage IV, for which we observed no significant differences between the treatment types. Patients with stage IV disease represent a heterogeneous group that observational studies cannot control for inherent selection bias, thus limiting the ability to discern which patients received primary treatment and which received palliative care. Our findings also suggest decreased survival outcomes with trimodal therapy when compared with radical cystectomy.
Second, we noticed lower cancer-specific survival among patients who underwent trimodal therapy compared with those who underwent radical cystectomy. These findings persisted across clinical stages except among patients with clinical stage IV disease, for whom no differences were observed. Although we are unable to eliminate selection bias, we believe the noted improved survival among patients with stage II and III disease suggests radical cystectomy may be best suited for these patients when feasible. As mentioned, patients with stage IV bladder cancer represent a heterogeneous population, and further research is needed to identify which patients are best suited for which type of local therapy. We also observed improved survival associated with the use of neoadjuvant chemotherapy, supporting guideline recommendations.2,4 Our findings provide additional information regarding cancer-specific survival that cannot be assessed using the National Cancer Database.5,11 In a recent propensity score–matched study, 112 patients in a single-center multidisciplinary clinic were analyzed, identified, and matched (56 who received trimodal therapy and 56 who received radical cystectomy).9 At a median follow-up of 4½ years, a total of 13 deaths from bladder cancer were noted in each group.9 That study had limited power, and our findings in a larger cohort suggest that trimodal therapy may have inferior oncological outcomes among older patients.
Third, although costs did not differ statistically at 30 days, trimodal therapy was associated with higher 90-day and 180-day costs. Extrapolating these figures to the total US population revealed $335 million in excess spending for trimodal therapy compared with the less costly radical cystectomy ($492 million) for patients who received a diagnosis in 2011. More recently, the health care costs associated with trimodal therapy have plateaued, whereas radical cystectomy costs have continued to rise. Possible explanations may include the centralization of care associated with radical cystectomy.22 Higher-volume hospitals committed to academic, research, and clinical excellence often accept patients regardless of clinical presentation and financial risk.24 Furthermore, these medical centers often have access to advanced technology-based subspecialist care.25,26,27
To our knowledge, this is the first study comparing costs between these 2 treatments. Given the current health care climate in the United States and the increased emphasis on high value–based cancer care over the entire care cycle, our findings regarding survival outcomes in the context of health care dollars is timely.28 The Institute of Medicine has proposed cost-containment measures (for inpatient and outpatient services) to include the limited use of advanced technology (robotics, radiotherapy, and advanced imaging) in the absence of less costly modalities.29 As depicted in the present study, further comparative effectiveness research into interventions to improve the quality and costs of the entire course of bladder cancer care is needed.
Limitations
Our findings must be interpreted within the context of the study design. First, the patients we identified were 66 years or older; thus, our findings may not be applicable to younger patients. However, most patients with bladder cancer are in the sixth decade of life, and we provided a contemporary analysis of treatment utilization rates for this disease.
Second, there is level 1 evidence supporting radical cystectomy plus neoadjuvant chemotherapy, linking this intervention with substantial downstaging and improved survival.30 In our study, only 99 patients (14.4%) who underwent radical cystectomy received neoadjuvant chemotherapy. In the present cohort, we noticed improved survival associated with neoadjuvant chemotherapy, which is consistent with findings in previous studies.5,31 Given the recent endorsements of this treatment by several urological associations, utilization trends may increase.2,32 Third, specifics regarding surgery, including the type of lymph node dissection, are not captured in the SEER database and may limit the interpretation of surgical quality.33
Fourth, this study is retrospective, with an inherent selection bias. Although we attempted to overcome this bias by using propensity score matching to control for potential confounders, we acknowledge the limitations in using this study design. We agree that clinical trials overcome concerns of internal validity, but there are often concerns regarding the external validity and generalizability of clinical trial enrollees.34,35 Our analysis has the advantage of providing a contemporary and generalizable assessment of treatment patterns among a large number of older adults.
Fifth, we provided a population-based assessment of trimodal therapy and radical cystectomy, but we did not assess which specific type of trimodal therapy was used. The SEER database lacks specifics on the dose or cycle of chemotherapy and dose of radiotherapy administered as well as the extent of transurethral resection. We attempted to account for fractions of radiotherapy delivered as a surrogate for radiotherapy dose, and we observed improved survival associated with the increased fractions administered. In addition, patients who underwent trimodal therapy with cisplatin or fluorouracil and mitomycin C had improved survival than those treated with other regimens. Selection of appropriate patients and adherence to guidelines-recommended trimodal protocols with meticulous follow-up are critical to achieving durable oncological outcomes.2,3,4
Sixth, a sample size or power calculation was not performed a priori; however, our sample size compares well with previous observational studies. Furthermore, no adjustment was made for within-center correlations or within-surgeon correlations of outcomes. Finally, we assessed costs up to 180 days after treatment. Further long-term cost-effectiveness research is needed, especially as we move toward a “global cost of care” payer system.36
Conclusions
Trimodal therapy was associated with significantly decreased overall and cancer-specific survival at increased costs when compared with radical cystectomy. Extrapolating these figures to the total US population revealed $335 million in excess spending for trimodal therapy compared with the less costly radical cystectomy ($492 million) for patients who received a diagnosis in 2011. Although observational and retrospective and with inherent selection bias, this study has important health policy implications regarding the appropriate use of high value–based care among patients who are candidates for either treatment.
eTable 1. Proportional Hazards Regression Model of Predictors for Time-to-Death According to Clinical Stage
eTable 2. Proportional Hazards Regression Model of Predictors for Time-to-Death According to Use of Chemotherapy Type in Trimodal Therapy
eTable 3. Proportional Hazards Regression Model of Predictors for Time-to-Death According to Fractions Delivered (<18 v >18) in Trimodal Therapy Cohort
eTable 4. Proportional Hazards Regression Model of Predictors for Time-to-Death on Use of Neoadjuvant Chemotherapy Among Radical Cystectomy Cohort
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30. doi: 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 2.Alfred Witjes J, Lebret T, Compérat EM, et al. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2017;71(3):462-475. doi: 10.1016/j.eururo.2016.06.020 [DOI] [PubMed] [Google Scholar]
- 3.Clark PE, Agarwal N, Biagioli MC, et al. ; National Comprehensive Cancer Network (NCCN) . Bladder cancer. J Natl Compr Canc Netw. 2013;11(4):446-475. doi: 10.6004/jnccn.2013.0059 [DOI] [PubMed] [Google Scholar]
- 4.Chang SS, Bochner BH, Chou R, et al. Treatment of non-metastatic muscle-invasive bladder cancer: AUA/ASCO/ASTRO/SUO guideline. J Urol. 2017;198(3):552-559. doi: 10.1016/j.juro.2017.04.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cahn DB, Handorf EA, Ghiraldi EM, et al. Contemporary use trends and survival outcomes in patients undergoing radical cystectomy or bladder-preservation therapy for muscle-invasive bladder cancer. Cancer. 2017;123(22):4337-4345. doi: 10.1002/cncr.30900 [DOI] [PubMed] [Google Scholar]
- 6.Gakis G, Efstathiou J, Lerner SP, et al. ; International Consultation on Urologic Disease-European Association of Urology Consultation on Bladder Cancer 2012 . ICUD-EAU International Consultation on Bladder Cancer 2012: radical cystectomy and bladder preservation for muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2013;63(1):45-57. doi: 10.1016/j.eururo.2012.08.009 [DOI] [PubMed] [Google Scholar]
- 7.Mitin T, George A, Zietman AL, et al. Long-term outcomes among patients who achieve complete or near-complete responses after the induction phase of bladder-preserving combined-modality therapy for muscle-invasive bladder cancer: a pooled analysis of NRG oncology/RTOG 9906 and 0233. Int J Radiat Oncol Biol Phys. 2016;94(1):67-74. doi: 10.1016/j.ijrobp.2015.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gofrit ON, Nof R, Meirovitz A, et al. Radical cystectomy vs. chemoradiation in T2-4aN0M0 bladder cancer: a case-control study. Urol Oncol. 2015;33(1):19.e1-19.e5. doi: 10.1016/j.urolonc.2014.09.014 [DOI] [PubMed] [Google Scholar]
- 9.Kulkarni GS, Hermanns T, Wei Y, et al. Propensity score analysis of radical cystectomy versus bladder-sparing trimodal therapy in the setting of a multidisciplinary bladder cancer clinic. J Clin Oncol. 2017;35(20):2299-2305. doi: 10.1200/JCO.2016.69.2327 [DOI] [PubMed] [Google Scholar]
- 10.Bekelman JE, Handorf EA, Guzzo T, et al. Radical cystectomy versus bladder-preserving therapy for muscle-invasive urothelial carcinoma: examining confounding and misclassification bias in cancer observational comparative effectiveness research. Value Health. 2013;16(4):610-618. doi: 10.1016/j.jval.2013.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seisen T, Sun M, Lipsitz SR, et al. Comparative effectiveness of trimodal therapy versus radical cystectomy for localized muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2017;72(4):483-487. doi: 10.1016/j.eururo.2017.03.038 [DOI] [PubMed] [Google Scholar]
- 12.Hu JC, Chughtai B, O’Malley P, et al. Perioperative outcomes, health care costs, and survival after robotic-assisted versus open radical cystectomy: a national comparative effectiveness study. Eur Urol. 2016;70(1):195-202. doi: 10.1016/j.eururo.2016.03.028 [DOI] [PubMed] [Google Scholar]
- 13.Weir HK, Johnson CJ, Mariotto AB, et al. Evaluation of North American Association of Central Cancer Registries’ (NAACCR) data for use in population-based cancer survival studies. J Natl Cancer Inst Monogr. 2014;2014(49):198-209. doi: 10.1093/jncimonographs/lgu018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams SB, Huo J, Chamie K, et al. Underutilization of radical cystectomy among patients diagnosed with clinical stage T2 muscle-invasive bladder cancer. Eur Urol Focus. 2017;3(2-3):258-264. doi: 10.1016/j.euf.2016.04.008 [DOI] [PubMed] [Google Scholar]
- 15.Jagsi R, Falchook AD, Hendrix LH, Curry H, Chen RC. Adoption of hypofractionated radiation therapy for breast cancer after publication of randomized trials. Int J Radiat Oncol Biol Phys. 2014;90(5):1001-1009. doi: 10.1016/j.ijrobp.2014.09.032 [DOI] [PubMed] [Google Scholar]
- 16.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258-1267. doi: 10.1016/S0895-4356(00)00256-0 [DOI] [PubMed] [Google Scholar]
- 17.America Cancer Society Cancer facts & figures 2011. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2011/cancer-facts-and-figures-2011.pdf. Published 2011. Accessed September 27, 2017.
- 18.Cipriano LE, Romanus D, Earle CC, et al. Lung cancer treatment costs, including patient responsibility, by disease stage and treatment modality, 1992 to 2003. Value Health. 2011;14(1):41-52. doi: 10.1016/j.jval.2010.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams SB, Huo J, Chu Y, et al. Cancer and all-cause mortality in bladder cancer patients undergoing radical cystectomy: development and validation of a nomogram for treatment decision-making. Urology. 2017;110:76-83. doi: 10.1016/j.urology.2017.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083-3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 22.Gore JL, Litwin MS, Lai J, et al. ; Urologic Diseases in America Project . Use of radical cystectomy for patients with invasive bladder cancer. J Natl Cancer Inst. 2010;102(11):802-811. doi: 10.1093/jnci/djq121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kulkarni GS, Klaassen Z. Trimodal therapy is inferior to radical cystectomy for muscle-invasive bladder cancer using population-level data: is there evidence in the (lack of) details? Eur Urol. 2017;72(4):488-489. doi: 10.1016/j.eururo.2017.04.028 [DOI] [PubMed] [Google Scholar]
- 24.Taheri PA, Butz DA, Dechert R, Greenfield LJ. How DRGs hurt academic health systems. J Am Coll Surg. 2001;193(1):1-8. doi: 10.1016/S1072-7515(01)00870-5 [DOI] [PubMed] [Google Scholar]
- 25.Miller DC, Ye Z, Gust C, Birkmeyer JD. Anticipating the effects of accountable care organizations for inpatient surgery. JAMA Surg. 2013;148(6):549-554. doi: 10.1001/jamasurg.2013.1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Regenbogen SE, Gust C, Birkmeyer JD. Hospital surgical volume and cost of inpatient surgery in the elderly. J Am Coll Surg. 2012;215(6):758-765. doi: 10.1016/j.jamcollsurg.2012.07.011 [DOI] [PubMed] [Google Scholar]
- 27.Williams SB, Amarasekera CA, Gu X, et al. Influence of surgeon and hospital volume on radical prostatectomy costs. J Urol. 2012;188(6):2198-2202. doi: 10.1016/j.juro.2012.08.012 [DOI] [PubMed] [Google Scholar]
- 28.Rocque G, Blayney DW, Jahanzeb M, et al. Choosing wisely in oncology: are we ready for value-based care? J Oncol Pract. 2017;13(11):e935-e943. doi: 10.1200/JOP.2016.019281 [DOI] [PubMed] [Google Scholar]
- 29.Smith GL, Ganz PA, Bekelman JE, et al. Promoting the appropriate use of advanced radiation technologies in oncology: summary of a National Cancer Policy Forum workshop. Int J Radiat Oncol Biol Phys. 2017;97(3):450-461. doi: 10.1016/j.ijrobp.2016.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349(9):859-866. doi: 10.1056/NEJMoa022148 [DOI] [PubMed] [Google Scholar]
- 31.Booth CM, Siemens DR, Li G, et al. Perioperative chemotherapy for muscle-invasive bladder cancer: a population-based outcomes study. Cancer. 2014;120(11):1630-1638. doi: 10.1002/cncr.28510 [DOI] [PubMed] [Google Scholar]
- 32.Chang SS, Bochner BH, Chou R, et al. Treatment of nonmetastatic muscle-invasive bladder cancer: American Urological Association/American Society of Clinical Oncology/American Society for Radiation Oncology/Society of Urologic Oncology clinical practice guideline summary. J Oncol Pract. 2017;13(9):621-625. doi: 10.1200/JOP.2017.024919 [DOI] [PubMed] [Google Scholar]
- 33.Froehner M, Novotny V, Heberling U, et al. Relationship of the number of removed lymph nodes to bladder cancer and competing mortality after radical cystectomy. Eur Urol. 2014;66(6):987-990. doi: 10.1016/j.eururo.2014.07.046 [DOI] [PubMed] [Google Scholar]
- 34.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720-2726. doi: 10.1001/jama.291.22.2720 [DOI] [PubMed] [Google Scholar]
- 35.Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21(7):1383-1389. doi: 10.1200/JCO.2003.08.010 [DOI] [PubMed] [Google Scholar]
- 36.Porter ME, Lee TH. From volume to value in health care: the work begins. JAMA. 2016;316(10):1047-1048. doi: 10.1001/jama.2016.11698 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Proportional Hazards Regression Model of Predictors for Time-to-Death According to Clinical Stage
eTable 2. Proportional Hazards Regression Model of Predictors for Time-to-Death According to Use of Chemotherapy Type in Trimodal Therapy
eTable 3. Proportional Hazards Regression Model of Predictors for Time-to-Death According to Fractions Delivered (<18 v >18) in Trimodal Therapy Cohort
eTable 4. Proportional Hazards Regression Model of Predictors for Time-to-Death on Use of Neoadjuvant Chemotherapy Among Radical Cystectomy Cohort