Abstract
Importance
Technical proficiency at robotic pancreaticoduodenectomy (RPD) and video assessment are promising tools for understanding postoperative outcomes. Delayed gastric emptying (DGE) remains a major driver of cost and morbidity after pancreaticoduodenectomy.
Objective
To determine if technical variables during RPD are associated with postoperative DGE.
Design, Setting, and Participants
A retrospective study was conducted of technical assessment performed in all available videos (n = 192) of consecutive RPDs performed at a single academic institution from October 3, 2008, through September 27, 2016.
Exposures
Video review of gastrojejunal anastomosis during RPD.
Main Outcomes and Measures
Delayed gastric emptying was classified according to International Study Group of Pancreatic Surgery criteria. Video analysis reviewed technical variables specific in the construction of the gastrojejunal anastomosis. Using multivariate analysis, DGE was correlated with known patient variables and technical variables, individually and combined.
Results
Of 410 RPDs performed, video was available for 192 RPDs (80 women and 112 men; mean [SD] age, 65.7 [11.1] years). Delayed gastric emptying occurred in 41 patients (21.4%; grade A, 15; grade B, 14; and grade C, 12). Patient variables contributing to DGE on multivariate analysis were advanced age (odds ratio [OR] 1.11; 95% CI, 1.05-1.16; P < .001), small pancreatic duct size (OR, 0.84; 95% CI, 0.72-0.98; P = .03), and postoperative pseudoaneurysm (OR, 17.29; 95% CI, 2.34-127.78; P = .005). However, technical variables contributing to decreased DGE on multivariate analysis included the flow angle (within 30° of vertical) between the stomach and efferent jejunal limb (OR, 0.25; 95% CI, 0.08-0.79; P = .02), greater length of the gastrojejunal anastomosis (OR, 0.40; 95% CI, 0.20-0.77; P = .006), and a robotic-sewn anastomosis (robotic suture vs stapler: OR, 0.30; 95% CI, 0.09-0.95; P = .04).
Conclusions and Relevance
This study examines modifiable technical factors through the use of review of video obtained at the time of operation and suggests ways by which the surgical construction of the gastrojejunal anastomosis during RPD may reduce the incidence of DGE as a framework for prospective quality improvement.
This study reviews video obtained at the time of robotic pancreaticoduodenectomy to determine if technical variables are associated with postoperative delayed gastric emptying among patients who underwent the surgery.
Key Points
Question
Can assessment of intraoperative video identify modifiable technical factors that prevent postoperative delayed gastric emptying in addition to identifying patient variables that prevent postoperative delayed gastric emptying?
Findings
During video review of 192 robotic pancreaticoduodenectomies, the angle of the gastrojejunal anastomosis, gastrostomy size, and surgical device style of gastrojejunal anastomosis were technical variables that were associated with postoperative delayed gastric emptying.
Meaning
Video assessment of technical factors is a powerful, yet underused, tool to assess perioperative outcomes, particularly delayed gastric emptying; targeting specific technical factors during surgery can potentially improve postoperative outcomes.
Introduction
Pancreaticoduodenectomy (PD) is a technically challenging procedure with low perioperative mortality (<3%) at high-volume centers.1,2,3 However, postoperative morbidity remains high among patients who undergo PD, with reported rates of 30% to 60%.4,5,6,7,8 Delayed gastric emptying (DGE) remains one of the most common causes of postoperative morbidity among patients who undergo PD, with a reported rate of 6% to 57%.9,10,11,12,13,14 Before 2007, there was wide variability in the definition of DGE. Consensus criteria set forth in 2007 by the International Study Group of Pancreatic Surgery12 for DGE standardized the definition in an attempt to study and mitigate this complication.
Delayed gastric emptying is not life threatening but can have significant consequences of patient discomfort, prolonged hospital stay, increased hospital cost, readmission4,5 and delay in initiation of adjuvant therapy.15,16 The pathogenesis of DGE is multifactorial and poorly understood. Delayed gastric emptying most commonly occurs in the presence of other intra-abdominal complications, such as pancreatic leak or intra-abdominal abscess (secondary DGE).17,18,19,20,21 Delayed gastric emptying can also occur in the absence of other intraabdominal complications (primary DGE). It has been hypothesized that pyloric denervation, lack of motilin from duodenectomy, loss of pyloric pump, gastric dysrhythmia, and inflammation may be underlying contributing mechanisms for DGE.18,22,23,24 Patient-related factors such as male sex, smoking history, and estimated blood loss have been shown to be associated with increased incidence of DGE.19 Currently, there are no modifiable procedure-related factors that have been shown to be associated with decreased or increased incidence of DGE after PD. It has been reported that some configurations of the gastrojejunal (GJ) anastomosis may interfere with food passage.25,26 Masui et al26 used contrast gastroradiography to show that the flow angle at the anastomosis of stomach to jejunum after Roux-en-Y reconstruction after distal gastrectomy correlated with postoperative DGE. The study did not include PD, and the methods required postoperative radiographic studies rather than intraoperative review of technique to obtain the data.
Our group has been performing robotic PD (RPD) since 2008 and has established its feasibility, safety, and the learning curve necessary to achieve competence equal to or superior to open PD techniques.1,27 The robotic approach, among its other putative advantages,28,29,30,31,32 allows for video recording of the procedure, which enables postoperative critical review. A study by Birkmeyer et al33 suggested that technical proficiency is an important factor associated with complications in patients undergoing bariatric surgery. Another study has previously demonstrated that technical scoring of a surgeon’s performance by video review for pancreaticojejunostomy was independently associated with outcomes of pancreatic fistulas in pancreatic surgery.32 However, intraoperative technical factors related to construction of the GJ anastomosis, which can contribute to DGE after PD, have not been systematically studied.
The goal of this study was to evaluate whether technical factors observed after RPD using video review retrospectively could be modified to reduce the risk of DGE independent of other patient-related variables. We hypothesized that the study of modifiable technical factors34 may estimate DGE and may help determine some of the yet unexplained factors that contribute to DGE.
Methods
Patient Selection
The present study was a retrospective review of a clinical database started with all consecutive RPDs performed at the University of Pittsburgh Medical Center beginning October 3, 2008, with video analysis of patients with available intraoperative videos first available after October 2011 (post–learning curve RPD31) through September 27, 2016, to include all patients with a minimum of 90 days of follow-up.31 The study was approved by the University of Pittsburgh Institutional Review Board (PRO15040497), which waived patient consent because data were deidentified.
Demographic and Clinical Data
Data from a database that contained demographic and clinical (preoperative, intraoperative, and 90 days postoperative) information were collected and analyzed for factors associated with DGE. Delayed gastric emptying was classified by the International Study Group of Pancreatic Surgery.12 Demographic and clinical variables included age, sex, body mass index, Charlson comorbidity index, prior abdominal surgery, American Society of Anesthesiologists classification, preoperative albumin, pathologic findings, neoadjuvant therapy, duct size, gland texture, operating time, estimated blood loss, transfusion, postoperative pancreatic fistula, pseudoaneurysm, Clavien-Dindo classification,35 fluid collection, length of hospital stay, readmission, reoperation, and 30-day and 90-day mortality.
Operative Procedure
There were subtle variations in the GJ anastomosis over time and by surgeon; however, a retromesenteric, antecolic, isoperistaltic GJ anastomosis with the efferent limb medial was typically performed (Figure 1). For the robotic-sewn anastomosis, the end of the stomach remnant was sutured to the side of the jejunum in 2 layers using running 3-0 V-loc sutures (Covidien) for the inner layer and interrupted 3-0 black silk sutures (Covidien) for the seromuscular layers. When a stapled anastomosis was used, the side-to-side GJ anastomosis used either the 45-mm stapler (EndoWrist Stapler 45 Blue Reload; Intuitive Surgical) or 60-mm stapler (EndoGIA, Tri-Staple Purple Reload; Covidien-Medtronic) for the inner layer, followed by common enterotomy closure using running 3-0 V-loc sutures for the inner layer and interrupted 3-0 black silk sutures for the seromuscular layer.
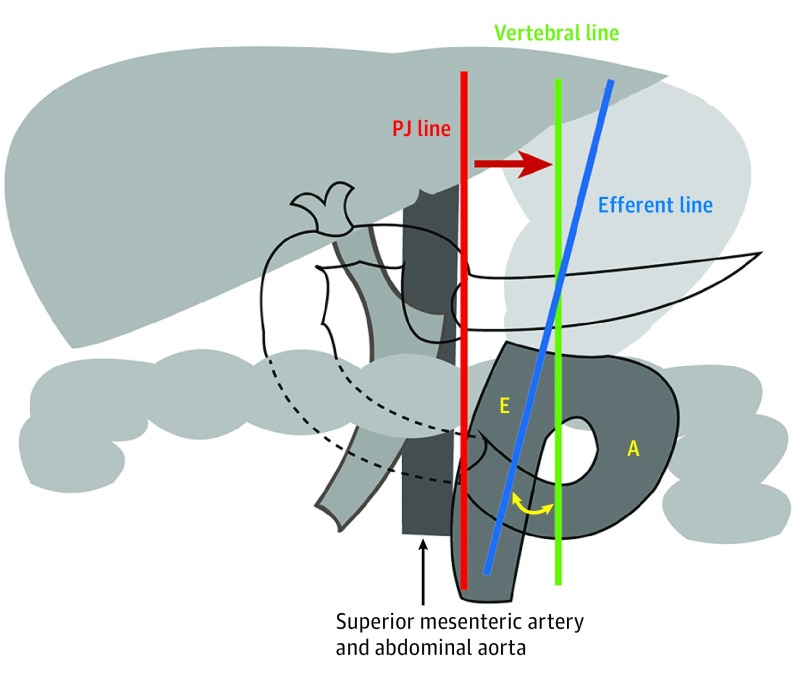
Figure 1. Schematic Diagram of Robotic Pancreaticoduodenectomy Gastrojejunostomy Reconstruction.
The gastrojejunal anastomosis flow angle is the angle between the green line (vertebral line) and blue line (efferent line). A indicates afferent limb of loop; E, efferent limb of loop; and PJ, pancreaticojejunostomy.
Video Review
All available RPD videos of completed GJ anastomosis with available 90-day postoperative clinical data were analyzed. Videos were reviewed retrospectively and independently by 2 experienced oncologic surgeons (J.P.J. and M.D.) blinded to patient identity, clinical outcome, and identity of the surgeon to minimize bias. The technical variables were added to the preexisting clinical database. Specific technical variables reviewed were GJ anastomosis flow angle to efferent limb (described below), length of the GJ anastomosis, GJ anastomotic method (robotic suture vs stapler), distance from pylorus to the site of gastric division, and time to completion. As part of a 5-step structured robotic curriculum,36 surgical oncology fellows are required to log the portions of the procedure completed from the console. From this procedure, the variable of “attending vs trainee” was added to the technical database after video review was complete.
Gastrojejunal Measurements
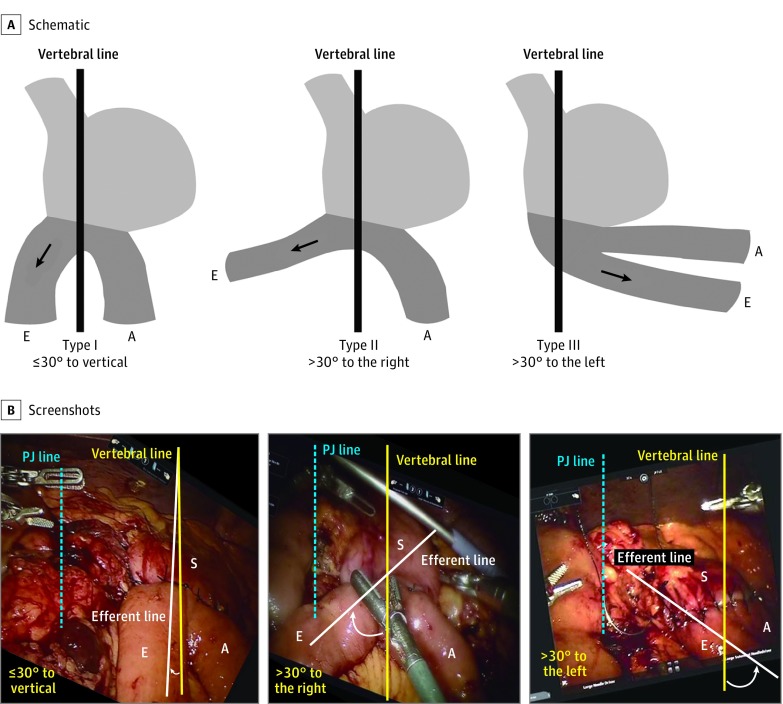
Masui et al26 had found that the flow angle between the stomach and the efferent limb at the completion of the GJ anastomosis (Roux-en-Y) was the most important factor associated with DGE after distal gastrectomy. Therefore, specific attention was dedicated to this variable. The flow angle between the efferent limb and vertebral line was measured on video review. As the video could not directly visualize the vertebral line, we used the pancreaticojejunostomy suture line as a vertebral line surrogate because they are parallel. As a result, we could measure the angle between the pancreaticojejunostomy suture line (red line) and efferent limb line (blue line) using the vertical vertebral line (green line) to calculate the flow angle (Figure 1), similar to the method used by Masui et al.26 The modified flow angle of the GJ anastomosis was categorized into the following 3 groups (Figure 2): type I, GJ anastomosis flow angle to efferent limb less than 30° on either side of the pancreaticojejunostomy suture line to vertical; type II, GJ anastomosis flow angle to efferent limb more than 30° to the right; and type III, GJ anastomosis flow angle to efferent limb more than 30° to the left.26 To estimate the length of the anastomosis, we used the working portion of a robotic scissor, 1 cm, and for the stapled GJ anastomosis, we used the 1-cm markers on the stapler as a ruler.
Figure 2. Classification of Gastrojejunostomy Flow Angle to Efferent Limb.
Modified categorization of gastrojejunal anastomosis angle in Roux-en-Y reconstruction after distal gastrectomy for gastric cancer.26 A, Schematic of gastrojejunostomy flow angle to efferent limb. B, Screenshots of gastrojejunostomy flow angle to efferent limb. A indicates afferent limb of loop; E, efferent limb of loop; PJ, pancreaticojejunostomy; and S, stomach.
Statistical Analysis
The distributional characteristics for variables were checked for normalcy. Continuous variable data were presented as means and SD or median and interquartile range. Categorical data were summarized using frequency and percentages. The t test was used to compare normally distributed continuous variables between groups, and the Wilcoxon rank sum test was used for nonnormally distributed continuous variables. The χ2 or Fisher exact test was used to compare categorical variables between groups. Pearson and Spearman correlations were used in obtaining correlation coefficients and related P values. Univariate and multivariate analysis were conducted using logistic regression to indicate factors associated with DGE for clinical and technical variables. Statistical analyses were performed using STATA, version 10 (StataCorp). All statistical tests were 2-tailed, and P < .05 was considered statistically significant.
Results
Entire Cohort vs Video Cohort
To date, a total of 410 RPDs (entire cohort) were performed, of which 192 (video cohort) had available video to review. There was no difference in DGE in the entire cohort (88 [21.5%]) compared with the video cohort (41 [21.4%]). The video cohort was chronologically later and after the learning curve, with representative differences including an increase in demographic variables of neoadjuvant therapy and a decrease in operative time, estimated blood loss, and conversion to open surgery for intraoperative variables. Also, a decrease in length of hospital stay and complications with a Clavien-Dindo score of 3 or more (severe) was seen for postoperative variables (eTable in the Supplement).
Patient Variables in the Video Cohort by Presence of DGE
Of the 192 patients with video, 41 (21.4%) developed DGE after RPD (International Study Group of Pancreatic Surgery grade A, 15; grade B, 14; and grade C, 12). Older age was the only demographic variable that was significantly associated with DGE (mean [SD] age: DGE group, 69.6 [10.3] years; non-DGE group, 64.6 [11.3] years; P = .01) (Table 1).35 None of the intraoperative variables were significantly different in patients with or without DGE. The patients with DGE were more likely than those without DGE to have pseudoaneurysms (4 of 41 [9.8%] vs 2 of 151 [1.3%]), severe Clavien-Dindo complications (score ≥3; 18 of 41 [43.9%] vs 27 of 151 [17.9%]), and a higher rate of reoperation (5 of 41 [12.2%] vs 2 of 151 [1.3%]). No difference was noted between the DGE and non-DGE groups in terms of deep space fluid collections or drain placement. In addition, a prolonged length of hospital stay and higher rate of readmission rate were associated with DGE.
Table 1. Descriptive Analysis of Patient Variables in the Video Cohort by Presence of Delayed Gastric Emptying (DGE)a.
| Variable | Video Cohort (N = 192) | Non-DGE (n = 151) | DGE (n = 41) | P Value |
|---|---|---|---|---|
| Demographic and preoperative clinical variables | ||||
| Age, mean (SD), y | 65.7 (11.1) | 64.6 (11.3) | 69.6 (10.3) | .01 |
| Sex | ||||
| Male | 112 (58.3) | 85 (56.3) | 27 (65.9) | .27 |
| Female | 80 (41.7) | 66 (43.7) | 14 (34.1) | |
| CCI score, mean (SD) | ||||
| Unadjusted for age | 2.56 (1.27) | 2.55 (1.25) | 2.63 (1.36) | .71 |
| Adjusted for age | 4.66 (1.91) | 4.53 (1.90) | 5.12 (1.90) | .08 |
| BMI, mean (SD) | 27.36 (5.52) | 27.29 (5.36) | 27.62 (6.10) | .73 |
| Prior abdominal surgery history | 103 (53.6) | 79 (52.3) | 24 (58.5) | .48 |
| ASA physical status classification | ||||
| 1 | 1 (0.5) | 1 (0.7) | 0 | .96 |
| 2 | 24 (12.5) | 19 (12.6) | 5 (12.2) | |
| 3 | 154 (80.2) | 120 (79.5) | 34 (82.9) | |
| 4 | 13 (6.8) | 11 (7.3) | 2 (4.9) | |
| Preoperative albumin, mean (SD), g/dL | 3.70 (0.55) | 3.69 (0.54) | 3.71 (0.59) | .85 |
| Cancer | 163 (84.9) | 131 (86.8) | 32 (78.0) | .17 |
| PDAC | 87 (45.3) | 68 (45.0) | 19 (46.3) | .88 |
| Neoadjuvant therapy | 67 (34.9) | 52 (34.4) | 15 (36.6) | .80 |
| Intraoperative clinical variables | ||||
| Duct size, median (IQR), mm | 3 (2.0-5.0) | 3 (2.0-5.0) | 3 (2.0-4.0) | .41 |
| Dilated duct >3 mm | 77 (40.1) | 63 (41.7) | 14 (34.1) | .32 |
| Soft pancreas texture | 116 (60.4) | 90 (59.6) | 26 (63.4) | .78 |
| Operation time, mean (SD), min | 374 (70.0) | 371 (70.0) | 384 (74.0) | .32 |
| GJ time, mean (SD), min | 42.0 (15.2) | 42.5 (15.8) | 40.1 (15.2) | .37 |
| Estimated blood loss, median (IQR), mL | 200 (114-350) | 200 (150-350) | 200 (100-350) | .94 |
| Estimated blood loss >500 mL | 26 (13.5) | 24 (15.9) | 2 (4.9) | .05 |
| RBC transfusion | 21 (10.9) | 15 (9.9) | 6 (14.6) | .40 |
| RBC units in patients who received transfusion, mean (SD) | 1.91 (0.83) | 2.0 (0.85) | 1.67 (0.82) | .42 |
| Postoperative clinical variables | ||||
| POPF (grades B and C) | 16 (8.3) | 11 (7.3) | 5 (12.2) | .34 |
| Pseudoaneurysm | 6 (3.1) | 2 (1.3) | 4 (9.8) | .02 |
| Maximum Clavien-Dindo score35 | ||||
| 0 | 53 (27.6) | 53 (35.1) | 0 | <.001 |
| 1 | 27 (14.1) | 21 (13.9) | 6 (14.6) | |
| 2 | 67 (34.9) | 50 (33.1) | 17 (41.5) | |
| 3 | 20 (10.4) | 12 (7.9) | 8 (19.5) | |
| 4 | 21 (10.9) | 13 (8.6) | 8 (19.5) | |
| 5 | 4 (2.1) | 2 (1.3) | 2 (4.9) | |
| Maximum Clavien-Dindo score ≥3 | 45 (23.4) | 27 (17.9) | 18 (43.9) | <.001 |
| Fluid collection | 30 (15.6) | 22 (14.6) | 8 (19.5) | .44 |
| Drain placement | 15 (7.8) | 11 (7.3) | 4 (9.8) | .53 |
| LOS, median (IQR), d | 7 (6-10) | 7 (6-9) | 13 (8-21) | <.001 |
| Readmission | 68 (35.4) | 44 (29.1) | 24 (58.5) | <.001 |
| Total LOS, median (IQR), d | 9 (7-15) | 8 (6-12) | 18 (11-31) | <.001 |
| Reoperation | 7 (3.7) | 2 (1.3) | 5 (12.2) | .005 |
| Mortality | ||||
| 30 d | 1 (0.5) | 1 (0.7) | 0 | >.99 |
| 90 d | 4 (2.1) | 2 (1.3) | 2 (4.9) | .20 |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CCI, Charlson comorbidity index; GJ, gastrojejunostomy; IQR, interquartile range; LOS, length of stay; PDAC, pancreatic ductal adenocarcinoma; POPF, postoperative pancreatic fistula; RBC, red blood cell.
SI conversion factor: To convert albumin to grams per liter, multiply by 10.0.
Data are presented as number (percentage) of patients unless otherwise indicated.
Technical Variables in the Video Cohort by Presence of DGE
Video review revealed several variations in the modifiable technical factors during the configurations of the GJ anastomosis. The cohort is composed of patients undergoing primarily non–pylorus-preserving reconstruction (174 of 192 [90.6%]) (Table 2). The distance from the pylorus to gastrectomy margin was about 3 cm. A stapled GJ anastomosis was used in 48 patients (25.0%) and a robotic suture technique was used in 144 patients (75.0%). Length of the GJ anastomosis was primarily 4.5 cm or greater (155 of 192 [80.7%]), and GJ anastomosis flow angle to efferent limb was mostly type I (114 of 192 [59.4%]). In addition, based on robotic surgery logs, fellows performed 76 (39.6%) of the GJ anastomosis procedures. When the stapled anastomosis was chosen, there were variations in the stapler used (robotic, 7 of 48 vs laparoscopic, 41 of 48) and connection of the GJ anastomosis (greater curvature, 22 of 48 vs posterior wall, 26 of 48) (Table 2).
Table 2. Descriptive Analysis of Technical Variables in the Video Cohort by Presence of Delayed Gastric Emptying (DGE).
| Variable | Patients, No. (%) | P Value | |
|---|---|---|---|
| Non-DGE | DGE | ||
| Intraoperative Technical Variables | |||
| Operation | |||
| RPPPD (n = 18) | 14 (77.8) | 4 (22.2) | >.99 |
| RPD (n = 174) | 137 (78.7) | 37 (21.3) | |
| Length of gastrectomy from pylorus to stomach, mean (SD), cm | 2.92 (1.48) | 2.79 (1.31) | .21 |
| GJ anastomotic method | |||
| Robotic suture (n = 144) | 116 (80.6) | 28 (19.4) | .26 |
| Stapler (n = 48) | 35 (72.9) | 13 (27.1) | |
| GJ direction | |||
| End to side (n = 143) | 115 (80.4) | 28 (19.6) | .31 |
| Side to side (n = 49) | 36 (73.5) | 13 (26.5) | |
| Length of GJ, mean (SD), cm | 5.04 (0.79) | 4.86 (0.82) | .21 |
| Length of GJ (cutoff) | |||
| ≥4.0 cm (n = 180) | 142 (78.9) | 38 (21.1) | .75 |
| <4.0 cm (n = 12) | 9 (75.0) | 3 (25.0) | |
| ≥4.5 cm (n = 155) | 125 (80.6) | 30 (19.4) | .17 |
| <4.5 cm (n = 37) | 26 (70.3) | 11 (29.7) | |
| ≥5.0 cm (n = 118) | 96 (81.4) | 22 (18.6) | .25 |
| <5.0 cm (n = 74) | 55 (74.3) | 19 (25.7) | |
| ≥5.5 cm (n = 72) | 59 (81.9) | 13 (18.1) | .39 |
| <5.5 cm (n = 120) | 92 (76.7) | 28 (23.3) | |
| ≥6.0 cm (n = 46) | 37 (80.4) | 9 (19.6) | .73 |
| <6.0 cm (n = 146) | 114 (78.1) | 32 (21.9) | |
| GJ flow angle class | |||
| Type I (n = 114) | 94 (82.5) | 20 (17.5) | .18 |
| Type II (n = 51) | 39 (76.5) | 12 (23.5) | |
| Type III (n = 27) | 18 (66.7) | 9 (33.3) | |
| Operator | |||
| Attending physician (n = 116) | 94 (81.0) | 22 (19.0) | .32 |
| Trainee [performed by fellow] (n = 76) | 57 (75.0) | 19 (25.0) | |
| Intraoperative Technical Variables in Stapled Gastrojejunostomies (n = 48) | |||
| Stapled with | |||
| Robotic stapler (n = 7) | 4 (57.1) | 3 (42.9) | .25 |
| Laparoscopic stapler (n = 41) | 31 (75.6) | 10 (24.4) | |
| Stapled area of stomach | |||
| Great curvature (n = 22) | 16 (72.7) | 6 (27.3) | .98 |
| Posterior wall (n = 26) | 19 (73.1) | 7 (26.9) | |
Abbreviations: GJ, gastrojejunostomy; RPD, robotic pancreaticoduodenectomy; RPPPD, robotic pylorus-preserving pancreaticoduodenectomy.
Multivariate Analysis of Patient and Technical Variables as Independent Factors Associated With DGE
First, patient-related variables (demographic, intraoperative, and postoperative) alone were included in multivariate modeling. Advanced age (odds ratio [OR], 1.08; 95% CI, 1.04-1.13; P < .001), smaller pancreatic duct (OR, 0.88; 95% CI, 0.77-1.005; P = .06), and postoperative pseudoaneurysm (OR, 13.53; 95% CI, 1.95-93.76; P = .008) were all associated with DGE on multivariate modeling.
Second, technical variables alone were included in multivariate modeling. The robotic suture technique yielded a significantly lower incidence of DGE (OR, 0.32; 95% CI, 0.12-0.86; P = .02) than the stapled anastomosis. In addition, improved gastric emptying was seen with a longer anastomosis (OR, 0.53; 95% CI, 0.31-0.91; P = .02).
Last, patient and technical variables were both included in multivariate modeling to assess whether technical variables could independently add to modeling of associations with DGE (Table 3). Statistically meaningful patient variables of DGE continued to be advanced age (OR, 1.11; 95% CI, 1.05-1.16; P < .001), smaller pancreatic duct size (OR, 0.84; 95% CI, 0.72-0.98; P = .03), and pseudoaneurysm (OR, 17.29; 95% CI, 2.34-127.78; P = .005). However, in contrast to when technical variables were analyzed alone, additional variables were associated with DGE when added to patient variables. Type I flow angle incidence of DGE was significantly lower (type I vs type III: OR, 0.25; 95% CI, 0.08-0.79; P = .02). In addition, the greater length of the GJ anastomosis (OR, 0.40; 95% CI, 0.20-0.77; P = .006) and the use of robotic suture technique (OR, 0.30; 95% CI, 0.09-0.95; P = .04) both led to a lower incidence of DGE than did a short anastomosis and the use of the stapler. Furthermore, there was a trend toward a lower incidence of DGE if the attending physician rather than the trainee performed the GJ anastomosis (OR, 0.43; 95% CI, 0.18-1.00; P = .05).
Table 3. Logistic Regression Analysis of Patient and Technical Variables as Independent Factors Associated With Delayed Gastric Emptying.
| Variable | Univariate Analysis | Multivariate Analysisa | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | |
| Patient variables | ||||
| Age, y | 1.05 (1.01-1.09) | .01 | 1.11 (1.05-1.16) | <.001 |
| Female sex | 0.67 (0.33-1.37) | .27 | NA | NA |
| CCI score, unadjusted for age | 1.05 (0.81-1.38) | .71 | NA | NA |
| BMI | 1.01 (0.95-1.08) | .73 | NA | NA |
| Prior abdominal surgery history | 1.29 (0.64-2.89) | .48 | NA | NA |
| Preoperative albumin, g/dL | 1.07 (0.56-2.01) | .85 | NA | NA |
| Neoadjuvant therapy | 1.10 (0.54-2.25) | .80 | NA | NA |
| Duct size, mm | 0.92 (0.82-1.04) | .18 | 0.84 (0.72-0.98) | .03 |
| Operation time, min | 1.00 (1.00-1.01) | .32 | NA | NA |
| GJ time, min | 0.99 (0.97-1.01) | .39 | NA | NA |
| Estimated blood loss, mL | 1.00 (1.00-1.00) | .28 | NA | NA |
| Cancer | 0.54 (0.23-1.30) | .17 | NA | NA |
| POPF | 2.09 (0.98-4.47) | .06 | NA | NA |
| Pseudoaneurysm | 8.05 (1.42-45.66) | .02 | 17.29 (2.34-127.78) | .005 |
| Intraoperative technical variables | ||||
| Length of GJ | 0.76 (0.49-1.17) | .21 | 0.40 (0.20-0.77) | .006 |
| GJ anastomotic method | ||||
| Robotic suture | 0.65 (0.72-3.29) | NA | 0.30 (0.09-0.95) | .04 |
| Stapler | 1 [Reference] | .27 | 1 [Reference] | NA |
| GJ flow angle class | ||||
| Type I | 0.43 (0.17-1.08) | .07 | 0.25 (0.08-0.79) | .02 |
| Type II | 0.62 (0.22-1.72) | .36 | 0.52 (0.16-1.71) | .28 |
| Type III | 1 [Reference] | NA | 1 [Reference] | NA |
| Operator | ||||
| Attending physician | 0.70 (0.35-1.41) | .32 | 0.43 (0.18-1.00) | .05 |
| Trainee (performed by fellow) | 1 [Reference] | NA | 1 [Reference] | NA |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CCI, Charlson comorbidity index; GJ, gastrojejunostomy; NA, not applicable; POPF, postoperative pancreatic fistula.
Logistic regression (n = 175, P value > χ2 = 0.0000, R2 = 0.21).
Discussion
In our study, we discovered that intraoperative techniques were associated with DGE and identified specific, modifiable technical factors for future DGE prevention strategies by using video review of RPD. Modifying known patient factors such as age, sex, smoking history, and preoperative comorbidity19,37 is not feasible; however, targeting intraoperative technical factors offers a unique opportunity to improve outcomes for patients. Our results show that the type I vertical GJ anastomosis flow angle to the efferent limb, greater length of the GJ anastomosis, and robotic-sewn GJ anastomosis were techniques for improving DGE independent from patient-related characteristics.
These 3 technical findings are supported by DGE studies in the literature. The GJ anastomosis flow angle to efferent limb, particularly type I, which is similar to vertical between the stomach and jejunum, has been evaluated in other studies as being associated with less DGE. This result has been supported by Sugiyama et al,25 Masui et al,26,38 and Murakami and Yasue,39 who proposed that the food is easily moved by gravity through the GJ anastomosis in this straightened position, as the stomach might act as a passive conduit into the jejunum owing to gastric atony, which may be present for 2 to 6 weeks after the procedure.22,23,24,25,26,40 In addition, greater length of the GJ anastomosis reduced DGE effectively. The mechanism is not known, but Khan et al41,42 commented that a long GJ anastomosis can avoid edema-induced strictures or passage problems. This finding was also supported by Walters et al,43 who concluded that a long GJ anastomosis (9 cm) closed by double stapling was more effective for reducing DGE than a routinely performed GJ anastomosis (4 cm). Also, the robotic-sewn GJ anastomosis decreased DGE on multivariate analysis compared with use of the stapler. Fujita et al44 also reported that patients had a higher frequency of DGE when the surgeons used a stapler for the GJ anastomosis because the inner mucosal healing occurred in a single phase after suturing in contrast to stapling, which caused a 2-phase healing owing to mucosal defect and epithelial healing over the granulation tissue. Consequently, this group postulated that delayed mucosal healing provoked DGE.
Other comparative technical studies of the GJ anastomoses have reported differences in rates of DGE. These reports indicated that standard PD had a trend toward a lower incidence of DGE than did a pylorus-preserving PD,45,46 that an antemesenteric method of jejunal reconstruction for pancreatobiliary limb had a lower risk of DGE than did a retromesenteric method,18,47 that an antecolic method of the GJ anastomosis decreased postoperative DGE compared with a retrocolic method,46,48,49,50 that Billroth II reconstruction of the GJ anastomosis decreased DGE compared with a Roux-en-Y and Billroth I reconstruction in meta-analysis,6,8,46 that adding a Braun jejunojejunostomy could also reduce DGE,46,51,52 and that a side-to-side anastomosis of the GJ anastomosis is associated with reducing DGE rather than an end-to-side anastomosis.53,54 Our basic GJ anastomosis techniques already incorporate many of these components at baseline: GJ anastomoses were performed with standard PD, retromesenteric jejunal reconstruction for pancreatobiliary limb, antecolic method of GJ anastomosis without a Braun jejunojejunostomy, and an end-to-side anastomosis of the GJ anastomosis by robotic suturing. Even though our technique incorporated most recommended GJ anastomosis reconstruction methods for improving DGE, the rate was still 21.4%. However, in these studies, all variables were not present, and those evaluated were assessed individually not in multivariate analysis.
In addition, as a surrogate for technical skill, our multivariate modeling showed a trend for increased rates of DGE (P = .05) when fellows performed the anastomosis, which shows that increased experience may correlate with decreased DGE. Because technical performance, such as a learning or proficiency curve, has been shown to correlate with the incidence of complications,33,55,56 robotic training programs and coaching on established intraoperative standard techniques are a useful way to optimize patient outcomes.31,32,57,58 Varban et al34,59 also noted that video-linked analysis with clinical outcomes can provide objective support for best practices, such as modifiable technical factors. We divided the GJ anastomosis into multiple ministeps to discern the best way of reducing DGE. Therefore, a combination of technical skill and modifiable technical factors may be necessary to improve patient outcomes.
Limitations
This study has many limitations. First, this was not a randomized clinical study but a retrospective observational study. Second, we did not have video for 100% of the cohort and the video was chronologically later, reflecting a mature program after its learning curve. Third, the rate of DGE was only 21.4%, and multiple variables were reviewed with only a small sample size of available videos; thus, the study potentially lacked power when performing analysis. There are also likely additional technical variables and other phenomena, such as the ileal brake,60 that we did not include in our analysis that could have affected the results. In addition, this analysis used the International Study Group of Pancreatic Surgery definition of DGE, which does not distinguish primary from secondary DGE and thus may overestimate DGE from technical considerations and not postoperative conditions. Also, because these were all RPD and not open procedures, direct extrapolation to a cohort that underwent open PD cannot be inferred, and a propensity-matched analysis was not able to be performed. This choice of RPD may include an inherent selection bias within the cohort in which the robotic cohort typically has fewer vascular resections and decreased pancreatic adenocarcinomas.1 Other published National Surgical Quality Improvement Program data on minimally invasive PD surgery by Nassour et al61 showed that incidences of DGE were 14.6% for RPD and 18.6% for laparoscopic PD. Despite these limitations, to our knowledge, this is the first study of technical review using operative video to identify important intraoperative techniques for improving DGE as a framework for prospective quality improvement.
As the next step, we are looking at studies of preoperative gastric emptying for patient variables, as gastric dysrhythmias and gastroparesis62 at baseline may be a contributing factor to postoperative DGE. Also, we are prospectively changing operative technique by measuring the GJ anastomosis to be 6 cm or greater and the flow angle of the efferent limb to be less than 30° to vertical, and we no longer staple our GJ anastomoses. If all 3 technical parameters are used, assuming a cohort with similar patient variables, it will take 80 potential patients to decrease the incidence of DGE by 50%.
Conclusions
To our knowledge, this is the first study evaluating intraoperative techniques for preventing DGE using video review as a study of modifiable technical factors. In the future, we will perform a prospective study based on flow angle, gastrotomy length, and sutured GJ anastomosis to improve our patient outcomes and be able to compare outcomes after incorporating quality improvement changes in 80 patients. This work is a proof of principle that modifiable technical factors can contribute to perioperative outcomes and that video review is a powerful tool to assess these techniques.
eTable. Descriptive Analysis of Patient Variables in the Entire Cohort With and Without Video
References
- 1.Zureikat AH, Postlewait LM, Liu Y, et al. . A multi-institutional comparison of perioperative outcomes of robotic and open pancreaticoduodenectomy. Ann Surg. 2016;264(4):640-649. doi: 10.1097/SLA.0000000000001869 [DOI] [PubMed] [Google Scholar]
- 2.McMillan MT, Zureikat AH, Hogg ME, et al. . A propensity score–matched analysis of robotic vs open pancreatoduodenectomy on incidence of pancreatic fistula. JAMA Surg. 2017;152(4):327-335. doi: 10.1001/jamasurg.2016.4755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winter JM, Brennan MF, Tang LH, et al. . Survival after resection of pancreatic adenocarcinoma: results from a single institution over three decades. Ann Surg Oncol. 2012;19(1):169-175. doi: 10.1245/s10434-011-1900-3 [DOI] [PubMed] [Google Scholar]
- 4.Emick DM, Riall TS, Cameron JL, et al. . Hospital readmission after pancreaticoduodenectomy. J Gastrointest Surg. 2006;10(9):1243-1252. doi: 10.1016/j.gassur.2006.08.016 [DOI] [PubMed] [Google Scholar]
- 5.Ahmad SA, Edwards MJ, Sutton JM, et al. . Factors influencing readmission after pancreaticoduodenectomy: a multi-institutional study of 1302 patients. Ann Surg. 2012;256(3):529-537. doi: 10.1097/SLA.0b013e318265ef0b [DOI] [PubMed] [Google Scholar]
- 6.Shimoda M, Kubota K, Katoh M, Kita J. Effect of Billroth II or Roux-en-Y reconstruction for the gastrojejunostomy on delayed gastric emptying after pancreaticoduodenectomy: a randomized controlled study. Ann Surg. 2013;257(5):938-942. doi: 10.1097/SLA.0b013e31826c3f90 [DOI] [PubMed] [Google Scholar]
- 7.Addeo P, Delpero JR, Paye F, et al. ; French Surgical Association (AFC) . Pancreatic fistula after a pancreaticoduodenectomy for ductal adenocarcinoma and its association with morbidity: a multicentre study of the French Surgical Association. HPB (Oxford). 2014;16(1):46-55. doi: 10.1111/hpb.12063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J, Wang C, Huang Q. Effect of Billroth II or Roux-en-Y reconstruction for the gastrojejunostomy after pancreaticoduodenectomy: meta-analysis of randomized controlled trials. J Gastrointest Surg. 2015;19(5):955-963. doi: 10.1007/s11605-015-2751-1 [DOI] [PubMed] [Google Scholar]
- 9.Yeo CJ, Cameron JL, Sohn TA, et al. . Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226(3):248-257. doi: 10.1097/00000658-199709000-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martignoni ME, Friess H, Sell F, et al. . Enteral nutrition prolongs delayed gastric emptying in patients after Whipple resection. Am J Surg. 2000;180(1):18-23. doi: 10.1016/S0002-9610(00)00418-9 [DOI] [PubMed] [Google Scholar]
- 11.Büchler MW, Wagner M, Schmied BM, Uhl W, Friess H, Z’graggen K. Changes in morbidity after pancreatic resection: toward the end of completion pancreatectomy. Arch Surg. 2003;138(12):1310-1314. doi: 10.1001/archsurg.138.12.1310 [DOI] [PubMed] [Google Scholar]
- 12.Wente MN, Bassi C, Dervenis C, et al. . Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007;142(5):761-768. doi: 10.1016/j.surg.2007.05.005 [DOI] [PubMed] [Google Scholar]
- 13.Cameron JL, He J. Two thousand consecutive pancreaticoduodenectomies. J Am Coll Surg. 2015;220(4):530-536. doi: 10.1016/j.jamcollsurg.2014.12.031 [DOI] [PubMed] [Google Scholar]
- 14.Chaudhary A, Barreto SG, Talole SD, Singh A, Perwaiz A, Singh T. Early discharge after pancreatoduodenectomy: what helps and what prevents? Pancreas. 2015;44(2):273-278. doi: 10.1097/MPA.0000000000000254 [DOI] [PubMed] [Google Scholar]
- 15.Bhatia S, Miller RC, Haddock MG, Donohue JH, Krishnan S. Adjuvant therapy for ampullary carcinomas: the Mayo Clinic experience. Int J Radiat Oncol Biol Phys. 2006;66(2):514-519. doi: 10.1016/j.ijrobp.2006.04.018 [DOI] [PubMed] [Google Scholar]
- 16.Marsh RDeW, Talamonti MS, Katz MH, Herman JM. Pancreatic cancer and FOLFIRINOX: a new era and new questions. Cancer Med. 2015;4(6):853-863. doi: 10.1002/cam4.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Berge Henegouwen MI, van Gulik TM, DeWit LT, et al. . Delayed gastric emptying after standard pancreaticoduodenectomy versus pylorus-preserving pancreaticoduodenectomy: an analysis of 200 consecutive patients. J Am Coll Surg. 1997;185(4):373-379. doi: 10.1016/S1072-7515(97)00078-1 [DOI] [PubMed] [Google Scholar]
- 18.Park YC, Kim SW, Jang JY, Ahn YJ, Park YH. Factors influencing delayed gastric emptying after pylorus-preserving pancreatoduodenectomy. J Am Coll Surg. 2003;196(6):859-865. doi: 10.1016/S1072-7515(03)00127-3 [DOI] [PubMed] [Google Scholar]
- 19.Eisenberg JD, Rosato EL, Lavu H, Yeo CJ, Winter JM. Delayed gastric emptying after pancreaticoduodenectomy: an analysis of risk factors and cost. J Gastrointest Surg. 2015;19(9):1572-1580. doi: 10.1007/s11605-015-2865-5 [DOI] [PubMed] [Google Scholar]
- 20.Park JS, Hwang HK, Kim JK, et al. . Clinical validation and risk factors for delayed gastric emptying based on the International Study Group of Pancreatic Surgery (ISGPS) classification. Surgery. 2009;146(5):882-887. doi: 10.1016/j.surg.2009.05.012 [DOI] [PubMed] [Google Scholar]
- 21.Welsch T, Borm M, Degrate L, Hinz U, Büchler MW, Wente MN. Evaluation of the International Study Group of Pancreatic Surgery definition of delayed gastric emptying after pancreatoduodenectomy in a high-volume centre. Br J Surg. 2010;97(7):1043-1050. doi: 10.1002/bjs.7071 [DOI] [PubMed] [Google Scholar]
- 22.Malfertheiner P, Sarr MG, Spencer MP, DiMagno EP. Effect of duodenectomy on interdigestive pancreatic secretion, gastrointestinal motility, and hormones in dogs. Am J Physiol. 1989;257(3, pt 1):G415-G422. [DOI] [PubMed] [Google Scholar]
- 23.Yeo CJ, Barry MK, Sauter PK, et al. . Erythromycin accelerates gastric emptying after pancreaticoduodenectomy: a prospective, randomized, placebo-controlled trial. Ann Surg. 1993;218(3):229-237. doi: 10.1097/00000658-199309000-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohwada S, Satoh Y, Kawate S, et al. . Low-dose erythromycin reduces delayed gastric emptying and improves gastric motility after Billroth I pylorus-preserving pancreaticoduodenectomy. Ann Surg. 2001;234(5):668-674. doi: 10.1097/00000658-200111000-00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugiyama M, Abe N, Ueki H, Masaki T, Mori T, Atomi Y. A new reconstruction method for preventing delayed gastric emptying after pylorus-preserving pancreatoduodenectomy. Am J Surg. 2004;187(6):743-746. doi: 10.1016/j.amjsurg.2003.10.013 [DOI] [PubMed] [Google Scholar]
- 26.Masui T, Kubora T, Nakanishi Y, et al. . The flow angle beneath the gastrojejunostomy predicts delayed gastric emptying in Roux-en-Y reconstruction after distal gastrectomy. Gastric Cancer. 2012;15(3):281-286. doi: 10.1007/s10120-011-0107-4 [DOI] [PubMed] [Google Scholar]
- 27.Girgis MD, Zenati MS, Steve J, et al. . Robotic approach mitigates perioperative morbidity in obese patients following pancreaticoduodenectomy. HPB (Oxford). 2017;19(2):93-98. doi: 10.1016/j.hpb.2016.11.008 [DOI] [PubMed] [Google Scholar]
- 28.Giulianotti PC, Coratti A, Angelini M, et al. . Robotics in general surgery: personal experience in a large community hospital. Arch Surg. 2003;138(7):777-784. doi: 10.1001/archsurg.138.7.777 [DOI] [PubMed] [Google Scholar]
- 29.Giulianotti PC, Sbrana F, Bianco FM, et al. . Robot-assisted laparoscopic pancreatic surgery: single-surgeon experience. Surg Endosc. 2010;24(7):1646-1657. doi: 10.1007/s00464-009-0825-4 [DOI] [PubMed] [Google Scholar]
- 30.Bao PQ, Mazirka PO, Watkins KT. Retrospective comparison of robot-assisted minimally invasive versus open pancreaticoduodenectomy for periampullary neoplasms. J Gastrointest Surg. 2014;18(4):682-689. doi: 10.1007/s11605-013-2410-3 [DOI] [PubMed] [Google Scholar]
- 31.Boone BA, Zenati M, Hogg ME, et al. . Assessment of quality outcomes for robotic pancreaticoduodenectomy: identification of the learning curve. JAMA Surg. 2015;150(5):416-422. doi: 10.1001/jamasurg.2015.17 [DOI] [PubMed] [Google Scholar]
- 32.Hogg ME, Zenati M, Novak S, et al. . Grading of surgeon technical performance predicts postoperative pancreatic fistula for pancreaticoduodenectomy independent of patient-related variables. Ann Surg. 2016;264(3):482-491. doi: 10.1097/SLA.0000000000001862 [DOI] [PubMed] [Google Scholar]
- 33.Birkmeyer JD, Finks JF, O’Reilly A, et al. ; Michigan Bariatric Surgery Collaborative . Surgical skill and complication rates after bariatric surgery. N Engl J Med. 2013;369(15):1434-1442. doi: 10.1056/NEJMsa1300625 [DOI] [PubMed] [Google Scholar]
- 34.Varban OA, Niemann A, Stricklen A, et al. . Far from standardized: using surgical videos to identify variation in technique for laparoscopic sleeve gastrectomy. J Laparoendosc Adv Surg Tech A. 2017;27(8):761-767. doi: 10.1089/lap.2017.0184 [DOI] [PubMed] [Google Scholar]
- 35.Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205-213. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tam V, Zenati M, Novak S, et al. . Robotic pancreatoduodenectomy biotissue curriculum has validity and improves technical performance for surgical oncology fellows. J Surg Educ. 2017;74(6):1057-1065. doi: 10.1016/j.jsurg.2017.05.016 [DOI] [PubMed] [Google Scholar]
- 37.Kunstman JW, Fonseca AL, Ciarleglio MM, Cong X, Hochberg A, Salem RR. Comprehensive analysis of variables affecting delayed gastric emptying following pancreaticoduodenectomy. J Gastrointest Surg. 2012;16(7):1354-1361. doi: 10.1007/s11605-012-1873-y [DOI] [PubMed] [Google Scholar]
- 38.Masui T, Doi R, Kawaguchi Y, Uemoto S. Delayed gastric emptying improved by straight stomach reconstruction with twisted anastomosis to the jejunum after pylorus-preserving pancreaticoduodenectomy (PPPD) in 118 consecutive patients at a single institution. Surg Today. 2012;42(5):441-446. doi: 10.1007/s00595-011-0097-1 [DOI] [PubMed] [Google Scholar]
- 39.Murakami H, Yasue M. A vertical stomach reconstruction after pylorus-preserving pancreaticoduodenectomy. Am J Surg. 2001;181(2):149-152. doi: 10.1016/S0002-9610(00)00556-0 [DOI] [PubMed] [Google Scholar]
- 40.Murakami Y, Uemura K, Sudo T, et al. . An antecolic Roux-en Y type reconstruction decreased delayed gastric emptying after pylorus-preserving pancreatoduodenectomy. J Gastrointest Surg. 2008;12(6):1081-1086. doi: 10.1007/s11605-008-0483-1 [DOI] [PubMed] [Google Scholar]
- 41.Khan AS, Hawkins WG, Linehan DC, Strasberg SM. A technique of gastrojejunostomy to reduce delayed gastric emptying after pancreatoduodenectomy. J Gastrointest Surg. 2011;15(8):1468-1471. doi: 10.1007/s11605-011-1471-4 [DOI] [PubMed] [Google Scholar]
- 42.Khan AS, Williams G, Woolsey C, et al. . Flange gastroenterostomy results in reduction in delayed gastric emptying after standard pancreaticoduodenectomy: a prospective cohort study. J Am Coll Surg. 2017;225(4):498-507. doi: 10.1016/j.jamcollsurg.2017.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walters DM, Shada AL, LaPar DJ, Adams RB, Bauer TW. A long gastrojejunostomy is associated with decreased incidence and severity of delayed gastric emptying after pancreaticoduodenectomy. Pancreas. 2015;44(8):1273-1279. doi: 10.1097/MPA.0000000000000415 [DOI] [PubMed] [Google Scholar]
- 44.Fujita T, Katai H, Morita S, Saka M, Fukagawa T, Sano T. Short-term outcomes of Roux-en-Y stapled anastomosis after distal gastrectomy for gastric adenocarcinoma. J Gastrointest Surg. 2010;14(2):289-294. doi: 10.1007/s11605-009-1082-5 [DOI] [PubMed] [Google Scholar]
- 45.Fujii T, Kanda M, Kodera Y, et al. . Preservation of the pyloric ring has little value in surgery for pancreatic head cancer: a comparative study comparing three surgical procedures. Ann Surg Oncol. 2012;19(1):176-183. doi: 10.1245/s10434-011-1901-2 [DOI] [PubMed] [Google Scholar]
- 46.Panwar R, Pal S. The International Study Group of Pancreatic Surgery definition of delayed gastric emptying and the effects of various surgical modifications on the occurrence of delayed gastric emptying after pancreatoduodenectomy. Hepatobiliary Pancreat Dis Int. 2017;16(4):353-363. doi: 10.1016/S1499-3872(17)60037-7 [DOI] [PubMed] [Google Scholar]
- 47.Butler TJ, Vair DB, Colohan S, McAlister VC. Multivariate analysis of technical variables in pancreaticoduodenectomy: the effect of pylorus preservation and retromesenteric jejunal position on early outcome. Can J Surg. 2004;47(5):333-337. [PMC free article] [PubMed] [Google Scholar]
- 48.Tani M, Terasawa H, Kawai M, et al. . Improvement of delayed gastric emptying in pylorus-preserving pancreaticoduodenectomy: results of a prospective, randomized, controlled trial. Ann Surg. 2006;243(3):316-320. doi: 10.1097/01.sla.0000201479.84934.ca [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eshuis WJ, de Bree K, Sprangers MA, et al. . Gastric emptying and quality of life after pancreatoduodenectomy with retrocolic or antecolic gastroenteric anastomosis. Br J Surg. 2015;102(9):1123-1132. doi: 10.1002/bjs.9812 [DOI] [PubMed] [Google Scholar]
- 50.Sahora K, Morales-Oyarvide V, Thayer SP, et al. . The effect of antecolic versus retrocolic reconstruction on delayed gastric emptying after classic non–pylorus-preserving pancreaticoduodenectomy. Am J Surg. 2015;209(6):1028-1035. doi: 10.1016/j.amjsurg.2014.04.015 [DOI] [PubMed] [Google Scholar]
- 51.Nikfarjam M, Houli N, Tufail F, Weinberg L, Muralidharan V, Christophi C. Reduction in delayed gastric emptying following non-pylorus preserving pancreaticoduodenectomy by addition of a Braun enteroenterostomy. JOP. 2012;13(5):488-496. [DOI] [PubMed] [Google Scholar]
- 52.Xu B, Meng H, Qian M, Gu H, Zhou B, Song Z. Braun enteroenterostomy during pancreaticoduodenectomy decreases postoperative delayed gastric emptying. Am J Surg. 2015;209(6):1036-1042. doi: 10.1016/j.amjsurg.2014.06.035 [DOI] [PubMed] [Google Scholar]
- 53.Nakamura T, Ambo Y, Noji T, et al. . Reduction of the incidence of delayed gastric emptying in side-to-side gastrojejunostomy in subtotal stomach-preserving pancreaticoduodenectomy. J Gastrointest Surg. 2015;19(8):1425-1432. doi: 10.1007/s11605-015-2870-8 [DOI] [PubMed] [Google Scholar]
- 54.Tsutaho A, Nakamura T, Asano T, et al. . Delayed gastric emptying in side-to-side gastrojejunostomy in pancreaticoduodenectomy: result of a propensity score matching. J Gastrointest Surg. 2017;21(10):1635-1642. doi: 10.1007/s11605-017-3540-9 [DOI] [PubMed] [Google Scholar]
- 55.Campbell DA Jr, Henderson WG, Englesbe MJ, et al. . Surgical site infection prevention: the importance of operative duration and blood transfusion—results of the first American College of Surgeons–National Surgical Quality Improvement Program Best Practices Initiative. J Am Coll Surg. 2008;207(6):810-820. doi: 10.1016/j.jamcollsurg.2008.08.018 [DOI] [PubMed] [Google Scholar]
- 56.Procter LD, Davenport DL, Bernard AC, Zwischenberger JB. General surgical operative duration is associated with increased risk-adjusted infectious complication rates and length of hospital stay. J Am Coll Surg. 2010;210(1):60-5.e1, 2. doi: 10.1016/j.jamcollsurg.2009.09.034 [DOI] [PubMed] [Google Scholar]
- 57.Zureikat AH, Nguyen KT, Bartlett DL, Zeh HJ, Moser AJ. Robotic-assisted major pancreatic resection and reconstruction. Arch Surg. 2011;146(3):256-261. doi: 10.1001/archsurg.2010.246 [DOI] [PubMed] [Google Scholar]
- 58.Zureikat AH, Moser AJ, Boone BA, Bartlett DL, Zenati M, Zeh HJ III. 250 Robotic pancreatic resections: safety and feasibility. Ann Surg. 2013;258(4):554-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Varban OA, Greenberg CC, Schram J, et al. ; Michigan Bariatric Surgery Collaborative . Surgical skill in bariatric surgery: does skill in one procedure predict outcomes for another? Surgery. 2016;160(5):1172-1181. doi: 10.1016/j.surg.2016.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barreto SG, Windsor JA. Does the ileal brake contribute to delayed gastric emptying after pancreatoduodenectomy? Dig Dis Sci. 2017;62(2):319-335. doi: 10.1007/s10620-016-4402-0 [DOI] [PubMed] [Google Scholar]
- 61.Nassour I, Wang SC, Porembka MR, et al. . Robotic versus laparoscopic pancreaticoduodenectomy: a NSQIP analysis. J Gastrointest Surg. 2017;21(11):1784-1792. doi: 10.1007/s11605-017-3543-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Grady G, Angeli TR, Du P, et al. . Abnormal initiation and conduction of slow-wave activity in gastroparesis, defined by high-resolution electrical mapping. Gastroenterology. 2012;143(3):589-598.e3. doi: 10.1053/j.gastro.2012.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Descriptive Analysis of Patient Variables in the Entire Cohort With and Without Video