Key Points
Question
Does increased daily water intake prevent cystitis in premenopausal women experiencing recurrent cystitis who drink low volumes of total fluid daily?
Findings
In this randomized clinical trial of 140 premenopausal women experiencing recurrent cystitis who report drinking less than 1.5 L of total fluid daily, cystitis episodes were significantly less frequent in women who drank more water for 12 months compared with women who maintained their usual fluid intake.
Meaning
Increasing daily water intake protects against recurrent cystitis in premenopausal women experiencing recurrent cystitis who drink low volumes of total fluid daily.
This randomized clinical trial assesses the efficacy of increased daily water intake vs usual fluid intake on the frequency of recurrent cystitis in premenopausal women experiencing recurrent cystitis who drink low volumes of total fluid daily.
Abstract
Importance
Increased hydration is often recommended as a preventive measure for women with recurrent cystitis, but supportive data are sparse.
Objective
To assess the efficacy of increased daily water intake on the frequency of recurrent cystitis in premenopausal women.
Design, Setting, and Participants
Randomized, open-label, controlled, 12-month trial at a clinical research center (years 2013-2016). Among 163 healthy women with recurrent cystitis (≥3 episodes in past year) drinking less than 1.5 L of fluid daily assessed for eligibility, 23 were excluded and 140 assigned to water or control group. Assessments of daily fluid intake, urinary hydration, and cystitis symptoms were performed at baseline, 6- and 12-month visits, and monthly telephone calls.
Interventions
Participants were randomly assigned to drink, in addition to their usual fluid intake, 1.5 L of water daily (water group) or no additional fluids (control group) for 12 months.
Main Outcomes and Measures
Primary outcome measure was frequency of recurrent cystitis over 12 months. Secondary outcomes were number of antimicrobial regimens used, mean time interval between cystitis episodes, and 24-hour urinary hydration measurements.
Results
The mean (SD) age of the 140 participants was 35.7 (8.4) years, and the mean (SD) number of cystitis episodes in the previous year was 3.3 (0.6). During the 12-month study period, the mean (SD) number of cystitis episodes was 1.7 (95% CI, 1.5-1.8) in the water group compared with 3.2 (95% CI, 3.0-3.4) in the control group, with a difference in means of 1.5 (95% CI, 1.2-1.8; P < .001). Overall, there were 327 cystitis episodes, 111 in the water group and 216 in the control group. The mean number of antimicrobial regimens used to treat cystitis episodes was 1.9 (95% CI, 1.7-2.2) and 3.6 (95% CI, 3.3-4.0), respectively, with a difference in means of 1.7 (95% CI, 1.3-2.1; P < .001). The mean time interval between cystitis episodes was 142.8 (95% CI, 127.4-160.1) and 84.4 (95% CI, 75.4-94.5) days, respectively, with a difference in means of 58.4 (95% CI, 39.4-77.4; P < .001). Between baseline and 12 months, participants in the water group, compared with those in the control group, had increased mean (SD) urine volume (1.4 [0.04] vs 0.1 [0.04] L; P < .001) and voids (2.4 [0.2] vs −0.1 [0.2]; P < .001) and decreased urine osmolality (−402.8 [19.6] vs −24.0 [19.5] mOsm/kg; P < .001).
Conclusions and Relevance
Increased water intake is an effective antimicrobial-sparing strategy to prevent recurrent cystitis in premenopausal women at high risk for recurrence who drink low volumes of fluid daily.
Trial Registration
ClinicalTrials.gov identifier: NCT02444975
Introduction
Acute uncomplicated cystitis is one of the most common infectious diseases in women, with a lifetime risk of greater than 50%.1 Approximately 27% of women with their first episode of cystitis will have at least 1 recurrence within 6 months,2 and among women with previous urinary tract infection (UTI), 44% to 70% will have a recurrence within 1 year.3,4 Many women with cystitis have significant morbidities such as pain, general discomfort, and decreased quality of life.5,6
It is estimated that approximately 15% of antimicrobial use in humans is for treatment of UTI.7 In addition, antimicrobials are often used to prevent recurrences of cystitis if antimicrobial-sparing approaches are ineffective.8 The degree to which antimicrobial use for treatment or prevention of cystitis contributes to the worldwide problem of antimicrobial resistance is unknown, but it is almost certainly substantial given the frequent occurrence of UTI and the strong correlation between antimicrobial use and resistance.9,10,11,12
The World Health Organization and others have highlighted the urgent need for novel antimicrobial-sparing approaches to infectious diseases.12,13,14,15 In this regard, women with recurrent cystitis are often counseled about behavioral approaches before antimicrobial prevention strategies are considered.8 One common recommendation is to increase hydration, based on the belief that dilution and flushing of bacteriuria is beneficial.16,17,18,19,20,21,22 However, published studies on the association between hydration status and risk of UTI are sparse and unconvincing.20,23,24,25,26,27,28,29 We therefore conducted a randomized clinical trial to determine whether increased daily water intake reduces the risk of recurrent cystitis in healthy premenopausal women with a history of recurrent cystitis.
Methods
Study Design and Oversight
This was a randomized, open-label, controlled study of increased water intake in women with recurrent cystitis. The study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki at COMAC Medical, a clinical research center based in Sofia, Bulgaria, whose ethics committee approved the study. Study methods and reporting follow recommended guidelines for randomized trials of nonpharmacological treatments. The investigators together with the sponsor designed the trial and had primary responsibility for protocol development. Trial oversight was provided by Pharm-Olam International Bulgaria Contract Research Organization (CRO). Analyses pertaining to the primary and secondary outcomes were performed by Lincoln CRO, Boulogne-Billancourt, France. The protocol is available in Supplement 1.
Participants
The COMAC principal investigator contacted local physicians in Sofia who were in their registry of referring physicians and who provide primary care to women with recurrent UTI and explained the requirements for study eligibility. Potential participants were asked to visit the COMAC facility for a screening visit during which inclusion and exclusion criteria were verified. Premenopausal women were eligible for the study if they were at least 18 years of age, in good general health, had no current UTI symptoms, reported at least 3 symptomatic episodes of cystitis in the past year resulting in a visit to a clinician (documentation required), at least 1 episode of which had to be culture confirmed (≥103 colony-forming units [CFU]/mL bacteria in a voided midstream urine culture), and self-reported drinking less than 1.5 L of fluid daily. Women were not eligible for enrollment if they had current symptoms of UTI, a history of pyelonephritis in the past 12 months, interstitial cystitis, symptomatic vulvovaginitis, or if they were pregnant, lactating, or planning to become pregnant in the following 12 months. All participants provided written informed consent.
Randomization
Eligible participants were randomly assigned (1:1) to drink either 1.5 L of water daily in addition to their usual fluid intake (water group) or no additional fluids (control group) for 12 months. Women in the water group were provided three 500-mL bottles of water (Evian) to be consumed daily, along with the suggestion to start a bottle at the beginning of every meal and fully drink it before the next meal. The intervention was assigned centrally by telephone, using a centralized interactive web response system that allocated participants using a computer-generated randomization list without stratification factors. The randomization list was prepared by an independent statistician and was concealed until allocation. Access was given to the COMAC research staff through login-based permission to randomize eligible participants.
Study Procedures
Before randomization, participants were asked to complete a 3-day fluid intake diary (eMethods 1 in Supplement 2) to record the type and amount of all beverages consumed during 3 consecutive days. Participants then returned for a prerandomization visit (baseline). Urine volume and osmolality were assessed with a 24-hour urine sample collected the day before the baseline visit. Participants were asked to start urine collections in the morning, after discarding the first-voided sample, and to collect all the voids in the following 24 hours including the first-voided urine the next morning. To continue in the study, participants had to have a 24-hour urine volume less than 1.2 L, and a 24-hour urine osmolality of at least 500 mOsm/kg. This latter inclusion criterion was added as an amendment to the protocol to refine our definition of a low-volume drinker.
After randomization, participants were contacted for telephone interviews by the COMAC research staff every month for 12 months. At each call, participants were asked about current or recent urinary symptoms, adverse events, medications taken, and adherence to the study protocol. Women in the water group were encouraged to adhere to the hydration protocol. Fluid intake was assessed monthly using the self-reported 3-day fluid intake diary, and 24-hour urine volume and osmolality were assessed at 6 and 12 months. During the 24-hour collection period, participants were asked to report their daily voids in a voiding diary (eMethods 2 in Supplement 2). Bottled water was delivered to the homes of participants assigned to the water group every 2 weeks.
Participants were instructed to contact the study staff at COMAC, or a clinician outside COMAC if they so chose, any time they experienced any urinary symptoms to perform a midstream urine culture. All clinical, microbiologic, and treatment data from outside facilities were requested to be provided to COMAC investigators once they were notified by study participants. Criteria for diagnosis of cystitis were the same for COMAC and non-COMAC facilities.
Outcomes
The primary study outcome was frequency of recurrent cystitis episodes over 12 months, defined as the presence of at least 1 UTI symptom (dysuria, frequency, urgency, and/or suprapubic pain) plus at least 103 CFU/mL uropathogens in a midstream urine culture.30,31 Uropathogens included gram-negative bacteria, staphylococci (including Staphylococcus aureus, Staphylococcus saprophyticus, and other coagulase-negative staphylococci), enterococci, and group B streptococci. Secondary study outcomes were the number of antimicrobial regimens used, mean time interval between cystitis episodes, and 24-hour urinary hydration measurements (volume, voids, and osmolality). We also assessed the time to first cystitis episode. Safety was evaluated by assessment of adverse events, defined as any unfavorable and unintended symptom or sign, and serious adverse events, defined as death, life-threatening event, hospitalization, or significant disability.
Sample Size
We assumed that the frequency of recurrent cystitis episodes in the control group would be unchanged (3 over a 12-month period) and that a reduction of at least 20% in the water group would be clinically meaningful. Based on these assumptions, a sample size of 42 evaluable participants per group was necessary to achieve 80% power to detect a difference with a 2-sided type I error rate of 5% in a Poisson model. Anticipating a 40% dropout rate, 70 participants per group were randomized.
Statistical Analyses
All continuous variables were calculated as mean (standard deviation), mean (range), mean (95% confidence interval), or median (range). Categorical data were calculated as frequency and percentage. The statistical analyses were performed on all participants who underwent randomization, according to the intention-to-treat principle. As a sensitivity analysis, the statistical analyses were also performed on the per-protocol population.
Descriptive analyses of urinary hydration measurements were performed on women who completed the 6- and 12-month follow-ups. All P values for analyses other than the primary outcome are nominal. Given the limited amount of missing data, no imputation method was used. Data management and statistical analyses were performed using SAS, version 9.2 (SAS Institute Inc).
Primary Outcome
An unadjusted Poisson model was used to compare the frequency of cystitis episodes over the study period between the study groups. The P value considered for statistical significance was .05.
Secondary Outcomes
The number of antimicrobial regimens used for cystitis during the study was compared using a Poisson model, adjusted for age. Time intervals between episodes of cystitis were compared using a γ model, adjusted for age. The 24-hour urinary hydration measurements were analyzed using repeated-measures analysis of covariance, adjusted for age, to compare the changes from baseline. Statistical analysis performed for urine voids was post hoc. Time to first cystitis episode was compared between the study groups using a Cox model adjusted for age (post hoc analysis).
Results
Study Participants
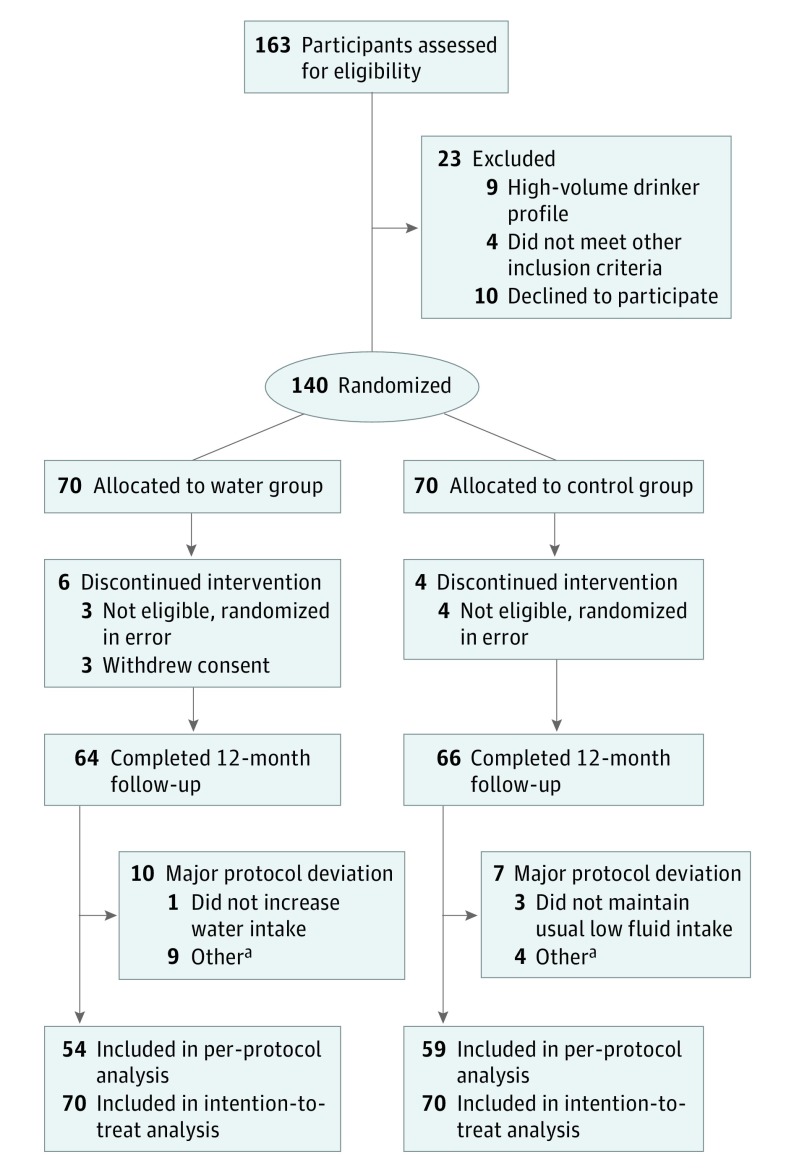
Patient enrollment and follow-up occurred between December 13, 2013, and July 13, 2016. In September 2014, we halted study enrollment to revise the protocol to add the 24-hour urine osmolality requirement to the inclusion criteria, and we restarted enrollment in June 2015. A total of 163 women were screened for participation; 23 were excluded owing to unwillingness to participate or not meeting inclusion criteria (Figure 1). The remaining 140 eligible women were randomly assigned to the water or control groups. After the protocol amendment to add the urine osmolality requirement, 7 participants (3 water group and 4 control group) were dropped from the study because they no longer met the enrollment criteria of a low-volume drinker. Three participants (all in the water group) withdrew their consent and were dropped from the study. In addition, 17 participants (10 water group and 7 control group) had major protocol deviations (Figure 1). Sixty-four (91%) participants in the water group and 66 (94%) in the control group completed the 12-month follow-up, and 54 (77%) and 59 (84%), respectively, completed the 12-month study without any major protocol deviation (per-protocol population) (Figure 1).
Figure 1. Study Flow Algorithm.
aOther major protocol deviations include time interval between randomization and first study product intake greater than 3 days, and 12-month visit occurred later than 389 days. Primary and secondary outcomes analyses are based on the intention-to-treat population (all 140 women who underwent randomization).
Demographic and behavioral characteristics at enrollment were similar between the 2 groups (Table 1). The mean (SD) age was 35.7 (8.4) years, 129 (92%) were sexually active, and the mean number of cystitis episodes in the previous 12 months was 3.3 (range, 3-6). Our study population was largely healthy with no significant comorbidities. The baseline daily fluid intake and urinary hydration measurements were similar between the 2 groups.
Table 1. Baseline Characteristics of the Intention-to-Treat Population.
| Parameter | Value | ||
|---|---|---|---|
| Water Group (n = 70) | Control Group (n = 70) | All (N = 140) | |
| Age, y, mean (SD) | 36.0 (7.8) | 35.3 (9.0) | 35.7 (8.4) |
| BMI, mean (SD) | 23.3 (3.5) | 23.2 (3.5) | 23.3 (3.5) |
| Sexually active during the past month, No. (%) | 64 (91) | 65 (93) | 129 (92) |
| No. of episodes of cystitis in past 12 mo, mean (SD) | 3.4 (0.6) | 3.3 (0.5) | 3.3 (0.6) |
| 3 Episodes in past 12 mo, No. (%) of women | 48 (69) | 53 (76) | 101 (72) |
| ≥4 Episodes in past 12 mo, No. (%) of women | 22 (31) | 17 (24) | 39 (28) |
| Daily fluid intake, mean (SD), L/da | 1.1 (0.1) | 1.1 (0.2) | 1.1 (0.2) |
| Water | 0.5 (0.2) | 0.5 (0.3) | 0.5 (0.2) |
| Hot drinks | 0.2 (0.1) | 0.2 (0.1) | 0.2 (0.1) |
| Alcohol | 0.1 (0.1) | 0.1 (0.1) | 0.1 (0.1) |
| Other | 0.3 (0.2) | 0.3 (0.2) | 0.3 (0.2) |
| 24-h urine volume, mean (SD), L | 0.9 (0.2) | 0.9 (0.2) | 0.9 (0.2) |
| No. of voids/d, mean (SD)a | 6.0 (1.6) | 6.2 (1.6) | 6.1 (1.6) |
| 24-h urine osmolality, mean (SD), mOsm/kg | 720.6 (169.2) | 728.1 (161.2) | 724.3 (164.7) |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
Day = 24 h.
Self-reported Adherence to Intervention
Self-reported daily fluid intake increased in the water group after randomization and remained elevated throughout the study. At month 12, mean daily fluid intake had increased by 1.7 L (range, 1.1-2.8 L) and mean daily water intake by 1.15 L (range, 0.48-1.63 L) above baseline in the water group. Neither parameter changed in the control group.
Outcomes
Primary
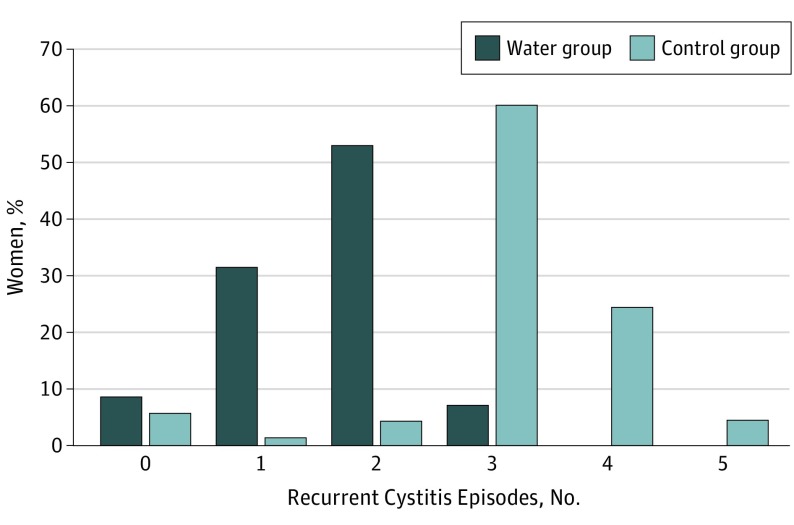
Over the 12-month study, the mean number of cystitis episodes was 1.7 (95% CI, 1.5-1.8) in the water group compared with 3.2 (95% CI, 3.0-3.4) in the control group, with a difference in means of 1.5 (95% CI, 1.2-1.8; P < .001). Overall, there were 327 cystitis episodes, 111 in the water group and 216 in the control group. Analyses performed on the per-protocol population showed similar results. A total of 93% of women in the water group had 2 or fewer episodes of cystitis whereas 88% in the control group had 3 or more episodes (median, 2 and 3, respectively) (Figure 2).
Figure 2. Recurrent Cystitis Episodes by Study Group.
Number of recurrent cystitis episodes during the 12-month follow-up, percent of women by study group. All 140 women who underwent randomization were included in the analysis.
Twenty-four (7%) of the 327 cystitis episodes were diagnosed and managed at non-COMAC facilities: 9 (8%) of 111 episodes in the water group and 15 (7%) of 216 in the control group.
Study participants reported only 1 episode of cystitis symptoms for which no urine culture was performed. Among the 416 episodes in which women presented with cystitis symptoms and had a urine culture performed, there was no difference between the study groups in the proportion that had positive results: 111 (77%) of 144 in the water group compared with 216 (79%) of 272 in the control group.
Escherichia coli was the causative uropathogen in 87 (78%) and 167 (77%), Klebsiella species in 9% and 10%, and Proteus species in 4% and 5% in the water and control groups, respectively.
Secondary
The mean number of antimicrobial regimens used to treat cystitis episodes was 1.9 (95% CI, 1.7-2.2) in the water group compared with 3.6 (95% CI, 3.3-4.0) in the control group, with a difference in means of 1.7 (95% CI, 1.3-2.1; P < .001). The mean time interval between cystitis episodes was 142.8 (95% CI, 127.4-160.1) and 84.4 (95% CI, 75.4-94.5) days, respectively, with a difference in means of 58.4 (95% CI, 39.4-77.4) days (P < .001). The median time to the first cystitis episode was 148.0 (range, 8.0-369.0) days in the water group compared with 93.5 (range, 7.0-291.0) days in the control group (hazard ratio, 0.51; 95% CI, 0.36-0.74; P < .001).
Over the study, mean 24-hour urine volumes increased by 1.3 L in the water group compared with 0.1 L in the control group (P < .001) (Table 2). The mean number of daily voids increased and urine osmolality decreased in the water group compared with no changes in the control group (P < .001 for both parameters). Data at 6 months for both groups were similar (Table 2).
Table 2. Urine Volume, Daily Voids, and Urine Osmolality Over 12-mo Follow-up.
| Visit | Water Group | Control Group | P Valuea | ||
|---|---|---|---|---|---|
| Mean (SD) | No. | Mean (SD) | No. | ||
| 24-h urine volume, L/db | |||||
| Baseline | 0.9 (0.2) | 70 | 0.9 (0.2) | 70 | |
| 6 mo | 2.2 (0.4) | 65 | 0.9 (0.2) | 66 | <.001 |
| 12 mo | 2.2 (0.3) | 64 | 1.0 (0.2) | 66 | <.001 |
| No. of voids/db | |||||
| Baseline | 6.0 (1.6) | 70 | 6.2 (1.6) | 70 | |
| 6 mo | 8.8 (2.4) | 65 | 5.9 (1.4) | 66 | <.001 |
| 12 mo | 8.2 (1.6) | 64 | 5.9 (1.1) | 66 | <.001 |
| 24-h urine osmolality, mOsm/kg | |||||
| Baseline | 720.6 (169.2) | 70 | 728.1 (161.2) | 70 | |
| 6 mo | 330.6 (108.4) | 65 | 742.2 (193.5) | 66 | <.001 |
| 12 mo | 329.2 (116.7) | 64 | 712.6 (193.3) | 66 | <.001 |
P values indicate the difference between water and control groups for the change from baseline to 6- and 12-mo visits.
Day = 24 h.
Adverse Events
Occurrences of adverse events were similar between the 2 groups. The most common were headache, reported by 12 women, and gastrointestinal symptoms, reported by 8, in each group. No serious adverse events occurred.
Discussion
Acute uncomplicated cystitis in women is one of the most common diagnoses, and its treatment accounts for considerable antimicrobial use.7 Given the association between antimicrobial use and the growing problem of antimicrobial resistance,9,10,11,12 novel antimicrobial-sparing strategies are needed to treat infections.12,13,14,15 Antimicrobial-sparing strategies for prevention of recurrent cystitis in women include education about risk factors such as sexual intercourse and behavioral counseling to liberalize fluid intake, not to delay urination, to urinate soon after intercourse, and to ensure good pelvic hygiene.8,20,32(pp344-345)33,34 Prior to this trial, however, there have been no prospective randomized clinical trials to assess the effectiveness of such measures.
The benefit of increased fluid intake for prevention of cystitis is thought to be from dilution and flushing of bacteriuria, thereby reducing attachment to uroepithelial cells, reducing nutrients for growth, and/or improving clearance.16,17,18,19,20,21,22 However, previously published evidence for a relationship between low fluid intake and/or low frequency of urination and cystitis is sparse and unconvincing.20,23,24,25,26,27,28,29 For example, case-control studies have not shown that low fluid intake is associated with recurrent UTIs, but these studies have not clearly defined low fluid intake.23,24 Several studies in women have shown an association between self-reported low fluid intake (usually less than 1 L daily) or low voiding frequency (usually <3 times daily) and UTI frequency, but they were nonrandomized and uncontrolled.25,26,27,28,29 Some of these studies demonstrated a reduced rate of self-reported UTI following educational campaigns to encourage women to increase water intake and not delay urination.26,28 The only published interventional study of hydration is limited by the small sample size, lack of definition for UTI, and no assessment of fluid intake.35
This study is the first randomized clinical trial to evaluate increased hydration for prevention of recurrent cystitis in women. We demonstrated that increasing daily water intake over a 12-month period resulted in an approximately 50% reduction in frequency of cystitis recurrences and a similar reduction in use of antimicrobial regimens. In addition, there were significant increases in days to first cystitis recurrence and between episodes. Although not as effective as antimicrobial prophylaxis, which has been shown to reduce the risk of cystitis recurrence by approximately 85% to 95%,8,36,37 the beneficial effects observed using water, which is safe, inexpensive, and does not select for antimicrobial resistance, are substantial and important.
We selected for this study women who self-reported daily fluid intake less than 1.5 L. These women are considered low-volume fluid drinkers based on recommendations of the European Food Safety Agency, which recommends a daily water intake from fluids of 1.6 L for women,38 and the Institute of Medicine, which recommends a daily water intake from fluids of 2.2 L.39 Daily water intake among women is often lower than these recommended amounts as shown in a study of 8696 women from 13 countries, which demonstrated that, on average, 40% of women (60% in some countries) report drinking less than 1.6 L daily.40
Strengths and Limitations
The strengths of our study are the similarities in demographic and behavioral characteristics between intervention and control groups, the extended study duration with 93% of participants completing all 12 months, the monthly telephone calls to query participants about symptoms and to encourage adherence to the protocol, use of objective measures for urine volume and osmolality to assess protocol adherence, and requirement for culture confirmation of cystitis. These strengths help to mitigate the unavoidable use of an open-label study design to study a hydration intervention. Although the study was conducted at a single site, the demographic characteristics of our study population, young healthy women with frequent recurrent UTIs, are similar to those in other studies of uncomplicated cystitis.41 We believe that our study findings are generalizable to this population of healthy premenopausal women.
Conclusions
Our data confirm the benefit of increased water intake in reducing the risk of recurrent cystitis in women with a history of frequent recurrent cystitis who are low-volume fluid drinkers. We did not perform a dose-response study, so we do not know what increment in daily water intake is sufficient for reducing risk of UTI. In addition, we do not know whether increased water intake is beneficial in women who are at lower risk for recurrent cystitis or who regularly drink higher quantities of fluid than women in this study. Of note, there are no published data on the proportion of women with recurrent UTI who are low-volume drinkers. Nevertheless, it seems appropriate for clinicians who counsel healthy women with recurrent cystitis to routinely ask about daily fluid intake and to recommend increased intake of water, especially in those who drink no more than 1.5 L of fluids daily, as a safe and inexpensive alternative to strategies that employ antimicrobials. The resulting reduction in antimicrobial use for treatment and prevention of cystitis in women is likely to have an important beneficial effect on antimicrobial resistance.42
Trial Protocol
eMethods 1. 3-days Fluid Intake Diary
eMethods 2. Voiding Diary
References
- 1.Foxman B, Barlow R, D’Arcy H, Gillespie B, Sobel JD. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol. 2000;10(8):509-515. doi: 10.1016/S1047-2797(00)00072-7 [DOI] [PubMed] [Google Scholar]
- 2.Foxman B. Recurring urinary tract infection: incidence and risk factors. Am J Public Health. 1990;80(3):331-333. doi: 10.2105/AJPH.80.3.331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hooton TM, Scholes D, Hughes JP, et al. . A prospective study of risk factors for symptomatic urinary tract infection in young women. N Engl J Med. 1996;335(7):468-474. doi: 10.1056/NEJM199608153350703 [DOI] [PubMed] [Google Scholar]
- 4.Ikäheimo R, Siitonen A, Heiskanen T, et al. . Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis. 1996;22(1):91-99. doi: 10.1093/clinids/22.1.91 [DOI] [PubMed] [Google Scholar]
- 5.Ellis AK, Verma S. Quality of life in women with urinary tract infections: is benign disease a misnomer? J Am Board Fam Pract. 2000;13(6):392-397. doi: 10.3122/15572625-13-6-392 [DOI] [PubMed] [Google Scholar]
- 6.Wagenlehner F, Wullt B, Ballarini S, Zingg D, Naber KG. Social and economic burden of recurrent urinary tract infections and quality of life: a patient web-based study (GESPRIT). Expert Rev Pharmacoecon Outcomes Res. 2018;18(1):107-117. [DOI] [PubMed] [Google Scholar]
- 7.Mazzulli T. Resistance trends in urinary tract pathogens and impact on management. J Urol. 2002;168(4 Pt 2):1720-1722. doi: 10.1097/00005392-200210020-00015 [DOI] [PubMed] [Google Scholar]
- 8.Hooton TM. Uncomplicated urinary tract infection. N Engl J Med. 2012;366(11):1028-1037. doi: 10.1056/NEJMcp1104429 [DOI] [PubMed] [Google Scholar]
- 9.Bronzwaer SL, Cars O, Buchholz U, et al. ; European Antimicrobial Resistance Surveillance System . A European study on the relationship between antimicrobial use and antimicrobial resistance. Emerg Infect Dis. 2002;8(3):278-282. doi: 10.3201/eid0803.010192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joseph NM, Bhanupriya B, Shewade DG, Harish BN. Relationship between antimicrobial consumption and the incidence of antimicrobial resistance in Escherichia coli and Klebsiella pneumoniae isolates. J Clin Diagn Res. 2015;9(2):DC08-DC12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096. doi: 10.1136/bmj.c2096 [DOI] [PubMed] [Google Scholar]
- 12.Harbarth S, Balkhy HH, Goossens H, et al. . Antimicrobial resistance: one world, one fight! Antimicrob Resist Infect Control. 2015;4:49. doi: 10.1186/s13756-015-0091-2 [DOI] [Google Scholar]
- 13.World Health Organization Antibacterial agents in clinical development: an analysis of the antibacterial clinical development pipeline, including tuberculosis. WHO/EMP/IAU/2017.12. Geneva, Switzerland: World Health Organization; 2017. [Google Scholar]
- 14.Mydock-McGrane L, Cusumano Z, Han Z, et al. . Antivirulence C-mannosides as antibiotic-sparing, oral therapeutics for urinary tract infections. J Med Chem. 2016;59(20):9390-9408. doi: 10.1021/acs.jmedchem.6b00948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brumbaugh AR, Mobley HL. Preventing urinary tract infection: progress toward an effective Escherichia coli vaccine. Expert Rev Vaccines. 2012;11(6):663-676. doi: 10.1586/erv.12.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts AP, Robinson RE, Beard RW. Some factors affecting bacterial colony counts in urinary infection. Br Med J. 1967;1(5537):400-403. doi: 10.1136/bmj.1.5537.400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Grady F, Cattell WR. Kinetics of urinary tract infection. II. the bladder. Br J Urol. 1966;38(2):156-162. doi: 10.1111/j.1464-410X.1966.tb09694.x [DOI] [PubMed] [Google Scholar]
- 18.Cattell WR, Fry IK, Spiro FI, Sardeson JM, Sutcliffe MB, O’Grady F. Effect of diuresis and frequent micturition on the bacterial content of infected urine: a measure of competence of intrinsic hydrokinetic clearance mechanisms. Br J Urol. 1970;42(3):290-295. doi: 10.1111/j.1464-410X.1970.tb11922.x [DOI] [PubMed] [Google Scholar]
- 19.Friedman SA, Gladstone JL. The effects of hydration and bladder incubation time on urine colony counts. J Urol. 1971;105(3):428-432. doi: 10.1016/S0022-5347(17)61542-8 [DOI] [PubMed] [Google Scholar]
- 20.Beetz R. Mild dehydration: a risk factor of urinary tract infection? Eur J Clin Nutr. 2003;57(suppl 2):S52-S58. doi: 10.1038/sj.ejcn.1601902 [DOI] [PubMed] [Google Scholar]
- 21.Cox CE, Hinman F Jr. Retention catheterization and the bladder defense mechanism. JAMA. 1965;191:171-174. doi: 10.1001/jama.1965.03080030015003 [DOI] [PubMed] [Google Scholar]
- 22.Tian Y, Cai X, Wazir R, Wang K, Li H. Water consumption and urinary tract infections: an in vitro study. Int Urol Nephrol. 2016;48(6):949-954. doi: 10.1007/s11255-016-1262-7 [DOI] [PubMed] [Google Scholar]
- 23.Remis RS, Gurwith MJ, Gurwith D, Hargrett-Bean NT, Layde PM. Risk factors for urinary tract infection. Am J Epidemiol. 1987;126(4):685-694. doi: 10.1093/oxfordjournals.aje.a114708 [DOI] [PubMed] [Google Scholar]
- 24.Scholes D, Hooton TM, Roberts PL, Stapleton AE, Gupta K, Stamm WE. Risk factors for recurrent urinary tract infection in young women. J Infect Dis. 2000;182(4):1177-1182. doi: 10.1086/315827 [DOI] [PubMed] [Google Scholar]
- 25.Vyas S, Varshney D, Sharma P, Juyal R, Nautiyal V, Shrotriya V. An overview of the predictors of symptomatic urinary tract infection among nursing students. Ann Med Health Sci Res. 2015;5(1):54-58. doi: 10.4103/2141-9248.149790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su SB, Wang JN, Lu CW, Guo HR. Reducing urinary tract infections among female clean room workers. J Womens Health (Larchmt). 2006;15(7):870-876. doi: 10.1089/jwh.2006.15.870 [DOI] [PubMed] [Google Scholar]
- 27.Nygaard I, Linder M. Thirst at work—an occupational hazard? Int Urogynecol J Pelvic Floor Dysfunct. 1997;8(6):340-343. doi: 10.1007/BF02765593 [DOI] [PubMed] [Google Scholar]
- 28.Adatto K, Doebele KG, Galland L, Granowetter L. Behavioral factors and urinary tract infection. JAMA. 1979;241(23):2525-2526. doi: 10.1001/jama.1979.03290490031020 [DOI] [PubMed] [Google Scholar]
- 29.Nielsen AF, Walter S. Epidemiology of infrequent voiding and associated symptoms. Scand J Urol Nephrol Suppl. 1994;157:49-53. [PubMed] [Google Scholar]
- 30.Grabe M, Bartoletti R, Bjerklund Johansen TE, et al. Guidelines on urological infections. European Association of Urology; 2015. https://uroweb.org/wp-content/uploads/19-Urological-infections_LR2.pdf.
- 31.Hooton TM, Roberts PL, Cox ME, Stapleton AE. Voided midstream urine culture and acute cystitis in premenopausal women. N Engl J Med. 2013;369(20):1883-1891. doi: 10.1056/NEJMoa1302186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kunin C. Urinary Tract Infections: Detection, Prevention and Management. 5th ed Baltimore, MD: Williams and Wilkins; 1997. [Google Scholar]
- 33.Beerepoot M, Geerlings S. Non-antibiotic prophylaxis for urinary tract infections. Pathogens. 2016;5(2):E36. doi: 10.3390/pathogens5020036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nosseir SB, Lind LR, Winkler HA. Recurrent uncomplicated urinary tract infections in women: a review. J Womens Health (Larchmt). 2012;21(3):347-354. doi: 10.1089/jwh.2011.3056 [DOI] [PubMed] [Google Scholar]
- 35.Eckford SD, Keane DP, Lamond E, Jackson SR, Abrams P. Hydration monitoring in the prevention of recurrent idiopathic urinary tract infections in pre-menopausal women. Br J Urol. 1995;76(1):90-93. doi: 10.1111/j.1464-410X.1995.tb07839.x [DOI] [PubMed] [Google Scholar]
- 36.Albert X, Huertas I, Pereiró II, Sanfélix J, Gosalbes V, Perrota C. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev. 2004;(3):CD001209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicolle LE. Uncomplicated urinary tract infection in adults including uncomplicated pyelonephritis. Urol Clin North Am. 2008;35(1):1-12. doi: 10.1016/j.ucl.2007.09.004 [DOI] [PubMed] [Google Scholar]
- 38.EFSA Panel on Dietetic Products Nutrition and Allergies (NDA) Scientific opinion on dietary reference values for water. EFSA J. 2010;8(3):1459. doi: 10.2903/j.efsa.2010.1459 [DOI] [Google Scholar]
- 39.Institute of Medicine Dietary reference intakes for water, potassium, sodium, chloride, and sulfate. Washington, DC: The National Academies Press; 2005. https://www.nap.edu/read/10925/chapter/1.
- 40.Ferreira-Pêgo C, Guelinckx I, Moreno LA, et al. . Total fluid intake and its determinants: cross-sectional surveys among adults in 13 countries worldwide. Eur J Nutr. 2015;54(suppl 2):35-43. doi: 10.1007/s00394-015-0943-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beerepoot MA, ter Riet G, Nys S, et al. . Cranberries vs antibiotics to prevent urinary tract infections: a randomized double-blind noninferiority trial in premenopausal women. Arch Intern Med. 2011;171(14):1270-1278. doi: 10.1001/archinternmed.2011.306 [DOI] [PubMed] [Google Scholar]
- 42.Gottesman BS, Carmeli Y, Shitrit P, Chowers M. Impact of quinolone restriction on resistance patterns of Escherichia coli isolated from urine by culture in a community setting. Clin Infect Dis. 2009;49(6):869-875. doi: 10.1086/605530 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods 1. 3-days Fluid Intake Diary
eMethods 2. Voiding Diary