Abstract
Glioblastoma multiforme is a highly malignant and aggressive primary brain tumor with a dismal prognosis. We studied the association of immunohistochemical expression of hypoxia inducible factor-1 alpha (HIF-1α), telomerase reverse transcriptase (TERT), isocitrate dehydrogenase 1 (IDH1) and tumor protein p53 with overall survival (OS) in glioblastoma patients uniformly treated by standard of care, with adequate follow-up. In 87 patient samples studied, 59 were male and 28 were female. The median age was 55 years. The median follow-up was 27.7 months and the median overall survival was 14.9 months. Nuclear staining of HIF-1α was expressed in all samples and scored as strong in 42 (48%) and weak in 45 (52%). Multivariable Cox regression revealed strong HIF-1α expression as an independent poor prognostic factor (Hazard Ratio 2.12, 95% CI 1.20 - 3.74, P = 0.01). There was a statistically significant difference in OS (9.8 months vs. 16.3 months) between the “HIF-1α - strong and TERT - strong” and the “HIF-1α - weak and TERT - weak” patient subgroups, as evaluated by Kaplan-Meier analysis (P = 0.005). In our study, HIF-1α expression was an independent predictor of OS. The subgroup of patients with strong expression of both HIF-1α and TERT had the poorest prognosis.
Keywords: glioblastoma, hypoxia, HIF-1α, TERT, IDH1, immunohistochemistry
Introduction
Tumour hypoxia plays a critical role in cancer metabolism triggering cancer aggressiveness, invasiveness and treatment resistance resulting in poor survival. Both normal and tumour cells respond to hypoxia by regulating the hypoxia inducible factor (HIF) family of transcription factors. In hypoxic conditions, both HIF-1α and HIF-2α proteins accumulate rapidly in the nucleus and disappear on re-oxygenation. HIF-1α activity is determined by its expression and is inversely proportional to the intracellular oxygen concentration 1. It is maintained at low levels under normoxic conditions due to continuous degradation via the ubiquitin-dependent proteasome pathway. Hypoxia via von Hippel-Lindau protein inhibits this pathway and leads to overexpression of HIF-1α protein levels. HIF-1α activates varied genes involved in glycolysis and angiogenesis like VEGF, glucose transporters, Insulin like growth factor 2 (IGF2) and IGF binding proteins 1,2 and 3 2,3. These genes synthesize proteins, which increase intracellular oxygen availability, or allow metabolic adaptation to hypoxia when HIF-1α is overexpressed 4.
Telomerase activity (TA) has been implicated in the tumorigenesis of many tumours including glioma. In normal somatic cells, telomeres progressively shorten with each cell division until it reaches a critical limit, ultimately triggering a DNA damage response leading to senescence or apoptotic cell death. Most human cancers adapt a telomere maintenance mechanism by the overexpression of telomerase while others may use alternative lengthening of telomeres (ALT). The telomerase complex consists of telomerase RNA component (TERC); a catalytic subunit called human telomerase reverse transcriptase (hTERT) and associated protein (hTP1). TERT expression is one of the critical determinants of TA. Promoter mutations in Telomerase reverse transcriptase (TERT) gene at specific hotspots (p. C228T and p. C250T) causes telomerase overexpression observed in nearly 80% of adult glioblastomas and is associated with poor prognosis 5,6. Meta-analysis of 28 studies of IDH and TERT mutations in 11,519 low-grade glioma patients showed that TERT promoter mutations were associated with a poor progression free survival (PFS) and overall survival (OS) but dependent on tumour grade and IDH mutation status 7. We studied the expression levels of IDH1, HIF-1α, TERT and p53 in human glioblastomas and their association with OS.
Methods
We examined formalin-fixed paraffin embedded (FFPE) tumour tissue sections from 87 patients diagnosed with GBM treated between June 2008 to January 2016 with the aim of studying immunohistochemical (IHC) expression of IDH1 (R132H), HIF-1α protein, TERT and p53 and their impact on OS. Inclusion criteria were patients age ≥ 18 years of age, 80-90 % viable tumor specimen in the paraffin block with more than 1 cm2 tissue by light microscope examination of haematoxylin and eosin (H & E) stained sections, availability of complete clinical data and adequate follow-up. All patients were treated with maximal safe surgical resection, adjuvant radiotherapy and temozolomide followed by 6 cycles of maintenance temozolomide. The study was carried out after approval from the Institutional ethics board. All relevant clinico-pathological information was obtained from the medical records. OS was defined as the time interval between surgery and death (because of any cause) or the date of last follow-up.
IHC expression was evaluated as immunoreactivity score (IRS) and is calculated from the percentage of stained cells and signal intensity using semi-quantitative microscopic analysis. Two spots were evaluated for each sample and a mean score was calculated. Staining was scored as 1 when ≤ 10% of cells were positive; 2 when 10-50% of cells were positive and 3 when ≥ 50% of cells were positive. Signal intensity was scored as negative (0), weak (1), moderate (2) and strong (3). IRS score was categorised as negative (0), weak (1 - 6) and strong (> 6).
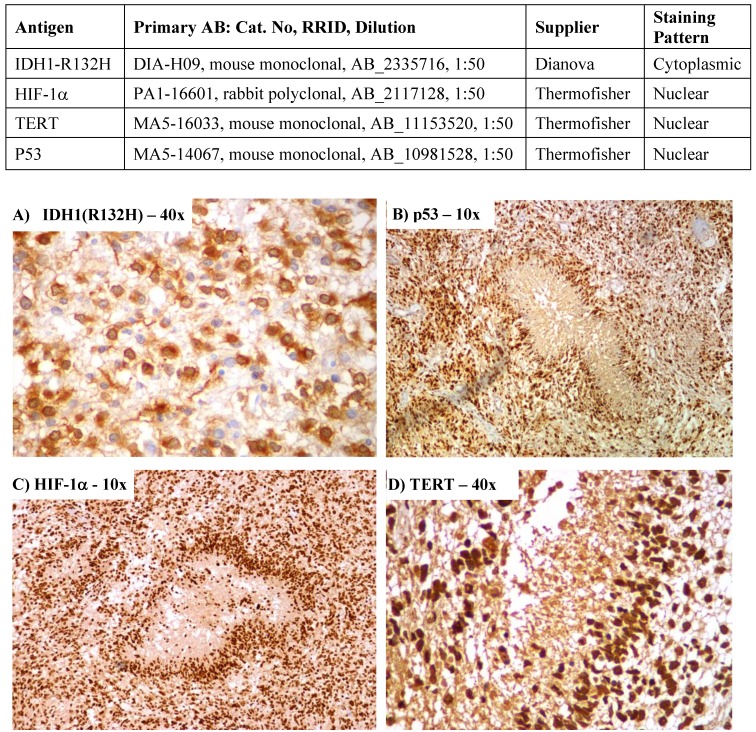
Serial sections of 3 microns were cut from each paraffin block, one of which was stained with H & E for histological assessment. For antibody validation, eight patient samples were studied. Two specimens each from IRS score 1-3, 4-6, 7-9 and 10-12 were analysed on samples obtained from the same block with two different antibodies. The first set of antibodies for IHC staining of the four biomarkers were HIF-1α (rabbit polyclonal, PA1-16601, Thermofisher), p53 (mouse cocktail, MA5-14067, Thermofisher), TERT mouse monoclonal, MA5-16033, Thermofisher) & IDH1-R132H (mouse monoclonal, DIA-H09, Dianova). The alternate antibodies were TERT - ab183105 (rabbit polyclonal, Abcam), HIF-1α - ab51608 (rabbit monoclonal, Abcam), IDH1-R132H - SAB4200548 (mouse monoclonal, Sigma-Aldrich) and p53 - ab131442 (rabbit polyclonal, Abcam). There was more than 90% concordance in the IRS scores between the antibodies. Hence, the first set of antibodies were used in the final analysis of the all the specimens. Previously confirmed p53-positive and HIF-1α-positive breast cancer tissue was used as positive control for p53 and HIF-1α. Previously confirmed TERT-positive liver cancer tissue was used as positive control for TERT. Negative controls were performed with phosphate buffered saline instead of primary antibody.
Sections were immunohistochemically stained for HIF-1α using the catalysed signal amplification system (Dako, Carpinteria, CA) based on a streptavidin-biotin-horseradish peroxidase (HRP) complex, employing rabbit polyclonal antibody against HIF-1α protein. After de-paraffinization and rehydration, slides were treated with target retrieval solution (CC1, pH 8.0) at 97°C for 45 minutes, and then manufacturer's instructions were followed. Nuclei were lightly counterstained with haematoxylin. The polyclonal antibody against HIF-1α was diluted to 1:50. Autoclave pre-treatment in 0.1M citrate buffer, pH 6.0, was performed for 10 minutes at 1050C. Automated immunohistochemistry analysis was performed on a VENTANA BENCHMARK XT (Ventana-BioTek Solutions, Inc., Tucson, AZ). Two independent experienced neuropathologists who were blinded to the specific diagnosis and prognosis for each individual case scored the IHC sample. For HIF-1α, cytoplasmic staining was ignored, as it is located only in the nucleus.
A statistical analysis was conducted using SPSS 17.0 software (SPSS, Chicago, IL, USA). Kaplan-Meier (KM) method was used to derive OS curves. Comparisons between survival curves were assessed using log-rank (LR) test. Univariate and multivariable analysis of the clinico-pathological parameters were performed using the Cox proportional hazards analysis. Chi-squared test was used to analyse the associations between HIF-1α and clinico-pathological variables. Differences were considered significant at P < 0.05.
Results
It was possible to evaluate the IHC tumour specific IDH1, HIF-1α, TERT and p53 expression in all the 87 specimens. Their distribution across various clinical subgroups is given in Table 1. Nuclear staining of HIF-1α expressed in all samples was scored as strong in 42 (48%) and weak in 45 (52%). Staining was seen predominantly in the perinecrotic tumour regions as shown in Figure 1 There were 52 (60%) patients with strong expression of TERT and in 35 (40%) patients it was absent/weakly expressed. There were 8 samples expressing IDH1 (R132H) mutant status and 79 were IDH1 wildtype. There was a high degree of concordance in the IHC interpretation between the investigators in 81 samples. In the remaining 6, a new sample was prepared from a different paraffin block and re-evaluated to reach a consensus.
Table 1.
Biomarker expression levels of 87 newly diagnosed histopathologically confirmed GBM patients
| Prognostic Factor | Number of Patients | IDH1-R132H | HIF-1α | TERT | P53 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Wildtype | Mutant | Weak (IRS ≤ 6) |
Strong (IRS 7-12) |
Weak (IRS ≤ 6) |
Strong (IRS 7-12) |
Weak (IRS ≤ 6) |
Strong (IRS 7-12) |
||
| Age | |||||||||
| 18 - 54 Years | 41 (47%) | 36 (88%) | 5 (12%) | 24 (58%) | 17 (42%) | 18 (44%) | 23 (56%) | 33 (80%) | 8 (20%) |
| >= 55 Years | 46 (53%) | 43 (94%) | 3 (6%) | 21 (46%) | 25 (54%) | 17 (37%) | 29 (63%) | 40 (87%) | 6 (13%) |
| Sex | |||||||||
| Male | 59 (68%) | 54 (91%) | 5 (9%) | 28 (48%) | 31 (52%) | 24 (41%) | 35 (59%) | 48 (81%) | 11 (19%) |
| Female | 28 (32%) | 25 (89%) | 3 (11%) | 17 (61%) | 11 (39%) | 11 (39%) | 17 (61%) | 25 (89%) | 3 (11%) |
| Karnofsky Performance Score | |||||||||
| >= 80 | 26 (30%) | 22 (85%) | 4 (15%) | 18 (69%) | 8 (31%) | 12 (46%) | 14 (54%) | 25 (96%) | 1 (4%) |
| >= 60 & < 80 | 30 (34%) | 27 (90%) | 3 (10%) | 13 (43%) | 17 (57%) | 12 (40%) | 18 (60%) | 21 (70%) | 9 (30%) |
| >= 40 & < 60 | 31 (36%) | 30 (97%) | 1 (3%) | 14 (45%) | 17 (55%) | 11 (36%) | 20 (64%) | 27 (87%) | 4 (13%) |
| Diabetes | |||||||||
| No | 65 (75%) | 58 (89%) | 7 (11%) | 33 (51%) | 32 (49%) | 26 (40%) | 39 (60%) | 51 (79%) | 14 (21%) |
| Yes | 22 (25%) | 21 (95%) | 1 (5%) | 11 (50%) | 11 (50%) | 9 (41%) | 13 (59%) | 22 (100%) | 0 (0%) |
| Hypertension | |||||||||
| No | 62 (71%) | 56 (90%) | 6 (10%) | 31 (50%) | 31 (50%) | 24 (39%) | 38 (61%) | 49 (79%) | 13 (21%) |
| Yes | 25 (29%) | 23 (92%) | 2 (8%) | 13 (52%) | 12 (48%) | 11 (44%) | 14 (56%) | 24 (96%) | 1 (4%) |
| Anemia | |||||||||
| No | 52 (60%) | 46 (88%) | 6 (12%) | 24 (46%) | 28 (54%) | 23 (44%) | 29 (56%) | 42 (81%) | 10 (19%) |
| Yes | 35 (40%) | 33 (94%) | 2 (6%) | 20 (57%) | 15 (43%) | 12 (34%) | 23 (66%) | 31 (89%) | 4 (11%) |
| Edema | |||||||||
| Moderate | 48 (55%) | 45 (94%) | 3 (6%) | 27 (56%) | 21 (44%) | 19 (40%) | 28 (60%) | 40 (85%) | 7 (15%) |
| Extensive | 39 (45%) | 34 (87%) | 5 (13%) | 18 (46%) | 21 (54%) | 16 (40%) | 24 (60%) | 33 (82%) | 7 (18%) |
| Extent of Resection | |||||||||
| Biopsy | 14 (16%) | 13 (93%) | 1 (7%) | 3 (21%) | 11 (79%) | 2 (14%) | 12 (86%) | 12 (86%) | 2 (14%) |
| Sub-total | 35 (40%) | 34 (97%) | 1 (3%) | 20 (57%) | 15 (43%) | 16 (46%) | 19 (54%) | 30 (86%) | 5 (14%) |
| Gross-total | 38 (44%) | 32 (84%) | 6 (16%) | 22 (58%) | 16 (42%) | 17 (45%) | 21 (55%) | 31 (82%) | 7 (18%) |
| Corpus callosum infiltration | |||||||||
| No | 64 (74%) | 56 (87%) | 8 (13%) | 39 (61%) | 25 (39%) | 30 (47%) | 34 (53%) | 54 (84%) | 10 (16%) |
| Yes | 23 (26%) | 23 (100%) | 0 (0%) | 6 (26%) | 17 (74%) | 5 (22%) | 18 (78%) | 19 (83%) | 4 (17%) |
HIF-1α: Hypoxia inducible factor-1 alpha, IDH1: Isocitrate deydrogenase 1, TERT: Telomerase reverse transcriptase, TP53: Tumor protein p53
Figure 1.
Immmunohistochemical expression of A) Isocitrate dehydrogenase 1 (IDH1), B) Tumor protein p53, C) Hypoxia inducible factor-1 alpha (HIF-1α) and D) Telomerase reverse transcriptase (TERT)
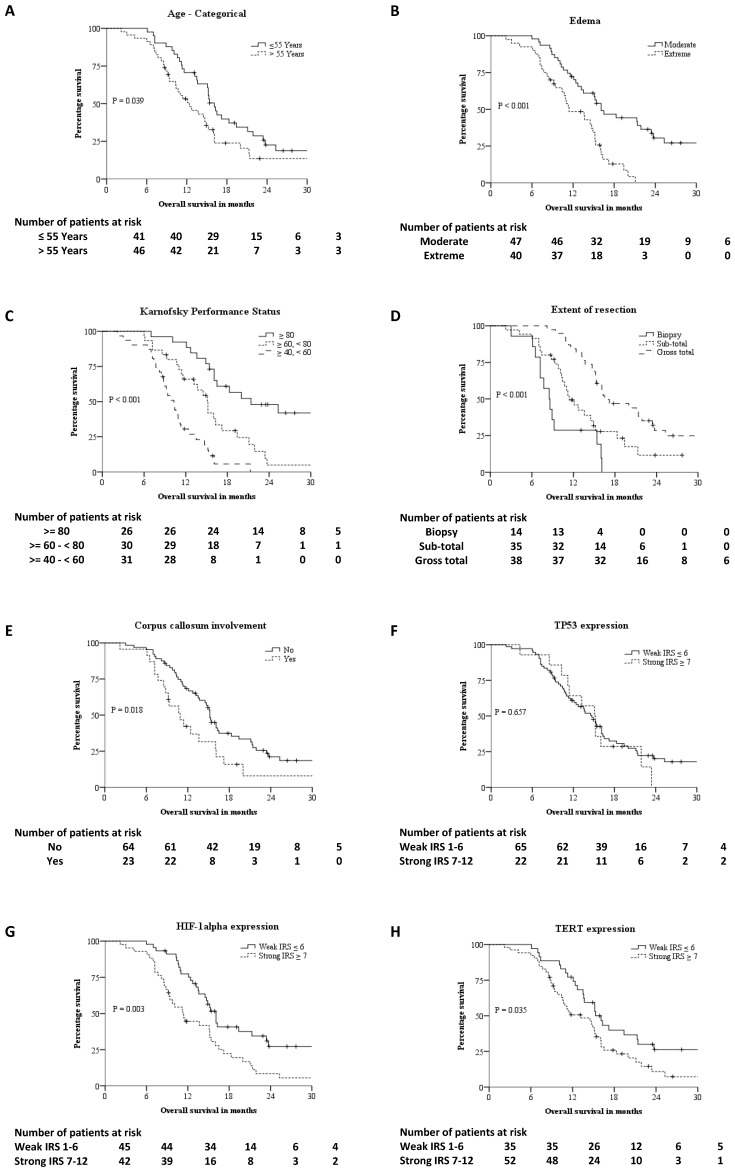
The median age was 55 years [Inter-quartile range- 22 years]. The median follow-up was 27.7 months and the median OS was 14.9 months. 70 (80.5%) patients died and 17 (19.5%) patients are alive with disease. No patients were lost to follow-up. Figure 2 summarizes the KM survival curves for age, edema, KPS, extent of resection, corpus callosum infiltration, p53, HIF-1α and TERT expression. The OS of patients showing strong expression of HIF-1α was significantly shorter than that of patients with tumours that stained weakly for HIF-1α, 11.3 months vs. 16.1 months respectively (P = 0.003) as evaluated by KM analysis. Age, KPS, extent of resection, edema, IDH1, HIF-1α, TERT expression and corpus callosum infiltration were all predictors for survival in unadjusted cox analysis (Table 2). Univariate cox proportional hazards analysis of HIF-1α expression gave a hazard ratio (HR) of 2.02 (95% Confidence Interval: 1.25 - 3.25, P = 0.004). Strong expression of HIF-1α as an independent adverse prognostic factor was maintained in multivariable analysis using cox proportional hazards model with a HR of 2.12 (95% CI: 1.20 - 3.74, P = 0.010). The OS of patients when TERT was absent or weakly expressed was significantly higher than tumours strongly expressing TERT, 15.3 months vs. 13.2 months respectively (P = 0.035) as evaluated by KM analysis. The HR for TERT expression was 1.69 (95% CI: 1.03 - 2.79, P = 0.038) in unadjusted Cox proportional hazards analysis. However, in adjusted analysis the HR was 1.65 (95% CI: 0.94 - 2.89, P = 0.082).
Figure 2.
Kaplan-Meier survival curves of different prognostic factor: A - Age-categorical, B - Edema, C - Karnofsky Performance Status (KPS), D - Extent of resection, E - Corpus callosum involvement, F - IHC expression of P53, G - IHC expression of HIF-1alpha, H - IHC expression of TERT.
Table 2.
Kalplan-Meier and Cox proportional hazards analysis
| Prognostic Factor | Kaplan-Meier | Unadjusted Cox | Adjusted Cox | |||||
|---|---|---|---|---|---|---|---|---|
| Median OS | P Value By Log Rank | P Value | Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | |||
| Age | ||||||||
| *18 - 54 Years | 16.1 | 0.039 | 0.041 | 1.64 (1.02-2.65) | 0.569 | 0.85 (0.47 - 1.51) | ||
| >= 55 Years | 12.4 | |||||||
| Sex | ||||||||
| *Male | 13.7 | 0.755 | ||||||
| Female | 16.1 | |||||||
| Karnofsky Performance Status | <0.001 | <0.001 | ||||||
| *>= 80 | 21.4 | < 0.001 | ||||||
| >= 60 & < 80 | 15.2 | 0.002 | 2.71 (1.42 - 5.17) | 0.173 | 1.64 (0.81 - 3.34) | |||
| >= 40 & < 60 | 10.3 | < 0.001 | 6.96 (3.49 - 13.87) | <0.001 | 4.84 (2.24 - 10.45) | |||
| Diabetes | ||||||||
| No | 15.1 | 0.892 | ||||||
| Yes | 12.4 | |||||||
| Hypertension | ||||||||
| *No | 15.1 | 0.874 | ||||||
| Yes | 14.5 | |||||||
| Edema | ||||||||
| Moderate | 16.1 | < 0.001 | <0.001 | 2.75 (1.62 - 4.66) | 0.004 | 2.46 (1.34 - 4.53) | ||
| Extensive | 11.4 | |||||||
| Extent of Resection | <0.001 | 0.016 | ||||||
| *Biopsy | 8.5 | < 0.001 | ||||||
| Sub-total | 11.4 | 0.027 | 0.46 (0.24 - 0.91) | 0.826 | 0.92 (0.43 - 1.96) | |||
| Gross-total | 16.5 | <0.001 | 0.20 (0.10 - 0.41) | 0.024 | 0.41 (0.19 - 0.89) | |||
| Corpus callosum infiltration | ||||||||
| No | 15.2 | 0.018 | 0.021 | 1.86 (1.10 - 3.16) | 0.911 | 0.96 (0.50 - 1.87) | ||
| Yes | 10.9 | |||||||
| IDH1 (R132H) | ||||||||
| *Wildtype | 13.6 | 0.040 | 0.048 | 0.40 (0.16 - 0.99) | 0.127 | 0.47 (0.18 - 1.24) | ||
| Mutant | 25.3 | |||||||
| HIF-1 α Expression | ||||||||
| *Weak (IRS 1-6) | 16.1 | 0.003 | 0.004 | 2.02 (1.25 - 3.25) | 0.010 | 2.12 (1.20 - 3.74) | ||
| Strong (IRS 7-12) | 11.3 | |||||||
| TERT Expression | ||||||||
| *Weak (IRS 1-6) | 15.3 | 0.035 | 0.038 | 1.69 (1.03 - 2.79) | 0.082 | 1.65 (0.94 - 2.89) | ||
| Strong (IRS 7-12) | 13.2 | |||||||
| TP53 Expression | ||||||||
| *Weak (IRS 1-6) | 14.7 | 0.657 | 0.659 | 1.15 (0.61 - 2.16) | ||||
| Strong (IRS 7-12) | 15.1 | |||||||
*Referent category. HIF-1α: Hypoxia inducible factor-1 alpha, IDH1: Isocitrate deydrogenase 1, TERT: Telomerase reverse transcriptase, TP53: Tumor protein p53
There were 79 patients with wild type IDH1 and 8 patients had IDH1 (R132H) mutation. The OS of IDH1 mutant patients was significantly higher than wild type IDH1 patients, 25.3 months vs. 13.6 months by KM analysis (P = 0.04). The HR for IDH1 expression was 0.40 (95% CI: 0.16 - 0.99, P = 0.048) in unadjusted Cox proportional hazards analysis. However, multivariable Cox proportional hazards analysis showed IDH1 was not an independent prognostic factor in our sample. The radiological edema was evaluated prior to surgery using baseline MRI. Edema extending ≤ 1cm from tumour margin was defined as moderate and edema extending for > 1cm from tumour margin was defined as extensive. The OS in patients with moderate and extensive edema was 16.1 months and 11.4 months respectively by KM analysis (P < 0.001). Univariate Cox proportional hazards analysis evaluated extent of edema as a prognostic variable with a HR of 2.75 (95% CI: 1.62 - 4.66, P < 0.001). This significance was retained in multivariable Cox proportional hazards analysis with a HR of 2.46 (95% CI: 1.34 - 4.53, P = 0.004). KPS and extent of surgical resection were also independent prognostic factors in KM and multivariable Cox proportional hazards analysis. Infiltration of tumour into corpus callosum, TERT expression, age-categorized (18-54 years, ≥ 55 years) were significant in KM analysis but not significant in multivariable Cox proportional hazards analysis. Sex, diabetes, hypertension and p53 expression had no significant effect on OS.
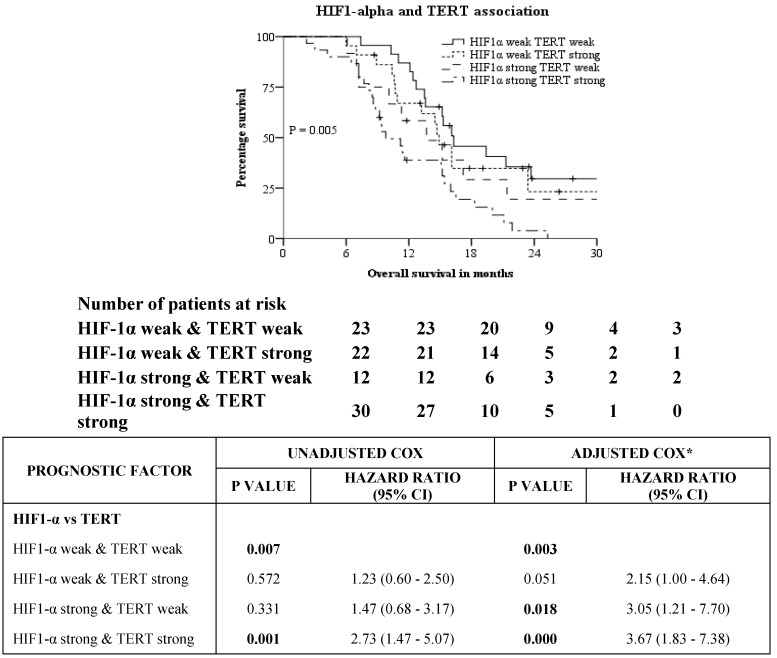
Survival analysis of HIF-1α expression against various subgroups of prognostic factors was studied using unadjusted Cox proportional hazards and the same has been represented in a Forest plot (Table 3). Chi-squared test of association to check for interaction of HIF-1α against the prognostic factors was significant for extent of resection, corpus callosum infiltration and TERT expression only. Chi squared test of association showed correlation between the presence of HIF-1α protein and expression of TERT (P = 0.032). A Kruskal-Wallis H test was conducted to determine if there were differences in OS between groups that differed in their biomarker expression: “HIF-1α - weak & TERT - weak” expression (n = 23), “HIF-1α - weak & TERT - strong” expression (n = 21), “HIF-1α - strong & TERT - weak” expression (n = 12) and “HIF-1α - strong & TERT - strong” expression (n = 31) groups (Figure 3). The mean ranks of OS were statistically significantly different between the groups, χ2(3) = 10.263, P = 0.016. Subsequently, pairwise comparisons were performed using Dunn's (1964) procedure with a Bonferroni correction for multiple comparisons. Statistical significance was accepted at P < 0.0083. This posthoc analysis revealed statistically significant difference in OS between the “HIF-1α - strong & TERT - strong” expression and “HIF-1α - weak & TERT - weak” expression groups only (P = 0.005). There was no difference in OS in any other group combination.
Table 3.
Forest-plot of patients categorised under subgroups of prognostic factors against HIF-1α expression using Cox proportional hazards.
| Prognostic Factor | Events*/Number of patients (%) | Estimated mortality at median follow-up of 27.7 months (%) | Unadjusted Cox Hazard Ratio (95% CI) | P value for interaction | |
|---|---|---|---|---|---|
| HIF-1α Weak (IRS ≤ 6) | HIF-1α Strong (IRS 7-12) |
||||
| Age | 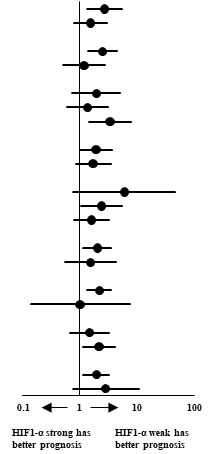 |
0.230 | |||
| 18 - 54 Years | 15/24 (63) | 17/17 (100) | 81.2 | ||
| >= 55 Years | 16/21 (76) | 22/25 (88) | 86.4 | ||
| Sex | 0.248 | ||||
| Male | 18/28 (64) | 29/31 (94) | 81.5 | ||
| Female | 13/17 (77) | 10/11 (91) | 88.8 | ||
| Karnofsky performance status | 0.102 | ||||
| >= 80 | 9/18 (50) | 8/8 (100) | 58.1 | ||
| >= 60 and < 80 | 10/13 (76) | 15/17 (88) | 95.1 | ||
| >= 40 and < 60 | 12/14 (86) | 16/17 (94) | 100.0 | ||
| Edema | 0.247 | ||||
| Moderate | 17/27 (63) | 19/21 (90) | 72.9 | ||
| Extensive | 14/18 (78) | 20/21 (95) | 100.0 | ||
| Resection | 0.046 | ||||
| Biopsy | 1/3 (33) | 11/11 (100) | 100.0 | ||
| Sub-total | 14/20 (70) | 12/15 (80) | 88.4 | ||
| Gross-total | 15/22 (68) | 16/16 (100) | 75.2 | ||
| Corpus callosum | 0.004 | ||||
| No infiltration | 26/39 (67) | 24/25 (96) | 81.6 | ||
| Infiltrated | 5/6 (83) | 15/17 (88) | 92.1 | ||
| IDH-1 | 0.522 | ||||
| Wildtype | 29/40 (72) | 36/39 (92) | 86.5 | ||
| Mutant (R132H) | 2/5 (40) | 3/3 (100) | 54.3 | ||
| TERT | 0.032 | ||||
| Weak IRS ≤ 6 | 17/23 (74) | 11/12 (92) | 73.8 | ||
| Strong 7-12 | 14/22 (64) | 28/30 (93) | 92.7 | ||
| TP53 | 0.469 | ||||
| Weak IRS ≤ 6 | 27/39 (69) | 31/34 (91) | 82.1 | ||
| Strong 7-12 | 4/6 (67) | 8/8 (100) | 100.0 | ||
*Event is death. HIF-1α: Hypoxia inducible factor-1 alpha, IDH1: Isocitrate deydrogenase 1, TERT: Telomerase reverse transcriptase, TP53: Tumor protein p53
Figure 3.
Kaplan-Meier and Cox proportional hazards analysis of association between IHC expression of HIF1-alpha and TERT. *Adjusted for Age-categorical, KPS, extent of resection, edema, corpus callosum infiltration & IDH1.
Discussion
Glioblastoma has a dismal prognosis with a median overall survival of 14.7 months. Genetic sequencing of this tumour has not provided a reliable marker to target owing to its intratumoral and inter-tumoral heterogeneity. In our study, we analysed the IHC expression of IDH1, HIF-1α, TERT and p53 to study their impact on OS and their correlation with clinico-pathological and radiological features. We confirmed the negative prognostic impact of strong expression of HIF-1α on OS of patients (11.3 months vs. 16.1 months, P = 0.003). Strong expression of HIF-1α was associated with TERT expression, type of surgery and involvement of corpus callosum but not with IDH1, P53, age and gender. KM analysis showed patients with weak expression of both HIF-1α and TERT had the best prognosis of 16.3 months while patients with strong expression of both HIF-1α and TERT had the worst prognosis of 9.8 months (P = 0.005 by LR). Our results indicate the significance of HIF-1α and TERT as potential prognostic markers in glioblastoma and provide a scope for targeted therapy in selected patients using HIF-1α and TERT inhibitors.
There are various factors responsible for treatment resistance in glioblastomas (Table 4) 8-15. Hypoxia has been correlated with poor prognosis in cervical cancers, head & neck cancers, soft tissue sarcomas, hepatocellular, gastric and invasive breast cancer 16-21. In our patient population, mainly from Southeast Asia, strong HIF-1α expression was associated with a poor prognosis of 11.3 months and weak HIF-1α expression with 16.1 months suggesting strong HIF-1α expression may lead to chemo-radioresistance and poor outcomes. Hypoxia has also been documented in malignant gliomas 22. Vaupel et al reported that even small alterations in oxygen tension in tumours could change the radiosensitivity 23. Many gliomas including glioblastomas overexpress HIF-1α possibly contributing to the poor prognosis 24.
Table 4.
Causes of treatment failure in glioblastoma
| Sl.No. | Cause of treatment resistance | Biomarkers | Treatment strategy | Challenges |
|---|---|---|---|---|
| 1 | Tumor hypoxia | HIF-1α, HIF-2α | Hyperbaric Oxygen, Allosteric hemoglobin modifiers, hypoxia activated prodrugs & Flurocarbons 8 | limited efficacy, side effects including seizures, chemo-radioresistance |
| 2 | Telomere dysfunction | TERC, TERT, TA | Telomerase inhibitor (Imetelstat) 9,10 | chemo-radioresistance |
| 3 | Immunosuppressive GBM microenvironment | CD19, EGFRvIII, HER2, IL-13 Rα2 | Car T-cell therapy 11 | Immunosuppressive effects of RT, TMZ and corticosteroids |
| CTLA4, PDL1, PD1 | Blockade checpoint inhibitors (Ex: PD1 checkpoint blockade), Vaccines, Adoptive cell transfer, Oncolytic viruses 12 | |||
| Tumor heterogeneity | EGFRvIII, SOX2, Olig2, MET, PDGFRA | Targeted therapy 13 | Identify and target all clonal sub-populations | |
| 4 | Tumor microtubules | Cx43, GAP-43, STMN1 | Anti-microtubule activity drugs 14 | Development of drug resistance |
| 5 | Inherent radioresistance | MGMT promoter methylation | Radiosensitizers (Ex: TMZ) | Unmethylated MGMT in GBM |
| 6 | Conventional fraction in RT | HIF-1α | Altered fractionation, Simultaneous integrated boost-RT | Microvascular damage, decreased vascular endothelial apoptosis 15 |
IL-13 Rα2: Interleukin-13 Receptor alpha 2; CTLA4: Cytotoxic T-Lymphocyte Associated Protein 4; PDL1: Programmed cell death ligand 1; PD1: Programmed cell death protein-1; Sox2: (sex determining region Y)-box 2; Olig2: Oligodendrocyte Transcription Factor 2; MET: MET Proto-Oncogene; PDGFRA: Platelet Derived Growth Factor Receptor Alpha; Cx43: Connexin 43; GAP-43: Gap associated protein 43; STMN1: Stathmin 1
Detecting hypoxia and strategies to overcome it have been attempted with conflicting results. Silencing HIF-1α inhibits proliferation, invasion and migration of glioblastoma cells in vitro and in vivo 25,26. Treatment modalities targeting hypoxia may improve therapeutic responses by acting as either radio/chemo sensitizer. Most of the solid tumours have a hypoxic microenvironment that can affect cell-mediated immunity resulting in ineffective immune response against the tumour or lead to immune suppression. The reasons for resistance to immunotherapy are varied. Intratumoral hypoxia induces excessive autophagy in cancer cells hampering natural killer (NK) cell mediated cytolysis. This leads to selective degradation of cytolytic effectors perforin and granzyme B making hypoxic tumour cells resistant to this mode of cell killing. The promise of immune checkpoint inhibitors too in preclinical studies has not been successful in clinical trials. It is unlikely that immunotherapy approach by itself will be effective because of the molecular heterogeneity of the tumour and involvement of multiple pathways in the maintenance and progression of this tumour. HIF-1α inhibitors with newer immunotherapy approaches supplementing the standard treatment of maximal safe resection, radiotherapy and temozolomide may stand a better chance of improving outcomes.
Telomere dysfunction and upregulation of TA have been implicated as a critical factor in carcinogenesis by maintaining the telomeres at the end of chromosomes and making them immortal 27, 28. TERT promoter mutation has been shown to promote hTERT gene expression selectively in tumour cells with more than 90% human cancers displaying high level of TA, whereas the other subunits are expressed both in normal and cancer cells 29, 20. Studies have shown IHC evaluation of TERT as a reliable representation of TA and function in cancer cells. Also, the nuclear overexpression of TERT has been associated with step-wise disease progression in breast cancer and with poor OS in Lung cancer, urothelial bladder cancer and head and neck squamous cell carcinoma 31-33. The OS of GBM patients with absent or weak expression of TERT was significantly higher than patients with strong expression of TERT, 15.3 months vs. 13.2 months respectively (P = 0.035) as evaluated by KM analysis. Spiegl-Kreinecker et al in his study on the prognostic potential of genomic alterations on telomerase activation in 126 glioblastoma patients showed TERT promoter mutation was associated with significantly shorter OS 35.
Hirotaka et al studies on human placenta have shown HIF-1α induces hTERT promoter activity and enhances endogenous TERT expression under hypoxic conditions 36. Glioblastomas are known to have extensive hypoxic regions within the tumour. We hypothesise that intratumoral low oxygen tension can induce increased HIF-1α expression and can directly upregulate hTERT transcription and TA resulting in increased proliferation and maintenance of cancer stem cells. The high levels of expression of HIF-1α and TERT in our samples suggest that this could be a generalized response to hypoxia in glioblastomas leading to chemo-radioresistance by upregulating telomerase. In our study population 30 (34.5%) patients with strong expression of both HIF-α and TERT had a median OS of 9.8 months. The high frequency of TERT mutations, increased TA and TERT expression gives an opportunity for exploring new approaches in treating glioblastoma. Since some normal tissues with high regenerative potential can also express TERT, clinical use of TERT inhibitors as anticancer drugs should be used with caution.
The main strength of our work is that it is a clinical study directly correlating HIF-1α and TERT immunohistochemical expression with OS in a group of patients uniformly treated by standard of care with adequate follow-up. There are several limitations in our study. MGMT methylation status was not available for most of the patients and hence was not considered in our analysis. MGMT is a known prognostic factor in glioblastomas and its impact, as a confounder on the role of HIF-1α in glioblastomas needs to be studied. Secondly, most of the patients are from South Asia and therefore the findings of this study need to be confirmed in patients from other parts of the world.
Conclusion
All glioblastomas exhibit some degree of activation of HIF-1α indicating that hypoxia plays an important role in its growth and response to treatment. A strong HIF-1α expression confers a worse prognosis in glioblastoma patients. Patients exhibiting a combination of weak HIF-1α and TERT expression have better prognosis implying that HIF-1α and TERT may play an important role as prognostic biomarkers and for development of targeted treatment strategies.
Acknowledgments
We gratefully acknowledge the generous grant from Board of Research in Nuclear Sciences (BRNS), India (Grant No.35/14/25/2016-BRNS). BRNS did not have any role in the design of study, collection, analysis, interpretation of data, writing of the report or in the decision to submit the article for publication.
References
- 1.Jiang BH, Semenza GL, Bauer C. et al. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol. 1996;271:C1172–80. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- 2.Tazuke SI, Mazure NM, Sugawara J. et al. Hypoxia stimulates insulin-like growth factor binding protein 1 (IGFBP-1) gene expression in HepG2 cells: A possible model for IGFBP-1 expression in fetal hypoxia. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(17):10188–10193. doi: 10.1073/pnas.95.17.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldser D, Agani F, Iyer NV, Reciprocal positive regulation of hypoxia-inducible factor 1α and insulin-like growth factor 2. Cancer Res; 1999. p. 59. 3915-3918. [PubMed] [Google Scholar]
- 4.Semenza GL. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev. 1998;8:588–94. doi: 10.1016/s0959-437x(98)80016-6. [DOI] [PubMed] [Google Scholar]
- 5.Killela PJ, Reitman ZJ, Jiao Y. et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(15):6021–6026. doi: 10.1073/pnas.1303607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckel-Passow JE, Lachance DH, Molinaro AM. et al. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. The New England journal of medicine. 2015;372(26):2499–2508. doi: 10.1056/NEJMoa1407279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vuong HG, Altibi AMA, Duong UNP. et al. TERT promoter mutation and its interaction with IDH mutations in glioma: Combined TERT promoter and IDH mutations stratifies lower-grade glioma into distinct survival subgroups - A meta-analysis of aggregate data. Critical Reviews in Oncology/Hematology. 2017;120:1–9. doi: 10.1016/j.critrevonc.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Graham K, Unger E. Overcoming tumor hypoxia as a barrier to radiotherapy, chemotherapy and immunotherapy in cancer treatment. Int J Nanomedicine. 2018;13:6049–6058. doi: 10.2147/IJN.S140462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrandon S, Malleval C, El Hamdani B. et al. Telomerase inhibition improves tumor response to radiotherapy in a murine orthotopic model of human glioblastoma. Mol Cancer. 2015;14:134. doi: 10.1186/s12943-015-0376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perry PJ, Gowan SM, Reszka AP. et al. 1,4- and 2,6-disubstituted amidoanthracene-9,10-dione derivatives as inhibitors of human telomerase. J Med Chem. 1998;41:3253–3260. doi: 10.1021/jm9801105. [DOI] [PubMed] [Google Scholar]
- 11.Bagley SJ. et al. CAR T-cell therapy for glioblastoma: recent clinical advances and future challenges. Neuro-Oncology. 2018;20(11):1429–1438. doi: 10.1093/neuonc/noy032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romani M, Pistillo MP, Carosio R, Morabito A, Banelli B. Immune Checkpoints and Innovative Therapies in Glioblastoma. Front Oncol. 2018;8:464. doi: 10.3389/fonc.2018.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garros-Regulez L, Garcia I, Carrasco-Garcia E. et al. Targeting SOX2 as a Therapeutic Strategy in Glioblastoma. Front Oncol. 2016;6:222. doi: 10.3389/fonc.2016.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calinescu AA, Castro MG. Microtubule targeting agents in glioma. Transl Cancer Res. 2016;5(Suppl 1):S54–S60. doi: 10.21037/tcr.2016.06.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Méndez O, Zavadil J, Esencay M. et al. Knock down of HIF-1α in glioma cells reduces migration in vitro and invasion in vivo and impairs their ability to form tumor spheres. Molecular Cancer. 2010;9:133. doi: 10.1186/1476-4598-9-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hockel M, Schlenger K, Aral B. et al. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56:4509–4515. [PubMed] [Google Scholar]
- 17.Graeber TG, Osmanian C, Jacks T. et al. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- 18.Brizel DM, Scully SP, Harrelson JM. et al. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 1996;56:941–3. [PubMed] [Google Scholar]
- 19.Nordsmark M, Overgaard J. A confirmatory prognostic study on oxygenation status and loco-regional control in advanced head and neck squamous cell carcinoma treated by radiation therapy. Radiother Oncol. 2000;57(1):39–43. doi: 10.1016/s0167-8140(00)00223-1. [DOI] [PubMed] [Google Scholar]
- 20.Zhu CL, Huang Q, Liu CH. et al. Prognostic value of HIF-1alpha expression in patients with gastric cancer. Mol Biol Rep. 2013;40:6055–6062. doi: 10.1007/s11033-013-2715-z. [DOI] [PubMed] [Google Scholar]
- 21.Papatheodorou H, Leotsinidis M, Kalofonos H, Hypoxia inducing factor 1a (HIF-1a) expression in invasive breast cancer: correlation with tumor clinicopathological parameters. Archiv für Pathologische Anatomie und Physiologie und für Klinische Medicin; 2011. p. 459. S79-S79. [Google Scholar]
- 22.Liu Q, Cao P. Clinical and prognostic significance of HIF-1α in glioma patients: a meta-analysis. International Journal of Clinical and Experimental Medicine. 2015;8(12):22073–22083. [PMC free article] [PubMed] [Google Scholar]
- 23.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res; 1989. p. 49. 6449-6465. [PubMed] [Google Scholar]
- 24.Mayer A, Schneider F, Vaupel P. et al. Differential expression of HIF-1 in glioblastoma multiforme and anaplastic astrocytoma. International Journal of Oncology. 2012;41(4):1260–1270. doi: 10.3892/ijo.2012.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen S, Kwan A, Chen Y. et al. Effect of silencing HIF-1α on proliferation, invasion and migration of glioblastoma U87 cells. Neurol Sci. 2013;34:365–371. doi: 10.1007/s10072-012-1010-4. [DOI] [PubMed] [Google Scholar]
- 26.Méndez O, Zavadil J, Esencay M. et al. Knock down of HIF-1α in glioma cells reduces migration in vitro and invasion in vivo and impairs their ability to form tumor spheres. Molecular Cancer. 2010;9:133. doi: 10.1186/1476-4598-9-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim NW, Piatyszek MA, Prowse KR. et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 28.Perrem K, Colgin LM, Neumann AA. et al. Coexistence of alternative lengthening of telomeres and telomerase in hTERT-transfected gm847 cells. Mol. Cell. Biol. 2001;21:3862–3875. doi: 10.1128/MCB.21.12.3862-3875.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakayama J, Tahara H, Tahara E. et al. Telomerase activation by hTERT in human normal fibroblasts and hepatocellular carcinomas. Nat Genet. 1998;18:65–68. doi: 10.1038/ng0198-65. [DOI] [PubMed] [Google Scholar]
- 30.Kyo S, Kanaya T, Takakura M. et al. Human telomerase reverse transcriptase as a critical determinant of telomerase activity in normal and malignant endometrial tissues. Int. J. Cancer. 1999;80:60–63. doi: 10.1002/(sici)1097-0215(19990105)80:1<60::aid-ijc12>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 31.Makki JS. Telomerase activity in breast cancer, promising marker of disease progression. Telomere and Telomerase. 2015;2:e681. [Google Scholar]
- 32.Lantuejoul S, Soria JC, Moro-Sibilot D. et al. Differential expression of telomerase reverse transcriptase (hTERT) in lung tumours. British Journal of Cancer. 2004;90(6):1222–1229. doi: 10.1038/sj.bjc.6601643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mavrommatis J, Mylona E, Gakiopoulou H. et al. Nuclear hTERT immunohistochemical expression is associated with survival of patients with urothelial bladder cancer. Anticancer Res. 2005;25:3109–3116. [PubMed] [Google Scholar]
- 34.Fabricius EM, Gurr U, Wildner GP. Telomerase activity levels in the surgical margin and tumour distant tissue of the squamous cell carcinoma of the head-and-neck. Anal. Cell. Pathol. 2002;24:25–39. doi: 10.1155/2002/452527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spiegl-Kreinecker S, Lötsch D, Ghanim B. et al. Prognostic quality of activating TERT promoter mutations in glioblastoma: interaction with the rs2853669 polymorphism and patient age at diagnosis. Neuro-Oncology. 2015;17(9):1231–1240. doi: 10.1093/neuonc/nov010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishi H, Nakada T, Kyo S. et al. Hypoxia-Inducible Factor 1 Mediates Upregulation of Telomerase (hTERT) Molecular and Cellular Biology. 2004;24(13):6076–6083. doi: 10.1128/MCB.24.13.6076-6083.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]