Abstract
Patient‐specific orthopaedic implants are emerging as a clinically promising treatment option for a growing number of conditions to better match an individual's anatomy. Patient‐specific implant (PSI) technology aims to reduce overall procedural costs, minimize surgical time, and maximize patient outcomes by achieving better biomechanical implant fit. With this commercially‐available technology, computed tomography or magnetic resonance images can be used in conjunction with specialized computer programs to create preoperative patient‐specific surgical plans and to develop custom cutting guides from 3‐D reconstructed images of patient anatomy. Surgeons can then place these temporary guides or “jigs” during the procedure, allowing them to better recreate the exact resections of the computer‐generated surgical plan. Over the past decade, patient‐specific implants have seen increased use in orthopaedics and they have been widely indicated in total knee arthroplasty, total hip arthroplasty, and corrective osteotomies. Patient‐specific implants have also been explored for use in total shoulder arthroplasty and spinal surgery. Despite their increasing popularity, significant support for PSI use in orthopaedics has been lacking in the literature and it is currently uncertain whether the theoretical biomechanical advantages of patient‐specific orthopaedic implants carry true advantages in surgical outcomes when compared to standard procedures. The purpose of this review was to assess the current status of patient‐specific orthopaedic implants, to explore their future direction, and to summarize any comparative published studies that measure definitive surgical characteristics of patient‐specific orthopaedic implant use such as patient outcomes, biomechanical implant alignment, surgical cost, patient blood loss, or patient recovery.
Keywords: Custom implants, Orthopaedic surgery, Patient‐specific implants
Introduction
Surgical implant devices have been used worldwide in orthopaedic surgery for over 100 years1. Today, modern implants are commonly used in joint arthroplasties, spine fixation, tissue reconstruction, as well as for fracture fixation2. The clinical need for such orthopaedic implants will continue to increase, as projections have demonstrated that the demand for total knee arthroplasties (TKAs) in the USA will grow by 484%, from 719,000 annual TKA procedures in 2015 to 3.48 million by 2030, while the demand for total hip arthroplasties (THAs) will grow by 172%, from 332,000 to 572,000 annual THA procedures over the same time period3. This is in part due to THAs and TKAs becoming increasingly common in younger patients, as joint arthroplasty procedures in patients under 65 years increased by 109% between 2000 and 2007, compared to an increase of just 46% over the same time period for patients over 65 years4.
This rise in joint arthroplasty coupled with a trend toward younger patients is noteworthy, as Harrysson et al. cite that over a preliminary 10‐year period, younger patients face a significantly higher risk of implant failure in undergoing a total joint arthroplasty (TJA)5. Several factors contribute to this joint arthroplasty failure, most notably micro‐motions of the implant due to uneven stress distribution on the bone surface6. This is primarily a concern in the increasingly prevalent younger and more active patient populations. The uneven stress distribution existing at the bone–implant interface is a result of the rigid bone preparation at the implant site in a conventional TJA7. In a traditional total joint arthroplasty, bones are uniformly prepared for implant component fit, resulting in a “squared‐off” bone end that lacks the roundness of the natural periarticular osseous anatomy7. This bone contouring can have a dramatic effect on weight distribution and can lead to the “corners” of the bone–implant interface taking on a disproportionate amount of stress, resulting in harmful bone remodeling and eventual loosening of the implant7.
In light of these insights, there has been increased attention paid to patient‐specific implants (PSIs) in an attempt to increase implant durability, while maintaining or decreasing associated implant costs8. PSIs utilize magnetic resonance imaging (MRI) or computed tomography (CT) scans of a joint or bone to create an alignment guide for each component of the implant that is specific to a patient's unique anatomy8. This improves implant fit and load distribution while minimizing the inefficiency and cost associated with sizing implants in the operating room7. Stress concentrations shown in Fig. 1 demonstrate the disproportionately localized stress along the sharp edges of a bone surface prepared for conventional implant (Fig. 1A), while bone surfaces of a PSI showed a more uniform stress distribution due to the pre‐planned surgical cuts, which better recreate the patient's natural contoured anatomy (Fig. 1B)7. The balanced load distribution of PSIs is consistent across implant types when compared to their conventional standardized counterparts. Despite this evidence for improved fit and load distribution, the assumed efficacy of patient‐specific implants is still controversial. According to a 2014 survey of nearly 15,000 AAOS (American Academy of Orthopaedic Surgeons) orthopaedic health professionals, only 47% of them see a benefit to using patient‐specific implants over traditional implants in orthopaedic surgery9. Given this divide in opinion in the field, further investigation into the biomechanics, patient recovery, cost, and true efficacy of PSI surgical options is essential.
Figure 1.
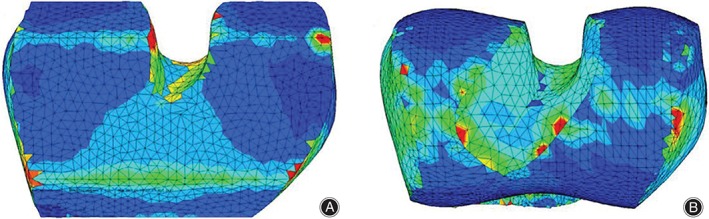
Representation of the stress distribution of bone surface for conventional (A) and patient‐specific (B) implant with both loading and reaction force applied in the center. Maximum stresses are shown in red color at a level of 5 MPa. Green contour stress levels are 2.5 MPa7.
Methods
For this review of the literature, related published reports were found via searches of PubMed using the following subject terminology: “Orthopaedic PSI,” “patient‐specific orthopaedic implants,” and “patient‐specific orthopaedic instruments”. In total, these searches returned 397 published articles. Of these, 11 non‐English articles were preliminarily excluded. Then, abstracts of all remaining articles were browsed for relevancy to the study and 279 non‐related publications were excluded. This left 107 articles for final review. Each article was strictly screened for quality via review of proper methodology, acceptable research design, and the reputability of its journal. Twenty‐seven articles were screened out due to unclear or improper study methodology, leaving 80 research articles for inclusion in this review. This entire process is depicted in Fig. 2.
Figure 2.
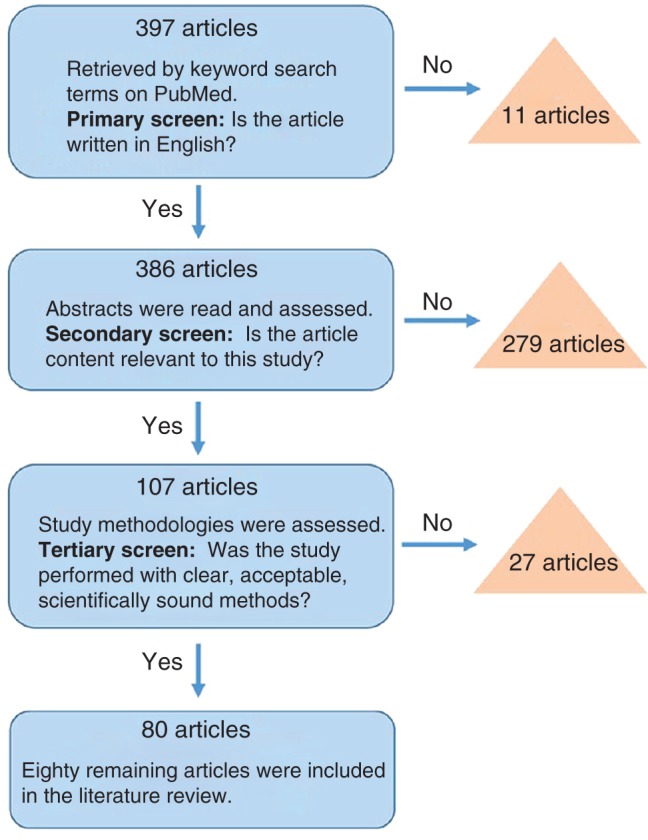
Flowchart depicting the selection process for articles included in this review.
Current Applications
Total Knee Arthroplasty
With the increasing popularity of TKA treatment for debilitating knee osteoarthritis and loss of joint function, investigating methods to improve the efficacy, accuracy, and reproducibility of TKAs has become an important goal in contemporary orthopaedic research, with patient‐specific TKA holding potential10. Biomechanical analysis cites correct implant component alignment as one of the most important features of a successful TKA11. TKA implant malpositioning relative to a patient's natural knee anatomy can lead to patellofemoral pain, knee instability, stiffness, inferior function, inferior range of motion, and wear and loosening of components: all precursors for implant failure (Fig. 3)13, 14, 15. Similarly, proper anatomical alignment correlates with better knee function, faster rehabilitation and recovery, less pain, increased TKA implant longevity, and improved quality of life12, 14, 16, 17, 18, 19, 20.
Figure 3.
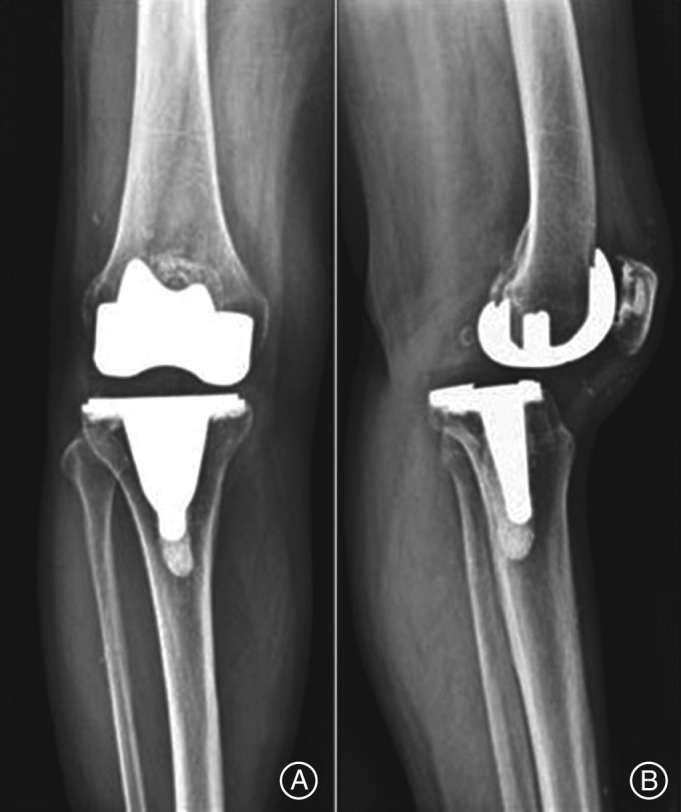
Anterior (A) and lateral (B) radiographs of knee, 2 years after TKA using a traditional cruciate‐retaining implant with notable loosening and polyethylene wear of the implant12.
Despite the importance of proper TKA implant alignment, conventional TKA surgical techniques have been associated with a high incidence of implant malalignment (20%–30%) independent of surgeon experience or US News and World Report hospital ranking21, 22, 23, 24. One of the primary objectives of PSI design is to reduce this malalignment in an effort to reduce associated complications or implant failure. In a PSI TKA, a commercial PSI program uses a MRI or CT scan to take patient‐specific measurements of the complete joint space in order to optimally guide the operative plan and the surgeon's specific bone cuts11. Ideal joint alignment theoretically results from the patient's postoperative mechanical leg axis, femoral component coronal alignment, tibial component coronal alignment, femoral component sagittal alignment, and tibial component sagittal alignment11. This includes the distal and posterior cutting planes for the femur and the proximal cutting plane for the tibia as well as the shape, size, and fit of the implant, which is displayed in a 3‐D anatomic model10. The program also provides the surgeon with on‐screen warnings if a cutting plane will result in an undesirable outcome such as “notching” (Fig. 4). After the surgeon reviews the preoperative plan, custom‐made jigs are manufactured based on the preoperatively planned bone resections (Fig. 5). These jigs act as temporary guidance to precisely reflect the preoperative plan and allow the surgeon to make the appropriate resections9.
Figure 4.
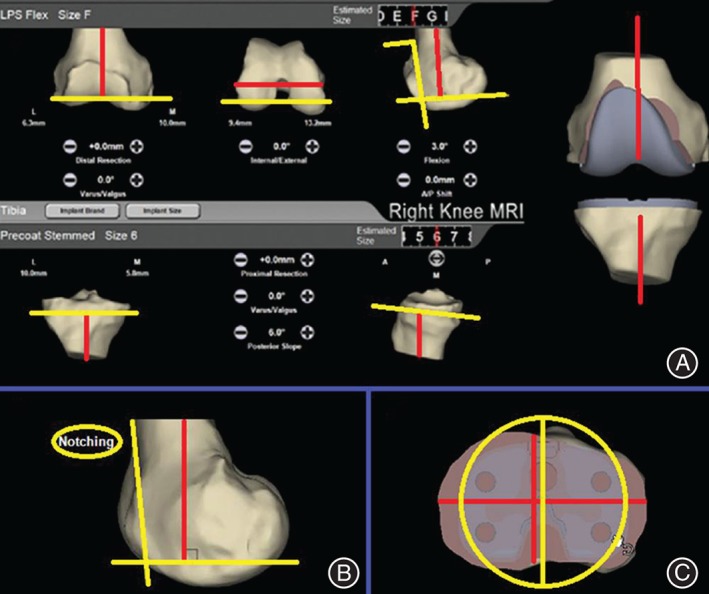
Adapted user interface showing the data necessary for preoperative planning of a patient‐specific total knee arthroplasty: (A) This image displays the distal and posterior cutting planes and the sagittal alignment of the femur (yellow lines), and the proximal cutting plane of the tibia with its slope (yellow lines). Red lines display the mechanical axis of the femur and tibia as well as the transepicondylar axis of the knee. The software displays a warning (B) if the anterior cutting plane exits the femoral cortical bone or (C) if tibial overhang occurs10.
Figure 5.
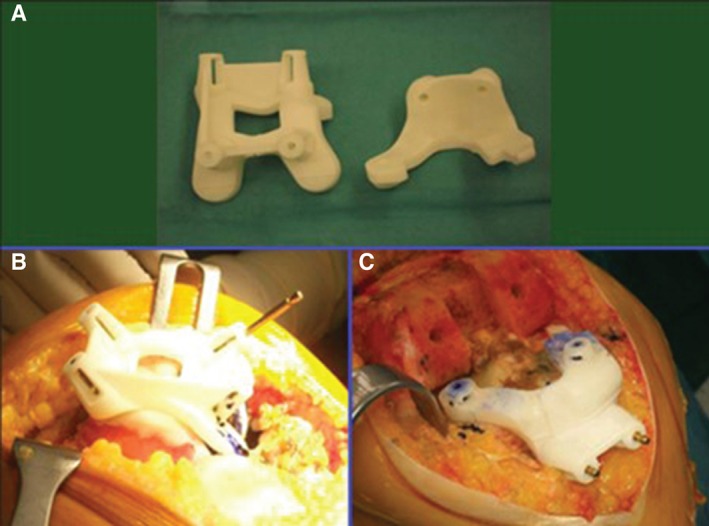
Femoral and tibial custom‐made jigs (A) used to guide the surgeon's cuts in the femur (B) and tibia (C).
Despite the theoretical advantages of PSIs in TKA, there is controversy regarding the clinical advantage of PSI use over traditional implants for TKA procedures. Currently, there is no reported clinical advantage of PSI use over traditional implants for outcomes in regards to blood loss, range of motion, length of hospital stay, postoperative Oxford knee score at 3 months, or postoperative UCLA activity score at 2 years8, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35. However, Ng et al. demonstrated that PSIs result in improved accuracy of biomechanical implant alignment when compared to standard TKA implants30. Furthermore, a 2015 cadaver study by Patil et al. showed that PSI TKAs allow for better knee kinematic function when compared to a standard TKA36. This was shown by testing active femoral rollback, active tibiofemoral adduction, and passive varus‐valgus laxity in cadaver knees that had either been implanted with patient‐specific implants using patient‐specific cutting guides, or with a standard implant using traditional intramedullary alignment cutting guides. The study concluded that PSI TKAs resulted in knee kinematics significantly closer to normal than the standard TKA implants were able to achieve, potentially suggesting improved function and patient satisfaction after a PSI TKA with the enhanced restoration of knee kinematics that PSIs allow for36. Patient‐specific TKA implants also carry a potential economic advantage, as DeHaan et al. found that a patient‐specific TKA carries a shorter average operating room time by 20.4 min and an average of four fewer instrument trays utilized per operation when compared to a traditional TKA procedure37. They argue that the shorter operating time and fewer instrument trays result in a net savings of US$736 per operation when compared to traditional TKA, even when they accounted for the additional cost of PSI imaging37. With the potential cost savings and improved alignment, PSI TKAs could be an advantageous treatment option in the future. However, studies are needed to assess long‐term PSI TKA patient outcomes at 10 or more years before definitive conclusions can be made.
Total Hip Arthroplasty
Traditional total hip arthroplasty (THA) consistently improves patient range of motion, decreases patient pain, and improves patients’ quality of life38, 39, 40. In a traditional THA, modular implants are placed using basic instrumentation and the surgeon's intraoperative assessment of anatomical landmarks in the patient40. One of two strategies is typically employed for a traditional THA: cemented or uncemented fixation of the femoral stem (Fig. 6)42. Figure 6 shows a comparison of cemented (Fig. 6A) fixation and press‐fit (Fig. 6B) fixation41. Without a clear advantage of using one fixation method over the other, orthopaedic surgeons are exploring PSIs as an alternative to traditional implants in THA43, 44, 45. Particular interest has been given to the improvement of acetabular cup alignment, as acetabular cup malpositioning has been demonstrated to be the most common cause of THA revision46. Accurate acetabular cup positioning in THA has been demonstrated to decrease the risk of dislocation, impingement, and implant wear rate47, 48, 49, 50, 51, 52, 53, 54, 55. Accurate acetabular cup placement could be an important advantage for using PSIs in THA procedures. In a PSI THA, preoperative computer‐assisted surgical planning software is used to plan the procedure and to fabricate patient‐specific instruments for use in guiding the procedure42, 56 (Fig. 7). The temporary PSI acetabular cup is placed within and around the acetabulum of the patient42 (Fig. 8). A peripheral guide‐wire is then drilled through the acetabular PSI cup, eventually serving as a reference for the trajectory of acetabular reaming. Then, the temporary PSI guide cup is removed, and an appropriately sized permanent acetabular component is chosen and inserted with the reference of the residual guide‐wire42. The PSIs are designed based on a CT scan or MRI of the patient's hip, with great attention given to the patient's unique bony morphologic features of the acetabulum and proximal femur42. This methodology theoretically minimizes the sources of error associated with conventional THA methodology, which has a much higher dependence on appropriate patient positioning, pelvis orientation, exposure, and surgeon experience42, 57, 58, 59, 60.
Figure 6.
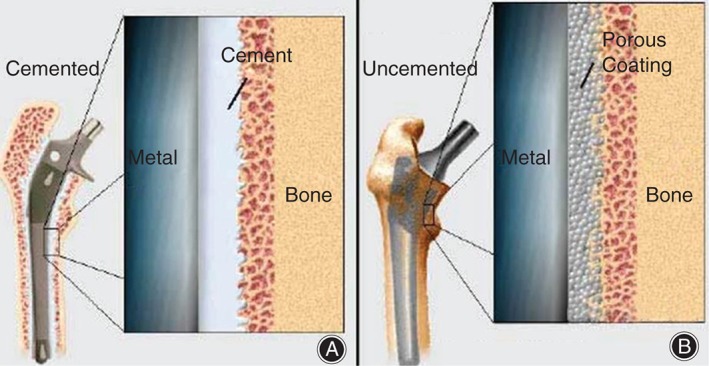
(A) A cemented implant is held by cement, which attaches the metal implant directly to the femur bone. (B) A press‐fit implant has a porous meshing between the implant and bone, allowing for the ingrowth of bone into the mesh41.
Figure 7.
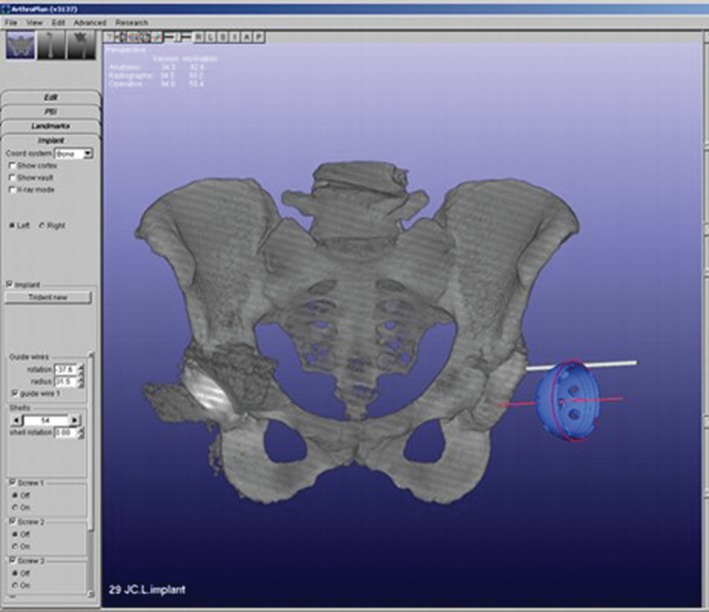
Screen shot of a surgical simulator software program used for preoperative planning and manufacturing of a 3D printed patient‐specific implant (PSI) acetabular cup42.
Figure 8.
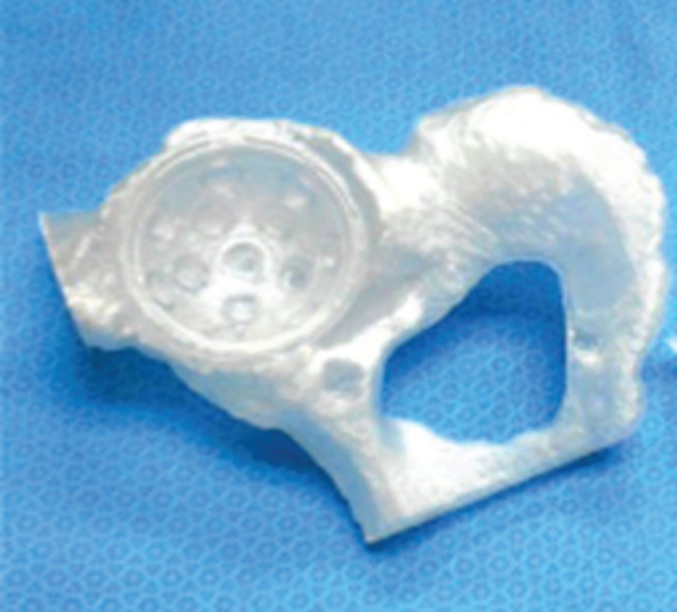
A 3D printed patient‐specific implant (PSI) right acetabular cup placed in a native acetabulum/pelvis patient‐specific model. Both the model and implant were manufactured based on a patient computed tomography (CT) scan, and were printed with an SLA 5000 3D printer from 3D systems using Watershed XC11122 resin42.
Similar to PSI usage in TKA procedures, there is a lack of literature comparing clinical outcomes of PSIs and conventional THAs. However, PSIs have been demonstrated to be more accurate at placing acetabular THA implants in their intended position and alignment, theoretically reducing the risk of implant wear, dislocations, impingement, and revision42, 61, 62, 63, 64, 65. PSI THA technology could also carry advantages for treating patients with a high body mass index (BMI) or unique morphologies. A higher BMI correlates with malpositioned acetabular cups in THAs, yet Small et al. found no difference in positioning for those patients with high BMI in patient‐specific THAs42, 66. This is attributed to the fact that traditional THA operative plans are impacted by soft tissue, patient positioning, and the degree of pathology. In comparison, PSI THA technology primarily relies on the imaging of the bony anatomy, allowing for objective measurements and preoperative customization42. Despite this benefit of PSIs, long‐term outcomes and cost analysis data for PSI THAs are required before a recommendation regarding the use of PSIs for hip arthroplasty can be made.
Bone Plates
A recently emerging indication for PSI use is in corrective osteotomies. 3‐D bone models are developed from CT scans, and a planned correctional osteotomy is simulated by manipulating the virtual bone models in computerized 3‐D space67, 68, 69, 70, 71, 72, 73, 74. Measurements are taken for the development of patient‐specific cutting guides and a patient‐specific osteosynthesis plate to stabilize postoperative bone healing (Fig. 9)75, 76. Accuracy in preoperative quantification of the surgical reconstruction is critical, as over‐correction and under‐correction in the correctional osteotomy is associated with loss of range‐of‐motion and muscle strength77. Therefore, computerized modeling, planning, and manufacturing of implants have seen increasing popularity75. Laboratory studies using patient‐specific guides have reported average residual errors less than 1° and 1 mm for simulated osteotomies, much lower than the 2.4° average error associated with a standard corrective osteotomy78, 79. However, as with joint arthroplasties, clinical outcome and cost studies are necessary prior to justifying the increased cost of PSIs for this application.
Figure 9.

Outline of a computer‐assisted planning approach for a corrective osteotomy with plate fixation. (A) First the degree of malunion is quantified by superimposing the proximal misaligned bone (orange) with the mirrored contralateral bone (green). (B) The distal fragment of bone is then reduced through simulation (violet) and the planned positioning of the patient‐specific fixation plate is calculated and displayed75.
Future Applications
With its increasing popularity in total knee arthroplasties, total hip arthroplasties, and corrective osteotomies, moving forward PSI technology has potential indications in many orthopaedic procedures. The idea of improved patient‐specific implants for spinal surgery is currently being explored, with Amendia, a provider of spinal technologies, recently acquiring Custom Spine and the rights to its 41 patents relating to patient‐specific spinal technologies80. The orthopaedic department at Cleveland Clinic has also explored the use of patient‐specific instruments in glenoid implant positioning in shoulder arthroplasty. Their results suggest that patient‐specific guiding instruments allow for a significant decrease in the average deviation of implant position for inclination and medial‐lateral offset in such procedures81.
There is controversy regarding the efficacy of patient‐specific orthopaedic implants, yet the theoretical alignment and precision of PSI methodology provide potential advantages when compared to standard procedures. However, the long‐term clinical efficacy of this custom technology must be demonstrated in further clinical study before the widespread application of PSI technology is fully supported and indicated for patient care.
Disclosure: No funding was received for this work.
References
- 1. Tezuka A. Total joint replacement in rheumatoid hip and knee. Orthop Traumatol, 1980, 29: 787–790. [Google Scholar]
- 2. Okazaki Y. Development trends of custom‐made orthopedic implants. J Artif Organs, 2012, 15: 20–25. [DOI] [PubMed] [Google Scholar]
- 3. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am, 2007, 89: 780–785. [DOI] [PubMed] [Google Scholar]
- 4. Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res, 2009, 467: 2606–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Harrysson OL, Robertsson O, Nayfeh JF. Higher cumulative revision rate of knee arthroplasties in younger patients with osteoarthritis. Clin Orthop Relat Res, 2004, 421: 162–168. [DOI] [PubMed] [Google Scholar]
- 6. Chaffin DB, Andersson GBJ, Martin BJ. Occupational Biomechanics. New York: John Wiley & Sons, 1999. [Google Scholar]
- 7. Harrysson OL, Hosni YA, Nayfeh JF. Custom‐designed orthopedic implants evaluated using finite element analysis of patient‐specific computed tomography data: femoral‐component case study. BMC Musculoskelet Disord, 2007, 8: 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schwarzkopf R, Brodsky M, Garcia GA, Gomoll AH. Surgical and functional outcomes in patients undergoing total knee replacement with patient‐specific implants compared with "off‐the‐shelf" implants. Orthop J Sports Med, 2015, 3, doi: 2325967115590379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prweb.com . Customized Joint Implants Popular Among Orthopedic Surgeons: Clinical Efficacy Important Internationally. Vicus, Inc, c1997–2015. Available from: http://www.prweb.com/releases/2015/02/prweb12509081.htm (accessed 3 May 2016). [Google Scholar]
- 10. Ferrara F, Cipriani A, Magarelli N, et al. Implant positioning in TKA: comparison between conventional and patient‐specific instrumentation. Orthopedics, 2015, 38: e271–e280. [DOI] [PubMed] [Google Scholar]
- 11. Cheng T, Zhao S, Peng X, Zhang X. Does computer‐assisted surgery improve postop‐erative leg alignment and implant positioning following total knee arthroplasty? A meta‐analysis of randomized controlled trials? Knee Surg Sports Traumatol Arthrosc, 2012, 20: 1307–1322. [DOI] [PubMed] [Google Scholar]
- 12. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res, 2002, 404: 7–13. [DOI] [PubMed] [Google Scholar]
- 13. Berger RA, Crossett LS, Jacobs JJ, Rubash HE. Malrotation causing patellofemoral complications after total knee arthroplasty. Clin Orthop Relat Res, 1998, 356: 144–153. [DOI] [PubMed] [Google Scholar]
- 14. Incavo SJ, Wild JJ, Coughlin KM, Beynnon BD. Early revision for component malrotation in total knee arthroplasty. Clin Orthop Relat Res, 2007, 458: 131–136. [DOI] [PubMed] [Google Scholar]
- 15. Choi YJ, Lee KW, Kim CH, et al. Long‐term results of hybrid Total knee arthroplasty: minimum 10‐years follow‐up. Knee Surg Relat Res, 2012, 24: 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Choong PF, Dowsey MM, Stoney JD. Does accurate anatomical alignment result in better function and quality of life? Comparing conventional and computer‐assisted total knee arthroplasty. J Arthroplasty, 2009, 24: 560–569. [DOI] [PubMed] [Google Scholar]
- 17. Longstaff LM, Sloan K, Stamp N, Scaddan M, Beaver R. Good alignment after total knee arthroplasty leads to faster rehabilitation and better function. J Arthroplasty, 2009, 24: 570–578. [DOI] [PubMed] [Google Scholar]
- 18. Czurda T, Fennema P, Baumgartner M, Ritschl P. The association between component malalignment and post‐operative pain following navigation‐assisted total knee arthroplasty: results of a cohort/nested case‐control study. Knee Surg Sports Traumatol Arthrosc, 2010, 18: 863–869. [DOI] [PubMed] [Google Scholar]
- 19. Nicoll D, Rowley DI. Internal rotational error of the tibial component is a major cause of pain after total knee replacement. J Bone Joint Surg Br, 2010, 92: 1238–1244. [DOI] [PubMed] [Google Scholar]
- 20. Fang DM, Ritter MA, Davis KE. Coronal alignment in total knee arthroplasty: just how important is it? J Arthroplasty, 2009, 24: 39–43. [DOI] [PubMed] [Google Scholar]
- 21. Bankes MJ, Back DL, Cannon SR, Briggs TW. The effect of component malalignment on the clinical and radiological outcome of the Kinemax total knee replacement. Knee, 2003, 10: 55–60. [DOI] [PubMed] [Google Scholar]
- 22. Mahaluxmivala J, Bankes MJ, Nicolai P, Aldam CH, Allen PW. The effect of surgeon experience on component positioning in 673 press fit condylar posterior cruciate‐sacrificing total knee arthroplasties. J Arthroplasty, 2001, 16: 635–640. [DOI] [PubMed] [Google Scholar]
- 23. Mihalko WM, Boyle J, Clark LD, Krackow KA. The variability of intramedullary alignment of the femoral component during total knee arthroplasty. J Arthroplasty, 2005, 20: 25–28. [DOI] [PubMed] [Google Scholar]
- 24. Siston RA, Patel JJ, Goodman SB, Delp SL, Giori NJ. The variability of femoral rotational alignment in total knee arthroplasty. J Bone Joint Surg Am, 2005, 87: 2276–2280. [DOI] [PubMed] [Google Scholar]
- 25. Bali K, Walker P, Bruce W. Custom‐fit total knee arthroplasty: our initial experience in 32 knees. J Arthroplasty, 2012, 27: 1149–1154. [DOI] [PubMed] [Google Scholar]
- 26. Boonen B, Schotanus MG, Kerens B, van der Weegen W, van Drumpt RA, Kort NP. Intra‐operative results and radiological outcome of conventional and patient‐specific surgery in total knee arthroplasty: a multicentre, randomised controlled trial. Knee Surg Sports Traumatol Arthrosc, 2013, 21: 2206–2212. [DOI] [PubMed] [Google Scholar]
- 27. Chareancholvanich K, Narkbunnam R, Pornrattanamaneewong C. A prospective randomised controlled study of patient‐specific cutting guides compared with conventional instrumentation in total knee replacement. Bone Joint J, 2013, 95: 354–359. [DOI] [PubMed] [Google Scholar]
- 28. Collins MJ. The impact patient‐specific instrumentation has on my practice in the last 5 years. Am J Orthop, 2014, 43: S14–S16. [PubMed] [Google Scholar]
- 29. Noble JW Jr, Moore CA, Liu N. The value of patient‐matched instrumentation in total knee arthroplasty. J Arthroplasty, 2012, 27: 153–155. [DOI] [PubMed] [Google Scholar]
- 30. Ng VY, DeClaire JH, Berend KR, Gulick BC, Lombardi AV Jr. Improved accuracy of alignment with patient‐specific positioning guides compared with manual instrumentation in TKA. Clin Orthop Relat Res, 2012, 470: 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Spencer BA, Mont MA, McGrath MS, Boyd B, Mitrick MF. Initial experience with custom‐fit total knee replacement: intra‐operative events and long‐leg coronal alignment. Int Orthop, 2009, 33: 1571–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stronach BM, Pelt CE, Erickson J, Peters CL. Patient‐specific total knee arthroplasty required frequent surgeon‐directed changes. Clin Orthop Relat Res, 2013, 471: 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lachiewicz PF, Henderson RA. Patient‐specific instruments for total knee arthroplasty. J Am Acad Orthop Surg, 2013, 21: 513–518. [DOI] [PubMed] [Google Scholar]
- 34. Abane L, Anract P, Boisgard S, Descamps S, Courpied JP, Hamadouche M. A comparison of patient‐specific and conventional instrumentation for total knee arthroplasty: a multicentre randomised controlled trial. Bone Joint J, 2015, 97: 56–63. [DOI] [PubMed] [Google Scholar]
- 35. Nam D, Park A, Stambough JB, Johnson SR, Nunley RM, Barrack RL. The mark Coventry award: custom cutting guides do not improve total knee arthroplasty clinical outcomes at 2 years follow up. Clin Orthop Relat Res, 2016, 474: 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Patil S, Bunn A, Bugbee WD, Colwell CW Jr, D'Lima DD. Patient‐specific implants with custom cutting blocks better approximate natural knee kinematics than standard TKA without custom cutting blocks. Knee, 2015, 22: 624–629. [DOI] [PubMed] [Google Scholar]
- 37. DeHaan AM, Adams JR, DeHart ML, Huff TW. Patient‐specific versus conventional instrumentation for total knee arthroplasty: peri‐operative and cost differences. J Arthroplasty, 2014, 29: 2065–2069. [DOI] [PubMed] [Google Scholar]
- 38. Eisler T, Svensson O, Tengström A, Elmstedt E. Patient expectation and satisfaction in revision total hip arthroplasty. J Arthroplasty, 2002, 17: 457–462. [DOI] [PubMed] [Google Scholar]
- 39. Furnes O, Lie SA, Espehaug B, Vollset SE, Engesaeter LB, Havelin LI. Hip disease and the prognosis of total hip replacements. A review of 53,698 primary total hip replacements reported to the Norwegian arthroplasty register 1987–99. J Bone Joint Surg Br, 2001, 83: 579–586. [DOI] [PubMed] [Google Scholar]
- 40. Older J. Charnley low‐friction arthroplasty: a worldwide retrospective review at 15 to 20 years. J Arthroplasty, 2002, 17: 675–680. [DOI] [PubMed] [Google Scholar]
- 41. Healthpages.org . Hip Joint Replacement Surgery. Healthpages.org Inc., c1995–2014. Available from: http://www.healthpages.org/surgical‐care/hip‐joint‐replacement‐surgery/ (accessed 1 May 2016). [Google Scholar]
- 42. Small T, Krebs V, Molloy R, Bryan J, Klika AK, Barsoum WK. Comparison of acetabular shell position using patient specific instruments vs. standard surgical instruments: a randomized clinical trial. J Arthroplasty, 2014, 29: 1030–1037. [DOI] [PubMed] [Google Scholar]
- 43. Havelin LI, Engesaeter LB, Espehaug B, Furnes O, Lie SA, Vollset SE. The Norwegian arthroplasty register: 11 years and 73,000 arthroplasties. Acta Orthop Scand, 2000, 71: 337–353. [DOI] [PubMed] [Google Scholar]
- 44. Mäkelä KT, Eskelinen A, Pulkkinen P, Paavolainen P, Remes V. Total hip arthroplasty for primary osteoarthritis in patients fifty‐five years of age or older. An analysis of the Finnish arthroplasty registry. J Bone Joint Surg Am, 2008, 90: 2160–2170. [DOI] [PubMed] [Google Scholar]
- 45. Morshed S, Bozic KJ, Ries MD, Malchau H, Colford JM Jr. Comparison of cemented and uncemented fixation in total hip replacement: a meta‐analysis. Acta Orthop, 2007, 78: 315–326. [DOI] [PubMed] [Google Scholar]
- 46. Wera GD, Ting NT, Moric M, Paprosky WG, Sporer SM, Della Valle CJ. Classification and management of the unstable total hip arthroplasty. J Arthroplasty, 2012, 27: 710–715. [DOI] [PubMed] [Google Scholar]
- 47. Ali Khan MA, Brakenbury PH, Reynolds IS. Dislocation following total hip replacement. J Bone Joint Surg Br, 1981, 63‐B: 214–218. [DOI] [PubMed] [Google Scholar]
- 48. Biedermann R, Tonin A, Krismer M, Rachbauer F, Eibl G, Stöckl B. Reducing the risk of dislocation after total hip arthroplasty: the effect of orientation of the acetabular component. J Bone Joint Surg Br, 2005, 87: 762–769. [DOI] [PubMed] [Google Scholar]
- 49. Colwell CW. Instability after total hip arthroplasty. Curr Orthop Pract, 2009, 20: 8–14. [Google Scholar]
- 50. Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip‐replacement arthroplasties. J Bone Joint Surg Am, 1978, 60: 217–220. [PubMed] [Google Scholar]
- 51. Patel AB, Wagle RR, Usrey MM, Thompson MT, Incavo SJ, Noble PC. Guidelines for implant placement to minimize impingement during activities of daily living after total hip arthroplasty. J Arthroplasty, 2010, 25: 1275–1281. [DOI] [PubMed] [Google Scholar]
- 52. Dandachli W, Islam SU, Liu M, Richards R, Hall‐Craggs M, Witt J. Three‐dimensional CT analysis to determine acetabular retroversion and the implications for the management of femoro‐acetabular impingement. J Bone Joint Surg Br, 2009, 91: 1031–1036. [DOI] [PubMed] [Google Scholar]
- 53. De Haan R, Pattyn C, Gill HS, Murray DW, Campbell PA, De Smet K. Correlation between inclination of the acetabular component and metal ion levels in metal‐on‐metal hip resurfacing replacement. J Bone Joint Surg Br, 2008, 90: 1291–1297. [DOI] [PubMed] [Google Scholar]
- 54. Gallo J, Havranek V, Zapletalova J. Risk factors for accelerated polyethylene wear and osteolysis in ABG I total hip arthroplasty. Int Orthop, 2010, 34: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Leslie IJ, Williams S, Isaac G, Ingham E, Fisher J. High cup angle and microseparation increase the wear of hip surface replacements. Clin Orthop Relat Res, 2009, 467: 2259–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hananouchi T, Saito M, Koyama T, Sugano N, Yoshikawa H. Tailor‐made surgical guide reduces incidence of outliers of cup placement. Clin Orthop Relat Res, 2010, 468: 1088–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. McCollum DE, Gray WJ. Dislocation after total hip arthroplasty. Causes and prevention. Clin Orthop Relat Res, 1990, 261: 159–170. [PubMed] [Google Scholar]
- 58. Beckmann J, Lüring C, Tingart M, Anders S, Grifka J, Köck FX. Cup positioning in THA: current status and pitfalls. A systematic evaluation of the literature. Arch Orthop Trauma Surg, 2009, 129: 863–872. [DOI] [PubMed] [Google Scholar]
- 59. Callanan MC, Jarrett B, Bragdon CR, et al. The John Charnley Award: risk factors for cup malpositioning: quality improvement through a joint registry at a tertiary hospital. Clin Orthop Relat Res, 2011, 469: 319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dorr LD, Wan Z, Malik A, Zhu J, Dastane M, Deshmane P. A comparison of surgeon estimation and computed tomographic measurement of femoral component anteversion in cementless total hip arthroplasty. J Bone Joint Surg Am, 2009, 91: 2598–2604. [DOI] [PubMed] [Google Scholar]
- 61. Haaker RG, Tiedjen K, Ottersbach A, Rubenthaler F, Stockheim M, Stiehl JB. Comparison of conventional versus computer‐navigated acetabular component insertion. J Arthroplasty, 2007, 22: 151–159. [DOI] [PubMed] [Google Scholar]
- 62. Kalteis T, Handel M, Bäthis H, Perlick L, Tingart M, Grifka J. Imageless navigation for insertion of the acetabular component in total hip arthroplasty: is it as accurate as CT‐based navigation? J Bone Joint Surg Br, 2006, 88: 163–167. [DOI] [PubMed] [Google Scholar]
- 63. Parratte S, Argenson JN. Validation and usefulness of a computer‐assisted cup‐positioning system in total hip arthroplasty. A prospective, randomized, controlled study. J Bone Joint Surg Am, 2007, 89: 494–499. [DOI] [PubMed] [Google Scholar]
- 64. Lazovic D, Kaib N. Results with navigated bicontact total hip arthroplasty. Orthopedics, 2005, 28: S1227–S1233. [DOI] [PubMed] [Google Scholar]
- 65. Wixson RL, MacDonald MA. Total hip arthroplasty through a minimal posterior approach using imageless computer‐assisted hip navigation. J Arthroplasty, 2005, 20: 51–56. [DOI] [PubMed] [Google Scholar]
- 66. McBride A, Flynn J, Miller G, Barnes M, Mackie S. Body mass index and acetabular component position in total hip arthroplasty. ANZ J Surg, 2013, 83: 171–174. [DOI] [PubMed] [Google Scholar]
- 67. Athwal GS, Ellis RE, Small CF, Pichora DR. Computer‐assisted distal radius osteotomy. J Hand Surg Am, 2003, 28: 951–958. [DOI] [PubMed] [Google Scholar]
- 68. Murase T, Oka K, Moritomo H, Goto A, Yoshikawa H, Sugamoto K. Three‐dimensional corrective osteotomy of malunited fractures of the upper extremity with use of a computer simulation system. J Bone Joint Surg Am, 2008, 90: 2375–2389. [DOI] [PubMed] [Google Scholar]
- 69. Schweizer A, Furnstahl P, Harders M, Szekely G, Nagy L. Complex radius shaft malunion: osteotomy with computer‐assisted planning. Hand, 2010, 5: 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dobbe JG, Strackee SD, Schreurs AW, et al. Computer‐assisted planning and navigation for corrective distal radius osteotomy, based on pre‐ and intraoperative imaging. IEEE Trans Biomed Eng, 2011, 58: 182–190. [DOI] [PubMed] [Google Scholar]
- 71. Kunz M, Ma B, Rudan JF, Ellis RE, Pichora DR. Image‐guided distal radius osteotomy using patient‐specific instrument guides. J Hand Surg Am, 2013, 38: 1618–1624. [DOI] [PubMed] [Google Scholar]
- 72. Leong NL, Buijze GA, Fu EC, et al. Computer‐assisted versus non‐computer‐assisted preoperative planning of corrective osteotomy for extra‐articular distal radius malunions: a randomized controlled trial. BMC Musculoskelet Disord, 2010, 11: 282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Miyake J, Murase T, Oka K, Moritomo H, Sugamoto K, Yoshikawa H. Computer‐assisted corrective osteotomy for malunited diaphyseal forearm fractures. J Bone Joint Surg Am, 2012, 94: e150. [DOI] [PubMed] [Google Scholar]
- 74. Schweizer A, Fürnstahl P, Nagy L. Three‐dimensional correction of distal radius intra‐articular malunions using patient‐specific drill guides. J Hand Surg Am, 2013, 38: 2339–2347. [DOI] [PubMed] [Google Scholar]
- 75. Vlachopoulos L, Schweizer A, Graf M, Nagy L, Fürnstahl P. Three‐dimensional postoperative accuracy of extra‐articular forearm osteotomies using CT‐scan based patient‐specific surgical guides. BMC Musculoskelet Disord, 2015, 16: 336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Croitoru H, Ellis RE, Prihar R, Small CF, Pichora DR. Fixation‐based surgery: a new technique for distal radius osteotomy. Comput Aided Surg, 2001, 6: 160–169. [DOI] [PubMed] [Google Scholar]
- 77. Schemitsch EH, Richards RR. The effect of malunion on functional outcome after plate fixation of fractures of both bones of the forearm in adults. J Bone Joint Surg Am, 1992, 74: 1068–1078. [PubMed] [Google Scholar]
- 78. Ma B, Kunz M, Gammon B, Ellis RE, Pichora DR. A laboratory comparison of computer navigation and individualized guides for distal radius osteotomy. Int J Comput Assist Radiol Surg, 2014, 9: 713–724. [DOI] [PubMed] [Google Scholar]
- 79. Oka K, Murase T, Moritomo H, et al. Accuracy of corrective osteotomy using a custom‐designed device based on a novel computer simulation system. J Orthop Sci, 2011, 16: 85–92. [DOI] [PubMed] [Google Scholar]
- 80. Prweb.com . Amendia Continues to Expand by Acquiring the Business of Custom Spine. Vicus, Inc., c1997–2015. . Available from: http://www.prweb.com/releases/amendia/acquires‐custom‐spine/prweb12870770.htm (accessed 30 March 2016). [Google Scholar]
- 81. Hendel MD, Bryan JA, Barsoum WK, et al. Comparison of patient‐specific instruments with standard surgical instruments in determining glenoid component position: a randomized prospective clinical trial. J Bone Joint Surg Am, 2012, 94: 2167–2175. [DOI] [PubMed] [Google Scholar]


