Abstract
One of its most serious complications associated with arthroplasty is the development of infections. Although its prevalence is only between 0.5% and 3%, in some cases it can lead to death. Therefore, an important challenge in joint surgery is the prevention of infections when an arthroplasty is performed. The use of antibiotic‐loaded cements could be a suitable tool due to numerous advantages. The main advantage of the use of antibiotic loading into bone cement derives directly from antibiotic release in the effect site, allowing achievement of high concentrations at the site of action, and minimal or no systemic toxicity. This route of administration was first described by Buchholz and Engelbrecht. In the case of infection treatment, this is an established method and its good results have been confirmed. However, its role in infection prevention, and, therefore, the use of these systems in clinical practice, has proved controversial because of the uncertainty about the development of possible antibiotic resistance after prolonged exposure time, their effectiveness, the cost of the systems, toxicity and loosening of mechanical properties. This review discusses all these topics, focusing on effectiveness and safety, antibiotic decisions, cement type, mixing method, release kinetics and future perspectives. The final objective is to provide the orthopaedic surgeons the right information in their clinical practice based on current evidence.
Keywords: Antibiotic, Arthroplasty, Bioactivity, Bone cement, Elution kinetics
Introduction
Total joint replacement is one of the most common and successful orthopaedic operations. The replacement is performed when there is irreversible damage in the joint, and, in general, it is recommended in the elderly, in whom wear of the prosthesis is much smaller due to low physical activity, reducing the possibility of failure. One of the most serious complications associated with total joint replacement is the development of infections. Although the prevalence of infection is only between 0.5% and 3%, in some cases, it can lead to death1. In cases of infection, a high dose of antibiotics is required to reach effective concentrations at the implantation site. Nevertheless, high doses of antibiotics can cause toxicity. To prevent the genesis of complications associated with the development of infections, the inclusion of antibiotics in the bone cement intended for mechanical attachment of the prosthesis to bone tissue has been suggested. The main advantage of this use of antibiotics derives directly from antibiotic release in the effect‐site, allowing achievement of high concentrations at the site of action, and minimal or no systemic toxicity2, 3. Currently, polymethylmethacrylate (PMMA) is the most widely used bone cement material for loading antibiotics and represents the current standard as an antibiotic delivery vehicle in orthopaedic surgery.
This route of administration was first described by Buchholz and Engelbrecht4. However, its role in infection prevention, and, therefore, the use of these systems in clinical practice, has proved controversial because of the uncertainty about the development of possible antibiotic resistance after prolonged exposure time, their effectiveness, the cost of these systems, toxicity, and loosening of mechanical properties 5, 6, 7, 8, 9. These aspects are reviewed in this document (Fig. 1).
Figure 1.
Flow chart showing the content of the article.
Method
A systematic review of the available literature was performed using the keyword terms “antibiotic loaded bone cement” and “arthroplasty”; there was no limit on the year of publication. The search was limited to English papers. The following databases were accessed on 9 June 2016: PubMed (http://www.ncbi.nlm.nih.gov/sites/entrez/). To to be considered eligible for inclusion, studies needed to focus on the prophylaxis of infection. Studies were excluded if: (i) outcomes of antibiotic‐loaded bone cements (ALBC) use in primary total knee arthroplasty were not reported; and (ii) it was impossible to extrapolate or calculate the necessary data from the published results.
Evidence of Effectiveness and Safety
A review of Kynaston‐Pearson et al. shows that 8% of all primary hip replacement prosthesis implanted in 2011 and recorded by the National Joint Registry had no readily available evidence relating to their safety or effectiveness10. This has led to further research in this field. However, such research is very difficult because there are many cement brands and prosthesis brands; for example, in the UK in 1996 there were 62 components in the market and in 2011 there were 265 different implants11. This fact added to the low number of patients included in the studies means that the studies cannot provide sufficient evidence.
In 2003, the Food and Drug Administration authorized antibiotic loading into bone cement for second‐stage reimplantation after infected arthroplasties. In contrast, the use of these delivery vehicles for prophylaxis in prosthesis surgery is off‐label use12. Nevertheless, the use of antibiotic‐loaded bone cement is recommended by most authors for joint arthroplasty revisions and in primary implants, which are at higher risk of infection7. Live audience polling at the 2009 American Association of Hip and Knee Surgeons Annual Meeting demonstrated that 37% of surgeons in attendance “always” used antibiotics in bone cement for routine primary total knee arthroplasty, while 45% used it on a more selective basis for high‐risk patients13. In the United States, off‐label use of antibiotic‐impregnated polymethylmethacrylate for primary joint replacement is increasing and multiple antibiotic‐containing polymethylmethacrylate products are commercially available. However, the use of antibiotic‐loaded bone cement in primary arthroplasty is controversial because its inclusion can reduce the mechanical properties of the cement and its use could produce bacteria resistance.
Currently, a few clinical assays evaluate the efficacy of antibiotic‐loaded cement in primary revision arthroplasty; there are only two meta‐analyses that evaluate their efficacy (Table 1).
Table 1.
Summary of meta‐analysis results
Parvizi et al. evaluated the efficacy of gentamicin‐loaded cement in primary revision arthroplasty14. A total of 21 444 knees arthroplasties impregnated with gentamicin or not were evaluated. Only one of the six studies evaluated by the authors reached statistical significance in regards to prophylaxis of infection. This paper concluded that the antibiotic‐loaded cement reduced the deep infection rate by approximately 50% (from 2.3% to 1.3% when antibiotic‐loaded cement was used) with statistical significance in favor of antibiotic loading into bone cement.
Wang et al. evaluated the deep and superficial infection rate when antibiotics were incorporated in bone cement (three studies with gentamicin included in Palacos, one with tobramycin included in Simplex P, one with cefuroxime included into Simplex P, one with erythromycin and colistine included in Simplex P and two with cefuroxime included in CMW) in primary revision arthroplasty15. In this study, the authors stated that the meta‐analysis reported by Parvizi et al. included some nonrandomized studies and their results should, thus, be treated with caution. Therefore, the inclusion criteria applied by Wang et al. were more restrictive and they evaluated a total of 6381 arthroplasties. The authors found statistically significant differences in the deep infection rate but not in the superficial infection rate. However, there were no statistically significant differences in aseptic loosening of prostheses (noninfectious loosening is defined as normal erythrocyte sedimentation rate, no pain, and bacteriologic cultures being negative) nor in clinical objectives (articular function evaluation).
In analyzing the meta‐analysis results, it can be concluded that antibiotic‐loaded bone cement would provide clinical benefit in primary surgery, as a prophylaxis to prevent deep infection.
Antibiotic Decisions
Dose of Antibiotic
The dose of antibiotic to be used in arthroplasty is not completely established; it depends if it is going to be used as treatment or prophylaxis. In most cases, it appears that the dose is set according to its influence on the mechanical properties of the cement, rather than to its therapeutic efficacy. It is established that to pursue therapeutic treatment, it is usually recommended to add 3.6 g of antibiotic to 40 g of acrylic cement to guarantee the correct drug levels16, 17. Conversely, for a prophylactic effect, a low dose of antibiotic appears to be sufficient. In this case, it is recommended to use 1 g of antibiotic per 40 g of cement; the lower proportion of antibiotic is less likely to alter the mechanical properties of the cement18.
Characteristics of the Antibiotic
Experience has shown that not all antibiotics satisfy the properties required to be incorporated into bone cements. At the moment, it is known that antibiotic election has to satisfy some criteria:
Stability at high temperature. The polymerization of PMMA increases the temperature of the cement mixture to 60°C–80°C19. Furthermore, it should be ensured that the degradation products, derivates of high temperature exposure, are not toxic drugs.
Different authors have reported that the inclusion of liquid antibiotics demonstrates a higher amount of antibiotic eluted but a loosening of the mechanical properties (these cements do not satisfy the ISO normative 5833 [Annex E])20, 21. In this way, the antibiotic included in bone cement must be in solid form. Nevertheless, the mechanical properties influenced by each antibiotic in solid form must be studied to guarantee that the corresponding ISO normative is accomplished.
The antibiotic must be effective against the microorganism that most frequently cause infection (i.e. have a wide antibacterial spectrum), specifically, methicillin‐resistant Staphylococcus aureus (MRSA) and gram negative aerobic bacillus22, 23.
Antibiotic elution from bone cement depends on the penetration of the surrounding media into the cement matrix, which is dictated by the wettability of the polymer, by the number and size of the pores in it, and by antibiotic solubility24. Therefore, antibiotics are required to have high solubility in water19.
Although antibiotic doses (1 g of antibiotic per 40 g of cement) are established to preserve mechanical integrity, the antibiotic doses are not equipotent. Thus, 1 g of gentamicin (habitual intravenous dose is 240 mg/24 h) is not the same as 1 g of amoxicillin (common intravenous dose is 3000 mg/24 h). For these reasons, another feature for the antibiotic inclusion is that it has to be effective at low doses.
However, to date, the antibiotic elution requirements are unknown.
Single Antibiotic Incorporation
The most commonly mixed antibiotics are gentamicin, tobramycin, vancomycin and clindamycin. These antibiotics satisfy the above criteria and are found on the market (including as ready‐mixed). The two antibiotics more readily available ready‐mixed are gentamicin and vancomycin. Ferraris et al. compared the commercial antibiotic‐loaded bone cement (Palacos R + G) and manually mixed bone cement (Palacos R and Palacos LV combined with gentamicin), evaluating their antibacterial behavior based on inhibition zones. They conclude that commercial formulation produces an inhibition zone that is a bit larger (23% greater, P < 0.05) and more regular than the manually‐mixed preparation. They attributed the differences to the lack of use of vacuum mixing techniques in manual mixtures25. A limitation of this study is that the antibiotic powder used in manual mixing is a commercial gentamicin sulfate, which is a mix of different substances; this limitation is present in many studies. Therefore, it can be concluded that the manual addition of commercial antibiotics to PMMA‐based bone cement produces inhibition zones that are moderately smaller and more irregular compared to commercial formulations of the same antibiotic‐loaded bone cements.
Other antibiotics, under research, that have been mixed by some authors are ciprofloxacin, cefazolin, moxifloxacin, and amoxicillin clavulanate26, 27, 28 (Table 2). Our research team examined ciprofloxacin release from three trademarks of bone cements (Simplex, Lima, and Palacos) and its bioactivity using as variables the mixing method, the chemical form of the antibiotic, and the antibiotic combination. The antibiotic amount released in base form represents 35% of the antibiotic amount released when hydrochloride is incorporated. Moreover, the combination (vancomycin and ciprofloxacin) shows a stronger release (132%) than hydrochloride ciprofloxacin alone. The three cements tested show equal drug release profiles (P > 0.05). A bioactivity simulation exercise showed that until 72 h post‐surgery, ciprofloxacin concentrations in the implant would be higher than 0.1 μg/mL in 100% of the patients. The limitations of this study are that no bending nor modulus strengths were calculated and the bioactivity was evaluated by means of a simulation exercise26.
Table 2.
Summary of clinical studies of antibiotic into bone cement
| Authors | Mixture | Antibiotic in cement (per 40 g) | Cement type | Percentage released (time) | Bioactivity | Mechanical properties (MPa) |
|---|---|---|---|---|---|---|
| Ferraris et al. 25 | Pre‐mixed | Gentamicin 0.5 g | Palacos R | N/S | Inh zone = 8.1 mm | N/S |
| Manual | Gentamicin 0.5 g | Palacos R + G | N/S | Inh zone = 10.0 mm | N/S | |
| Neut et al. 29 | Pre‐mixed | Gentamicin 0.5 g | Refobacin Palacos R | 8.6% ± 0.6% (168 h) | A gentamicin‐sensitive bacterium did not survive. Survival was independent of the level of burst release by the bone cement. | N/S |
| Gentamicin 0.5 g | Refobacin Bone Cement R | 12.2% ± 0.8% (168 h) | N/S | |||
| Gentamicin 0.5 g | Palacos R + G |
12.5% ± 3.6% (168 h) | N/S | |||
| Gentamicin 0.5 g | SmartSet GHV | 3.6% ± 0.4% (168 h) | N/S | |||
| Martinez‐Moreno et al. 26 | Manual | Ciprofloxacin hydrochloride (1 g) | Simplex | 2.65% (1344 h) | The concentrations of ciprofloxacin reachable in the implant would be higher than 0.1 μg/mL in 100% of patients, decreasing the coverage when higher concentrations are need. | N/S |
| Lima | 2.42% (1344 h) | N/S | ||||
| Palacos | 3.50% (1344 h) | N/S | ||||
| Ciprofloxacin base (1 g) | Lima | 0.75% (1344 h) | N/S | |||
| Vacuum | Ciprofloxacin hydrochloride (1 g) | Simplex | 2.85% (1344 h) | N/S | ||
| Lima | 5.40% (1344 h) | N/S | ||||
| Palacos | 4.63% (1344 h) | N/S | ||||
| Paz et al. 27 | Vacuum | Vaconmycin (1 g) | Palacos | 8.58% (672 h) | N/S | ND |
| Vaconmycin (4 g) | Palacos | 2.89% (672 h) | N/S | ND | ||
| Cefazolin (1 g) | Palacos | 27.14% (672 h) | N/S | ND | ||
| Galvez‐Lopez. et al. 28 | Manually | Vancomycin (1 g) | CMW | 31.32% (720 h) | N/S | 586.2 |
| Gentamycin (1 g) | CMW | 13.31% (720 h) | N/S | 166.27 | ||
| Moxifloxacin (1 g) | CMW | 50.40% (720 h) | N/S | 383 | ||
| Rifampicin (1 g) | CMW | 41.24% (720 h) | N/S | 42 | ||
| Daptomycin (1 g) | CMW | 17.09% (720 h) | N/S | 78.5 | ||
| Ertapenem (1 g) | CMW | 22.54% (720 h) | N/S | 121 | ||
| Meropenem (1 g) | CMW | 27.24% (720 h) | N/S | 342 | ||
| Cefotaxime (1 g) | CMW | 26.50% (720 h) | N/S | 75.82 | ||
| Ampicilin (1 g) | CMW | 0.99% (720 h) | N/S | N/S | ||
| Cefepime (1 g) | CMW | 1.49% (720 h) | N/S | 144 | ||
| Hsu et al. 30 | Manual | Daptomycn (0.5 g) | Osteobond | 9.59% | All bone cements of the three daptomycin preparations (low, mid, and high) produced detectable bacterial inhibition on day 1. However, growth inhibition for all groups rapidly declined from day 2. | 112.39 |
| Daptomycn (1 g) | Osteobond | 15.25% | 112.97 | |||
| Daptomycn (2 g) | Osteobond | 20.64% | 112.97 | |||
| Snir et al. 31 | Manual | — | Smart Set GHV and CMW1 | — | — | 2285 N† |
| Manual | Linezolid (1 g) | Smart Set GHV and CMW1 | N/S | MRSA MIC = 0.625 mcg/mL S. epidermidis MIC = 0.312 mcg/mL |
2552 N† | |
| Vancomycin(1 g) | Smart Set GHV and CMW1 | N/S | MRSA MIC = 1.25 mcg/mL S. epidermidis MIC = 1.25 mcg/mL |
2344 N† | ||
| Gentamicin (1 g) | Smart Set GHV and CMW1 | N/S | MRSA MIC = 0.1 mcg/mL S. epidermidis MIC = 7.81 mcg/mL |
2301 N† | ||
| Linezolid (1 g) + Vancomycin(1 g) | Smart Set GHV and CMW1 | N/S | MRSA MIC = 0.625 mcg/mL S. epidermidis MIC = 0.312 mcg/mL |
2480 N† | ||
| Linezolid (1 g) + Gentamicin (1 g) | Smart Set GHV and CMW1 | N/S | MRSA MIC = 0.625 mcg/mL S. epidermidis MIC = 0.312 mcg/mL |
2513 N† | ||
| Van de Belt et al. 18 | Manual | Gentamicin (1 g) | CMW1 | 3.52% (168 h) | Reduction on biofilm formation only before 6 h | N/S |
| CMW3 | 3.16% (168 h) | Reduction on biofilm formation from 24 to 72 h | N/S | |||
| CMW Endurance | 3.40% (168 h) | Reduction on biofilm formation only after 48–72 h | N/S | |||
| CMW2000 | 2.64% (168 h) | Reduction on biofilm formation only after 48–72 h | N/S | |||
| Gentamicin (0.82 g) | Palacos | 3.43% (168 h) | Reduction on biofilm formation only before 6 h | N/S | ||
| Palamed | 6.86% (168 h) | Reduction on biofilm formation only after 48–72 h | N/S | |||
| Cerretani et al. 32 | Manual | Vancomycin (2 g) | CMW1 | 2.00% (840 h) | N/S | N/S |
| Vancomycin (2 g) | Simplex P | 1.69% (840 h) | N/S | N/S | ||
| Vancomycin (2 g) | Palacos‐R | 1.94% (840 h) | N/S | N/S | ||
| Vancomycin (2 g) + Imipenem‐cilastatin (2 g) | CMW1 | 2.61% (840 h) | N/S | N/S | ||
| Vancomycin (2 g) + Imipenem‐cilastatin (2 g) | Simplex P | 2.54% (840 h) | N/S | N/S | ||
| Vancomycin (2 g) + Imipenem‐cilastatin (2 g) | Palacos‐R | 2.91% (840 h) | N/S | N/S |
Axial compression testing
MRSA, methicillin‐resistant Staphylococcus aureus; ND, no differences; N/S, unknown.
Paz et al. studied the inclusion of vancomycin or cefazolin at prophylaxis doses (1 g of antibiotic per 40 g of bone cement) into bone cement Palacos R + G; vancomycin and cefazolin release, fluid absorption, and mechanical properties were evaluated under physiological conditions. Cefazolin at 672 h showed higher release (227.28 ± 23.91 μg/mL) compared to vancomycin (71.86 ± 25.34 μg/mL) (P < 0.01). However, the differences in release between both antibiotics were not so marked during the first 24 h, being 44.26 ± 3.37 μg/mL and 32.46 ± 9.70 μg/mL for cefazolin and vancomycin, respectively (P = 0.281). The compressive strength of cements added of the two antibiotics without aging and after aging for 1 month in phosphate buffered saline (PBS) at 37°C was calculated. All cements without aging showed no statically significant difference to the control cement (P > 0.01). However, cefazolin aged in PBS at 37°C experienced significant reductions in compressive properties (P < 0.01). The limitation of this study is that there is no data on bioactivity and, therefore, whether the differences are clinically significant cannot be assessed27.
Galvez‐Lopez et al. evaluated different ALBC for elution kinetics, thermal stability, and mechanical properties. A 10% or 20% mixture (w/w) of beads of medium viscosity bone cement (DePuy) and vancomycin, gentamycin, daptomycin, moxifloxacin, rifampicin, cefotaxime, cefepime, ampicillin, meropenem, and ertapenem were evaluated. Elution kinetic profiles of all antibiotics tested, with the exception of ampicillin and cefepime, demonstrated a triphasic pattern of release with a progressive increase in the first 24 h followed by a rapid decrease and a final phase with a low and steady decline through the rest of the experiment. Three particular behaviors of elution were identified depending on the antibiotics tested. Vancomycin, gentamycin, moxifloxacin, and rifampicin, loaded at 10% (w/w), demonstrated constant elution kinetics through the 30‐day duration of the experiment. Daptomycin, meropenem, ertapenem, and cefotaxime, although also having the triphasic pattern, showed a lower peak and a faster decrease of elution between days 3 and 30, but eluted concentrations remained above the minimum inhibitory concentration (MIC) of susceptible organisms, according to antimicrobial susceptibility testing (EUCAST) clinical breakpoints. Finally, ampicillin and cefepime showed minimal elution with eluted concentrations being almost undetectable at day 4 and always below the MIC of susceptible organisms, according to the European Committee on EUCAST clinical breakpoints. The percentage eluted from each ALBC is shown in Table 2. Presence of antibiotics did not affect the strength of ALBC with mean compression values greater than 70 MPa, except for rifampicin‐loaded bone cement, for which the compression strength did not exceed 42.9 MPa28. A limitation of this study is the measurement of antimicrobial properties; the antibacterial activity was only measured at 30 min from the beginning of the assay.
Hsu et al. incorporated 0.5, 1, and 2 g of daptomicin (Cubicin, the commercial antibiotic, which is more than 90% pure antibiotic) per 40 g of PMMA; in this study, the authors showed that the mechanical strength is not compromised by daptomycin at any concentration, because all samples had a compressive strength higher than 100 MPa. The percentage of daptomcin eluted during 2 weeks was 9.59% ± 0.85%, 15.25% ± 0.69%, and 20.64% ± 20.33% from 0.5, 1, and 2 g of daptomycin, respectively. The bioactivity of the cements was also confirmed including MSSA, MRSA, Staphylococcus epidermidis, Enterococcus faecium, and Enterococcus faecalis. The authors concluded that the inclusion of commercial daptomycin at a low dose in bone cement was satisfactory; both bioactivity and resistance tests were adequate30.
Snir et al. analyzed 1 g of linezolid vancomycin, or gentamicin per 40 g included into PMMA (Smart Set GHV and CMW1). There were no differences between cement brands. The study demonstrated that linezolid shows a minimum inhibitory concentration (MIC) of 0.625, 0.312, 1, 250, and 250 mcg/mL to methicillin‐resistant S. aureus (MRSA), S. epidermidis, vancomycin‐resistant enterococci (VRE), Escherichia coli and Klebsiella pneumoniae, respectively. Vancomycin shows an MIC of 1.25, 1.25, 0.4, 125, and 125 mcg/mL to MRSA, S. epidermidis, VRE, E. coli, and K. pneumoniae, respectively. Finally, gentamicin shows an MIC of 0.1, 7.81, 23.43, 1, and 0.625 mcg/mL to MRSA, S. epidermidis, VRE, E. coli and K. pneumoniae, respectively. Table 3 shows the growth inhibitory time (GIT) of beads impregnated with antibiotics. In conclusion, the authors showed that the GIT of linezolid was significantly longer than that of vancomycin and gentamicin for MRSA and S. epidermidis. An axial compression test was performed to verify whether the mechanical strength of PMMA was compromised because of the addition of antibiotics. The results revealed no reduction in the mechanical strength of PMMA beads (P > 0.2) with the concentration of antibiotics used in this study (maximum 5% weight/weight antibiotic per PMMA packet). Both types of cements maintained similar mechanical properties. With this study, it can be said that linezolid is more effective than gentamicin and vancomycin against MRSA and S. epidermidis. Table 2 shows that the combinations of gentamicin plus linezolid or vancomycin plus linezolid do not provide a greater bactericidal potency. It can be concluded that PMMA impregnated with linezolid has the potential to be efficacious in the prevention and treatment of bone and joint infections31. Anguita‐Alonso et al. found that linezolid used at three different concentrations (2.5%, 5%, and 7.5% weight/weight) maintained excellent stability and elution after PMMA polymerization in vitro 33. The PMMA used was Simplex P in the form of beads, and the indicator microorganism was Bacillus subtilis. They also reported that compared with other antibiotics (ie, cefazolin, ciprofloxacin, gatifloxacin, levofloxacin, and rifampicin), the elution of linezolid from PMMA was less affected by impregnated antibiotic concentration.
Table 3.
Growth inhibitory time of beads impregnated with antibiotics
| Antibiotic | MRSA | S epidermidis | VRE | K pneumoniae | E coli |
|---|---|---|---|---|---|
| Linezolid | 21 ± 0.75* | 29 ± 0.5* | 15 ± 4.6* | Resistant | Resistant |
| Gentamicin | Resistant | 5 ± 1.7 | Resistant | 10 ± 1.73 | 16 ± 2 |
| Vancomycin | 8 ± 0.5 | 19 ± 1.9 | Resistant | Resistant | Resistant |
| Linezolid ± gentamicin | >45† | 38 ± 0.95† | 32† | >45 | 40 ± 0.5 |
| Linezolid ± vancomycin | 31±10‡ | >45‡ | 17 ± 1.15 | Resistant | Resistant |
These values are significantly longer (P < 0.01) compared with those of vancomycin for respective bacteria
These values are significantly longer (P < 0.01) compared with those of vancomycin, linezolid or gentamicin alone for respective bacteria
These values are significantly longer (P < 0.01) compared with those of either vancomycin or linezolid alone for respective bacteria
MRSA, methicillin‐resistant Staphylococcus aureus; VRE, vancomycin‐resistant enterococci.
Another important aspect related with the antibiotic loaded into bone cement is whether the exposure to antibiotic causes resistance; Corona et al. have seen in their study that the inclusion of gentamicin or tobramycin in cement spacers (4 g of antibiotic/40 g of PMMA) seems to increase the gram‐positive cocci resistance. They analyzed 113 chronic hip and knee prosthesis joint infections and observed that aminoglycoside‐resistance in gram‐positive cocci was significantly higher when aminoglycosides were incorporated in cement spacers than with no use of them. Gentamicin resistance after previous aminoglycoside‐cement spacer use was significantly higher (49.2% vs. 19.3%; P < 0.0001) as well as resistance to tobramycin (52.7% vs. 30.9%; P = 0.014)34. There is little evidence of this aspect.
In conclusion, the commercial formulations produce a greater and more regular release of antibiotic from bone cement than the manually mixed preparations. One of the biggest issues of most of the studies is that the commercial form of the antibiotic, which comes with excipients in many occasions, is used. This fact may explain the differences between pre‐mixed and manually mixed ALBC. Finally, currently there are a large number of combinations of bone cements with antibiotics, for which much remains to be elucidated, and it cannot be concluded that a perfect unique combination exists; each one adapts to the requirements of the clinical condition.
Two Antibiotics Combination
It has been reported that the simultaneous incorporation of two antibiotics or more into bone cement results in a higher rate of elution compared to bone cement loaded with one antibiotic. When two antibiotics are incorporated, more voids and cracks are present in bone cements as the drugs are released, thus increasing the release of the remaining antibiotics. Moreover, authors have described a synergic effect between some antibiotics (e.g. between aminoglycosides and glycopeptides)19. A study about the optimal antibiotic combination for the antibiotics gentamicin, vancomycin, and teicoplanin in cements showed that the combination of gentamicin and teicoplanin had a bactericidal activity more prolonged than gentamicin alone. Moreover, the synergic effect of teicoplanin and gentamicin had superior bactericidal activity compared to gentamicin and vancomycin35, 36. Bertazzoni Minelli et al. compared gentamicin plus vancomycin spacers to gentamicin alone spacers. The study showed that the combination was more effective than gentamicin alone37. These results concur with those mentioned above.
To date, there is no ideal combination of antibiotic and cement that allow covering all possible infections and, therefore, the antibiotic election must be effective against most microorganisms that cause infection.
Cement Type
Polymethylmethacrylate (PMMA) is the main component used in the fixation of joint prosthesis. It is prepared in the operating room, mixing the solid and liquid components. As bone cements have some disadvantages, these systems are fragile and produce necrosis due to exothermic reaction during the polymerization38, 39, 40, 41. There have been reports of thermal damage of cartilage and periosteum, leading to non‐union of fractures and loosening of implants40, 41.
Viscosity of the cement is very important in the mixing moment. Low viscosity promotes the mixing process; however, its mechanical strength is worse than that of high viscosity cements. Clinical outcomes of low viscosity bone cement demonstrate that they have higher risk of revision and loosening42, 43.
The cause of the loosening is unclear to date; Ayre et al. studied the mechanism that causes the aseptic loosening. To explain the aseptic loosening, two commercial high viscosity bone cements (Palacos and Cemex Isoplastic) were aged in an isotonic fluid at physiological temperatures. After 30 days, aging cements increased in weight by approximately 2% and the outermost layers of the cement were hydrolysed. This study concluded that this molecular change and the plasticizing effect of water resulted in reduced mechanical and fatigue properties over time and, therefore, cement aging contributes to the long‐term failure of cemented joint replacements44. These studies are important to simulate the evolution of bone cement into the organism.
The addition of barium sulfate and zirconium oxide (for radiological detection) increases the risk of loosening45. These radiopacifiers are hydrophilic and promote the hydrolysis of ester groups of methyl methacrylate (MMA) and PMMA. The previous study suggests using hydrophobic radiopacifiers, such as iodine‐based ones, developed by Lewis et al., to decrease the risk of loosening46. Shearwood et al. studied the effect of barium sulfate agglomerates on mechanical characterization of bone cement. They evaluated the effect of barium sulfate agglomeration on crack initiation processes in conventional, vacuum‐mixed acrylic cement. The tendency of barium sulfate particles to agglomerate is clearly evidenced to be detrimental to the fatigue performance of the cement47. Gomoll et al. studied the effect of replacing barium sulfate microparticles that are usually present in commercial PMMA cements with barium sulfate nanoparticles. They conclude that the nanoparticulate substitution of radio‐opacifiers substantially improved the in vitro mechanical properties of PMMA bone cement without changing the known chemical composition48. Ultimately, the use of the hydrophilic radio‐opacifiers damages the mechanical properties of bone cements, so there is more investigation required to find alternatives for the future.
Antibiotic elution from bone cement depends on cement composition and physicochemical characteristics of antibiotics. About gentamicin, Van de Belt et al. studied the formation of an S. aureus biofilm on six gentamicin‐loaded bone cements (CMW1, CMW3, CMW Endurance and CMW2000 with 2.5% of gentamicin; Palacos and Palamed with 1.25% of gentamicin). None of the gentamicin‐loaded cements showed a reduction in biofilm formation relative to unloaded cements within 6 h after inoculation, whereas only gentamicin‐loaded CMW1 and Palacos reduced biofilm formation 24 h after inoculation. Alternatively, CMW Endurance, CMW2000 and Palamed did not exhibit any initial reductions in biofilm formation, but effects started after 48 and 72 h, respectively. Biofilm reduction by gentamicin‐loaded CMW3 lasted the longest from 24 to 72 h. Biofilm formation on all cements follows a similar pattern in time, but the gentamicin‐loaded cements demonstrate different reductions of biofilm formation, which seems unrelated with the gentamicin‐release kinetics from the cements previously measured (Table 2). The authors conclude that biofilm formation on bone cements is not only related to gentamicin release but may also be dependent on other properties of the cement surface, such as its roughness18. Scott et al. compared the bioactivity of the two most used aminoglycosides (tobramycin and gentamicin) from different cements (Palacos and Simplex), and showed that tobramycin incorporated into Simplex has antibacterial activity against 98% of P. aeruginosa while gentamicin into Palacos against 93% of the same bacteria (P < 0.001). In this study, the authors compared the zone of inhibition of gentamicin and tobramycin loaded into bone cement at prophylaxis doses against 100 clinical isolates of P. aeruginosa collected from sputum, urine, and ear, but none that has caused a prosthetic infection. Results are consistent with the type of antibiotic, because tobramycin is slightly more effective than gentamicin against P. aeruginosa 49. With respect to vancomycin, Cerretani et al. compared the 2 g of vancomycin elution from 40 g of CMW1, Palacos‐R and Simplex‐P with a pharmacokinetic study. The authors performed a pharmacokinetic study which evaluated the area under the concentration‐time curve against time (AUC), which represents: the amount of drug released and pharmacologically available; the half‐life of release (t1/2); peak concentration (Cmax); and time at which Cmax is obtained (Tmax). The cements released 2.00%, 1.94%, and 1.69% of antibiotic incorporated after 35 days, respectively. Only t1/2 showed statistically significant differences between bone cements brands; with CMW1, there was a significantly longer release half‐life. Although there are significant differences, the clinical implications that this may involve are not clarified; bioactivity studies are needed to determine the clinical impact of differences32.
In regards to the comparison of pre‐mixed commercial ALBC, Neut et al. investigated differences in gentamicin release and the antibacterial efficacy of the eluent between four cement brands (Refobacin Palacos R, Refobacin Bone Cement R, Palacos R + G and SmartSet GHV). Table 2 shows the differences in the amount of antibiotic eluted and the bioactivity. Although the cements Refobacin Bone Cement R and Palacos R + G provided higher release of antibiotic, there was no colony growth in any cement sample during the 1‐week study, so it can be said that all commercial cements with gentamicin had adequate bioactivity during the first week29.
Mixing Method
The mixing method characterizes the antibiotic elution. The best antibiotic elution is associated to high cement porosity. The problem of high porosity is the loss of mechanical properties35, 50, 51. The presence of air trapped in cement decreased its resistance. The vacuum mixing decreases the air trapped in cement from 25% to 1%. Therefore, this mixing method provides advantages: the resistance increases from 70 to 90 MPa and fatigue resistance rises from 10 to 30 MPa51, 52. Nevertheless, the preparation under vacuum conditions causes a major reduction of bone cement and then a worse adhesion from bone cement‐to bone is obtained43, 53. There is a division of opinions according to the authors54. Meyer et al. compared 6 commercial bone cements (Cemex Genta Gentamicin 1.0 g/40 g, Cobalt G‐HV Gentamicin 0.5 g/40 g, Palacos R + G Gentamicin 0.5 g/40 g, Simplex P Tobramycin 1.0 g/40 g, SmartSet GMV Gentamicin 1.0 g/40 g and VersaBond AB Gentamicin 1.0 g/40 g) mixed at atmospheric pressure and under vacuum conditions55. A standard Kirby–Bauer bioassay technique was subsequently used to quantify antibiotic elution from the products. The results from the study demonstrated that vacuum mixing produced lower antibiotic release from Cemex, SmartSet and Versabond and increased the release of antibiotic from Palacos, Simplex and Cobalt (Fig. 2). According to these statements, the study concluded that the effect of vacuum‐mixing on antibiotic elution is product‐specific55. Our research team, compared the manual and vacuum mixing when ciprofloxacin hydrochloride was mixed with different bone cements (Simplex, Palacos and Lima CMT1). When comparing the two mixing procedures, no statistically significant differences were found between vacuum and manual mixing with respect to the drug release rate from Simplex and Palacos bone cements. In contrast, when Lima CMT1 bone cement was used, significant differences were observed for up to 697 h. However, no statistically significant differences in the percentage of amount released were observed at subsequent testing times. This significant difference can be explained if the high variability of the manual batches tested is considered. It should be emphasized that variability of the percentage of drug released from the vacuum‐mixed samples was much lower than that seen with manually‐mixed ones. Ultimately, vacuum mixing reduces variability in the release profiles, but the influence on kinetic properties is product‐specific.
Figure 2.
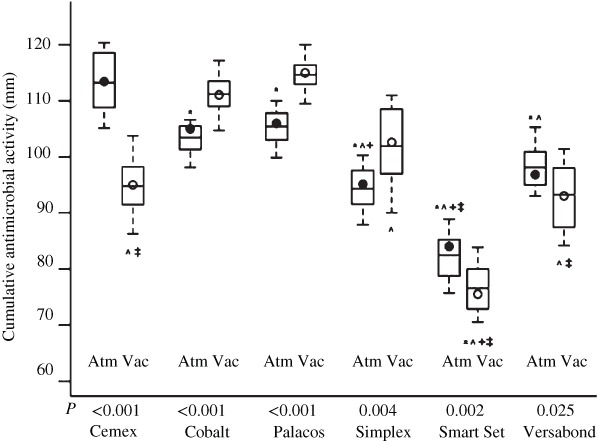
Summary of use of vacuum mixing when Cemex, SmartSet, Versabond, Palacos, Simplex, and Cobalt was used.
Some authors advocate vacuum mixing because the surgeons have less exposure to cement vapors; to date, several studies have shown that exposure to vapors from bone cements results in undetectable plasma levels. Homlar et al. studied the effect of exposure to PMMA. Twenty healthy volunteers were exposed during the mixing of polymethylmethacrylate cement in a simulated operating room environment (this study was purposefully designed using non‐laminar flow rooms, an open bowl mixing technique, and without the use of personal exhaust hoods to simulate a worst case scenario exposure). Methyl methacrylate was not detected in any of experimental specimens56.
Another important aspect is the mixing speed; Pithankuakul et al. evaluated the effect of the mixing speed of hand‐mixing bone cement. In the study, the antibiotic‐loaded bone cement used was Vancomycin‐Palacos LV. The authors concluded that preparing bone cement with high‐speed hand mixing and delaying antibiotic addition can increase vancomycin release57.
As shown, antibiotic elution depends on many factors (e.g. cement characteristics, physicochemical properties of the antibiotic, and mixing procedure), and there is no absolute best option; however, there is an optimal combination (antibiotic plus cement brand) for each microorganism. The continuing emergence of new commercially‐available brands of ALBC makes it important to establish which one will provide the most favorable antibiotic release, and, consequently, yield the best antibacterial efficacy.
Release Kinetics
Different authors have indicated that the inclusion of the antibiotic into bone cement provides a high antibiotic level in the first days, followed by a sustained release19. There are different studies showing evidence that the release can last for a few hours in some cases or for several weeks in others58, 59. First the antibiotic is eluted from the cement surface and then from the cement inside. The fluids in contact penetrate into the cement and dissolve the antibiotic. Then, the antibiotic dissolved is eluted from voids and cracks of bone cements60, 61. Various authors have stated that antibiotic elution from bone cement is conditioned by cement type and porosity, antibiotic molecular weight and physico‐chemical properties, surface in contact with the liquid of the environment, and amount of antibiotic incorporated16, 20, 21, 62, 63. The problem is that the PMMA is a highly hydrophobic polymer, which limits the elution. For this reason, some antibiotics are only eluted during the first hours; that is, only the antibiotic on the surface is released64. Only high solubility and low molecular weight antibiotics would be eluted through voids and cracks16, 20, 21, 62, 63.
Because antibiotics dissolved from the cement surface represent the highest amount released, cement surface in contact with fluids conditions efficacy. Moojen et al. and Bertazzoni et al. showed that the initial release is proportional to the rugosity and then to the surface37, 65, while release in the following days is proportional to cement porosity. This statement is logical and it should always be extrapolated into clinical practice.
As stated above, currently, the use of premixed antibiotic loaded bone cement has been approved. Only commercially available antibiotic PMMA can be used for reconstruction of a previously septic total knee or total hip replacement. Antibiotic incorporation into bone cement by surgeons is not permitted and, therefore, the only antibiotics available are vancomycin, clindamycin, tobramycin and gentamicin. Meta‐analysis previously referenced showed that the inclusion of antibiotics into bone cement demonstrated its efficacy in deep infection but not in superficial infection15, 66. This result was expected because the antibiotic released out of cement would stay at the cement–bone interface. In any case, the antibiotic release from bone cement would be an effective system for deep infection, which is more complicated due to poor blood supply.
In summary, the PMMA highly hydrophobic polymer limits the elution, and makes it dependent on features of the antibiotic and the surface in contact. Some authors discuss the possible systemic bioavailability of antibiotics from bone cement. Kendoff et al. evaluated the systemic bioavailability of antibiotics from bone cement after implantation, determining the concentrations of gentamicin and vancomycin in plasma and urine of patients receiving a novel bone cement during one‐stage revision in periprosthetic hip infections. The mean postoperative maximum gentamicin plasma concentration at 5.85 h was 209.65 ng/mL. For vancomycin, a mean postoperative maximum plasma concentration of 134.64 ng/mL was determined at 20.03 h. The authors concluded that there was slow absorption of both antibiotics after release from the cement, resulting in plasma concentrations well below toxic levels, which would not result in a critical systemic concentration potentially inducing bacterial resistance67. In any case, ALBC are safe from the pharmacotherapeutical point of view, with a very low systemic absorption.
Perspectives and Conclusions
Currently, researchers are looking for ways to increase and improve these systems’ release. In this manner, there are studies where some substances are included in bone cement to improve the elution. As an example, it has been observed that vitamin E is a scavenger of free radicals in the oxidative process. Moreover, its inclusion in bone cement reduces the temperature of the hardening process (62 to 36°C) and, therefore, increases cytocompatibility. Up to 25% of vitamin E does not decrease the mechanical strength68. Penalba et al. studied the effect of bone cement loaded with daptomycin alone or in combination with gentamicin or PEG600 in the prevention of biofilm formation of S. epidermidis. For comparison, PMMA loaded with gentamicin or vancomycin was tested. The study showed that vancomycin was superior to daptomycin and gentamicin inhibiting staphylococcal adherence in vitro. However, PMMA loaded with daptomycin combined with gentamicin or PEG600 completely inhibited S. epidermidis‐biofilm formation69.
It has been demonstrated that the inclusion of chitosan nanoparticles has activity against S. aureus and S. epidermidis, without a decrease in mechanical strength compared to PMMA alone70. The inclusion of this polysaccharide would have antimicrobial activity per se. These findings support the possibility of combining in cements this polymer with antibiotics. Another improvement is the inclusion of silver nanoparticles71. When this metal is included in cements it is eluted and has antimicrobial activity against A. baumanii, P. aeruginosa, P. mirabilisy, and S. aureus, but its inclusion reduces the mechanical strength of cement72.
Although there are still many variables to elucidate, antibiotic‐loaded bone cements are a successful alternative to decrease the infection rate. Many questions, including what is the optimal dose, which patients would benefit from it and which is the optimal antibiotic–cement combination to eradicate microorganisms specifically, are still open. Nevertheless, there are a many ways to improve these delivery systems that can lead in the future to ALBC able to provide clinical benefit in primary surgery, as a prophylaxis to prevent deep infection.
Disclosure: This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors.
References
- 1. Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res, 2010, 468: 52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Regis D, Sandri A, Samaila E, Benini A, Bondi M, Magnan B. Release of gentamicin and vancomycin from preformed spacers in infected total hip arthroplasties: measurement of concentrations and inhibitory activity in patients’ drainage fluids and serum. ScientificWorldJournal, 2013, 2013: 752184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hsieh PH, Huang KC, Tai CL. Liquid gentamicin in bone cement spacers: in vivo antibiotic release and systemic safety in two‐stage revision of infected hip arthroplasty. J Trauma, 2009, 66: 804–808. [DOI] [PubMed] [Google Scholar]
- 4. Buchholz HW, Engelbrecht H. Depot effects of various antibiotics mixed with Palacos resins. Chirurg, 1970, 41: 511–515. [PubMed] [Google Scholar]
- 5. Arciola CR, Campoccia D, Montanaro L. Effects on antibiotic resistance of Staphylococcus epidermidis following adhesion to polymethylmethacrylate and to silicone surfaces. Biomaterials, 2002, 23: 1495–1502. [DOI] [PubMed] [Google Scholar]
- 6. Joseph TN, Chen AL, Di Cesare PE. Use of antibiotic‐impregnated cement in total joint arthroplasty. J Am Acad Orthop Surg, 2003, 11: 38–47. [DOI] [PubMed] [Google Scholar]
- 7. Hanssen AD. Prophylactic use of antibiotic bone cement: an emerging standard‐‐in opposition. J Arthroplasty, 2004, 19: 73–77. [DOI] [PubMed] [Google Scholar]
- 8. Gutowski CJ, Zmistowski BM, Clyde CT, Parvizi J. The economics of using prophylactic antibiotic‐loaded bone cement in total knee replacement. Bone Joint J, 2014, 96: 65–69. [DOI] [PubMed] [Google Scholar]
- 9. Thomes B, Murray P, Bouchier‐Hayes D. Development of resistant strains of Staphylococcus epidermidis on gentamicin‐loaded bone cement in vivo . J Bone Joint Surg Br, 2002, 84: 758–760. [DOI] [PubMed] [Google Scholar]
- 10. Kynaston‐Pearson F, Ashmore AM, Malak TT, et al Primary hip replacement prostheses and their evidence base: systematic review of literature. BMJ, 2013, f6956: 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. National Joint Registry for England and Wales . 9th annual report. National Joint Registry, 2012. Available from: http://www.njrcentre.org.uk/njrcentre/Reports,PublicationsandMinutes/Annualreports/tabid/86/Default.aspx (accessed 1 September 2016).
- 12. Bourne RB. Prophylactic use of antibiotic bone cement: an emerging standard‐‐in the affirmative. J Arthroplasty, 2004, 19: 69–72. [DOI] [PubMed] [Google Scholar]
- 13. Parvizi J, Della Valle CJ. AAOS clinical practice guideline: diagnosis and treatment of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg, 2010, 18: 771–772. [DOI] [PubMed] [Google Scholar]
- 14. Parvizi J, Saleh KJ, Ragland PS, Pour AE, Mont MA. Efficacy of antibiotic‐impregnated cement in total hip replacement. Acta Orthop, 2008, 79: 335–341. [DOI] [PubMed] [Google Scholar]
- 15. Wang J, Zhu C, Cheng T, et al A systematic review and meta‐analysis of antibiotic‐impregnated bone cement use in primary total hip or knee arthroplasty. PLoS One, 2013, 8: e82745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Penner MJ, Duncan CP, Masri BA. The in vitro elution characteristics of antibiotic‐loaded CMW and Palacos‐R bone cements. J Arthroplasty, 1999, 14: 209–214. [DOI] [PubMed] [Google Scholar]
- 17. Steinbrink K. The case for revision arthroplasty using antibiotic‐loaded acrylic cement. Clin Orthop Relat Res, 1990, 261: 19–22. [PubMed] [Google Scholar]
- 18. van de Belt H, Neut D, Schenk W, van Horn JR, van Der Mei HC, Busscher HJ. Staphylococcus aureus biofilm formation on different gentamicin‐loaded polymethylmethacrylate bone cements. Biomaterials, 2001, 22: 1607–1611. [DOI] [PubMed] [Google Scholar]
- 19. Anagnostakos K, Kelm J. Enhancement of antibiotic elution from acrylic bone cement. J Biomed Mater Res B Appl Biomater, 2009, 90: 467–475. [DOI] [PubMed] [Google Scholar]
- 20. Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone‐cement. J Arthroplasty, 1996, 11: 939–944. [DOI] [PubMed] [Google Scholar]
- 21. Lawson KJ, Marks KE, Brems J, Rehm S. Vancomycin vs tobramycin elution from polymethylmethacrylate: an in vitro study. Orthopedics, 1990, 13: 521–524. [DOI] [PubMed] [Google Scholar]
- 22. Patel A, Calfee RP, Plante M, Fischer SA, Arcand N, Born C. Methicillin‐resistant Staphylococcus aureus in orthopaedic surgery. J Bone Joint Surg Br, 2008, 90: 1401–1406. [DOI] [PubMed] [Google Scholar]
- 23. Dirschl DR, Almekinders LC. Osteomyelitis. Common causes and treatment recommendations. Drugs, 1993, 45: 29–43. [DOI] [PubMed] [Google Scholar]
- 24. Xu J, Liu Y, He J, Zhang R, Zuo B, Wang X. Surface structures of poly(methyl methacrylate) films influenced by chain entanglement in the corresponding film‐formation solution. Soft Matter, 2014, 10: 8992–9002. [DOI] [PubMed] [Google Scholar]
- 25. Ferraris S, Miola M, Bistolfi A, et al In vitro comparison between commercially and manually mixed antibiotic‐loaded bone cements. J Appl Biomater Biomech, 2010, 8: 166–174. [PubMed] [Google Scholar]
- 26. Martinez‐Moreno J, Mura C, Merino V, Nacher A, Climente M, Merino‐Sanjuan M. Study of the influence of bone cement type and mixing method on the bioactivity and the elution kinetics of ciprofloxacin. J Arthroplasty, 2015, 30: 1243–1249. [DOI] [PubMed] [Google Scholar]
- 27. Paz E, Sanz‐Ruiz P, Abenojar J, Vaquero‐Martin J, Forriol F, Del Real JC. Evaluation of elution and mechanical properties of high‐dose antibiotic‐loaded bone cement: comparative “in vitro” study of the influence of vancomycin and cefazolin. J Arthroplasty, 2015, 30: 1423–1429. [DOI] [PubMed] [Google Scholar]
- 28. Galvez‐Lopez R, Pena‐Monje A, Antelo‐Lorenzo R, et al Elution kinetics, antimicrobial activity, and mechanical properties of 11 different antibiotic loaded acrylic bone cement. Diagn Microbiol Infect Dis, 2014, 78: 70–74. [DOI] [PubMed] [Google Scholar]
- 29. Neut D, Kluin OS, Thompson J, van der Mei HC, Busscher HJ. Gentamicin release from commercially‐available gentamicin‐loaded PMMA bone cements in a prosthesis‐related interfacial gap model and their antibacterial efficacy. BMC Musculoskelet Disord, 2010, 11: 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hsu YM, Liao CH, Wei YH, et al Daptomycin‐loaded polymethylmethacrylate bone cement for joint arthroplasty surgery. Artif Organs, 2014, 38: 484–492. [DOI] [PubMed] [Google Scholar]
- 31. Snir N, Meron‐Sudai S, Deshmukh AJ, Dekel S, Ofek I. Antimicrobial properties and elution kinetics of linezolid from polymethylmethacrylate. Orthopedics, 2013, 36: e1412–e1417. [DOI] [PubMed] [Google Scholar]
- 32. Cerretani D, Giorgi G, Fornara P, et al The in vitro elution characteristics of vancomycin combined with imipenem‐cilastatin in acrylic bone‐cements: a pharmacokinetic study. J Arthroplasty, 2002, 17: 619–626. [DOI] [PubMed] [Google Scholar]
- 33. Anguita‐Alonso P, Rouse MS, Piper KE, Jacofsky DJ, Osmon DR, Patel R. Comparative study of antimicrobial release kinetics from polymethylmethacrylate. Clin Orthop Relat Res, 2006, 445: 239–244. [DOI] [PubMed] [Google Scholar]
- 34. Corona PS, Espinal L, Rodriguez‐Pardo D, Pigrau C, Larrosa N, Flores X. Antibiotic susceptibility in gram‐positive chronic joint arthroplasty infections: increased aminoglycoside resistance rate in patients with prior aminoglycoside‐impregnated cement spacer use. J Arthroplasty, 2014, 29: 1617–1621. [DOI] [PubMed] [Google Scholar]
- 35. Lewis G, Janna S. Estimation of the optimum loading of an antibiotic powder in an acrylic bone cement: gentamicin sulfate in SmartSet HV. Acta Orthop, 2006, 77: 622–627. [DOI] [PubMed] [Google Scholar]
- 36. Anagnostakos K, Kelm J, Regitz T, Schmitt E, Jung W. In vitro evaluation of antibiotic release from and bacteria growth inhibition by antibiotic‐loaded acrylic bone cement spacers. J Biomed Mater Res B Appl Biomater, 2005, 72: 373–378. [DOI] [PubMed] [Google Scholar]
- 37. Bertazzoni Minelli E, Benini A, Magnan B, Bartolozzi P. Release of gentamicin and vancomycin from temporary human hip spacers in two‐stage revision of infected arthroplasty. J Antimicrob Chemother, 2004, 53: 329–334. [DOI] [PubMed] [Google Scholar]
- 38. Whitehouse MR, Atwal NS, Pabbruwe M, Blom AW, Bannister GC. Osteonecrosis with the use of polymethylmethacrylate cement for hip replacement: thermal‐induced damage evidenced in vivo by decreased osteocyte viability. Eur Cell Mater, 2014, 27: 50–62. [DOI] [PubMed] [Google Scholar]
- 39. Stanczyk M, van Rietbergen B. Thermal analysis of bone cement polymerisation at the cement‐bone interface. J Biomech, 2004, 37: 1803–1810. [DOI] [PubMed] [Google Scholar]
- 40. Radev BR, Kase JA, Askew MJ, Weiner SD. Potential for thermal damage to articular cartilage by PMMA reconstruction of a bone cavity following tumor excision: a finite element study. J Biomech, 2009, 42: 1120–1126. [DOI] [PubMed] [Google Scholar]
- 41. Boner V, Kuhn P, Mendel T, Gisep A. Temperature evaluation during PMMA screw augmentation in osteoporotic bone‐‐an in vitro study about the risk of thermal necrosis in human femoral heads. J Biomed Mater Res B Appl Biomater, 2009, 90: 842–848. [DOI] [PubMed] [Google Scholar]
- 42. Stone JJ, Rand JA, Chiu EK, Grabowski JJ, An KN. Cement viscosity affects the bone‐cement interface in total hip arthroplasty. J Orthop Res, 1996, 14: 834–837. [DOI] [PubMed] [Google Scholar]
- 43. Lewis G, Janna S, Bhattaram A. Influence of the method of blending an antibiotic powder with an acrylic bone cement powder on physical, mechanical, and thermal properties of the cured cement. Biomaterials, 2005, 26: 4317–4325. [DOI] [PubMed] [Google Scholar]
- 44. Ayre WN, Denyer SP, Evans SL. Ageing and moisture uptake in polymethyl methacrylate (PMMA) bone cements. J Mech Behav Biomed Mater, 2014, 32: 76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sylvain GM, Kassab S, Coutts R, Santore R. Early failure of a roughened surface, precoated femoral component in total hip arthroplasty. J Arthroplasty, 2001, 16: 141–148. [DOI] [PubMed] [Google Scholar]
- 46. Lewis G, van Hooy‐Corstjens CS, Bhattaram A, Koole LH. Influence of the radiopacifier in an acrylic bone cement on its mechanical, thermal, and physical properties: barium sulfate‐containing cement versus iodine‐containing cement. J Biomed Mater Res B Appl Biomater, 2005, 73: 77–87. [DOI] [PubMed] [Google Scholar]
- 47. Shearwood‐Porter N, Browne M, Sinclair I. Micromechanical characterisation of failure in acrylic bone cement: the effect of barium sulphate agglomerates. J Mech Behav Biomed Mater, 2012, 13: 85–92. [DOI] [PubMed] [Google Scholar]
- 48. Gomoll AH, Fitz W, Scott RD, Thornhill TS, Bellare A. Nanoparticulate fillers improve the mechanical strength of bone cement. Acta Orthop, 2008, 79: 421–427. [DOI] [PubMed] [Google Scholar]
- 49. Scott CP, Higham PA. Antibiotic bone cement for the treatment of Pseudomonas aeruginosa in joint arthroplasty: comparison of tobramycin and gentamicin‐loaded cements. J Biomed Mater Res B Appl Biomater, 2003, 64: 94–98. [DOI] [PubMed] [Google Scholar]
- 50. Lewis G. Properties of antibiotic‐loaded acrylic bone cements for use in cemented arthroplasties: a state‐of‐the‐art review. J Biomed Mater Res B Appl Biomater, 2009, 89: 558–574. [DOI] [PubMed] [Google Scholar]
- 51. Wixson RL, Lautenschlager EP, Novak MA. Vacuum mixing of acrylic bone cement. J Arthroplasty, 1987, 2: 141–149. [DOI] [PubMed] [Google Scholar]
- 52. Eveleigh R. Mixing systems and the effects of vacuum mixing on bone cement. Br J Perioper Nurs, 2001, 11: 135–140. [DOI] [PubMed] [Google Scholar]
- 53. Jiranek WA, Hanssen AD, Greenwald AS. Antibiotic‐loaded bone cement for infection prophylaxis in total joint replacement. J Bone Joint Surg Am, 2006, 88: 2487–2500. [DOI] [PubMed] [Google Scholar]
- 54. Laine JC, Nguyen TQ, Buckley JM, Kim HT. Effects of mixing techniques on vancomycin‐impregnated polymethylmethacrylate. J Arthroplasty, 2011, 26: 1562–1566. [DOI] [PubMed] [Google Scholar]
- 55. Meyer J, Piller G, Spiegel CA, Hetzel S, Squire M. Vacuum‐mixing significantly changes antibiotic elution characteristics of commercially available antibiotic‐impregnated bone cements. J Bone Joint Surg Am, 2011, 93: 2049–2056. [DOI] [PubMed] [Google Scholar]
- 56. Homlar KC, Sellers MH, Halpern JL, Seeley EH, Holt GE. Serum levels of methyl methacrylate following inhalational exposure to polymethylmethacrylate bone cement. J Arthroplasty, 2013, 28: 406–409. [DOI] [PubMed] [Google Scholar]
- 57. Pithankuakul K, Samranvedhya W, Visutipol B, Rojviroj S. The effects of different mixing speeds on the elution and strength of high‐dose antibiotic‐loaded bone cement created with the hand‐mixed technique. J Arthroplasty, 2015, 30: 858–863. [DOI] [PubMed] [Google Scholar]
- 58. Elson RA, Jephcott AE, McGechie DB, Verettas D. Antibiotic‐loaded acrylic cement. J Bone Joint Surg Br, 1977, 59: 200–205. [DOI] [PubMed] [Google Scholar]
- 59. Stevens CM, Tetsworth KD, Calhoun JH, Mader JT. An articulated antibiotic spacer used for infected total knee arthroplasty: a comparative in vitro elution study of Simplex and Palacos bone cements. J Orthop Res, 2005, 23: 27–33. [DOI] [PubMed] [Google Scholar]
- 60. Torrado S, Frutos P, Frutos G. Gentamicin bone cements: characterisation and release (in vitro and in vivo assays). Int J Pharm, 2001, 217: 57–69. [DOI] [PubMed] [Google Scholar]
- 61. Duey RE, Chong AC, McQueen DA, et al Mechanical properties and elution characteristics of polymethylmethacrylate bone cement impregnated with antibiotics for various surface area and volume constructs. Iowa Orthop J, 2012, 32: 104–115. [PMC free article] [PubMed] [Google Scholar]
- 62. Baker AS, Greenham LW. Release of gentamicin from acrylic bone cement. Elution and diffusion studies. J Bone Joint Surg Am, 1988, 70: 1551–1557. [PubMed] [Google Scholar]
- 63. DeLuise M, Scott CP. Addition of hand‐blended generic tobramycin in bone cement: effect on mechanical strength. Orthopedics, 2004, 27: 1289–1291. [DOI] [PubMed] [Google Scholar]
- 64. Powles JW, Spencer RF, Lovering AM. Gentamicin release from old cement during revision hip arthroplasty. J Bone Joint Surg Br, 1998, 80: 607–610. [PubMed] [Google Scholar]
- 65. Moojen DJ, Hentenaar B, Charles Vogely H, Verbout AJ, Castelein RM, Dhert WJ. In vitro release of antibiotics from commercial PMMA beads and articulating hip spacers. J Arthroplasty, 2008, 23: 1152–1156. [DOI] [PubMed] [Google Scholar]
- 66. Hinarejos P, Guirro P, Leal J, et al The use of erythromycin and colistin‐loaded cement in total knee arthroplasty does not reduce the incidence of infection: a prospective randomized study in 3000 knees. J Bone Joint Surg Am, 2013, 95: 769–774. [DOI] [PubMed] [Google Scholar]
- 67. Kendoff DO, Gehrke T, Stangenberg P, Frommelt L, Bosebeck H. Bioavailability of gentamicin and vancomycin released from an antibiotic containing bone cement in patients undergoing a septic one‐stage total hip arthroplasty (THA) revision: a monocentric open clinical trial. Hip Int, 2016, 26: 90–96. [DOI] [PubMed] [Google Scholar]
- 68. Mendez JA, Aguilar MR, Abraham GA, et al New acrylic bone cements conjugated to vitamin E: curing parameters, properties, and biocompatibility. J Biomed Mater Res, 2002, 62: 299–307. [DOI] [PubMed] [Google Scholar]
- 69. Penalba Arias P, Furustrand Tafin U, Betrisey B, Vogt S, Trampuz A, Borens O. Activity of bone cement loaded with daptomycin alone or in combination with gentamicin or PEG600 against Staphylococcus epidermidis biofilms. Injury, 2015, 46: 249–253. [DOI] [PubMed] [Google Scholar]
- 70. Shi Z, Neoh KG, Kang ET, Wang W. Antibacterial and mechanical properties of bone cement impregnated with chitosan nanoparticles. Biomaterials, 2006, 27: 2440–2449. [DOI] [PubMed] [Google Scholar]
- 71. Alt V, Bechert T, Steinrucke P, et al Nanoparticulate silver. A new antimicrobial substance for bone cement. Orthopade, 2004, 33: 885–892. [DOI] [PubMed] [Google Scholar]
- 72. Morones JR, Elechiguerra JL, Camacho A, et al The bactericidal effect of silver nanoparticles. Nanotechnology, 2005, 16: 2346–2353. [DOI] [PubMed] [Google Scholar]


