Abstract
Objective
To demonstrate the effect of the screw‐home motion on the stability of the patellofemoral joint, and investigate its mechanism of regulation of patellar tracking.
Methods
Twenty volunteers who met the criteria were examined. All subjects had axial computed tomography (CT) scanning performed on bilateral knees at 0° and 30° of flexion. Scanning began above the femorotibial articulation and femoral trochlear groove, and moved sequentially down to the level of the anterior tibial tubercle. The following measurements were obtained: tibial rotation relative to the femur (TRRF), tibial tuberosity–trochlear groove (TT–TG) distance, lateral patellar displacement (LPD), patellar tilt angle (PTA), and congruence angle (CA). We assessed the change (Δ) in each variable at both flexion angles, and analyzed this to investigate the corresponding relationship between the patella, the femur, and the screw‐home mechanism. The differences between the values measured at 0° and those measured at 30° flexion were analyzed using the paired sample t‐test. The differences between men and women were analyzed using the t‐test. Pearson’s correlations were performed to determine the relationship between ΔTT–TG distance and ΔLPD, ΔPTA and ΔTRRF, and ΔCA and ΔTRRF.
Results
There were 10 women and 10 men enrolled in the present study, with an average age of 25 years and an average body mass index of 21.8 kg/m2, and all volunteers had no history of knee injuries. Compared with measurements taken at 0° flexion, TRRF at 30° flexion was significantly increased, and the PTA, CA, LPD, and TT–TG distance were significantly decreased (all P < 0.01). There was no difference between men and women at 0° and 30° flexion, respectively (P < 0.01). In this respect, there was no sex difference, but the change was greater for men than for women. Both ΔPTA and ΔCA demonstrated significant correlation with the ΔTRRF (both P < 0.01); a significant correlation between ΔLPD and ΔTT–TG distance was also demonstrated (P < 0.01).
Conclusions
As the tibiofemoral joint rotated, the patellofemoral joint became more stable and aligned, which indicates that the screw‐home mechanism plays an important role in regulating patellofemoral joint alignment.
Keywords: Kinematics, Patellar tracking, Patellofemoral joint, “Screw‐home” mechanism, Tibiofemoral joint
Introduction
Patellar tracking is defined as the motion of the patella relative to the femur or the femoral groove during knee flexion and extension. Patellar maltracking is thought to relate to many disorders of the patellofemoral joint1. Consequently, normal patellar tracking is the basis of the kinematics of knee. Normal patellar tracking depends on a variety of dynamic and static structures around the patella, and previous studies have investigated patellar tracking with varying results2, 3. In general, the patella is gradually aligned to the femoral trochlear groove during knee flexion from 0° to 30°. In this range of flexion, the patella is not yet confined to its femoral groove, thus giving room for free patellar movement; this is referred to as “capture.” “Capture” is affected by tibial rotation, through tensioning of the patellar ligament and the lateral and medial retinacula, which is an important premise for normal patellar tracking4, 5.
The effects of longitudinal tibial rotation on the patellofemoral joint have attracted increasing attention over the past two decades5, 6; however, the specific mechanism remains unclarified. In the normal knee, internal rotation of the tibia relative to the femur commonly occurs during knee flexion. However, Coughlin et al. report that 81% of the rotation movement occurred during knee flexion from 0° to 30°7; this particular motion was referred to as the “screw‐home” motion. Research shows that the screw‐home mechanism is coupled internal–external rotation, which could improve the mechanical efficiency of the knee joint4, 8, 9. Some authors report that screw‐home motion is characteristic of healthy knee motion, and its absence is often thought to be an indication of instability or joint disease4, 8, 10, 11.
Interestingly, both of these important components of knee movement, the capture mechanism and the screw‐home motion of the patellofemoral joint, are exhibited in the early stage of knee flexion. Time synchronization suggests that there must be intrinsic biomechanical and kinematical relationships between the screw‐home mechanism and the capture mechanism of the patellofemoral joint. Computed tomography (CT) is useful in the diagnosis of patellar malalignment, and many studies have carried out CT examination at varying degrees of knee flexion12, 13.
The aim of the present study was to investigate the effect of the screw‐home motion on the stability of the patellofemoral joint, and to explore its mechanism of regulation of patellar tracking so as to further enhance our understanding of knee kinematics.
Materials and Methods
Participants
Volunteers were screened based on the following criteria. Inclusion criteria: (i) the human age ranges from 18 to 40 years; and (ii) 19 kg/m2 ≤ body mass index ≤ 25 kg/m2. 32 Volunteers with any of the following conditions were excluded: (i) symptoms such as knee pain, swelling, and/or dislocation; (ii) varus or valgus deformity of the knee and patella alta; (iii) joint hypermobility syndrome and joint hyperextension; (iv) suppurative, rheumatoid, or tuberculosis arthritis; (v) history of knee surgery or trauma; (vi) intra‐articular tumor or tumor‐like lesion; (vii) radiologic evidence of asymptomatic patellar instability such as patellar tilt and/or http://dict.baidu.com/s?wd=subluxation%20of%20the%20patella. A total of 32 volunteers were excluded. The present study was approved by the local ethics committee of our institution. All subjects provided written informed consent.
Computed Tomography Measurements
All subjects had axial CT scanning performed on bilateral knees at 0° and 30° of flexion in the supine position, with the limbs fixed by equipment to support the joint at the correct angle14. The flexion angle was monitored using a handheld goniometer. The CT scanning plane was perpendicular to the longitudinal axis of the femur at full extension or flexion of 30°.
Computed Tomography Protocol
For all diagnostic examinations, a Phillips LXC CT Scanner (Phillips Medical System, Best, Netherlands) was used to create transaxial images (1‐mm slice thickness, 1‐mm slice gap, 3.8‐s scan time, 150 mA, 100 kVp, 250 FOV, 570 mAs) in all participants. Scanning began above the femorotibial articulation and femoral trochlear groove, and moved sequentially down to the level of the anterior tibial tubercle.
Parameters for Measurement
Dejour et al. report that a tibial tuberosity trochlear groove distance (TT–TG) > 20 mm is generally considered pathological and as an indication for medial tibial tubercle transfer in symptomatic participants15. Tibial rotation angle relative to the femur was defined as the relative rotational difference between the femur and the tibia16. Patellar tilt angle, congruence angle and lateral patellar displacement could indicate the matching index of the patellofemoral joint17, 18. As a result, the following measurements were obtained.
Tibia Tuberosity–Trochlear Groove (TT–TG) Distance
It was measured by superimposing the axial section depicting the deepest part of the trochlear groove upon the center of the tibial tuberosity, while ensuring that the measurement was parallel to the posterior condylar axis of the femur19 (Fig. 1A).
Figure 1.
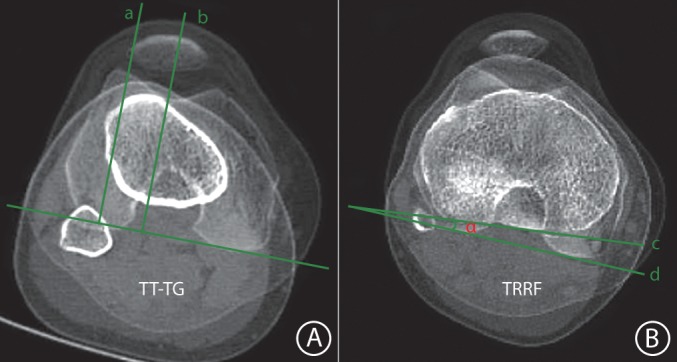
Measurement of tibia tuberosity‐trochlear groove (TT–TG) distance and tibial rotation relative to the femur (TRRF). (A) TT–TG distance: the distance between the axial section depicting the deepest part of the trochlear groove (a) and the center of the tibial tuberosity (b), while ensuring that the measurement was parallel to the posterior condylar axis of the femur; (B) TRRF on axial computed tomography scan: the angle (α) between the line (c) drawn through the posterior border of the proximal tibia and the line (d) drawn through the two most posterior points of the posterior femoral condyles. F, femur; T, tibia.
Tibial Rotation Relative to the Femur (TRRF)
It measured the angle between two lines drawn through the two most posterior points of the posterior femoral condyles and the posterior border of the proximal tibia, with positive values indicating internal rotation (Fig. 1B).
Patellar Tilt Angle (PTA)
It measured the angle subtended by a line through the medial and lateral edge of the patella and another line through the anterior border of both femoral condyles20 (Fig. 2A).
Figure 2.
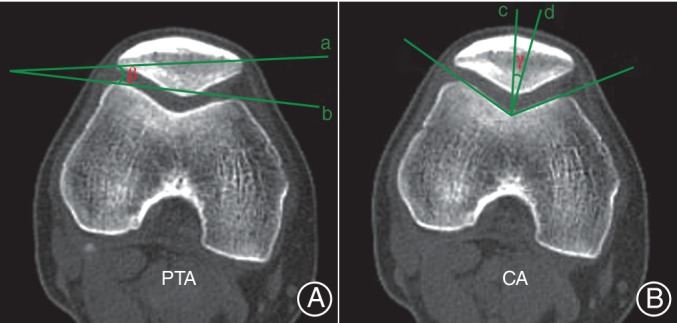
Measurement of patellar tilt angle (PTA) and congruence angle (CA). (A) PTA, the angle (β) between the line intersecting the widest part of the patella (a) and the line tangential to the anterior surfaces of the femoral condyles (b); (B) CA, the angle (γ) between the line bisecting the sulcus angle (c) and the line connecting the apex of the sulcus to the lowest aspect of the patella ridge (d).
Congruence Angle (CA)
It measured the angle between the line bisecting the sulcus angle and the line connecting the apex of the sulcus to the lowest aspect of the patella ridge, based on the method described by Kujala et al. 21 (Fig. 2B).
Lateral Patellar Displacement (LPD)
It measured the negative values indicating medial translation, as described by Kujala et al. 21 (Fig. 3).
Figure 3.
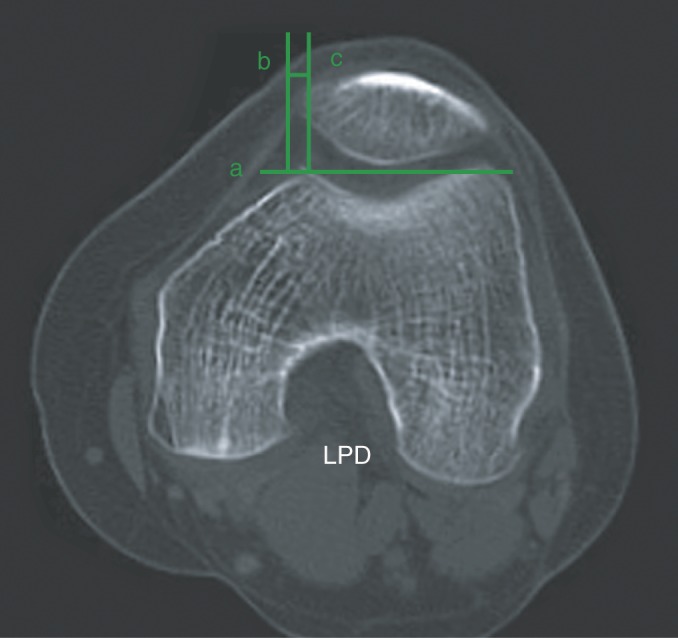
Measurement of lateral patella displacement (LPD). Line a connects the lateral and medial femoral anterior condyles. Line c is perpendicular to line a through the peak of femoral condyle, and line b is parallel with line a through medial edge of the patella, and LPD is defined as the distance from b to c.
A single experienced orthopedic surgeon (X.M.W.) performed all measurements. Each value was measured three times, and the average was calculated. The subjects’ identifying information (name, age, and sex) was blinded.
Statistical Analysis
The data obtained were statistically analyzed using SPSS 21.0 software (Chicago, IL, USA). All data were expressed as the mean ± standard deviation. The differences between the values measured at 0° and those measured at 30° flexion were analyzed using the paired sample t‐test. P < 0.01 was considered statistically significant. The differences between men and women were analyzed using the t‐test. Pearson’s correlations were performed to determine the relationship between ΔTT–TG distance and ΔLPD, as well as the relationship between ΔPTA and ΔTRRF, and that between ΔCA and ΔTRRF. A Shapiro–Wilk normality test confirmed that all variables were normally distributed.
Results
Characteristics of Participants
Twenty volunteers (10 women and 10 men) who met the criteria were examined. The subject age was 25 ± 2.3 years (range, 21–34 years), the body mass index was 21.8 ± 2.1 kg/m2, and the height was 172.0 ± 5.5 cm.
Measurement Results
All planned measurements were obtained for each knee in every subject, and all measured values were significantly different between 0° and 30° of knee flexion. (both P < 0.01, Table 1).
Table 1.
Measurement results at 0° and 30° flexion (mean±SD)
| Indexes | 0°(40 cases) | 30°(40 cases) | t | P |
|---|---|---|---|---|
| TFFR (°) | −1.8 ± 1.3 | 8.1 ± 1.5** | −25.3 | 0.000 |
| CA (°) | 23.6 ± 2.7 | −7.4 ± 1.8** | 46.7 | 0.000 |
| PTA (°) | 14.5 ± 1.1 | 8.0 ± 1.2** | 22.5 | 0.000 |
| TT–TG (mm) | 15.4 ± 1.3 | 9.2 ± 1.4** | 18.5 | 0.000 |
| LPD (mm) | 5.8 ± 1.3 | −0.8 ± 1.5** | 15.4 | 0.000 |
Significant difference between 30° and 0° (P < 0.01).
CA, congruence angle; LPD, lateral patella displacement; PTA, patella tilt angle; TRRF, tibia rotation relative to femur; TT–TG, tibia tuberosity‐trochlear groove distance.
The TRRF measurements showed that at knee flexion of 30°, the angle significantly increased (550%) from −1.8° ± 1.3° to 8.1° ± 1.5° (P < 0.01). The TRRF angles was −2.3° ± 2.1° and 7.1° ± 1.2° in male participants, and −1.1° ± 1.7° and 8.7° ± 1.6° in female participants at the knee flexion of 0° and 30°, respectively. There was no difference between male and female participants at 0° and 30° flexion, respectively (P < 0.01). Their changed trend was the same as the overall trend (Fig. 4).
Figure 4.
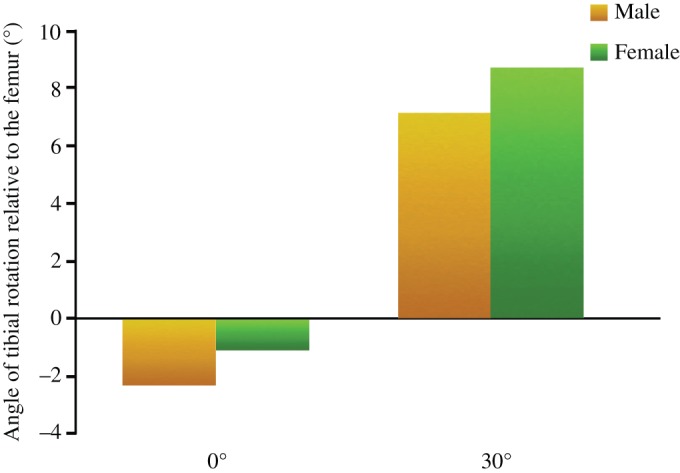
Bar chart illustrates the angle of tibial rotation relative to the femur (TRRF) at 0° and 30° flexion between men and women.
With the increase in the flexion angle from 0° to 30°, the PTA significantly decreased (45%), from 14.5° ± 1.1° to 8.0° ± 1.2° (P < 0.01). The patellar tilt angle was 15.1° ± 2.3° and 7.2° ± 1.8° in male participants, and 12.7° ± 1.1° and 8.5° ± 1.6° in female participants at the knee flexion of 0° and 30°, respectively. In this respect, there was no sex difference, but the change was greater for men (ΔPTA = 7.9°) than for women (ΔPTA = 4.2°) (Fig. 5).
Figure 5.
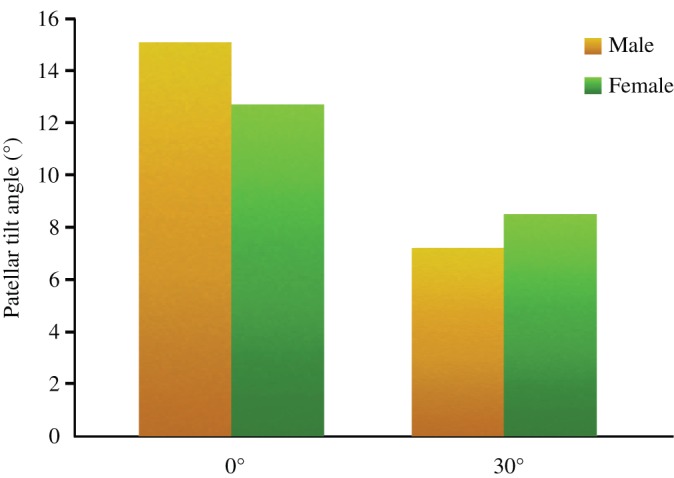
Bar chart illustrates the patellar tilt angle (PTA) at 0° and 30° flexion between men and women.
The CA significantly decreased (131%), from 23.6° ± 2.7° to −7.4° ± 1.8° with the increase in the flexion angle from 0° to 30° (P < 0.01). The congruence angle was 22.9° ± 2.1° and −7.0° ± 1.3° in male participants, and 24.5° ± 1.2° and −8.2° ± 2.5° in female participants at the knee flexion of 0° and 30°, respectively. There was no difference between male and female participants at 0° and 30° flexion and the numerical value changed trend (P < 0.01) (Fig. 6).
Figure 6.
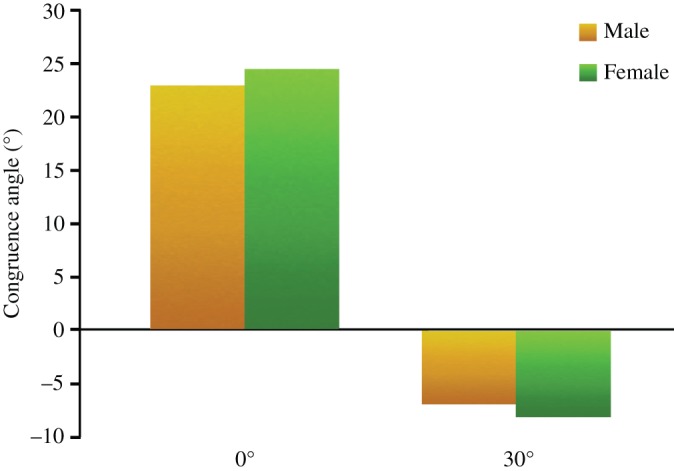
Bar chart illustrates the congruence angle (CA) at 0° and 30° flexion between men and women.
The average TT–TG distance and LPD at 0° flexion were 15.4 ± 1.3 mm and 5.8 ± 1.3 mm, respectively, while at 30° flexion they significantly reduced (40% and 114%) to 9.2 ± 1.4 mm and −0.8 ± 1.5 mm, respectively (both P < 0.01). The TT–TG distance was 15.9 ± 1.3 mm and 10.3 ± 2.1 mm in male participants, and 15.1 ± 2.7 mm and 8.7 ± 1.4 mm in female participants at the knee flexion of 0° and 30°, respectively (Fig. 7). The LPD distance was 5.2 ± 2.5 mm and −1.2 ± 1.2 mm in male participants, and 5.9 ± 1.3 mm and −0.5 ± 1.6 mm in female participants at the knee flexion of 0° and 30°, respectively. There was no difference between male and female participants at 0° and 30° flexion (P < 0.01). Their changed trend was the same as the overall trend (Fig. 8).
Figure 7.
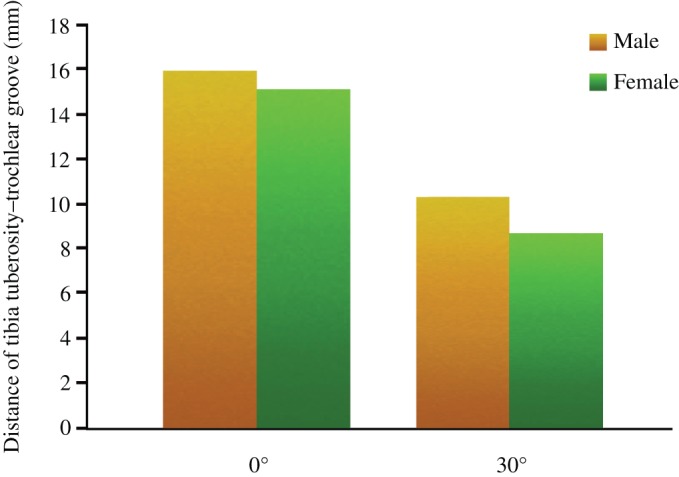
Bar chart illustrates the distance of tibia tuberosity–trochlear groove (TT–TG) at 0° and 30° flexion between men and women.
Figure 8.
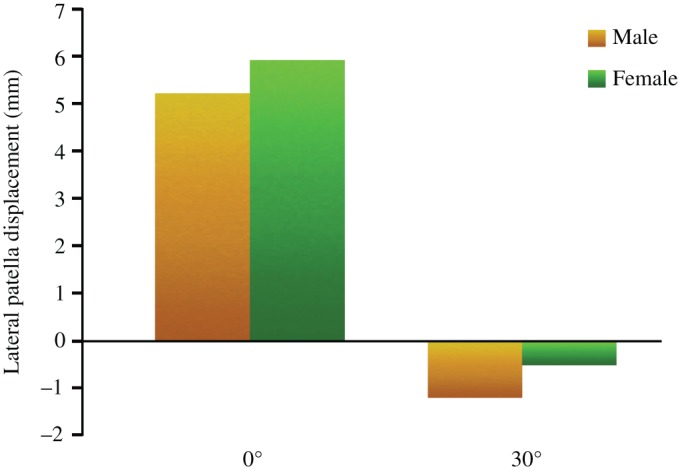
Bar chart illustrates the lateral patella displacement (LPD) at 0° and 30° flexion between men and women.
Correlation between Patella Position and Tibial Rotation
The TT–TG distance and LPD significantly decreased between the knee flexion of 0° to 30°. When all knees were analyzed together, a significant correlation was found between ΔTT–TG distance and ΔLPD (r = 0.905, P < 0.01, Fig. 4). Along with the decrease of the TT–TG, LPD also decreases between the knee flexion of 0° to 30°.
While at 0°–30° flexion, the TRRF significantly fell. With the increase in the flexion angle from 0° to 30°, the PTA and CA significantly decreased. Significant correlations were also found between ΔTRRF and both ΔCA (r = 0.869, P < 0.01, Fig. 5) and ΔPTA (r = 0.885, P < 0.01, Fig. 6).
Discussion
The most important finding of this study was the special relationship between the screw‐home mechanism and the “capture” mechanism. It was confirmed that the tibiofemoral joint rotation could regulate patellar tracking in order to guarantee the balance of patellofemoral joint alignment during flexion–extension movement of the knee.
As the medial femoral condyle is longer than the lateral condyle, the tibia rotates internally on the femur during the first stage of flexion; this well‐known kinematic phenomenon is called the screw‐home movement4. Coughlin et al. report that 81% of rotational movement occurs during knee flexion from 0° to 30°7. Karrholm et al. report that the rotational movement of the tibia ranges from 1.6° (external rotation) to 9.0° (internal rotation) during active flexion of the healthy knee joint20. The present study demonstrated that tibial internal rotation occurred with knee flexion, and the average angle was 9.9° ± 1.9° during knee flexion at 30°, which was similar to the findings of Karrholm et al. 20 Furthermore, rotation of the knee joint reportedly significantly alters the TT–TG distance22, 23. In the present study, the TT–TG distance significantly reduced during knee flexion from 0° to 30°; this indicates that screw‐home movement has a large influence on patellar position.
Previous research suggests that patellar tracking is very complex, and can undergo shift, rotation, and tilt relative to the femur24, 25; as the knee flexes, the patella gradually shifts laterally after a slight medial shift in the early phase of knee flexion. In the range of 0° to 30° knee flexion, patellar translation motion causes the patella to shift towards the medial femoral condyle26, 27. The present study revealed that the patella shifted internally relative to the femur in the knees during flexion from 0° to 30°. Our data showed that LPD significantly reduced, and that the average patellar medial displacement was 6.6 mm21. The present results confirm that the patella showed a slight medial shift during early flexion, similar to other studies28, 29, 30.
Patellar stability depends mainly on the static and dynamic structures around it. During knee flexion from 0° to 30°, the patella is not yet confined to its femoral groove. It is affected by tibial rotation, through tensioning of the patellar ligament and of the lateral and medial retinacula. Hence, these structures play an important role in regulating patellar tracking during knee flexion from 0° to 30°, and the position of the tibial tubercle has a greater influence on patellar position during the early stage of knee flexion31. In the present study, LPD decreased as the TT–TG distance decreased. The decrease in LPD represents the medial shift of the patella, and the TT–TG distance represents the tibial internal rotation32. The present study found a significant correlation between ΔTT–TG distance and ΔLPD, which suggests that the patellar medial–lateral displacement was affected by the tibial tubercle displacement. Therefore, the greater the angle of internal tibial rotation, the greater the medial patellar translation. The present study also demonstrated that the screw‐home mechanism could displace the tibial tubercle and cause medial translation of the patella, aided by the traction of the patellar ligament. A series of actions caused by the tibiofemoral joint rotation causes the patella to gradually slide into the femoral trochlea.
In the normal patellofemoral joint, the patella is aligned to the femur, which ensures normal knee flexion–extension movement and avoids the problems caused by patellar instability and dislocation, such as anterior knee pain and patellofemoral arthritis33, 34. On CT scans, PTA and CA effectively express the patellofemoral alignment35; when the PTA and CA decrease, the stability of the patellofemoral joint improves. The present study showed that PTA and CA significantly decreased at 30° of knee flexion, suggesting that the patellar stability was markedly improved. However, ΔPTA and ΔCA were both correlated with ΔTRRF, which indicates that patellar stability was affected by the tibial rotation, and improved with the increase in flexion and rotation angle. Several studies have reported the relationship between patellar dislocation and tibial torsional deformity, and that good results can be obtained by performing corrective osteotomy36, 37.
During knee flexion, the patella moves into the femoral trochlear groove while rotational movement adjusts the patellofemoral joint stability; this is known as the capture mechanism of the femur, and is very important clinically. The capture mechanism prevents abnormal patellar tracking in the early stage of knee flexion38. Notably, as an efficient regulating mechanism, the screw‐home mechanism is the insurance of the capture mechanism, and plays an important role in guiding patellar sliding into the femoral trochlear groove. We think that this is a dynamic regulation mechanism of the kinematics of the patellofemoral joint.
There are several limitations to the current study. As this research causes radiation exposure, the sample size was small. We chose to use a static measurement (CT) to measure the changes during knee flexion. Although this cannot reflect the dynamic activity of the knee joint, it could provide more precise measurement data. We only examined knee rotation between the knee flexion from 0° to 30°; thus, our findings were insufficient to discuss total flexion angles. However, as the main purpose of the present study was to evaluate the screw‐home mechanism affecting patellar tracking, not patellar tracking itself, it was not essential to assess patellar tracking during the whole range of knee flexion, which did not have a direct effect on the experiment.
This study analyzed the movement of the knee joint as a whole. We believe that the screw‐home mechanism could significantly influence the patellofemoral stability. The dynamic regulation mechanism was crucial to knee flexion and contributed to smooth capture movement.
Conclusions
Although current studies show that many factors could affect patellar tracking, the present data demonstrated a special role of the screw‐home mechanism in regulating patellofemoral joint movement. The screw‐home mechanism http://dict.baidu.com/s?wd=not%20only ensures the stability of knee extension, but also regulates the patellofemoral alignment during knee flexion–extension. As a bridge, it could combine the patellofemoral joint and the tibiofemoral joint and maintain stability of the normal knee kinematics.
Disclosure: The authors of this study declare no conflicts of interest.
Contributor Information
Xiao‐meng Wang, Email: wangxiaomeng0318@126.com.
Fei Wang, Email: wangxiaomeng0318@126.com.
References
- 1. Distefano MD, Michael C. Disorders of the patellofemoral joint. 4th edn. J Bone Joint Surg Br, 2005, 87: 482. [Google Scholar]
- 2. Senavongse W, Amis AA. The effects of articular, retinacular, or muscular deficiencies on patellofemoral joint stability: a biomechanical study in vitro. J Bone Joint Surg Br, 2005, 87: 577–582. [DOI] [PubMed] [Google Scholar]
- 3. Katchburian MV, Bull AM, Shih YF, Heatley FW, Amis AA. Measurement of patellar tracking: assessment and analysis of the literature. Clin Orthop Relat Res, 2003, 412: 241–259. [DOI] [PubMed] [Google Scholar]
- 4. Kim HY, Kim KJ, Yang DS, Jeung SW, Choi HG, Choy WS. Screw‐home movement of the tibiofemoral joint during normal gait: three‐dimensional analysis. Clin Orthop Surg, 2015, 7: 303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Parikh S, Noyes FR. Patellofemoral disorders: role of computed tomography and magnetic resonance imaging in defining abnormal rotational lower limb alignment. Sports Health, 2011, 3: 158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clementz BG. Assessment of tibial torsion and rotational deformity with a new fluoroscopic technique. Clin Orthop Relat Res, 1989, 245: 199–209. [PubMed] [Google Scholar]
- 7. Coughlin KM, Incavo SJ, Churchill DL, Beynnon BD. Tibial axis and patellar position relative to the femoral epicondylar axis during squatting. J Arthroplasty, 2003, 18: 1048–1055. [DOI] [PubMed] [Google Scholar]
- 8. Hallén LG, Lindahl O. The“screw‐home”movement in the knee‐joint. Acta Orthop Scand, 1966, 37: 97–106. [DOI] [PubMed] [Google Scholar]
- 9. Moglo KE, Shirazi‐Adl A. Cruciate coupling and screw‐home mechanism in passive knee joint during extension‐flexion. J Biomech, 2005, 38: 1075–1083. [DOI] [PubMed] [Google Scholar]
- 10. Freeman MAR. How the knee moves. Curr Orthop, 2001, 15: 444–450. [Google Scholar]
- 11. Sanfridsson J, Arnbjörnsson A, Fridén T, Ryd L, Svahn G, Jonsson K. Femorotibial rotation and the Q‐angle related to the dislocating patella. Acta Radiol, 2001, 42: 218–224. [PubMed] [Google Scholar]
- 12. Wagenaar FC, Koëter S, Anderson PG, Wymenga AB. Conventional radiography cannot replace CT scanning in detecting tibial tubercle lateralisation. Knee, 2007, 14: 51–54. [DOI] [PubMed] [Google Scholar]
- 13. Abadie P, Galaud B, Michaut M, Fallet L, Boisrenoult P, Beaufils P. Distal femur rotational alignment and patellar subluxation: a CT scan in vivo assessment. Orthop Traumatol Surg Res, 2009, 95: 267–271. [DOI] [PubMed] [Google Scholar]
- 14. Samukawa M, Yamamoto T, Miyamoto S, Yamaguchi A, Katayose M. Analysis of tibial rotation using magnetic resonance imaging. Man Ther, 2009, 14: 712–715. [DOI] [PubMed] [Google Scholar]
- 15. Dejour H, Walch G, Nove‐Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc, 1994, 2: 19–26. [DOI] [PubMed] [Google Scholar]
- 16. Eckhoff DG, Johnston RJ, Stamm ER, Kilcoyne RF, Wiedel JD. Version of the osteoarthritic knee. J Arthroplasty, 1994, 9: 73–79. [DOI] [PubMed] [Google Scholar]
- 17. Laurin CA, Dussault R, Levesque HP. The tangential x‐ray investigation of the patellofemoral joint: x‐ray technique, diagnostic criteria and their interpretation. Clin Orthop Relat Res, 1979, 144: 16–26. [PubMed] [Google Scholar]
- 18. Merchant AC, Mercer RL, Jacobsen RH, Cool CR. Roent‐genographic analysis of patellofemoral congruence. J Bone Joint Surg Am, 1974, 56: 1391–1396. [PubMed] [Google Scholar]
- 19. Goutallier D, Bernageau J, Lecudonnec B. The measurement of the tibial tuberosity. Patella groove distanced technique and results (author's transl). Rev Chir Orthop Reparatrice Appar Mot, 1978, 64: 423–428. [PubMed] [Google Scholar]
- 20. Karrholm J, Brandsson S, Freeman MA. Tibiofemoral movement 4: changes of axial tibial rotation caused by forced rotation at the weight‐bearing knee studied by RSA. J Bone Joint Surg Br, 2000, 82: 1201–1203. [DOI] [PubMed] [Google Scholar]
- 21. Kujala UM, Osterman K, Kormano M, Komu M, Schlenzka D. Patellar motion analyzed by magnetic resonance imaging. Acta Orthop Scand, 1989, 60: 13–16. [DOI] [PubMed] [Google Scholar]
- 22. Kessler O, Patil S, Colwell CW Jr, D'Lima DD. The effect of femoral component malrotation on patellar biomechanics. J Biomech, 2008, 41: 3332–3339. [DOI] [PubMed] [Google Scholar]
- 23. Nagamine R, Whiteside LA, Otani T, White SE, McCarthy DS. Effect of medial displacement of the tibial tubercle on patellar position after rotational malposition of the femoral component in total knee arthroplasty. J Arthroplasty, 1996, 11: 104–110. [DOI] [PubMed] [Google Scholar]
- 24. Bull AM, Katchburian MV, Shih YF, Amis AA. Standardisation of the description of patellofemoral motion and comparison between different techniques. Knee Surg Sports Traumatol Arthrosc, 2002, 10: 184–193. [DOI] [PubMed] [Google Scholar]
- 25. Nagamine R, Otani T, White SE, McCarthy DS, Whiteside LA. Patellar tracking measurement in the normal knee. J Orthop Res, 1995, 13: 115–122. [DOI] [PubMed] [Google Scholar]
- 26. Ahmed AM, Duncan NA, Tanzer M. In vitro measurement of the tracking pattern of the human patella. J Biomech Eng, 1999, 121: 222–228. [DOI] [PubMed] [Google Scholar]
- 27. Grelsamer RP, Weinstein CH. Applied biomechanics of the patella. Clin Orthop Relat Res, 2001, 389: 9–14. [DOI] [PubMed] [Google Scholar]
- 28. Laprade J, Lee R. Real‐time measurement of patellofemoral kinematics in asymptomatic subjects. Knee, 2005, 12: 63–72. [DOI] [PubMed] [Google Scholar]
- 29. Patel VV, Hall K, Ries M, et al. Magnetic resonance imaging of patellofemoral kinematics with weight‐bearing. J Bone Joint Surg Am, 2003, 85: 2419–2424. [DOI] [PubMed] [Google Scholar]
- 30. MacIntyre NJ, Hill NA, Fellows RA, Ellis RE, Wilson DR. Patellofemoral joint kinematics in individuals with and without patellofemoral pain syndrome. J Bone Joint Surg Am, 2006, 88: 2596–2605. [DOI] [PubMed] [Google Scholar]
- 31. Kwak SD, Colman WW, Ateshian GA, Grelsamer RP, Henry JH, Mow VC. Anatomy of the human patellofemoral joint articular cartilage: surface curvature analysis. J Orthop Res, 1997, 15: 468–472. [DOI] [PubMed] [Google Scholar]
- 32. Tensho K, Akaoka Y, Shimodaira H, et al. What components comprise the measurement of the tibial tuberosity‐trochlear groove distance in a patellar dislocation population? J Bone Joint Surg Am, 2015, 97: 1441–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hinterwimmer S, Gotthardt M, von Eisenhart‐Rothe R, et al. In vivo contact areas of the knee in patients with patellar subluxation. J Biomech, 2005, 38: 2095–2101. [DOI] [PubMed] [Google Scholar]
- 34. Heegaard J, Leyvraz PF, Van Kampen A, Rakotomanana L, Rubin PJ, Blankevoort L. Influence of soft structures on patellar three‐dimensional tracking. Clin Orthop Relat Res, 1994, 299: 235–243. [PubMed] [Google Scholar]
- 35. Sasaki T, Yagi T. Subluxation of the patella: investigation by computerized tomography. Int Orthop, 1986, 10: 115–120. [PubMed] [Google Scholar]
- 36. Cameron JC, Saha S. External tibial torsion: an underrecognized cause of recurrent patellar dislocation. Clin Orthop Relat Res, 1996, 328: 177–184. [PubMed] [Google Scholar]
- 37. Drexler M, Dwyer T, Dolkart O, et al. Tibial rotational osteotomy and distal tuberosity transfer for patella subluxation secondary to excessive external tibial torsion: surgical technique and clinical outcome. Knee Surg Sports Traumatol Arthrosc, 2014, 22: 2682–2689. [DOI] [PubMed] [Google Scholar]
- 38. Quintelier J, Skalski K, Verdonk P, De Baets P, Almqvist F. Patellofemoral contact pressures. Acta Bioeng Biomech, 2008, 10: 23–28. [PubMed] [Google Scholar]


