Abstract
Objective
Anterior cervical discectomy and fusion is the most commonly employed surgical technique for treating cervical spondylosis. Although autologous bone grafts are considered the gold standard in achieving fusion, associated short‐ and long‐term morbidities have led to a search for alternative materials. These have included carbon‐fiber, titanium alloy (Ti) and ceramic and polyetheretherketone (PEEK) based implants. Recent attempts to optimize cage implants through using composite designs have combined Ti and PEEK. However, there are few published reports on the clinical and radiological outcomes of commercially available composite cages. Our study aimed to provide and evaluate initial outcomes of a composite Ti/PEEK cage.
Methods
In this prospective single senior surgeon cohort study, 31 consecutive patients underwent a modified Smith–Robinson technique under general anesthesia and relevant data were collected. The study patients were aged between 18 and 75 years and underwent surgery from November 2013 to May 2014. Indications for surgery included traumatic and degenerative cervical disease that was unsuitable for or unresponsive to conservative management. All cages were between 5 and 8 mm and packed with super critical fluid sterilized allograft and bone marrow aspirate before insertion. Patients were followed‐up for a minimum of 12 months. Fusion was assessed using fine cut CT and anteroposterior and lateral radiographs. Clinical outcomes were measured using a Visual Analogue Scale, Neck Oswestry Disability Index and Patient's Satisfaction Index.
Results
Six of the original cohort were unavailable for adequate follow‐up. The remaining 25 patients (17 men, 8 women; 33 operative levels) were observed for a mean of 14.6 months (range, 12–16 months). All operation levels were between C4 and C7. Single‐level operations were performed in 19 patients and additional plating in 14 patients. A fusion rate of 96% was achieved. Patients in both plated and non‐plated groups experienced statistically significant improvements; good to excellent outcomes being seen in 92% of patients. There was one complication, namely recurrent laryngeal nerve palsy, which had partially resolved at 6 months follow‐up.
Conclusion
The present study shows that enhancement of PEEK cages with Ti endplates is a safe and effective treatment with the potential for early osseointegration and early radiological evidence of fusion.
Keywords: Anterior cervical discectomy and fusion, Composite Titanium/PEEK, Interbody cage
Introduction
Age‐related degeneration of the cervical spine is present in over 50% of middle‐aged individuals and is the most common cause of neural dysfunction. First line treatment is conservative; however surgery is indicated in symptomatic patients who are unresponsive to conservative management.1 Since its description by Robinson and Smith in 1955, anterior cervical discectomy and fusion (ACDF) has been widely utilized to treat symptomatic cervical spondylosis and disc herniation that is unresponsive to conservative management.2 Though the original form of the Smith–Robinson technique has undergone technical modification, this procedure is still standard today, leaving improvements in fusion rates and clinical outcomes to be generated through development and improvement of implant designs and materials. In the original Smith–Robinson technique, a bone block made from autograft harvested from the iliac crest was implanted. Although autologous bone grafts remain the gold standard today, the morbidity associated with harvesting of grafts, including allograft, synthetic and factor/cell‐based grafts, has led to an exploration of possible implant alternatives.
Initially, alternative graft‐only options, including allografts, were explored; however, these yielded their own complications of increased rates of graft collapse and pseudarthrosis in addition to standard concerns of disease transmission, infection and histocompatibility differences. In 1988, a cage fusion technology was proposed by Bagby and since then stand‐alone cage designs, with or without additional fixation, have become the mainstay of ACDF, achieving excellent safety, primary stability and long‐term fusion without the limitations and morbidity associated with graft options. Cage interbody implants have improved biomechanical properties, designs having improved year by year with to maximization of biocompatibility and osseointegration.3 The basic design of cage implants is a small, hollow implant featuring lateral, upper or lower windows or both to a central cavity filled with either autologous bone, allograft bone or osteoinductive materials.4 Historically, cage designs varied, both threaded and non‐threaded designs being available. Since their introduction, optimization of ACDF procedures has been achieved by research in broad fields encompassing ideal shape, dimensions, materials and enhancement with biological growth factors.
Historically, three main materials have been utilized in the creation of cervical cages: Titanium (Ti) and its alloys, polyetheretherketone (PEEK) and carbon fiber‐PEEK. Ti and PEEK are preferred in current designs because of the synovitis and lymphatic spread of fiber debris associated with radiolucent carbon fiber–PEEK cages. However, all have advantages and disadvantages.5, 6, 7, 8 Ti and its alloys were one of the first materials to be utilized for cages in the 1980s. Used in the orthopedic world since the 1940s, Ti is a biocompatible, robust material with excellent corrosion resistance and a low density.9 PEEK cages were introduced in the 1990s by AcroMed as an alternative to Ti cages; they provide the advantages of radiolucency and an elastic modulus close to bone, thereby avoiding the stress shielding associated with Ti.10 Today, there is controversy over the utilization of Ti versus PEEK cages. Although PEEK has theoretical advantages, studies have rarely controlled for the roles of endplate preparation, area of contact and over‐distraction. A majority of studies have reported improved fusion rates, lower subsidence rates and radiolucency with PEEK than with Ti cages,11, 12, 13, 14 one long‐term study by Chen et al. reporting minimal differences in the early postoperative period, but better maintenance of intervertebral height, cervical lordosis and clinical outcomes by PEEK cages over a 7‐year follow‐up.15 Although Ti has shown extensive ability to support osseointegration,16 PEEK is radiolucent, enabling easier assessment of fusion and has an elastic modulus closer to that of bone, theoretically reducing levels of subsidence.9
An ideal cage design would restore healthy alignment and disc height and achieve immediate post‐operative stability, high‐fusion rates and low complication rates. Recent cage designs have attempted to promote osseointegration and thus fusion through modification of cage surfaces. Ti and its alloys can be modified to increase surface roughness by plasma beam and electron spray techniques.9 In vitro experimentation has shown this increases amounts of total protein and alkaline phosphatase, thereby increasing osteogenic cell differentiation.17 Composite Ti/PEEK spacers take advantage of the superior bioactivity of Ti and the elastic modulus and radioluminescence of PEEK.18, 19 Clinically available composite spacers combine PEEK bodies with Ti‐endplates to theoretically augment bone–implant fusion; however, there are few published reports comparing their efficacy with that of established clinical and radiographic baselines for Ti or PEEK cages. To the authors’ knowledge, there are no published studies evaluating their usage.
This study reports early clinical and radiological outcomes of a Ti/PEEK cage, both with and without anterior plating. The purposes of this study were: (i) to report on early patient clinical and radiological outcomes of Ti/PEEK spacers; (ii) to assess their clinical safety and efficacy and compared it with that of reported single‐material implants; and (iii) to provide initial data for the conduct of further long‐term trials.
Materials and Methods
Ethical Approval
Approval for this study was obtained from the South Eastern Sydney Local Health District‐Northern Sector (SESLHD‐NS) ethics committee, Ref: HREC 11/183.
Patient Data
Over a 5‐month period from October 2013 through April 2014, the study procedure was performed by a single senior surgeon (RJM) on 31 patients and data were prospectively collected from. Inclusion criteria were patients aged 18–75 years with cervical traumatic or degenerative disease that was unsuitable for or unresponsive to conservative treatment. Patients with significant comorbidities, including systemic infection and terminal cancer, and those with posterior longitudinal ligament ossification were excluded.
The participants were followed up for a minimum of 12 months, with assessments preoperatively and postoperatively on Day 1, Week 6, Months 6 and 12. A composite Ti/PEEK Combo cage (A‐Spine ASIA, Taipei, Taiwan) was utilized in all patients (Fig. 1). This cage features Ti‐endplate inserts and a PEEK body and is available in the dimensions 14 mm × 15 mm (depth × width) and ranges in height from 5 mm to 8 mm.
Figure 1.

Combo (A‐Spine) composite Ti/PEEK spacer featuring ridged titanium alloy endplates in combination with a PEEK body.
Surgical Technique
All surgeries were conducted under general anesthesia by a single senior surgeon author using a modified Smith–Robinson technique (Memphis, Tennessee, USA). Following a linear right anterolateral incision, adequate distraction of the musculo‐visceral column was achieved using a Trim‐Line (Center Valley, Pennsylvania, USA) retractor system with distraction of the vertebral bodies using Caspar retraction pins. Pathological disc material was then removed using a combination of rongeurs and curettes under the direct observation of an operating microscope. Any visible osteophytes were also removed using a high speed drill and the posterior longitudinal ligament was resected. Complete decompression and visualization of the dura and nerve roots was achieved in all cases. Decortication of the vertebral endplates was performed to optimize the bone‐cage/graft interface.
The appropriate size cage was determined in all cases by using a trial spacer to confirm the height of the disc space. Allograft Supercritical CO2 sterilized “crunch” (SCCO2) from Australian Biotechnologies (Sydney, NSW, Australia) was used along with bone marrow aspirate from the right iliac crest for interbody grafting. The allograft was firmly packed into the cage with the aim of distributing the axial loading through the implant. Cages were inserted using standard instrumentation and tapped into place (Fig. 2A–C).
Figure 2.
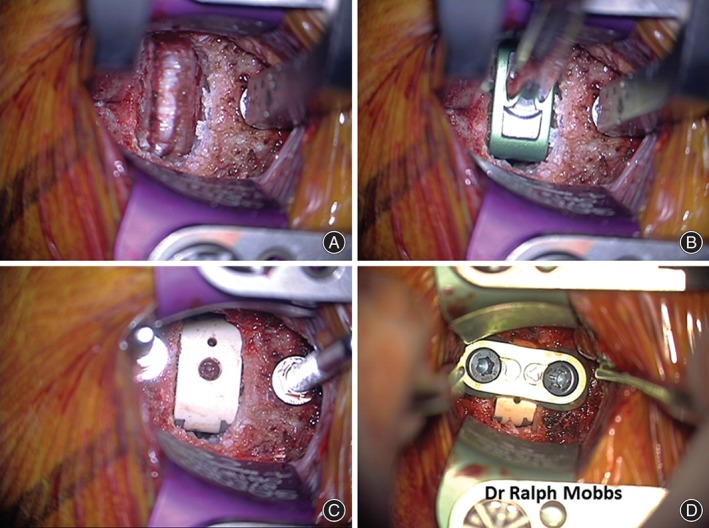
Sequence of anterior cervical discectomy and fusion (ACDF). (A) Exposure with Trim Line and Casper retractor followed by discectomy and decompression of the neurological elements. (B) Trial implant to determine height of final prosthesis. (C) Implantation of Combo Ti/PEEK cage with allograft. (D) Anterior plate fixation (Uniplate, Depuy Spine, Raynham, MA, USA).
In cases where additional stabilization was considered necessary, anterior plate fixation was applied following implant impaction and verification on lateral X‐ray films (Fig. 2D). Prior to wound closure, intraoperative anteroposterior and lateral plain radiographs were obtained to confirm the correct implant and fixation plate positioning. All non‐plated patients were advised to wear a cervical orthosis postoperatively for 6 weeks. Postoperative pain relief was achieved with a low dose of non‐steroidal anti‐inflammatory drug and paracetamol.
Outcome Measures
Radiographic fusion was assessed by an independent radiologist with no conflict of interest with regards to the study outcome. Anteroposterior and lateral cervical radiographs were performed on Day 1 and Week 6 postoperatively to radiographically check for implant failure or movement from the original implantation position. CT scans were performed at 6 months to assess early fusion status of bone through the implant and the presence or absence of lucency. Fusion was considered to have occurred if bridging bone incorporating the graft and adjoining the Ti endplates was apparent (Figs 3 and 4) with additional loss of radiolucency, restoration of interbody space and no evidence of hardware failure.20
Figure 3.
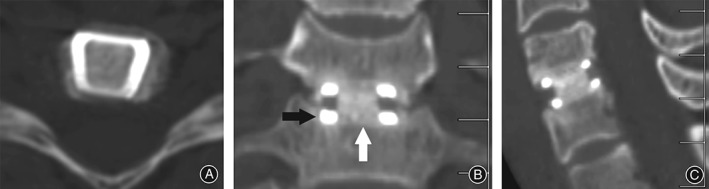
CT images showing (A) solid fusion 6 months postoperatively. (B) White arrow indicates incorporation of allograft/BMA at graft/endplate junction. Black arrow indicates absence of halo/lucency at Ti/bone junction consistent with incorporation of the Ti into the bone endplate (C) Demonstrates sagittal view.
Figure 4.
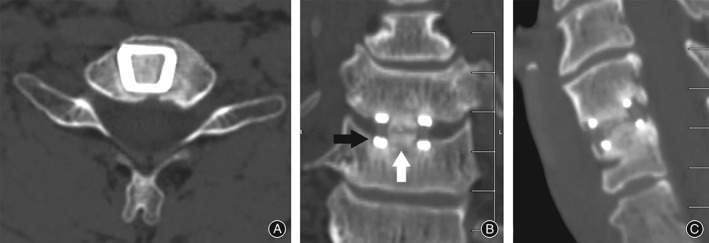
CT images showing (A, B, C) non‐union of graft 6 months postoperatively with union of Ti endplate. White arrow indicates lucency through the allograft/bone marrow aspirate at mid‐graft level. Black arrow indicates absence of halo/lucency at Ti/bone junction consistent with incorporation of the Ti into the bone endplate.
Clinical outcomes were assessed preoperatively and 6 and 12 months postoperatively. Patients were asked to quantify neck and arm pain on a Visual Analogue Scale (VAS) ranging from 0 (no pain/discomfort) to 10 (worst pain/discomfort imaginable) pre‐ and post‐operatively. Functional outcomes were measured using the Oswestry Disability Index (ODI). Additionally, patients were assessed according to the Quality of Life 12‐item Short Form (SF‐12) Patient satisfaction with their procedure was elicited using the Patient Satisfaction Index as described by Palit et al.21 at final follow‐up.
Statistical Analysis
Descriptive data are represented as means ± standard deviation (range, minimum–maximum). All data sets were tested for normality with the D'Agostino and Pearson omnibus normality test. Unpaired nonparametric data was analyzed using the Mann–Whitney U test and parametric data with an unpaired t‐test for comparison of results between the plated and non‐plated groups. The Wilcoxon signed rank test was used for nonparametric, symmetrically distributed data and the paired t‐test for parametric data to compare pre and postoperative variables within patient groups. Statistical significance was set at P < 0.05. All analyses and graphs were generated using a commercial software package (GraphPad Prism version 5.01, GraphPad Software).
Results
Patient Characteristics
Of the 31 consecutive patients in the original data set, 25 patients with 33 operative levels met the inclusion criteria. Two patients died of complications associated with their initial multisystem trauma presentation. Six patients were excluded because of inadequate clinical and/or radiological data. There were 17 men and 8 women, with a mean age of 55.1 ± 13.4 years (range, 30–72 years) and a mean duration of follow‐up of 14.6 months (range, 12–16 months). Seven patients had been smokers and had ceased tobacco usage prior to surgery. Two patients had diabetes and eight were receiving workers compensation coverage for their surgery. Neck pain was present in almost all patients, the main indication for surgery being cervical disc herniation associated with radiculopathy or spinal stenosis and cord compression. Within the non‐trauma cohort, the mean preoperative duration of symptoms was 2.9 years (range, 6 weeks to 15 years). All patients were operated on between the surgical levels C4 and C7, 6 being multiple level and 19 single level ACDF procedures. Fourteen patients received additional anterior plating.
Radiological Outcomes
A fusion rate of 96% (24/25) was achieved, one case of non‐union occurring in the non‐plated group. In this case, although bridging of bone occurred outside of the implant, the patient still experienced an excellent clinical outcome. It was also noted that the presence of Ti‐endplates did not interfere with fusion assessments: there was 100% reliability between X‐ray and CT assessments and an absence of the PEEK‐associated halo effect in all cases of successful fusion.
Clinical Outcomes
VAS scores for neck and arm pain showed significant improvement (P < 0.001) between pre‐ (7.1 ± 1.9) and post‐operative (2.0 ± 1.7) scores (average improvement 4.6 ± 2.1). There was no significant difference in VAS score improvement between the plated and non‐plated groups.
Preoperative SF‐12 scores were physical component summary score (PCS) 38.8 ± 5.9 and mental component summary score (MCS) 37.0 ± 8.1, with mean postoperative improvement in scores by 4.2 for PCS to 43.4 ± 8.2 and by 11.0 for MCS to 50.1 ± 9.3. The difference was statistically significant (P = 0.025) for MCS, but not for PCS. Mean preoperative neck ODI (NODI) scores were 44.0 (SD ± 15.2) with a mean improvement of 24.7 (SD ± 8.8) to an average postoperative score of 26.4 (SD ± 21.7) (P = 0.039) (Fig. 5).
Figure 5.
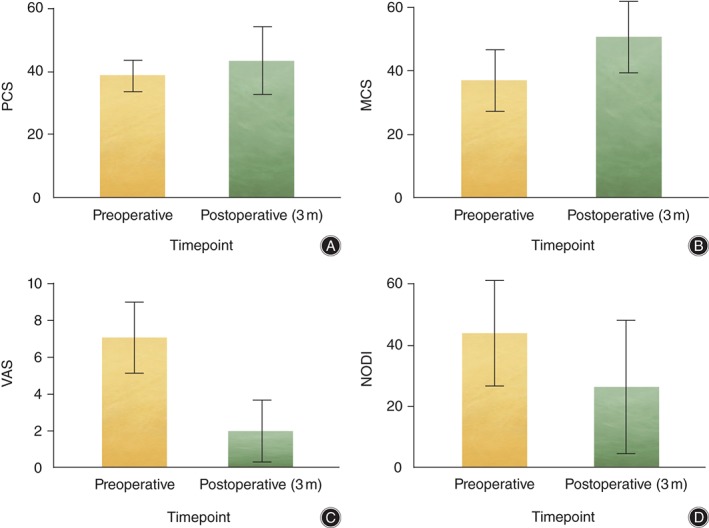
Patient clinical outcomes. Statistically significant improvements are seen between preoperative and postoperative (3 months [3m]) SF‐12 MCS, VAS and NODI scores. (A) SF‐12 PCS. (B) SF‐12 MCS. (C) VAS. (D) NODI.
According to Odom's criteria, there were 16 excellent, 7 good, 2 fair and no poor outcomes, 92% of patients achieving a good or excellent clinical outcome. There was no significant difference between the plated and non‐plated groups (Table 1).
Table 1.
Patient clinical outcomes (mean ± standard deviation)
| Evaluation criterion | Preoperative score | Postoperative score | Improvement |
|---|---|---|---|
| VAS | 7.1 ± 1.9 | 2.0 ± 1.7 | 4.6 ± 2.1** |
| SF‐12 | |||
| PCS | 38.8 ± 5.1 | 43.3 ± 10.7 | 4.2 ± 11.1 |
| MCS | 37.0 ± 9.7 | 50.1 ± 11.3 | 11.0 ± 9.6* |
| NODI | 44.0 ± 17.2 | 26.4 ± 21.7 | 24.7 ± 8.8* |
, P < 0.05;
, P < 0.001;
VAS, Visual Analogue Scale; SF‐12, Quality of Life 12‐item Short Form; PCS, physical component summary score; MCS, mental component summary score; NODI, neck Oswestry Disability Index.
Complications
Complications were classified as approach‐related or implant‐related. There were no implant‐related complications. Six month fine‐cut CTs were examined carefully for any evidence presence of implant failure at the Ti/PEEK junction; no such evidence was found. Approach‐related complications included one case of recurrent laryngeal nerve palsy that had partially resolved at 6 months follow‐up. There were no cases of postoperative hematoma or infection.
Discussion
A Cochrane systematic review concluded that fusion techniques utilizing autografts yielded higher fusion rates than allograft and synthetic bone substitute techniques; however, donor site morbidity associated with autograft has fueled a growing interest in alternative materials.22, 23 In this study, the combination of a Ti/PEEK cage with allograft proved to be an effective and safe combination of materials, resulting in statistically significant improvements in pain and function, both with and without plate fixation.
Interbody Cage Material Properties
PEEK is a semicrystalline polyaromatic linear polymer and thermoplastic material of high molecular weight that is biologically inert, radiolucent and non‐resorbable.24 It elicits minimal cytotoxic and inflammatory responses25 and is also compatible with many reinforcing agents (e.g., titanium, carbon fiber).10 For interbody spacers, PEEK provides a hard frame able to resist spinal loading, thereby providing initial stability whilst having an elastic modulus similar to that of bone and thus minimizing graft subsidence.26 Titanium can be modified to improve both ongrowth and ingrowth. Ongrowth of bone is the direct apposition of bone to the surface, whereas ingrowth involves the interlocking or bone growth into the surface of a material and requires a 3‐D structure with pores open to the outside. These modifications are aimed at influencing the way tissues incorporate the implant material.27
Previous studies have compared the efficacy of Ti and PEEK cages in both single and multi‐level ACDF. Studies have shown that PEEK achieves better long‐term maintenance of clinical height, lower rates of subsidence and better clinical outcomes.19 These advantages are attributed to PEEK's elastic modulus, however Ti implants are exceptionally capable of supporting osseointegration, as well as having a surface structure that is comparatively resistant to microbial adhesion.14, 28 Combining these materials in a composite cage theoretically utilizes the advantages of both materials. This study provides initial data showing that composite cages achieve comparable radiological and clinical outcomes to single material devices within 1 year follow‐up (Table 2).11, 12, 13, 24, 29, 30, 32, 33, 34, 35, 36
Table 2.
Clinical and radiological outcomes of Ti, PEEK and Ti/PEEK cages11, 15, 22, 23, 24, 25, 26, 27, 28, 29, 30
| Cage material | Good to excellent clinical outcome (%) | Fusion rate at 3 months (%) | Fusion rate at 6 months (%) | Fusion rate at 12 months (%) | Subsidence (%) |
|---|---|---|---|---|---|
| Titanium11, 22, 24, 29, 31 | 46–95 | — | 37.2–97 | 86.5–99 | 13–45 |
| PEEK11, 15, 22, 25, 26, 27, 28, 29, 30 | 74–100 | — | 61.1–96 | 93–100 | 5–15 |
| Ti/PEEK (Current study) | 92 | 96 | — | — | — |
Graft Choice
Although there are numerous published reports comparing implant materials and designs in ACDF, there is little information on the clinical impact of interbody graft choice. Successful fusion is dependent on not only osteogenic potential and osteoinductive factors, but also the structural scaffold, which aids neovascularization and bony ingrowth.37 Interbody grafts are utilized to promote osseointegration by surrounding the cage body with an osteoinductive and osteoconductive material.38 However, interbody grafts may also play an additional role in mechanical load distribution. Subsidence is thought to occur in relation to high pressures delivered through interbody spacers over a small surface area. Thus, PEEK cages which have an elastic modulus closer to that of cancellous bone, have lower rates of subsidence.15 There are very few data comparing the rates of subsidence between patients with and without grafting or between graft types; however, it is known that autograft, allograft and synthetic bone graft substitutes each have different mechanical and osteo‐integrative profiles. The mechanical properties of allograft bone depend on the type of sterilization treatment. It has been shown that ionizing radiation sterilization of allografts increases their brittleness and affects mechanical load bearing, whereas SCCO2 treatment maintains the graft's intrinsic mechanical properties.39, 40 All implants in our study were grafted with SCCO2 allograft crunch; hypothetically, this would have enables improved load distribution and therefore prevention of subsidence. However, further studies are required to determine the role of this interbody graft choice.
Anterior Cervical Plating
Fixation plates reduce the amount of micro‐motion at the graft‐host interface, graft settling and kyphotic deformity, but also add to costs, risks and operative time.41 Anterior cervical plating is often utilized in multi‐level ACDF procedures because it provides additional stability and is associated with lower rates of subsidence; however, a high rate of dysphagia is associated with its use. The largest study reviewing the rates of dysphagia after ACDF reported an overall incidence of 30% at three months postoperatively, the risk increasing with number of operated levels and operative time.42
Anterior plating in single level ACDF is a controversial topic.43, 44, 45, 46 Nevertheless, it has been found to be safe, not incurring an increased rate of complications or an increased tendency to adjacent segment disease.47 Plates circumvent the need for cumbersome external immobilization collars postoperatively and may hasten patient recovery. In addition, because they alter load distributions and provide additional stability, they are also reportedly associated with lower rates of graft subsidence.48 Studies have also noted relatively high rates of subsidence without plate use.5, 31 A meta‐analysis of 21 studies by Fraser and Härtl revealed that anterior plate systems significantly improve fusion rates in one‐ and two‐level disease (P < 0.0001).49 However, the improved rates of fusion and lower rates of subsidence have not been associated with statistically improved clinical outcomes, leaving the role of plate fixation in one and two level ACDF uncertain.
The role of integrated plate devices as provided by low‐profile designs including the Zero‐P (Synthes CmbH Switzerland, Oberdorf, Switzerland) and the ROI‐C cervical cage (LDR Holding Global, Troyes, France) is also of interest. By streamlining anterior plating into a stand‐alone device, these designs aim to minimize implant‐to‐soft tissue impact, reducing dysphagia rates and other plate‐related complications, whilst still reducing the risk of subsidence, pseudarthrosis and cervical kyphosis.50 Early results have shown that both designs achieve good clinical outcomes, with a lower incidence of dysphagia and shorter operation times, and could provide a compromise concerning whether or not to plate in single level ACDF procedures.50, 51
A primary limitation of this study is its relatively small number of subjects. Although we reported plated versus non‐plated data, the cohort sizes were too small for power (α = 0.8) to be reached, a minimum of 30 patients in each cohort being requirement for a valid comparison to be made. Incomplete follow‐up data on patients was a difficulty in our study, as is common for clinical studies; in our particular cohort this was primarily related to patients from rural areas being unable to access medical imaging centers in the required timeframe.
In addition, the assessment of interbody fusion and integration of the Ti endplate remains a challenge. Because there are no universally accepted criteria for determining radiological fusion, it is often difficult to make a true assessment of fusion based on plain radiography alone, particularly when synthetic cages have been utilized. Our study utilized fine‐cut CT scans with reconstructions; this has been shown to be more reliable and sensitive for the detection of pseudarthrosis than plain radiography.52, 53 In addition, we noted that the Ti‐endplates did not interfere with fusion assessments on either radiographs or CT images.
Conclusions
In this study, we found that utilizing Ti/PEEK interbody cages containing allograft in anterior cervical discectomy and fusion is a safe and effective treatment for degenerative and traumatic cervical pathologies. There was only one case of lucency or halos adjacent to the Ti endplates at 6 months follow‐up. Enhancement of PEEK cages with Ti endplates is likely to assist with early integration of prostheses with the surrounding bone and vertebral endplate. Further studies are required to determine if the usage of a composite design improves implant longevity by limiting subsidence as well as stress shielding and associated complications.
Acknowledgement
We wish to thank Dr. Vedran Lovric for providing information on SCCO2 allografts.
Disclosure: Implants used in this study were provided free of charge by A‐Spine Asia (Taipei, Taiwan). No other conflicts of interest are reported. All authors are in the agreement with the manuscript and meet the authorship criteria according to the latest guidelines of the International Committee of Medical Journal Editors.
References
- 1. Chong E, Pelletier MH, Mobbs RJ, Walsh WR. The design evolution of interbody cages in anterior cervical discectomy and fusion: a systematic review. BMC Musculoskelet Disord, 2015, 16: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mobbs RJ, Rao P, Chandran NK. Anterior cervical discectomy and fusion: analysis of surgical outcome with and without plating. J Clin Neurosci, 2007, 14: 639–642. [DOI] [PubMed] [Google Scholar]
- 3. McConnell JR, Freeman BJ, Debnath UK, Grevitt MP, Prince HG, Webb JK. A prospective randomized comparison of coralline hydroxyapatite with autograft in cervical interbody fusion. Spine (Phila Pa 1976), 2003, 28: 317–323. [DOI] [PubMed] [Google Scholar]
- 4. Wilke HJ, Kettler A, Claes L. Primary stabilizing effect of interbody fusion devices for the cervical spine: an in vitro comparison between three different cage types and bone cement. Eur Spine J, 2000, 9: 410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bartels RH, Donk RD, Feuth T. Subsidence of stand‐alone cervical carbon fiber cages. Neurosurgery, 2006, 58: 502–508. [DOI] [PubMed] [Google Scholar]
- 6. Vavruch L, Hedlund R, Javid D, Leszniewski W, Shalabi A. A prospective randomized comparison between the cloward procedure and a carbon fiber cage in the cervical spine: a clinical and radiologic study. Spine (Phila Pa 1976), 2002, 27: 1694–1701. [DOI] [PubMed] [Google Scholar]
- 7. Gercek E, Arlet V, Delisle J, Marchesi D. Subsidence of stand‐alone cervical cages in anterior interbody fusion: warning. Eur Spine J, 2003, 12: 513–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Parsons JR, Bhayani S, Alexander H, Weiss AB. Carbon fiber debris within the synovial joint. A time‐dependent mechanical and histologic study. Clin Orthop Relat Res, 1985, 196: 69–76. [PubMed] [Google Scholar]
- 9. Rao PJ, Pelletier MH, Walsh WR, Mobbs RJ. Spine interbody implants: material selection and modification, functionalization and bioactivation of surfaces to improve osseointegration. Orthop Surg, 2014, 6: 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kurtz SM, Devine JN. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials, 2007, 28: 4845–4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chou YC, Chen DC, Hsieh WA, et al Efficacy of anterior cervical fusion: Comparison of titanium cages, polyetheretherketone (PEEK) cages and autogenous bone grafts. J Clin Neurosci, 2008, 15: 1240–1245. [DOI] [PubMed] [Google Scholar]
- 12. Niu CC, Liao JC, Chen WJ, Chen LH. Outcomes of interbody fusion cages used in 1 and 2‐levels anterior cervical discectomy and fusion: titanium cages versus polyetheretherketone (PEEK) cages. J Spinal Disord Tech, 2010, 23: 310–316. [DOI] [PubMed] [Google Scholar]
- 13. Liao JC, Niu CC, Chen WJ, Chen LH. Polyetheretherketone (PEEK) cage filled with cancellous allograft in anterior cervical discectomy and fusion. Int Orthop, 2008, 32: 643–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cabraja M, Oezdemir S, Koeppen D, Kroppenstedt S. Anterior cervical discectomy and fusion: comparison of titanium and polyetheretherketone cages. BMC Musculoskelet Disord, 2012, 13: 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen Y, Wang X, Lu X, et al Comparison of titanium and polyetheretherketone (PEEK) cages in the surgical treatment of multilevel cervical spondylotic myelopathy: a prospective, randomized, control study with over 7‐year follow‐up. Eur Spine J, 2013, 22: 1539–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Svehla M, Morberg P, Zicat B, Bruce W, Sonnabend D, Walsh W. Morphometric and mechanical evaluation of titanium implant integration: comparison of five surface structures. J Biomed Mater Res, 2000, 51: 15–22. [DOI] [PubMed] [Google Scholar]
- 17. Rosa AL, Beloti MM. Effect of cpTi surface roughness on human bone marrow cell attachment, proliferation, and differentiation. Braz Dent J, 2003, 14: 16–21. [DOI] [PubMed] [Google Scholar]
- 18. Olivares‐Navarrete R, Gittens RA, Schneider JM, et al Osteoblasts exhibit a more differentiated phenotype and increased bone morphogenetic protein production on titanium alloy substrates than on poly‐ether‐ether‐ketone. Spine J, 2012, 12: 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Han CM, Lee EJ, Kim HE, et al The electron beam deposition of titanium on polyetheretherketone (PEEK) and the resulting enhanced biological properties. Biomaterials, 2010, 31: 3465–3470. [DOI] [PubMed] [Google Scholar]
- 20. van Jonbergen HP, Spruit M, Anderson PG, Pavlov PW. Anterior cervical interbody fusion with a titanium box cage: early radiological assessment of fusion and subsidence. Spine J, 2005, 5: 645–649. [DOI] [PubMed] [Google Scholar]
- 21. Palit M, Schofferman J, Goldthwaite N, et al Anterior discectomy and fusion for the management of neck pain. Spine (Phila Pa 1976), 1999, 24: 2224–2228. [DOI] [PubMed] [Google Scholar]
- 22. Vaccaro AR, Singh K, Haid R, et al The use of bioabsorbable implants in the spine. Spine J, 2003, 3: 227–237. [DOI] [PubMed] [Google Scholar]
- 23. Jacobs WC, Anderson PG, Limbeek J, Willems PC, Pavlov P. Single or double‐level anterior interbody fusion techniques for cervical degenerative disc disease. Cochrane Database Syst Rev, 2004, 4: CD004958. [DOI] [PubMed] [Google Scholar]
- 24. Mastronardi L, Ducati A, Ferrante L. Anterior cervical fusion with polyetheretherketone (PEEK) cages in the treatment of degenerative disc disease. Preliminary observations in 36 consecutive cases with a minimum 12‐month follow‐up. Acta Neurochir, 2006, 148: 307–312. [DOI] [PubMed] [Google Scholar]
- 25. Moore R, Beredjiklian P, Rhoad R, et al A comparison of the inflammatory potential of particulates derived from two composite materials. J Biomed Mater Res, 1997, 34: 137–147. [DOI] [PubMed] [Google Scholar]
- 26. Cho DY, Liau WR, Lee WY, Liu JT, Chiu CL, Sheu PC. Preliminary experience using a polyetheretherketone (PEEK) cage in the treatment of cervical disc disease. Neurosurgery, 2002, 51: 1343–1349, discussion 1349–1350. [PubMed] [Google Scholar]
- 27. Pelletier M, Cordaro N, Lau A, Walsh WR. PEEK versus Ti interbody fusion devices: resultant fusion, bone apposition, initial and 26 week biomechanic. J Spinal Disord Tech, 2012, Jul 13. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 28. Johansson CB, Röser K, Bolind P, Donath K, Albrektsson T. Bone‐tissue formation and integration of titanium implants: an evaluation with newly developed enzyme and immunohistochemical techniques. Clin Implant Dent Relat Res, 1999, 1: 33–40. [DOI] [PubMed] [Google Scholar]
- 29. Moreland DB, Asch HL, Clabeaux DE, et al Anterior cervical discectomy and fusion with implantable titanium cage: initial impressions, patient outcomes and comparison to fusion with allograft. Spine J, 2004, 4: 184–191, discussion 191. [DOI] [PubMed] [Google Scholar]
- 30. Majd ME, Vadhva M, Holt RT. Anterior cervical reconstruction using titanium cages with anterior plating. Spine (Phila Pa 1976), 1999, 24: 1604–1610. [DOI] [PubMed] [Google Scholar]
- 31. Dai L, Jiang LS. Anterior cervical fusion with interbody cage containing beta‐tricalcium phosphate augmented with plate fixation: a prospective randomized study with 2‐year follow‐up. Eur Spine J, 2008, 17: 698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eastlack RK, Garfin SR, Brown CR, Meyer S. Osteocel plus cellular allograft in anterior cervical discectomy and fusion: evaluation of clinical and radiographic outcomes from a prospective multicenter study. Spine (Phila Pa 1976), 2014, 39: E1331–E1337. [DOI] [PubMed] [Google Scholar]
- 33. Faldini C, Chehrassan M, Miscione MT, et al Single‐level anterior cervical discectomy and interbody fusion using PEEK anatomical cervical cage and allograft bone. J Orthop Traumatol, 2011, 12: 201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Faldini C, Miscione MT, Acri F, et al Single level cervical fusion by an anterior approach using autologous bone graft influences the adjacent levels degenerative changes: clinical and radiographic results at 10‐year minimum follow‐up. Eur Spine J, 2012, 21 (Suppl. 1): S90–S93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ha SK, Park JY, Kim SH, Lim DJ, Kim SD, Lee SK. Radiologic assessment of subsidence in stand‐alone cervical polyetheretherketone (PEEK) cage. J Korean Neurosurg Soc, 2008, 44: 370–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang JJ, Yu CH, Chang BS, Yeom JS, Lee JH, Lee CK. Subsidence and nonunion after anterior cervical interbody fusion using a stand‐alone polyetheretherketone (PEEK) cage. Clin Orthop Surg, 2011, 3: 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bruce C, Chin KR, Cumming V, Crawford NR. Stabilizing effects of a particulate demineralized bone matrix in the L4 interbody space with and without PEEK cage: a literature review and preliminary results of a cadaveric biomechanical study. West Indian Med J, 2013, 62: 748–751. [DOI] [PubMed] [Google Scholar]
- 38. Park HW, Lee JK, Moon SJ, Seo SK, Lee JH, Kim SH. The efficacy of the synthetic interbody cage and Grafton for anterior cervical fusion. Spine (Phila Pa 1976), 2009, 34: E591–E595. [DOI] [PubMed] [Google Scholar]
- 39. Currey JD, Foreman J, Laketić I, Mitchell J, Pegg DE, Reilly GC. Effects of ionizing radiation on the mechanical properties of human bone. J Orthop Res, 1997, 15: 111–117. [DOI] [PubMed] [Google Scholar]
- 40. Russell N, Rives A, Bertollo N, Pelletier MH, Walsh WR. The effect of sterilization on the dynamic mechanical properties of paired rabbit cortical bone. J Biomech, 2013, 46: 1670–1675. [DOI] [PubMed] [Google Scholar]
- 41. Rhee JM, Park JB, Yang JY, Riew DK. Indications and techniques for anterior cervical plating. Neurol India, 2005, 53: 433–439. [DOI] [PubMed] [Google Scholar]
- 42. Riley LH 3rd, Skolasky RL, Albert TJ, Vaccaro AR, Heller JG. Dysphagia after anterior cervical decompression and fusion: prevalence and risk factors from a longitudinal cohort study. Spine (Phila Pa 1976), 2005, 30: 2564–2569. [DOI] [PubMed] [Google Scholar]
- 43. Wang JC, McDonough PW, Endow K, Kanim LE, Delamarter R. The effect of cervical plating on single‐level anterior cervical discectomy and fusion. J Spinal Disord, 1999, 12: 467–471. [PubMed] [Google Scholar]
- 44. Caspar W, Geisler FH, Pitzen T, Johnson T. Anterior cervical plate stabilization in one‐ and two‐level degenerative disease: overtreatment or benefit? J Spinal Disord, 1998, 11: 1–11. [PubMed] [Google Scholar]
- 45. Kaiser MG, Haid RW Jr, Subach BR, Barnes B, Rodts GE Jr. Anterior cervical plating enhances arthrodesis after discectomy and fusion with cortical allograft. Neurosurgery, 2002, 50: 229–236, discussion 236–238. [DOI] [PubMed] [Google Scholar]
- 46. Song KJ, Taghavi CE, Lee KB, Song JH, Eun JP. The efficacy of plate construct augmentation versus cage alone in anterior cervical fusion. Spine (Phila Pa 1976), 2009, 34: 2886–2892. [DOI] [PubMed] [Google Scholar]
- 47. Rao RD, Wang M, McGrady LM, Perlewitz TJ, David KS. Does anterior plating of the cervical spine predispose to adjacent segment changes? Spine (Phila Pa 1976), 2005, 30: 2788–2792, discussion 2793. [DOI] [PubMed] [Google Scholar]
- 48. Song KJ, Taghavi CE, Hsu MS, Lee KB, Kim GH, Song JH. Plate augmentation in anterior cervical discectomy and fusion with cage for degenerative cervical spinal disorders. Eur Spine J, 2010, 19: 1677–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fraser JF, Härtl R. Anterior approaches to fusion of the cervical spine: a metaanalysis of fusion rates. J Neurosurg Spine, 2007, 6: 298–303. [DOI] [PubMed] [Google Scholar]
- 50. Miao J, Shen Y, Kuang Y, et al Early follow‐up outcomes of a new zero‐profile implant used in anterior cervical discectomy and fusion. J Spinal Disord Tech, 2013, 26: E193–E197. [DOI] [PubMed] [Google Scholar]
- 51. Wang Z, Jiang W, Zhang Z, et al Comparison of ROI‐C and traditional cage with anterior plating for anterior cervical discectomy and fusion. Zhonghua Wai Ke Za Zhi, 2014, 52: 425–430. [PubMed] [Google Scholar]
- 52. Carreon L, Glassman S, Djurasovic M. Reliability and agreement between fine‐cut CT scans and plain radiography in the evaluation of posterolateral fusions. Spine (Phila Pa 1976), 2007, 7: 39–43. [DOI] [PubMed] [Google Scholar]
- 53. Santos E, Goss D, Morcom R, Fraser R. Radiologic assessment of interbody fusion using carbon fiber cages. Spine(Phila Pa 1976), 2003, 28: 997–1001. [DOI] [PubMed] [Google Scholar]
- 54. Chong E, Pelletier MH, Mobbs RJ, Walsh WR. The design evolution of interbody cages in anterior cervical discectomy and fusion: a systematic review. BMC musculoskeletal disorders, 2015, 16(1): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bagby GW, inventor ; Washington State University Research Foundation, Inc., assignee. Process for fusing bone joints. United States patent US 4,501,269, 1985.


