Abstract
Purpose: To evaluate the prognostic value of early postoperative prostate specific antigen (PSA) levels after radical prostatectomy (RP) and pelvic lymph node dissection (PLND) in prostate cancer patients with lymph node invasion (LNI).
Materials and Methods: The retrospective analysis involved 96 patients who had a diagnosis of LNI with available data on the first PSA level at postoperative 6 weeks after RP and PLND between 2002 and 2014 at our institution. PSA persistence was defined as PSA ≥ 0.1 ng/ml at 6 weeks after surgery. Radiographic progression was defined as positive imaging during follow-up after the onset of biochemical recurrence, consisting of a bone scan and/or computed tomography (CT) and/or magnetic resonance imaging and/or 18F-(2-deoxy-2-fluoro-D-glucose positron emission tomography/CT scan. Comparative analysis of patients with and without PSA persistence was done, and Kaplan-Meier curve analysis with log-rank test and Cox proportional hazard regression models assessed radiographic progression free survival (PFS).
Results: Fifty two (54.2%) patients displayed PSA persistence. Kaplan-Meier curve analysis showed significantly decreased 5-year radiographic PFS (64.2% vs. 93.2%, log-rank, p=0.009) in the PSA persistence group compared to the no PSA persistence group. In a multivariate analysis, PSA persistence was a statistically significant predictor of radiographic PFS.
Conclusions: Early assessment of PSA after surgery is important for predicting radiographic progression in node-positive prostate cancer patients. Risk stratification based on the early PSA value after surgery would be helpful to identify patients who may benefit from early adjuvant therapies.
Keywords: Lymph node invasion, Pelvic lymph node dissection, Progression, PSA, Radical prostatectomy
Introduction
Pelvic lymph node dissection (PLND) is usually recommended for a subset of intermediate-risk group of nodal metastases > 5% according to the preoperative nomograms, and in all high-risk cases of prostate cancer (PCa) 1-3. The prevalence of lymph node invasion (LNI) is approximately 10% (up to 14%) after radical prostatectomy (RP) and extended PLND (ePLND) 4-6. However, not all node-positive patients have the same long-term prognosis 7,8. Accordingly, several prediction tools have been developed to assess the outcome of patients with LNI based on clinicopathological parameters 2,3,6,9.
RP and ePLND should theoretically result in an undetectable level of prostate specific antigen (PSA) within 21-30 days after surgery considering PSA's half-life of 3.15 days 9,10. Therefore, early PSA assessment after surgery could be a beneficial indicator of optimal cancer control, and a surrogate for residual tumor burden and/or micro-metastasis. Bianchi et al. 6 showed that PSA persistence (PSA ≥ 0.1 ng/ml) 6 weeks after RP and ePLND was an independent predictor of both clinical recurrence and cancer-specific mortality in patients with LNI. Accordingly, PSA persistence could be an important surrogate marker in planning the optimal treatment strategy. Conversely, adjuvant therapies like adjuvant radiotherapy could be waived or delayed in case of PSA that is not detectable (< 0.1 ng/ml) 6 weeks after surgery. Other studies also showed that patients with low volume positive LN (single or ≤ 2) can display good long-term results 8,11. Subsequently, the authors recommended that close observation with delayed adjuvant hormone therapy could be considered in these groups. Conversely, the foregoing indicated to us that risk stratification of node-positive patients based on early PSA value after surgery is helpful to identify patients who may benefit from early adjuvant therapies.
To assess this, we evaluated the prognostic value of early postoperative PSA assessment after RP and PLND in node-positive PCa patients, using RP data of a Korean large tertiary referral center.
Materials and Methods
Ethics statement
Institutional Review Boards of the Seoul National University Hospital approved this study (Approval number: H-1703-128-841). As the present study was carried out retrospectively, written informed consent from patients was waived. Personal identifiers were completely removed and the data were analyzed anonymously. Our study was conducted according to the ethical standards of the 1964 Declaration of Helsinki and its later amendments.
Study cohort
Clinical data of 2670 patients who underwent RP for clinically localized or locally advanced PCa between 2002 and 2014 at our institution were reviewed retrospectively. Patients with preoperative evidence of distant metastases were excluded. PLND was performed according to the surgeon's preference. Subsequently, a total of 740 patients (27.7%) underwent PLND, with standard PLND (sPLND; external iliac, obturator and internal iliac areas) for 593 patients and ePLND (adding the common iliac area and the presacral area to sPLND) for 147 1,12. LNI was reported in 114 (15.4%) patients. Patients had received adjuvant therapy according to the surgeon's discretion within 3 months after RP without any clinical evidence of residual or recurrent disease at the time of treatment.
Among them, patients who had no data of PSA value at 6 weeks postoperatively (n=6), who received adjuvant treatment prior to the assessment of PSA at 6 weeks (n=4), and who received salvage treatment due to the high PSA nadir level (> 0.2 ng/mL, n=8) were excluded. Ninety six patients were included in final analysis.
Data acquisition and definitions
Surgeries were conducted by several surgeons using open, laparoscopic, or robotic modality. All pathological specimens were evaluated by a staff pathologist who had genitourinary expertise. Biochemical recurrence (BCR) was defined as two consecutive increases in PSA > 0.2 ng/mL. Radiographic progression was defined as positive imaging during follow-up after the onset of BCR, with imaging comprising bone scan and/or computed tomography (CT) and/or magnetic resonance imaging, and/or 18F-fluoro-2-deoxy-D-glucose positron emission tomography (18F-FDG PET)/CT scan. PSA persistence was defined as PSA ≥ 0.1 ng/ml 6 weeks after surgery 6,7. The following variables were used for a comparative analysis between PSA persistence and no persistence groups: age, body mass index (BMI), preoperative PSA level, PSA level at 6 weeks, operative type (open vs. robotic), pathologic T stage and Gleason score (GS), total number of removed LNs, total number of positive LNs, total LN density (i.e., number of positive LNs divided by total number of LNs removed), extracapsular extension (ECE), seminal vesicle invasion (SVI), positive surgical margin (PSM), lymphovascular invasion (LVI), status of adjuvant therapy, BCR, and radiographic progression-free survival (PFS). The pathologic T stage was categorized as ≤ pT2 or ≥ pT3 (organ confined disease or not), and pathologic GS was classified as pGS < 8 or pGS ≥ 8. Subgroup analysis was also performed in the patients who underwent ADT only as an adjuvant therapy to adjust for potential confounding factors.
Statistical analyses
The clinicopathological characteristics were compared between PSA persistence and no persistence groups using chi-squared test for categorical variables, and independent t-test or Mann-Whitney U test for continuous variables. Kaplan-Meier curve analysis was used to calculate survival estimates for radiographic PFS and log-rank test was used to conduct comparisons between the groups. Univariate and multivariate Cox-proportional hazard regression analyses were performed to evaluate significant variables associated with radiographic PFS. Age, BMI, preoperative PSA, operative type, pathologic T stage and GS, total number of removed LNs, number of positive LNs, LN density, ECE, SVI, PSM, LVI, status of adjuvant therapy, BCR, and PSA persistence were included in the analyses. The variables that were included in the multivariable Cox proportional-hazard models were selected from the univariate analyses based on p-values of <0.05, and final model was derived using the backward selection method with an elimination criterion of P>0.05. All statistical analyses were performed using commercially available software (IBM SPSS Statistics ver. 21.0, Armonk, NY, USA). Twosided pvalue of <0.05 was considered statistically significant.
Results
Mean patient age and follow-up was 67.0 ± 6.7 years and 45.0 (range 2-136) months, respectively. A total of 52 (54.2%) patients showed PSA persistence. At the last follow-up, 8 (8.3%) patients had died of any cause. They comprised 6 (11.5%) patients in the PSA persistence group and 2 (4.5%) in the no PSA persistence group. Seven deaths were due to PCa, comprising 6 (11.5%) patients in the PSA persistence group and 1 (2.3%) patient in the no PSA persistence group. Radiographic progression occurred in 21 (21.9%) patients overall, with 16 (30.8%) cases in the PSA persistence group and 5 (11.4%) cases in the no PSA persistence group.
Comparison of clinicopathological features
Results of the comparative analysis of clinicopathological features between PSA persistence group and no PSA persistence group are summarized in Table 1. Those with PSA persistence showed significantly higher number of positive LNs (p=0.037), LN density (p=0.012), and radiographic PFS (p=0.022). There were no significant differences between the two groups in terms of age, BMI, preoperative and at 6-week PSA, operative type, pathologic T stage and GS, total number of removed LNs, ECE, SVI, PSM, LVI, status of adjuvant therapy, and BCR.
Table 1.
Comparative analyses results of variables between PSA persistence group and no PSA persistence group
| PSA persistence (n=52) | No PSA persistence (n=44) | p | |
|---|---|---|---|
| Age, years, mean (SD) | 66.9 (7.0) | 67.1 (6.4) | 0.892 |
| BMI, kg/m2, mean (SD) | 23.9 (2.5) | 24.2 (2.4) | 0.509 |
| Preoperative PSA, ng/mL, mean (SD) | 30.6 ± 29.1 | 30.5 ± 37.2 | 0.996 |
| PSA at 6 weeks, ng/mL, mean (SD) | 3.2 ± 9.1 | 0.02 ± 0.03 | 0.014 |
| Operative type, n (%) | 0.476 | ||
| Open | 36 (69.2) | 28 (63.6) | |
| Robotic | 16 (30.8) | 16 (36.4) | |
| Pathologic stage, n | 0.704 | ||
| ≤ pT2 | 3 (6.1%) | 4 (9.1%) | |
| ≥ pT3 | 49 (93.9%) | 40 (90.9%) | |
| Pathologic GS, n | 0.517 | ||
| 7 | 29 (55.3%) | 28 (64.3%) | |
| ≥8 | 23 (44.7%) | 16 (35.7%) | |
| Total number of removed LNs, mean (SD, range) | 13.8 (7.8, 2-41) | 13.8 (5.9, 3-29) | 0.992 |
| Total number of positive LNs, mean (SD, range) | 2.9 (3.4, 1-18) | 1.8 (1.2, 1-5) | 0.037 |
| Total number of positive LNs ≥ 2 | 29 (55.8%) | 21 (47.7%) | 0.432 |
| LN density, mean (SD) | 0.25 (0.24) | 0.15 (0.10) | 0.012 |
| ECE, yes | 41 (78.8%) | 36 (81.8%) | 1.000 |
| SVI, yes | 33 (63.5%) | 29 (65.9%) | 1.000 |
| PSM, yes | 41 (79.6%) | 32 (72.7%) | 0.472 |
| LVI, yes | 23 (44.2%) | 21 (47.7%) | 0.767 |
| Adjuvant therapy, n | 0.934 | ||
| None | 7 (14.3%) | 9 (20.0%) | |
| ADT only | 34 (64.3%) | 26 (60.0%) | |
| Radiotherapy only | 0 (0%) | 0 (0%) | |
| ADT + Radiotherapy | 11 (21.4%) | 9 (20.0%) | |
| BCR, yes | 10 (19.2%) | 15 (34.1%) | 0.159 |
| Radiographic PFS, yes | 16 (30.8%) | 5 (11.4%) | 0.022 |
ADT: androgen deprivation therapy, BMI: body mass index, ECE: extracapsular extension, GS: Gleason score, LN: lymph node, LVI: lymphovascular invasion, PFS: progression-free survival, PSM: positive surgical margin, SVI: seminal vesicle invasion
Survival outcomes and Cox-proportional hazard regression analyses
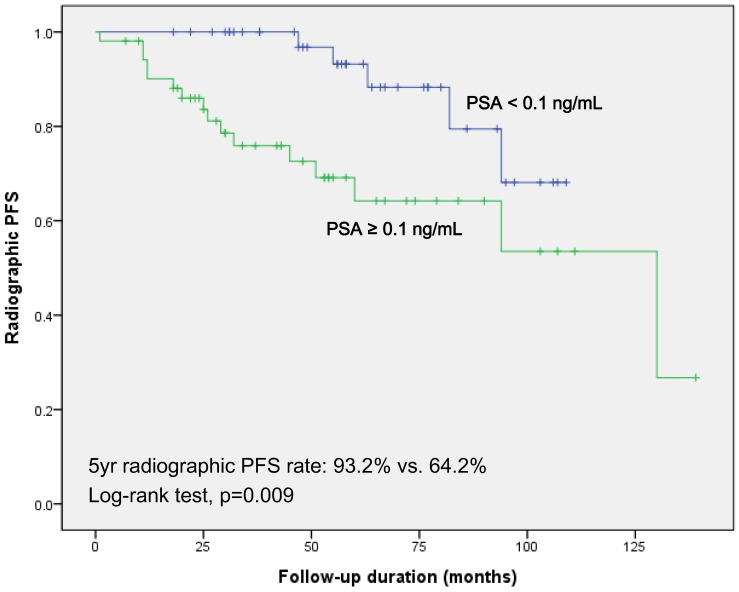
Kaplan-Meier survival analysis in the two groups revealed significantly decreased 5-year radiographic PFS in patients with persistent PSA (64.2% vs. 93.2%; log-rank test, p=0.009; Fig 1). In subgroup of patients who underwent androgen deprivation therapy (ADT) only as an adjuvant therapy, Kaplan-Meier survival analysis showed results concordant with those of the total cohort (74.7% vs. 100%; log-rank test, p=0.026; Fig. S1).
Fig 1.
Kaplan-Meier survival curves for radiographic progression free survival according to the PSA value at 6-week after radical prostatectomy and pelvic lymph node dissection
Table 2 summarizes the results of univariate and multivariate Cox regression analyses based on the total study cohort. Univariate analysis revealed pathologic GS (p=0.035), adjuvant therapy (p=0.030), and PSA persistence (p=0.015) as significant predictors of radiographic PFS. Only PSA persistence was identified as a single significant predictor of radiographic PFS in the multivariate analysis (p=0.046). The first site of radiographic progression was local (prostatic bed), pelvic LNs, and distant (skeletal and/or visceral metastases) in 19.0%, 14.3%, and 66.7% patients, respectively (Table S1), with no statistically significant difference between the two groups.
Table 2.
Uni- and multivariate Cox regression analyses evaluating variables associated with radiographic PFS
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Age | 0.975 | 0.913-1.042 | 0.454 | |||
| BMI | 1.029 | 0.871-1.216 | 0.735 | |||
| Preoperative PSA | 0.990 | 0.971-1.009 | 0.283 | |||
| Operative type | ||||||
| Open | Reference | |||||
| Robotic | 0.737 | 0.243-2.240 | 0.591 | |||
| Pathologic T stage | ||||||
| ≤ pT2 | Reference | |||||
| ≥ pT3 | 2.244 | 0.277-18.161 | 0.449 | |||
| Pathologic GS | ||||||
| 7 | Reference | Reference | ||||
| ≥8 | 2.799 | 1.077-7.274 | 0.035 | 2.539 | 0.976-6.603 | 0.056 |
| Total number of removed LNs | 0.962 | 0.896-1.032 | 0.280 | |||
| Total number of positive LNs | 1.068 | 0.909-1.256 | 0.423 | |||
| LN density | 0.762 | 0.110-5.275 | 0.783 | |||
| ECE | 0.846 | 0.276-2.592 | 0.769 | |||
| SVI | 1.958 | 0.652-5.883 | 0.231 | |||
| PSM | 1.303 | 0.431-3.942 | 0.639 | |||
| LVI | 1.272 | 0.432-3.743 | 0.662 | |||
| Adjuvant therapy | 2.690 | 1.099-6.583 | 0.030 | 1.227 | 0.397-3.794 | 0.722 |
| BCR | 1.812 | 0.727-4.512 | 0.202 | |||
| PSA persistence | 3.527 | 1.278-9.737 | 0.015 | 2.927 | 1.021-8.393 | 0.046 |
BCR: biochemical recurrence, BMI: body mass index, ECE: extracapsular extension, GS: Gleason score, LN: lymph node, PFS: progression-free survival, PSM: positive surgical margin, SVI: seminal vesicle invasion
Discussion
PSM, high number of positive LNs (a potential proxy for systemic dissemination), biopsy and pathologic GS 8-10, and pathologic stage >T3a have been significantly associated with PSA persistence 6,9,13-16. Previous authors have hypothesized that those with PSA persistence either had residual PCa in the prostatic bed/pelvic areas (PSM) or already had occult micro-metastases (positive LNs) at the time of surgery. Current study also showed that patients with persistent PSA had a significantly higher number of positive LNs (2.9 vs. 1.8, p=0.037) and LN density (0.25 vs. 0.15, p=0.012) (Table 1). However, no significant differences were evident between two groups in terms of pathologic GS or T stage; this may reflect the small number of patients, especially ≤ pT2 patients (7.3%). In addition, PSM also did not affect PSA persistence. Consequently, it may be provisionally considered that occult micro-metastasis through positive LNs is a more powerful factor for PSA persistence than residual tumor (even including benign tissue) through PSM 13-16.
In PCa patients with LNI, the optimal postoperative management is still controversial 17. Even in the presence of high level evidence of adjuvant hormone therapy 18,19, several studies showed favorable long-term outcomes for selected patients without adjuvant hormone therapy in these groups 8,11,20. In addition, the recent EAU-ESTRO-SIOG guidelines described that improving local control with early adjuvant hormone therapy combined with adjuvant radiotherapy appears beneficial in node-positive PCa patients treated with an ePLND 1. Men with minimal-volume nodal disease (≤ 3 LNs) and pathologic GS 7-10 and pT3-4 or R1 as well as men with 3-4 LN appear more likely to benefit from combined therapy after surgery 21. Notably, the authors described that the very low risk group (≤ 2 LNs, GS 2-6) did not benefit from combined therapy. For such patients, early postoperative management may be deferred until the development of recurrence or clinical progression. Accordingly, potential overtreatment and related adverse events are not issues for these patients. The potential benefit of adjuvant therapy on survival outcomes might differ depending on the primary PCa tumor characteristics. Not all node-positive patients share the same long-term prognosis. Consequently, risk stratification and identification of potential candidates for adjuvant therapy is necessary in an optimum postoperative management strategy.
Presently, PSA persistence was significant predictive factor for radiographic PFS (Table 2 and Fig 1). Wiegel et al. 13 showed that patients with undetectable PSA after RP followed by a surveillance or received adjuvant radiotherapy with 60Gy had a better prognosis including recurrence-free survival, metastasis-free survival, and overall survival in comparison with those who had a detectable PSA followed by immediate salvage radiotherapy with 66Gy. Bianchi et al. 6 also reported that node-positive patients with PSA persistence (PSA ≥ 0.1 ng/ml 6 weeks after surgery) showed less favorable outcomes over time. The authors suggested that risk stratification of node-positive patients based on early PSA value after surgery is helpful to identify patients who may benefit from early adjuvant therapies. Multi-institutional large retrospective or prospective studies should be conducted to ultimately allocate patients individually to the appropriate treatment strategy.
In the current study no significant differences were evident between patients with or without PSA persistence in terms of the first site of radiographic progression: local (prostatic bed, 18.6% vs. 20.0%, respectively), pelvic LNs (12.5%, vs. 20.0%, respectively), and distant (skeletal and/or visceral, 68.8% vs. 60.0%, respectively) (all p > 0.05, Table S1). This is consistent with a prior description of no significant differences in the first site of relapse; local, pelvic LNs, retroperitoneal LNs, and systemic (skeletal and/or visceral metastases) in 9%, 28%, 12%, and 51%, respectively 6. Notably, both studies showed concordance in terms of recurrence pattern, with over 50% of patients harboring distant metastases. However, a substantial number of patients also experienced local recurrence or pelvic LNs metastases. The data support the view that further lymphatic dissemination occurs in some node-positive patients 22.
The current study has several limitations. Due to the retrospective nonrandomized design, the extent of PLND and was at the discretion of the individual clinicians, not using standardized approaches. A previous systematic review and meta-analysis showed a significant difference in BCR between ePLND and sPLND in PCa patients; thus, ePLND could be an oncological benefit 23. Also, the rate of PLND was relatively lower than other studies 24-26. It might be derived from a large portion of robot-assisted laparoscopic RP (RARP) in our study population (32/96, 33.3%, Table 1). As known by previous studies 24-26, the rate of PLND was significantly lower in RARP than open RP (17% vs. 83% for laparoscopic RP/RARP vs. open RP, respectively, p<0.001) 25. In addition, due to the small number of patients, significant differences could not be discerned according to the adjuvant therapy strategies between the two groups (Table 1). Notably, only 20 (21.5%) patients had received combined therapy (ADT with adjuvant radiotherapy). Of patients with PSA persistence, 7 (14.3%) patients had never received adjuvant therapy; conversely, majority of patients with no PSA persistence received adjuvant therapies (Table 1). However, our study cohort had a long-standing history of PCa (>10 years). Consequently, this reflects the real-world clinical practice of a single tertiary institution. Further analyses involving large multi-institutional cohorts are needed to show the differences between the two groups in terms of potential benefit, or even harm from related adverse event after early combined adjuvant therapies.
In conclusion, Early PSA assessment at 6-week after surgery has an important prognostic value for predicting radiographic progression in node-positive PCa patients. Risk stratification based on the early PSA value after surgery would be one of the potential surrogate markers to identify patients who may benefit from early adjuvant therapies.
Supplementary Material
Supplementary figures and tables.
Acknowledgments
This work was funded by the Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Science, ICT and Future Planning (grant 2015R1A2A2A01006813) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2015R1C1A2A01051546).
References
- 1.Mottet N, Bellmunt J, Bolla M. et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 2017;4:618–29. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Briganti A, Larcher A, Abdollah F. et al. Updated nomogram predicting lymph node invasion in patients with prostate cancer undergoing extended pelvic lymph node dissection: the essential importance of percentage of positive cores. Eur Urol. 2012;3:480–7. doi: 10.1016/j.eururo.2011.10.044. [DOI] [PubMed] [Google Scholar]
- 3.Briganti A, Karakiewicz PI, Chun FK. et al. Percentage of positive biopsy cores can improve the ability to predict lymph node invasion in patients undergoing radical prostatectomy and extended pelvic lymph node dissection. Eur Urol. 2007;6:1573–81. doi: 10.1016/j.eururo.2007.01.108. [DOI] [PubMed] [Google Scholar]
- 4.Abdollah F, Suardi N, Gallina A. et al. Extended pelvic lymph node dissection in prostate cancer: a 20-year audit in a single center. Ann Oncol. 2013;6:1459–66. doi: 10.1093/annonc/mdt120. [DOI] [PubMed] [Google Scholar]
- 5.Abdollah F, Sun M, Thuret R. et al. Decreasing rate and extent of lymph node staging in patients undergoing radical prostatectomy may undermine the rate of diagnosis of lymph node metastases in prostate cancer. Eur Urol. 2010;6:882–92. doi: 10.1016/j.eururo.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 6.Bianchi L, Nini A, Bianchi M. et al. The Role of Prostate-specific Antigen Persistence After Radical Prostatectomy for the Prediction of Clinical Progression and Cancer-specific Mortality in Node-positive Prostate Cancer Patients. Eur Urol. 2016;6:1142–8. doi: 10.1016/j.eururo.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Briganti A, Karnes JR, Da Pozzo LF. et al. Two positive nodes represent a significant cut-off value for cancer specific survival in patients with node positive prostate cancer. A new proposal based on a two-institution experience on 703 consecutive N+ patients treated with radical prostatectomy, extended pelvic lymph node dissection and adjuvant therapy. Eur Urol. 2009;2:261–70. doi: 10.1016/j.eururo.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 8.Schumacher MC, Burkhard FC, Thalmann GN. et al. Good outcome for patients with few lymph node metastases after radical retropubic prostatectomy. Eur Urol. 2008;2:344–52. doi: 10.1016/j.eururo.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 9.Audenet F, Seringe E, Drouin SJ. et al. Persistently elevated prostate-specific antigen at six weeks after radical prostatectomy helps in early identification of patients who are likely to recur. World J Urol. 2012;2:239–44. doi: 10.1007/s00345-011-0707-y. [DOI] [PubMed] [Google Scholar]
- 10.Semjonow A, Hamm M, Rathert P. Prediction of tumor recurrence after radical prostatectomy using elimination kinetics of prostate-specific antigen. World J Urol. 1993;11:218–20. doi: 10.1007/BF00185073. [DOI] [PubMed] [Google Scholar]
- 11.Kim DK, Koo KC, Abdel Raheem A. et al. Single Positive Lymph Node Prostate Cancer Can Be Treated Surgically without Recurrence. PLoS One. 2016;3:e0152391. doi: 10.1371/journal.pone.0152391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feifer AH, Elkin EB, Lowrance WT. et al. Temporal trends and predictors of pelvic lymph node dissection in open or minimally invasive radical prostatectomy. Cancer. 2011;117:3933–42. doi: 10.1002/cncr.25981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiegel T, Bartkowiak D, Bottke D. et al. Prostate-specific antigen persistence after radical prostatectomy as a predictive factor of clinical relapse-free survival and overall survival: 10-year data of the ARO 96-02 trial. Int J Radiat Oncol Biol Phys. 2015;2:288–94. doi: 10.1016/j.ijrobp.2014.09.039. [DOI] [PubMed] [Google Scholar]
- 14.Boorjian SA, Thompson RH, Siddiqui S. et al. Long-term outcome after radical prostatectomy for patients with lymph node positive prostate cancer in the prostate specific antigen era. J Urol. 2007;178:864–70. doi: 10.1016/j.juro.2007.05.048. [DOI] [PubMed] [Google Scholar]
- 15.Daneshmand S, Quek ML, Stein JP. et al. Prognosis of patients with lymph node positive prostate cancer following radical prostatectomy: long-term results. J Urol. 2004;172:2252–5. doi: 10.1097/01.ju.0000143448.04161.cc. [DOI] [PubMed] [Google Scholar]
- 16.Bader P, Burkhard FC, Markwalder R. et al. Disease progression and survival of patients with positive lymph nodes after radical prostatectomy. Is there a chance of cure? J Urol. 2003;169:849–54. doi: 10.1097/01.ju.0000049032.38743.c7. [DOI] [PubMed] [Google Scholar]
- 17.Briganti A, Passoni NM, Abdollah F. et al. Treatment of lymph node-positive prostate cancer: teaching old dogmas new tricks. Eur Urol. 2014;1:26–7. doi: 10.1016/j.eururo.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Messing EM, Manola J, Yao J. et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006;6:472–9. doi: 10.1016/S1470-2045(06)70700-8. [DOI] [PubMed] [Google Scholar]
- 19.Messing EM, Manola J, Sarosdy M. et al. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med. 1999;24:1781–8. doi: 10.1056/NEJM199912093412401. [DOI] [PubMed] [Google Scholar]
- 20.Touijer KA, Mazzola CR, Sjoberg DD. et al. Long-term outcomes of patients with lymph node metastasis treated with radical prostatectomy without adjuvant androgen-deprivation therapy. Eur Urol. 2014;1:20–5. doi: 10.1016/j.eururo.2013.03.053. [DOI] [PubMed] [Google Scholar]
- 21.Abdollah F, Karnes RJ1, Suardi N. et al. Impact of adjuvant radiotherapy on survival of patients with node-positive prostate cancer. J Clin Oncol. 2014;35:3939–47. doi: 10.1200/JCO.2013.54.7893. [DOI] [PubMed] [Google Scholar]
- 22.Moschini M, Sharma V, Zattoni F. et al. Natural History of Clinical Recurrence Patterns of Lymph Node-Positive Prostate Cancer After Radical Prostatectomy. Eur Urol. 2016;69:135–42. doi: 10.1016/j.eururo.2015.03.036. [DOI] [PubMed] [Google Scholar]
- 23.Choo MS, Kim M, Ku JH. et al. Extended versus Standard Pelvic Lymph Node Dissection in Radical Prostatectomy on Oncological and Functional Outcomes: A Systematic Review and Meta-Analysis. Ann Sug Oncol. 2017;24:2047–54. doi: 10.1245/s10434-017-5822-6. [DOI] [PubMed] [Google Scholar]
- 24.Prasad SM, Shalhav AL. Comparative effectiveness of minimally invasive versus open lymphadenectomy in urological cancers. Curr Opin Urol. 2013;23:57–64. doi: 10.1097/MOU.0b013e32835af286. [DOI] [PubMed] [Google Scholar]
- 25.Prasad SM, Keating NL, Wang Q. et al. Variations in surgeon volume and use of pelvic lymph node dissection with open and minimally invasive radical prostatectomy. Urology. 2008;72:647–52. doi: 10.1016/j.urology.2008.03.067. [DOI] [PubMed] [Google Scholar]
- 26.Ku JH, Jeong CW, Park YH. et al. Biochemical recurrence after radical prostatectomy with or without pelvic lymphadenectomy in Korean men with high-risk prostate cancer. Jpn J Clin Oncol. 2011;41:656–62. doi: 10.1093/jjco/hyr030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures and tables.