Abstract
Stem cell research has been a popular topic in the past few decades. This review aims to discuss factors that help regulate, induce, and enhance mesenchymal stem cell (MSC) differentiation into osteoblasts for bone regeneration. The factors analyzed include bone morphogenic protein (BMP), transforming growth factor β (TGF‐β), stromal cell‐derived factor 1 (SDF‐1), insulin‐like growth factor type 1 (IGF‐1), histone demethylase JMJD3, cyclin dependent kinase 1 (CDK1), fucoidan, Runx2 transcription factor, and TAZ transcriptional coactivator. Methods promoting bone healing are also evaluated in this review that have shown promise in previous studies. Methods tested using animal models include low intensity pulsed ultrasound (LIPUS) with MSC, micro motion, AMD3100 injections, BMP delivery, MSC transplantation, tissue engineering utilizing scaffolds, anti‐IL‐20 monoclonal antibody, low dose photodynamic therapy, and bone marrow stromal cell transplants. Human clinical trial methods analyzed include osteoblast injections, bone marrow grafts, bone marrow and platelet rich plasma transplantation, tissue engineering using scaffolds, and recombinant human BMP‐2. These methods have been shown to promote and accelerate new bone formation. These various methods for enhanced bone regeneration have the potential to be used, following further research, in clinical practice.
Keywords: Animal trials, Bone regeneration, Clinical trials, Mesenchymal stem cells, Osteoblasts, Related factors
Introduction
Mesenchymal stem cells (MSC) are involved in bone regeneration after injury. These are pluripotent cells that have the potential to differentiate into a limited number of cell types, including adipocytes, osteoblasts, chondrocytes, and debatably endothelial cells1, 2. Divergent differentiation of MSC toward each individual cell type is dependent on the effects of several different growth factors3. MSC that are found in bone marrow, periosteum, vessel walls, muscle, circulation, and other tissues migrate to the site of bone injury4. Chemokines play a critical role in recruiting MSC; however, the mechanism of recruitment remains unclear. Recruiting MSC from surrounding tissues could be a beneficial way of inducing or supporting bone regeneration5.
Mesenchymal stem cells have been shown to enhance bone regeneration and treat critical sized defects. Enhancing fracture healing can be critical for recovery as the healing process can take several weeks to complete and can result in complications such as delayed unions and non‐unions in 10%–20% of cases6. New methods to accelerate recovery can help combat the increasing rate of patients with bone fractures, shortages of bone matrixes, and higher percentages of osteoporosis due to aging7. MSC bone regeneration in past experimental and clinical studies has been hindered by possible vulnerability to infection, variable differentiation of MSC in different in vivo situations, uncertain compatibly of MSC, high cost of ex vivo handling, limited number of obtainable cells, and possible malignant transformation during ex vivo cell expansion5, 8, 9.
Mesenchymal stem cells play a pivotal role in the initial formation and maintenance of bone. Endochondral ossification, a type of bone healing mechanism, involves MSC differentiating into chondrocytes to lay down cartilage, which is calcified, and then remodeled into bone10. Intramembranous ossification, another type of bone healing mechanism, involves MSC or undifferentiated bone‐forming cells directly differentiating into osteoblasts11. Osteoblasts are of particular importance in the initial formation of bone, for maintaining bone ossification, and for fracture repair12. Osteoblasts are involved in repairing the constant micro fractures that occur from daily use and contribute to the dynamic property of bone13. The wide range of bioactive molecules secreted by MSC also help in creating an optimal regenerative microenvironment14. This field is rapidly progressing, with the potential to be used in clinical application. This updated prospective review article aims to identify methods used to differentiate MSC into osteoblasts and compare current proposed methods for fracture repair. Benefits will be highlighted in this review to compare the potential of the various proposed methods to enhance bone regeneration. This review aims to bring together past studies in this field to provide a useful update on new discoveries and advancements.
Factors in Mesenchymal Stem Cell Differentiation and Migration
Methods to induce bone proliferation at the fracture site include increasing the number of MSC or increasing differentiation factors at the site to induce differentiation of MSC into osteoblasts. MSC differentiation into osteoblasts involves a complex interaction between many paracrine and autocrine signals that initiate molecular mechanisms allowing full osteogenic differentiation15.
Normal Fracture Healing with Bone Morphogenic Protein and Transforming Growth Factor β
Bone morphogenic protein (BMP) is involved in the differentiation of undifferentiated mesenchymal cells into chondrocytes and osteoblasts as well as osteoprogenitors into osteoblasts. BMP arise from osteoprogenitor cells, mesenchymal cells, osteoblasts, and chondrocytes, and mainly reside in the bone extracellular matrix. They target mesenchymal cells, osteoprogenitor cells, and osteoblasts. BMP heterodimers, including BMP‐4/‐7 and BMP‐2/‐7, more efficiently regulate the differentiation and proliferation of MSC into osteoblasts in vitro and in vivo, thus enhancing the osteoinductive activity10. Another study identified BMP‐2/‐6/‐9 as the most potent factors to induce MSC differentiation into osteoblasts based on a comprehensive analysis of the 14 types of BMP molecules. BMP‐2 is expressed on Day 1 of fracture healing to stimulate MSC differentiation. BMP‐6 and ‐9 are expressed at later stages in the animal model16. BMP induce a cascade resulting in chemotaxis, mesenchymal and osteoprogenitor cell proliferation and differentiation, angiogenesis, and controlled synthesis of extracellular matrix17. Low levels of BMP will, in turn, promote differentiation of MSC into adipocytes. BMP play other roles in the healing process, such as stimulating the synthesis and secretion of other bone and angiogenic growth factors, directly activing endothelial cells for angiogenesis, and regulating callus formation10.
Transforming growth factor β (TGF‐β) is a potent chemotactic stimulator of MSC, enhancing proliferation of MSC, pre‐osteoblasts, chondrocytes, and osteoblasts. TGF‐β initiates signaling for BMP synthesis in osteoprogenitor cells and inhibits osteoclast activation and stimulates osteoclast apoptosis. TGF‐β and PDGF that are released by activated platelets in early stages of fracture healing induce MSC migration, activation, and proliferation along with angiogenesis and inflammatory reactions. TGF‐β's osteoinductive potential, however, is limited and has shown various side effects, thus limiting its clinical use for bone regeneration aside from enhancing proliferation10, 18.
Transforming growth factor β and BMP2 are required for normal fracture healing. Without these factors, MSC do not differentiate toward the osteogenic lineage, inhibiting healing (Fig. 1)19. Studies have shown that TGF‐β and BMP receptors increase early in the repair process but decrease as the callus cells differentiate and bone formation starts20.
Figure 1.
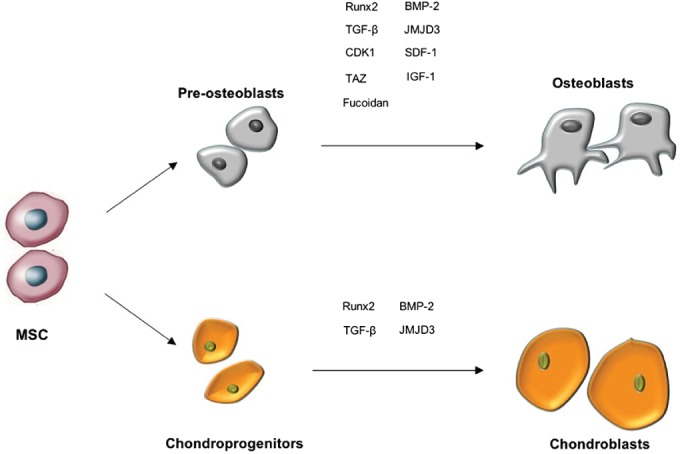
Effects of different factors on mesenchymal stem cell differentiation.
Transcriptional Regulation of Bone Formation
Histone demethylase JMJD3 positively regulates MSC differentiation into osteoblasts in both intramembranous and endochondral bone formation. JMJD3, which is expressed in osteoblasts, directly promotes Runx2 transcription and interacts with Runx2 to form new bone and increase osteoblast differentiation. This enzyme is required for intramembranous ossification in vivo. JMJD3 is also involved in chondrocyte proliferation and hypertrophy, macrophage differentiation, lung development, and neurogenesis in vivo. Homozygous deletion of JMJD3 severely delayed osteoblast differentiation and bone ossification in mice21, 22.
Insulin‐like growth factor type 1 (IGF‐1), which is released from bone matrix, is also crucial for terminal osteoblast differentiation of MSC. IGF‐1 plays a part in regulating early differentiation of MSC into osteoblasts23. IGF‐1 is also positively associated with maintenance of bone mineral density and acquisition of a higher peak bone mass (PBM), which reduces subsequent fracture risk24. Xian et al. report that IGF‐1 secreted by bone matrix specifically activates mammalian target of rapamycin (mTOR) during the bone remodeling process to stimulate osteoblastic differentiation of MSC25. Impaired IGF‐1 signaling in MSC has been shown to impair bone mass acquisition. Knockout of IGF‐1 in MSC of mice impaired osteoblast differentiation and decreased trabecular bone formation24.
Cyclin dependent kinase 1 (CDK1) promotes osteogenic differentiation of human mesenchymal stem cells by phosphorylating Enhancer of zeste homologue 2 (EZH2), which regulates stem cell differentiation. EZH2 catalyzes the trimethylation of histone H3 on H3K27, repressing gene transcription. Knockdown of CDK1 by three different shRNAs blocked osteogenic differentiation. The knockdown led to decreased EZH2 phosphorylation, increased H3K27 trimethylation, and repression of osteogenic markers, including Runx2 and osteopontin. Thus, CDK1 inhibition of EZH2 methyltransferase activity promotes MSC differentiation into osteoblasts26.
Fucoidan, a polysaccharide, induces MSC proliferation and promotes osteoblast differentiation via a JNK‐ and ERK‐dependent BMP2‐Smad 1/5/8 signaling in hMSC. Fucoidan significantly increased ALP activity and levels of osteocalcin and BMP‐2 related to bone mineralization27. It facilitated calcium accumulation and upregulation of osteoblast specific genes, including ALP, Runx2, type I collagen‐α 1, and osteocalcin. Fucoidan also induced BMP2 expression and stimulated activation of extracellular signal‐related kinase (ERK), c‐Jun N‐terminal kinase (JNK), and Smad 1/5/8 by increasing phosphorylation. Phosphorylation of Smad 1/5/8 and BMP2 mRNA expression was markedly increased by fucoidan. Smad signaling is known to mediate the effects of BMP, which are involved in bone formation signaling pathways. The effect of fucoidan on osteogenic differentiation was inhibited by BMP2 knockdown and by ERK and JNK inhibitors, indicating that fucoidan influences differentiation through the BMP2‐Smad 1/5/8 signaling pathway by activating ERK and JNK28.
Stromal cell‐derived factor 1 (SDF‐1) has been shown to enhance MSC differentiation into osteoblasts, which is mediated by the BMP signaling pathway. Cells cultured in SDF‐1 and osteoinductive medium showed higher ALP activity than cells cultured in osteoinductive medium alone, supporting its role in enhancing osteogenic differentiation5. In addition, disrupting SDF‐1 signaling‐impaired bone nodule mineralization and inhibited BMP‐2‐induced early expression of Runx2 and osterix (Osx) which are regulators of osteogenesis. Blocking the SDF‐1/CXCR4 axis or adding SDF‐1 significantly affected BMP‐2‐induced ALP activity and osteocalcin synthesis, markers of preosteoblasts and mature osteoblasts, respectively29. SDF‐1 is also upregulated at sites of injury, specifically the periosteum, and activates the CXCR4 receptor on MSC aiding in regeneration5. This factor binds to the CXCR4 (C‐X‐C chemokine receptor type 4) receptor, forming a complex that promotes proliferation and increased metabolic activity of cells at the injury site30. Using a mouse model, Granero‐Molto et al. determined that MSC migration to fracture sites was time‐dependent and dose‐dependent, and exclusively CXCR4‐dependent31. SDF‐1 is also a potent chemoattractant for MSC migration to the site of injury32. In the Ho et al. study, SDF‐1 was transfected into MSC. MSC with this SDF‐1 gene secreted increased levels of SDF‐1, leading to increased MSC migration20. Transplanting these MSC cells into the fracture site enhanced fracture healing in the rat model by increasing new bone formation and bone mineral content and density.
Runt‐related transcription factor 2 (Runx2) is a key transcription factor involved in regulating MSC differentiation into osteoblasts. Expression of Runx2 increases gene expression of osteoblast‐specific genes and initiates mineralization33, 34. Cfba1 knockout mice lack expression of the Cfba1/Runx2 transcription factor, resulting in a diminished number of functional differentiated osteoblasts and lacking mineralized bone35. Runx2 also plays a role in regulating chondrocyte hypertrophy, bone formation, and angiogenesis. Kang et al. transduced the gene Runx2 into human MSC using a lentiviral vector and transplanted them into mice. Superior bone healing was demonstrated in mice with modified hMSC containing Runx2 compared to controls with unmodified hMSC. Transplanted cells migrated to the fracture site and differentiated into osteoblasts to form new bone. Runx2 can, therefore, increase the efficiency of bone healing, which may be clinically applicable, but overexpression of Runx2 has been linked to human malignancies in various cancers, demonstrating the need for caution21.
TAZ is a Runx2 transcriptional coactivator for the osteocalcin gene in MSC and represses PPAR γ‐dependent gene transcription. TAZ promotes MSC differentiation into osteoblasts, and inhibits PRARγ from facilitating MSC differentiation into adipocytes36. TAZ co‐activates Runx2 in cells, which is critical to osteoblast differentiation. Stimuli promoting bone formation upregulates TAZ and Runx2 concurrently. Hong et al. experiments showed that MSC differentiation into osteoblasts is dependent on Runx2 and TAZ. Zebrafish without TAZ showed defective bone formation37. When the murine cells with upregulated TAZ expression were exposed to BMP‐2, a protein naturally present at bone fracture sites, there was a 400‐fold increase in the osteocalcin gene expression. The osteocalcin gene is a maker of osteoblast development, and upregulation of this marker is hypothesized to correlate to higher osteoblast proliferation. Upregulating TAZ and Runx2 may be a future avenue for bone formation.
Animal Trials: Accelerating Recovery
Ultrasound Stimulation
Fracture healing can be enhanced by combining MSC with low intensity pulsed ultrasound (LIPUS) which provides local acoustic mechanical stimulation. This combination enhanced callus formation, new bone formation, and accelerated bone remodeling in a rat model. This can be used as a potential interventional approach for delayed union or nonunion fractures, accelerating proper fracture healing in both animal models and randomized clinical trials38. Individually, MSC and LIPUS have shown to help with bone healing. Transplanting MSC enhanced callus volume, new bone volume, hydroxyapatite content and biomechanical properties in mice. However, treating with MSC alone enhances fracture repair to a lesser extent than using a combination of MSC and LIPUS. LIPUS has shown to accelerate healing by about 30% in many clinical trials. The effects of LIPUS can be explained by its ability to enhance cell proliferation and differentiation of periosteal cells into an osteogenic lineage. LIPUS has also been proven to induce production of cytokines that enhance fracture healing, increase blood flow at the fracture site, and help with MSC migration6. LIPUS upregulates SDF‐1 and CXCR4 expression levels in MSC. Increased SDF‐1 protein levels from LIPUS promotes MSC migration to the fracture site, improving callus microarchitecture and mechanical properties. Blocking SDF‐1 signaling with AMD3100 reduced the healing effects of LIPUS39.
Micro Motion Stimulation
In secondary fractures, micro motion is necessary for fracture healing because it induces the electric polarization necessary for bone healing. Up to a certain threshold micro motion has positive effects on bone ossification, and above it deleterious effects begin to occur40. Micro motion mechanical stimulation showed increased mineralization and almost no traces of cartilage in a rat model when applied 10 days post fracture. Ten days was determined to be the optimal time to start stimulation to increase bone formation. However, micro motion decreased mineralization and mechanical properties if stimulated during the initial response to the fracture possibly due to motion induced rupture of newly forming blood vessels. Delaying treatment after the inflammatory phase can give time for new vessels and soft tissue to form41.
Stem Cell Therapy
Mobilizing endogenous stem cells may provide an alternative strategy to enhance bone regeneration. Toupadakis et al. injected mice with AMD3100 that is an antagonist of the chemokine receptor 4 (CXCR4) that has been shown to mobilize stem cells from the blood. Injected mice displayed higher numbers of circulating MSC, hematopoietic stem cells and progenitor cells, and endothelial progenitor cells42. The fracture callus was significantly larger at day 21 and significantly smaller at day 84 due to remodeling compared to saline injected mice. AMD3100 injected mice also showed significantly higher bone mineral density. However, AMD3100 may disrupt stem cell homing to the site of injury by interfering with the CXCR4/SDF‐1 pathway. AMD3100 disrupts the anchoring of CXCR4+ cells in SDF‐1 rich bone marrow for cell mobilization. The periosteum expresses SDF‐1 during injury which has been proven to be a pivotal molecule for bone formation and chemo‐attraction of stem cells to the site of injury. Administering injections three times during the first 3 days of healing showed benefits in this study but continuing injections for the remainder of the healing process impaired recovery43.
Recombinant Bone Morphogenetic Proteins (BMP), an osteoinductive signal, are FDA approved to help with spine fusions but require large doses to be effective when collagen carriers are used as the delivery mechanism. Adipose tissue contains large numbers of MSC and does not decrease with age unlike bone marrow derived MSC. Adipose derived MSC can only treat a bone defect if they are genetically modified by adenoviral gene transfer to overexpress BMP‐2 as shown in a rat model by Peterson et al. These cells can potentially be used to treat delayed unions or nonunions44. Hashimoto et al. showed that administration of recombinant human BMP‐2 (rhBMP‐2) stimulated ectopic ossification in rabbit tendons45. Although the osteogenic potential of recombinant BMP has been shown, further research is needed to optimize the delivery method for BMP and determine the best source of MSC for ex vivo gene transfer strategies.
Transplanting MSC in mice at the fracture site improved the biomechanical properties of the fracture callus site, the callus size and morphology, and made the callus less brittle. Significant increases were shown in new bone, soft tissue, callus volume and callus mineralization content compared to the control group12. Dreger et al. systemically transplanted human bone marrow derived MSC into the tail vein of mice 1 and 3 days after fracture46. Significantly more MSC at the fracture site from the day 1 injection as compared to the day 3 injection was found and lasted at least 7 days. While both fractures healed by 3 weeks, the optimal time for intravenous injections of MSC was determined to be around 24 h post fracture in mice. Another study transfected MSC with IGF‐I, transplanted them into mice with fractured femurs, and traced their migration to the fracture site. These mice had greater matrix mineralization and osseous progression within the callus47.
Tissue engineering involving a coral scaffold infused with rhBMP‐2 and MSC in the rabbit model showed greater bone formation than the coral scaffold and rhBMP‐2 alone as shown in Hou et al. The coral‐MSC‐rhBMP‐2 engineered bone showed comparable results to the auto‐bone‐graft for repairing critical‐sized bone defects, demonstrating a possible alternative to autologous bone grafts48.
Nair et al. tested the uses of a triphasic ceramic‐coated hydroxypatite (HASi) scaffold loaded with MSC for bone fracture healing in a goat model49. The seeded scaffold was treated to induce MSC osteoblast differentiation, transplanted into the 2 cm femoral fracture and evaluated at 4 months. Good osteoinduction and integration were present in the MSC HASi when compared to the unseeded HASi group. Furthermore, it was determined the MSC HASi group had faster healing based on evidence of lamellar bone in the scaffold at 4 months. The unseeded scaffold still had immature woven bone present and less of the scaffold was degraded. The seeded HASi scaffold shows promise as a method for treating bone defects in long bones.
Mankani et al. used autologous bone marrow stromal cell‐hydroxyapatite/tricalcium phosphate transplants to treat critical sized calvarial defects in dogs50. Each dog also received a hydroxyapaptite/tricalcium phosphate (HA/TCP) transplant without bone marrow stromal cells (BMSC) in an identical contralateral calvarial defect. BMSC containing transplants formed bone faster and more extensively than transplants without BMSC. This study showed that autologous cultured bone marrow stromal cell transplantation is a possible therapy for bone defects.
Antibody Therapy
Anti‐IL‐20 monoclonal antibody 7E in a mouse model increased bone formation at the fracture site, displaying its potential to be a therapeutic agent for bone fractures. IL‐20 is involved in inhibiting osteoblast differentiation and maturation and increasing osteoclast differentiation. In vitro, IL‐20 upregulated sclerostin and downregulated osterix (OSX), Runx2, and osteoprotegerin (OPG) thus inhibiting osteoblast formation. A deficiency in IL‐20 decreased fracture healing time by limiting the inhibitory effects of IL‐20 on osteoblast differentiation from MSC and osteoprogenitor cells. IL‐20 has shown a significant correlation with sclerostin in patients with bone fractures and osteoporosis. Sclerostin also inhibits osteoblast differentiation, proliferation, and function. Overexpression of sclerostin showed an osteoporotic phenotype in mice. An anti‐sclerostin antibody was shown in reverse bone loss due to estrogen deficiency by increasing bone formation in a rat model51.
Phototherapy
Low dose Photodynamic therapy (PDT) accelerates osteoblast differentiation by activating activator protein‐1 (AP‐1) in mouse osteoblast precursor cells and rat primary MSC. This involves injection of a photosensitizing compound systemically or at the site of injury, light irradiation that activates the photosensitizing compound, and generation of reactive oxygen species (ROS) photochemically. ROS are involved in signaling pathways and act as second messengers in proliferation or differentiation of bone marrow derived stem cells. AP‐1 is a transcription factor that mediates induction of osteoblast differentiation after upregulation of its activity by ROS from low dose PDT. Although low dose PDT did not produce significant cytotoxicity in this study, the cytotoxic effects of high levels of ROS should be considered if PDT is used clinically to aid with fracture healing52.
Blue laser irradiation promotes extracellular calcification of MSC matrix by facilitating the translocation of the circadian rhythm protein cryptochrome 1 (CRY1) from the cytoplasm to the nucleus. The CRY1 protein likely has a regulatory role in the balance between bone formation and resorption. Immersion of murine MSC in blue laser light induced transcription of CRY1, disrupting the normal homeostatic sequence and promoting matrix mineralization. This control over MSC fate could lead to beneficial treatment options53.
Somatic Cell Nuclear Transfer
The use of somatic cell nuclear transfer (SCNT) is a controversial but potentially promising method for the treatment of a variety of defects. Unlike previous methods, this one does not use mesenchymal stem cells. Instead it combines the isolated nucleus of a somatic cell with a de‐nucleated embryonic stem cell. This modified cell retains the cellular identity of the nuclear donor and has the capability to develop into any cell type with proper stimulation and guidance (Fig. 2). One drawback to this treatment is its lack of efficiency; many of the modified cells are not viable, and those that are have limited proliferation. Yet SCNT methods are being constantly refined, and could provide many benefits for bone regeneration. Aside from mitochondria, the modified cells would be identical to those of their nuclear donor. This would eliminate worries about rejection in the patients, assuming they are the nuclear donor54. Additionally, somatic cells are much easier than MSC to obtain from patients. This would result in much less pain and other complications during the cellular harvesting process55. Interestingly, several studies concluded that MSC nuclear donors provide better success rates in SCNT than somatic cells. These studies found that in pigs, using nuclei from the less differentiated MSC produced better survival outcomes for the modified cells during both pre‐implantation and post‐implantation development56, 57. Paired with a reliable method for harvesting circulating MSC, this technique could provide useful strategies for therapeutic treatment in humans.
Figure 2.
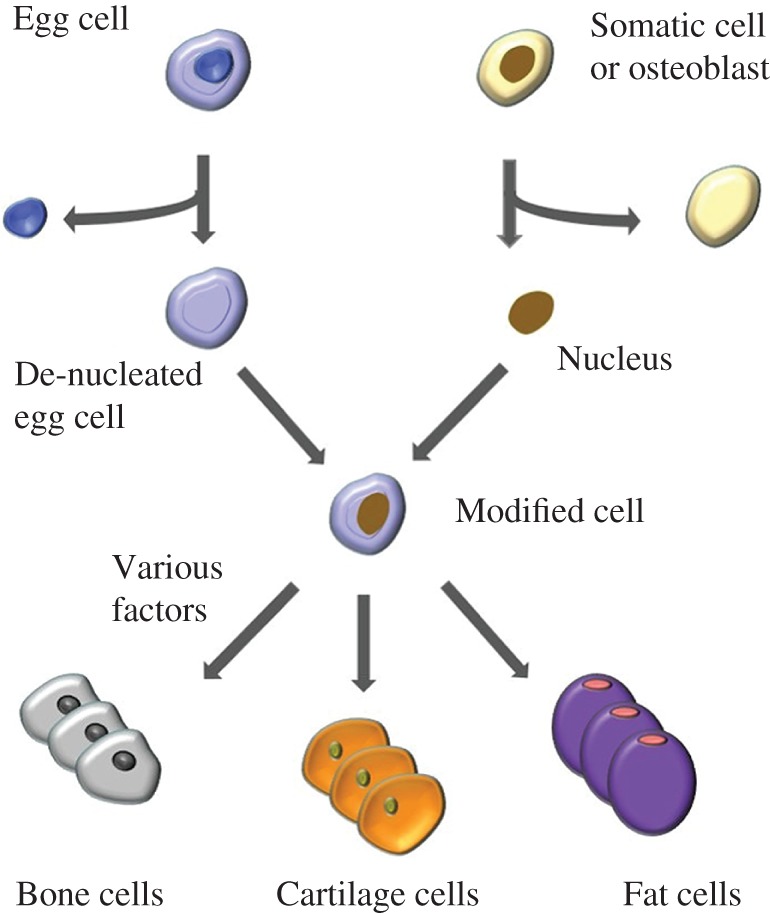
Process of somatic nuclear cell transfer, with ability to differentiate into multiple different cell types.
Clinical Trials
Injection of Autologous Osteoblasts and Bone Marrow Grafts
Autologous cultured osteoblast injections have shown success in treating long‐bone fractures in a study conducted by Kim et al. 58. Thirty‐one patients that were given the treatment showed statistically significant healing acceleration. Bone marrow was aspirated from the iliac spine and treated with L‐ascorbic acid and dexamethasone to facilitate osteoblast differentiation. Cultured osteoblasts were mixed with fibrin at a 1:1 ratio and injected into the non‐union site. At 1 and 2 months post injection the experimental patient group experienced more callus formation than the control group but the difference was only significant at 2 months. The injection accelerated the healing process and created no specific patient complications. Less pain was experienced compared to a bone transplant and patients were less likely to need follow‐up surgery with the osteoblast injections.
Percutaneous autologous concentrated bone marrow grafts (PABMB) enabled healing in non‐union fractures in two out of the three cases studied in Gross et al. Bone marrow was harvested from the iliac crest, concentrated, and injected into the non‐union site in 45 non‐union long bone cases. An average of 62% of patients experienced union, 18 of the 28 tibia (69%), and 10 of the 16 femurs (63%), but none of the humeri non‐unions healed. Factors such as smoking, diabetes, and history of infection at the non‐union site are thought to be major contributors to the failed cases. The procedure was deemed invasive yet promising and safe. It resulted in less morbidity than standard non‐union procedures59. Hernigou et al. saw similar results when using PABMB to treat non‐union fractures. Bone marrow from the iliac crest was injected into 60 patients with tibial non‐union. Bone union was successfully obtained in 53 of these patients (88%), showing the clinical benefit of this treatment method60.
Transplantation of Bone Marrow
Culture expanded bone marrow cells and platelet rich plasma (PRP) transplantation during limb lengthening increased bone healing as transplanted bone marrow cells differentiated into osteoblasts as shown in Kitoh et al. Limb lengthening treatment is used to treat bone loss after trauma, congenital deficiencies, or tumor resection to lengthen limbs and correct angular/rotational deformities61. These transplantations helped accelerate callus formation in patients, preventing complications from the typically long treatment periods. Treating bone marrow derived MSC with dexamethasone can commit MSC to the osteogenic lineage ex vivo. PRP also contains osteoinductive growth factors that accelerate bone regeneration pathways. This method of cell therapy showed faster femoral than tibial lengthening, possibly due to differences in the microenvironment and local blood supply at transplanted sites. Favorable results are seen when transplanted areas have sufficient blood supply and soft tissue. These transplantations have also been shown to improve bone repair by accelerating vascular invasion, stimulating osteoprogenitor cells in surrounding soft tissue, and promoting osteogenesis at the transplant site.
Marcacci et al. used tissue engineering to treat four patients with large bone diaphysis defects62. Cells were isolated from the patient's bone marrow stroma, expanded, and seeded into porous hydroxyapatite ceramic scaffolds. These were surgically inserted into areas of bone loss. Complete fusion between the implant and the patient's bone happened about 5–7 months after surgery with no complications. Implants were still well integrated 6–7 years post‐surgery and had no signs of reabsorption. The presence of MSC/progenitor cells in the implant helped vascularize the implant which is needed for its survival. Using these porous bioceramics along with culture expanded osteoprogenitor cells can be a potential method to treat critical‐sized long bone defects. Other types of scaffolds should be investigated in which initial support is provided for cells followed by slow reabsorption of the scaffold.
Allografts with rhBMP‐2
The use of recombinant BMP‐2 has shown some promise in humans. The widely accepted method of autogenous bone grafting for treatment of some fractures often comes with a high morbidity rate. It was shown that compared to this method, cancellous allograft with recombinant human BMP‐2 (rhBMP‐2) is just as safe and more practical in treatment of tibial fractures. This treatment resulted in better healing and improvement in skeletal function without need for secondary intervention63. Govender et al. looked at the efficacy of using rhBMP‐2 for treatment of open tibial fractures in 421 patients64. This study found that 44% of patients receiving this treatment experienced a reduction in the risk of failure and need for secondary interventions, as well as faster fracture healing.
Conclusions
Many factors play a role in promoting or regulating MSC differentiation into osteoblasts for new bone formation, such as, BMP, TGF‐β, SDF‐1, IGF‐1, Histone demethylase JMJD3, CDK1, Fucoidan, Runx2, TAZ, etc. Targeting these factors to accelerate bone healing via osteoblast formation may be a method to treat complex fractures and minimize consequences of a prolonged nonunion. This prospective study reviewed the potential use of bone regeneration therapies in clinical practice. Further research, however, is needed to compare them for efficacy.
Disclosure: The authors report no conflicts of interest. This project received no funding.
References
- 1. Ponte AL, Marais E, Gallay N, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells, 2007, 25: 1737–1745. [DOI] [PubMed] [Google Scholar]
- 2. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol, 2008, 8: 726–736. [DOI] [PubMed] [Google Scholar]
- 3. Kratchmarova I, Blagoev B, Haack‐Sorensen M, Kassem M, Mann M. Mechanism of divergent growth factor effects in mesenchymal stem cell differentiation. Science, 2005, 308: 1472–1477. [DOI] [PubMed] [Google Scholar]
- 4. Wynn RF, Hart CA, Corradi‐Perini C, et al. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood, 2004, 104: 2643–2645. [DOI] [PubMed] [Google Scholar]
- 5. Ito H. Chemokines in mesenchymal stem cell therapy for bone repair: a novel concept of recruiting mesenchymal stem cells and the possible cell sources. Mod Rheumatol, 2011, 21: 113–121. [DOI] [PubMed] [Google Scholar]
- 6. Cheung WH, Chin WC, Wei FY, Li G, Leung KS. Applications of exogenous mesenchymal stem cells and low intensity pulsed ultrasound enhance fracture healing in rat model. Ultrasound Med Biol, 2013, 39: 117–125. [DOI] [PubMed] [Google Scholar]
- 7. Kang JW, Park KD, Choi Y, et al. Biodistribution and in vivo efficacy of genetically modified human mesenchymal stem cells systemically transplanted into a mouse bone fracture model. Arch Pharm Res, 2013, 36: 1013–1022. [DOI] [PubMed] [Google Scholar]
- 8. Dimitriou R, Jones E, McGonagle D, Giannoudis PV. Bone regeneration: current concepts and future directions. BMC Med, 2011, 9: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abdallah BM, Kassem M. Human mesenchymal stem cells: from basic biology to clinical applications. Gene Ther, 2008, 15: 109–116. [DOI] [PubMed] [Google Scholar]
- 10. Dimitriou R, Tsiridis E, Giannoudis PV. Current concepts of molecular aspects of bone healing. Injury, 2005, 36: 1392–1404. [DOI] [PubMed] [Google Scholar]
- 11. Thompson Z, Miclau T, Hu D, Helms JA. A model for intramembranous ossification during fracture healing. J Orthop Res, 2002, 20: 1091–1098. [DOI] [PubMed] [Google Scholar]
- 12. Tosounidis T, Kontakis G, Nikolaou V, Papathanassopoulos A, Giannoudis PV. Fracture healing and bone repair: an update. Trauma, 2009, 11: 145–156. [Google Scholar]
- 13. Bielby R, Jones E, McGonagle D. The role of mesenchymal stem cells in maintenance and repair of bone. Injury, 2007, 38: 526–532. [DOI] [PubMed] [Google Scholar]
- 14. Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol, 2007, 213: 341–347. [DOI] [PubMed] [Google Scholar]
- 15. Jaiswal RK, Jaiswal N, Bruder SP, Mbalaviele G, Marshak DR, Pittenger MF. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen‐activated protein kinase. J Biol Chem, 2000, 275: 9645–9652. [DOI] [PubMed] [Google Scholar]
- 16. Cheng H, Jiang W, Phillips FM, et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J Bone Joint Surg Am, 2003, 85: 1544–1552. [DOI] [PubMed] [Google Scholar]
- 17. Bessa PC, Casal M, Reis RL. Bone morphogenetic proteins in tissue engineering: the road from the laboratory to the clinic, Part I (basic concepts). J Tissue Eng Regen Med, 2008, 2: 1–13. [DOI] [PubMed] [Google Scholar]
- 18. Pelissier P, Masquelet AC, Bareille R, Pelissier SM, Amedee J. Induced membranes secrete growth factors including vascular and osteoinductive factors and could stimulate bone regeneration. J Orthop Res, 2004, 22: 73–79. [DOI] [PubMed] [Google Scholar]
- 19. Simmons CA, Alsberg E, Hsiong S, Kim WJ, Mooney DJ. Dual growth factor delivery and controlled scaffold degradation enhance in vivo bone formation by transplanted bone marrow stromal cells. Bone, 2004, 35: 562–569. [DOI] [PubMed] [Google Scholar]
- 20. Ho CY, Sanghani A, Hua J, Coathup M, Kalia P, Blunn G. Mesenchymal stem cells with increased SDF‐1 expression enhanced fracture healing. Tissue Eng Part A, 2015, 21: 594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang F, Xu L, Xu L, Xu Q, Karsenty G, Chen CD. Histone demethylase JMJD3 is required for osteoblast differentiation in mice. Sci Rep, 2015, 5: 13418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ye L, Fan Z, Yu B, Chang J, et al. Histone demethylases KDM4B and KDM6B promotes osteogenic differentiation of human MSCs. Cell Stem Cell, 2012, 11: 50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koch H, Jadlowiec JA, Campbell PG. Insulin‐like growth factor‐i induces early osteoblast gene expression in human mesenchymal stem cells. Stem Cells Dev, 2005, 14: 621–631. [DOI] [PubMed] [Google Scholar]
- 24. Crane JL, Zhao L, Frye JS, Xian L, Qiu T, Cao X. IGF‐1 signaling is essential for differentiation of mesenchymal stem cells for peak bone mass. Bone Res, 2013, 28: 186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xian L, Wu X, Pang L, et al. Matrix IGF‐1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat Med, 2012, 18: 1095–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wei Y, Chen YH, Li LY, et al. CDK1‐dependent phosphorylation of EZH2 suppresses methylation of H3K27 and promotes osteogenic differentiation of human mesenchymal stem cells. Nat Cell Biol, 2011, 13: 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cho YS, Jung WK, Kim JA, Choi IW, Kim SK. Beneficial effects of fucoidan on osteoblastic MG‐63 cell differentiation. Food Chem, 2009, 116: 990–994. [Google Scholar]
- 28. Kim BS, Kang HJ, Park JY, Lee J. Fucoidan promotes osteoblast differentiation via JNK‐ and ERK‐dependent BMP2‐Smad 1/5/8 signaling in human mesenchymal stem cells. Exp Mol Med, 2015, 47: e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hosogane N, Huang Z, Rawlins BA, et al. Stromal derived factor‐1 regulates bone morphogenetic protein 2‐induced osteogenic differentiation of primary mesenchymal stem cells. Int J Biochem Cell Biol, 2010, 42: 1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Teicher BA, Fricker SP. CXCL12 (SDF‐1)/(CXCR4) pathway in cancer. Clin Cancer Res, 2010, 16: 2927–2931. [DOI] [PubMed] [Google Scholar]
- 31. Granero‐Moltó F, Weis JA, Miga MI, et al. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells, 2009, 27: 1887–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kitaori T, Ito H, Schwarz EM, et al. Stromal cell‐derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum, 2009, 60: 813–823. [DOI] [PubMed] [Google Scholar]
- 33. Komori T. Regulation of osteoblast differentiation by transcription factors. J Cell Biochem, 2006, 99: 1233–1239. [DOI] [PubMed] [Google Scholar]
- 34. Ding J, Ghali O, Lencel P, et al. TNF‐α and IL‐1β inhibit RUNX2 and collagen expression but increase alkaline phosphatase activity and mineralization in human mesenchymal stem cells. Life Sci, 2009, 84: 499–504. [DOI] [PubMed] [Google Scholar]
- 35. Franceschi RT, Xiao G. Regulation of the osteoblast‐specific transcription factor, Runx2: responsiveness to multiple signal transduction pathways. J Cell Biochem, 2003, 88: 446–454. [DOI] [PubMed] [Google Scholar]
- 36. Hong JH, Yaffe MB. TAZ: a β‐catenin‐like molecule that regulates mesenchymal stem cell differentiation. Cell Cycle, 2006, 5: 176–179. [DOI] [PubMed] [Google Scholar]
- 37. Hong JH, Hwang ES, McManus MT, et al. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science, 2005, 309: 1074–1078. [DOI] [PubMed] [Google Scholar]
- 38. Romano CL, Romano D, Logoluso N. Low‐intensity pulsed ultrasound for the treatment of bone delayed union or nonunion: a review. Ultrasound Med Biol, 2009, 35: 529–536. [DOI] [PubMed] [Google Scholar]
- 39. Wei FY, Leung KS, Li G, et al. Low intensity pulsed ultrasound enhanced mesenchymal stem cell recruitment through stromal derived factor‐1 signaling in fracture healing. PLoS One, 2014, 9: e106722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wazen RM, Currey JA, Guo H, Brunski JB, Helms JA, Nanci A. Micromotion‐induced strain fields influence early stages of repair at bone‐implant interfaces. Acta Biomater, 2013, 9: 6663–6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Weaver AS, Su YP, Begun DL, Miller JD, Alford AI, Goldstein SA. The effects of axial displacement on fracture callus morphology and MSC homing depend on the timing of application. Bone, 2010, 47: 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Toupadakis CA, Granick JL, Sagy M, et al. Mobilization of endogenous stem cell populations enhances fracture healing in a murine femoral fracture model. Cytotherapy, 2013, 15: 1136–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kumar S, Ponnazhagan S. Mobilization of bone marrow mesenchymal stem cells in vivo augments bone healing in a mouse model of segmental bone defect. Bone, 2012, 50: 1012–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Peterson B, Zhang J, Iglesias R, et al. Healing of critically sized femoral defects, using genetically modified mesenchymal stem cells from human adipose tissue. Tissue Eng, 2005, 11: 120–129. [DOI] [PubMed] [Google Scholar]
- 45. Hashimoto Y, Yoshida G, Toyoda H, Takaoka K. Generation of tendon‐to‐bone interface “enthesis” with use of recombinant BMP‐2 in a rabbit model. J Orthop Res, 2007, 25: 1415–1424. [DOI] [PubMed] [Google Scholar]
- 46. Dreger T, Watson JT, Akers W, et al. Intravenous application of CS271‐selected mesenchymal stem cells during fracture healing. J Orthop Trauma, 2014, 28: 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shen FH, Visger JM, Balian G, Hurwitz SR, Diduch DR. Systemically administered mesenchymal stromal cells transduced with insulin‐like growth factor‐i localize to a fracture site and potentiate healing. J Orthop Trauma, 2002, 16: 651–659. [DOI] [PubMed] [Google Scholar]
- 48. Hou R, Chen F, Yang Y, et al. Comparative study between coral‐mesenchymal stem cells‐rhBMP‐2 composite and auto‐bone‐graft in rabbit critical‐sized cranial defect model. J Biomed Mater Res A, 2007, 80: 85–93. [DOI] [PubMed] [Google Scholar]
- 49. Nair MB, Varma HK, Menon KV, Shenoy SJ, John A. Tissue regeneration and repair of goat segmental femur defect with bioactive triphasic ceramic‐coated hydroxyapatite scaffold. J Biomed Mater Res A, 2009, 91: 855–865. [DOI] [PubMed] [Google Scholar]
- 50. Mankani MH, Kuznetsov SA, Shannon B, et al. Canine cranial reconstruction using autologous bone marrow stromal cells. Am J Pathol, 2006, 168: 542–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hsu YH, Chiu YS, Chen WY, et al. Anti‐IL‐20 monoclonal antibody promotes bone fracture healing through regulating IL‐20‐mediated osteoblastogenesis. Sci Rep, 2016, 6: 24339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kushibiki T, Tu Y, Abu‐Yousif AO, Hasan T. Photodynamic activation as a molecular switch to promote osteoblast cell differentiation via AP‐1 activation. Sci Rep, 2015, 5: 13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kushibiki T, Awazu K. Blue laser irradiation enhances extracellular calcification of primary mesenchymal stem cells. Photomed Laser Surg, 2009, 27: 493–498. [DOI] [PubMed] [Google Scholar]
- 54. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 2006, 126: 663–676. [DOI] [PubMed] [Google Scholar]
- 55. Yu J, Vodyanik MA, Smuga‐Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science, 2007, 318: 1917–1920. [DOI] [PubMed] [Google Scholar]
- 56. Faast R, Harrison SJ, Beebe LF, McIlfatrick SM, Ashman RJ, Nottle MB. Use of adult mesenchymal stem cells isolated from bone marrow and blood for somatic cell nuclear transfer in pigs. Cloning Stem Cells, 2006, 8: 166–173. [DOI] [PubMed] [Google Scholar]
- 57. Li Z, He X, Chen L, et al. Bone marrow mesenchymal stem cells are an attractive donor cell type for production of cloned pigs as well as genetically modified cloned pigs by somatic cell nuclear transfer. Cell Reprogram, 2013, 15: 459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kim SJ, Shin YW, Yang KH, et al. A multi‐center randomizes clinical study to compare the effect and safety of autologous cultured osteoblast (OssronTM) injection to treat fractures. BMC Musculoskelet Disord, 2009, 10: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gross JB, Diligent J, Bensoussan D, Galois L, Stoltz JF, Mainard D. Percutaneous autologous bone marrow injection for treatment of delayed and non‐union of long bone: a retrospective study of 45 cases. Biomed Mater Eng, 2015, 25: 187–197. [DOI] [PubMed] [Google Scholar]
- 60. Hernigou P, Poignard A, Beaujean F, Rouard H. Percutaneous autologous bone‐marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am, 2005, 87: 1430–1437. [DOI] [PubMed] [Google Scholar]
- 61. Kitoh H, Kawasumi M, Kaneko H, Ishiguro N. Differential effects of culture‐expanded bone marrow cells on the regeneration of bone between the femoral and the tibial lengthenings. J Pediatr Orthop, 2009, 29: 643–649. [DOI] [PubMed] [Google Scholar]
- 62. Marcacci M, Kon E, Moukhachev V, et al. Stem cells associated with marcoporous bioceramics for long bone repair: 6‐ to 7‐ year outcome of a pilot clinical study. Tissue Eng, 2007, 13: 947–955. [DOI] [PubMed] [Google Scholar]
- 63. Jones AL, Bucholz RW, Bosse MJ, et al. Recombinant human BMP‐2 and allograft compared with autogenous bone graft for reconstruction of diaphyseal tibial fractures with cortical defects. A randomized, controlled trial. J Bone Joint Surg Am, 2006, 88: 1431–1441. [DOI] [PubMed] [Google Scholar]
- 64. Govender S, Csimma C, Genant HK, et al. Recombinant human bone morphogenetic protein‐2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am, 2002, 84: 2123–2134. [DOI] [PubMed] [Google Scholar]


