Abstract
Objective
Improvements in cancer treatment have resulted in an increased number of patients with metastatic spinal cord compression (MSCC). Because patients with MSCC often have a limited expected survival time, maintenance of a high functional level and quality of life are important. However, there is limited information about health‐related quality of life (HRQoL) in patients with MSCC. The aim of this study was to examine the feasibility of routine assessment of HRQoL based on the Euroqol‐5 dimensions (EQ‐5D) questionnaire in a cohort of patients consecutively admitted for evaluation of acute symptoms of MSCC.
Methods
From 1 January to 31 December 2011, 544 patients diagnosed with acute symptoms of MSCC were consecutively enrolled in a cohort study. All patients were evaluated through a centralized referral system at one treatment facility. Data were prospectively registered, the variables age, sex, primary oncologic diagnosis, Tokuhashi Revised score, EQ‐5D score and treatment modality being recorded on admission. The study patients were treated conservatively with radiotherapy alone or with surgery and subsequent radiotherapy. The EQ‐5D questionnaire was administered on admission (baseline) and 6, 12, 26 and 52 weeks after admission. Response rates, completion rates and HRQoL scores were analyzed by relevant subgroups. Response rates were based on all questionnaires returned regardless of whether or not they had been completed, whereas completion rates were based on fully completed questionnaires (i.e., containing responses to all five questions.
Results
The mean age was 65 years (range, 20–95 years); 57% of the patients were men. The overall response rate to the Euroqol‐5 dimensions (EQ‐5D) questionnaires was 84% and the overall completion rate 72%. At baseline, mean EQ‐5D scores were significantly lower for patients treated with surgery and subsequent radiotherapy 0.28 (95% CI, 0.19–0.36) than for those treated with radiotherapy alone 0.42 (95% CI, 0.38–0.46). At the one‐year follow‐up, the mean EQ‐5D scores had improved to 0.71 (95% CI, 0.64–0.77) for patients treated with surgery and subsequent radiotherapy and 0.63 (95% CI, 0.56–0.70) for patients treated with radiotherapy alone.
Conclusions
Measurement of HRQoL in patients consecutively admitted for evaluation of acute symptoms of MSCC is feasible and detects significant changes over time between treatment modalities and different strata of expected survival.
Keywords: EQ‐5D, Health‐related quality of life, Spinal metastases, Spine surgery
Introduction
Cancer treatment has continued to improve, resulting in an increased survival among cancer patients1, 2, 3. Consequently, there has been an increase in the prevalence of patients with spinal metastases and hence with metastatic spinal cord compression (MSCC). There are very limited data regarding health‐related quality of life (HRQoL) in such patients; however, this is becoming increasingly relevant because HRQoL is used for selecting optimal treatment and for prioritization of treatment modalities by decision‐makers4, 5.
Because a diagnosis of MSCC usually implies a life expectancy of less than 6, the primary aim of treatment is generally to improve the quality of life rather than to improve survival. Most patients with MSCC are offered radiotherapy only, or surgical treatment followed by radiotherapy with the aim of reducing symptoms like pain and neurologic impairment6. Previous randomized studies and systematic reviews have predominantly estimated the effectiveness of the treatment by examining neurologic status and pain7, 8. Since the goal of treatment is often to improve the quality of life, it is important to assess HRQoL as a measure of the treatment effect. Karnofsky and colleagues were the first to describe a clinical evaluation with well‐being as an endpoint; this was an early version of what was later defined as quality of life9. The precise definition of quality of life has been debated; however, it can be defined as a person's subjective, personal feeling and experience of well‐being, including physical, emotional, and social well‐being10. Quality of life measurements have many potential applications in aiding routine clinical practice, including facilitating communication between the practitioner and patient, help in prioritizing within the healthcare system, identifying patients' preferences and monitoring changes or responses to a given treatment.
The economic evaluation of new healthcare technologies is a critical element in allocation of health resources. These economic evaluations are based on analyses of costs and outcomes attributable to the treatment. Generic health profile classifications like the Euroqol‐5 dimensions (EQ‐5D) allow determination of a single index of health status11. The EQ‐5D comprises five dimensions (mobility, self‐care, usual activities, pain/discomfort and anxiety/depression), each with three levels (no problems, some problems, extreme problems/unable), thus generating 243 possible health states (Fig. 1).
Figure 1.
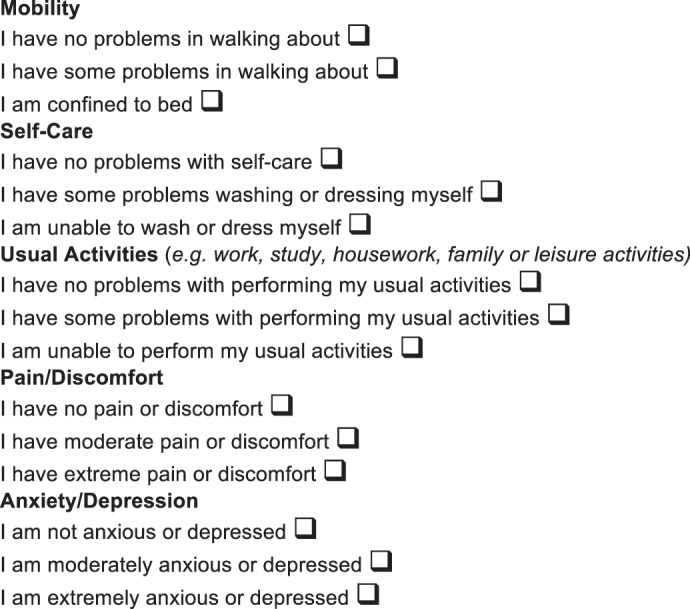
The EuroQol EQ‐5D questionnaire.
A single index score can be derived for each of these health states by applying preference weights obtained from the general population. Danish EQ‐5D preference weights have previously been generated using the Time Trade‐Off valuation technique in a large sample from the Danish general population11.
Such scores provides an opportunity for measurement of the benefits of treatment (utility) and can act as a single “currency” for assessing the value for money generated by treatments both across and within the same disease. Measures of HRQoL could be valuable in cost utility analyses examining the cost of both surgical and non‐surgical treatment of patients with MSCC; for this reason, measures of HRQoL are sometimes requested4, 12.
The EQ‐5D is a brief and easy‐to‐use patient‐based questionnaire developed for reporting generic outcomes across different health problems. Previous studies have shown that the EQ‐5D questionnaire is better able to capture relevant changes at a disease‐specific level than measures such as the Oswestry Disability Index13. This questionnaire may therefore also be suitable for assessing HRQoL of patients with MSCC treated with or without surgery. However, it could be speculated that late‐stage cancer patients may be unable to complete such questionnaires because of their general health. The feasibility of obtaining routine measures of HRQoL with the EQ‐5D in this group of patients therefore remains to be clarified14, 15, 16.
To our knowledge, only few studies have reported HRQoL and utility scores when assessing effects of treatment in patients with MSCC or with solely spinal metastases15, 16, 17, 18.
Therefore, the overall aim of this study was to measure HRQoL in a large consecutive cohort of patients with acute symptoms of MSCC admitted to one center for evaluation.
Specifically we aimed: (i) to examine whether it is feasible to obtain routine measurements of HRQoL based on the EQ‐5D in a cohort of consecutive patients with acute symptoms of MSCC admitted for evaluation; (ii) to assess the HRQoL in such a cohort; and (iii) to use HRQoL to assess effects of treatment.
Materials and Methods
All patients from Eastern Denmark with acute symptoms of MSCC are evaluated at the university hospital of Copenhagen (Rigshospitalet) through a centralized referral system. From 1 January to 31 December 2011, 622 patients with acute symptoms of MSCC were admitted for evaluation. Of these, 78 patients did not have MSCC; accordingly, 544 patients were enrolled in this study. Patients entered the cohort on the day of admission and were followed until 1 year after admission or the time of death; whichever came first.
The on‐call oncologist evaluated all patients on admission and a treatment strategy was decided within 24 h. Diagnoses were based on MRI combined with clinical symptoms of back pain and/or neurologic impairment.
Treatment Regimens
Patients were offered surgical treatment in combination with radiotherapy or radiotherapy alone in those with short estimated survival. Patients with an expected survival of less than 3 months were generally not offered surgical treatment. The indications for surgical treatment weres severe pain and/or neurological impairment caused by spinal cord compression or spinal instability. Most surgically treated patients were offered posterior decompression/laminectomy on relevant spinal levels depending on neurological symptoms. If spinal stabilization was needed after decompression, patients underwent posterior instrumentation with pedicle screws and titanium rods. Instrumentation was performed two or three levels above and below each level with metastatic disease. Postoperative radiotherapy commenced between 10 and 21 days after decompressive surgery. The radiation target included the entire affected vertebral body and the vertebral arch at the operated level of the vertebral column. Patients receiving postoperative radiotherapy were planned to receive 30 Gy in 10 fractions (i.e., 3 Gy/fraction). Patients who were not candidates for surgical treatment received short‐course radiotherapy regimes with less than 10 fractions (in most cases five or fewer fractions).
Data Analysis
Data were prospectively registered and the variables age, sex, primary oncologic diagnosis, Tokuhashi Revised score19 and treatment modality recorded on admission (baseline). Patients were asked to self‐report their HRQoL using the Danish version of the EQ‐5D 3‐level questionnaire on admission and after 6, 12, 26 and 52 weeks20. The EQ‐5D questionnaire was sent to the patients by regular mail 1 week before the time of follow‐up. Patients who did not return the questionnaire at the time of follow‐up were contacted until 2 weeks after follow‐up for an assisted interview.
Response and completion rates and HRQoL scores were analyzed for relevant subgroups, patients being grouped according to treatment and survival. Division into survival groups was in accordance with the Tokuhashi Revised score prognostic groups; <6 months survival, 6–12 months survival and ≥12 months survival. Response rates were based on all questionnaires returned regardless of whether or not they had been completed. Completion rates were based on fully completed questionnaires, that is, responses to all five questions. HRQoL scores were based on an EQ‐5D scoring algorithm derived from a Danish national valuation study using the Time Trade‐Off technique11, 21.
Parametric statistics were applied for the reporting of means and confidence intervals (CIs). Delta scores were calculated as the difference between baseline and a given follow‐up score. Probability values less than 0.05 were considered statistically significant. All analyses were performed using the stata software package version 12.1.
Ethics and Data Protection
This study was conducted in accordance with the ethical principles for medical research published by the World Medical Association in the Helsinki Declaration. According to the National Committee on Health Research Ethics, this study did not require ethical approval but only approval for data collection. Permission to register the variables in this study has been obtained through the Danish Data Protection Agency22. Official approval was obtained for usinge the EQ‐5D for research purposes23.
Results
There were 544 patients eligible for inclusion; 94 were excluded in the final analysis. The reasons for exclusion included residence outside Denmark, dementia, language problems and refusal to fill out the questionnaire. These patients were registered with all study variables except for EQ‐5D scores.
Significantly more men than women were enrolled. The average age at referral was 65 years (SD, 11; range, 20–95 years). The most frequent primary oncologic diagnoses were lung, breast and prostate cancer. Tokuhashi Revised scores were significantly higher in the 19% of patients who underwent surgery than in those treated with radiotherapy alone (9.7 vs. 8.8; P = 0.014). The patients excluded in the study (94 cases) did not differ from the included patients regarding subsequent treatment and sex, but did differ significantly regarding mean age, mean survival and mean Tokuhashi score (Table 1).
Table 1.
Characteristics of consecutive patients diagnosed with metastatic spinal cord compression
| Treatment groups | Subgroups | Cases | Men (cases [%]) | Age (years, mean [SD]) | Tokuhashi score (mean [CI]) | Survival days (median [CI]) |
|---|---|---|---|---|---|---|
| Patients who agreed to participate (450 cases) | SR | 69 | 36 (52) | 64 (10) | 9.7 (8.9–10.2) | 323 (145–365) |
| RA | 381 | 224 (59) | 66 (11) | 8.8 (8.5–9.1) | 124 (102–145) | |
| Patients who did not agree to participate (94 cases) | SR | 18 | 12 (67) | 62 (11) | 9.4 (7.9–11.0) | 150 (52–365) |
| RA | 76 | 45 (59) | 70 (12) | 7.7 (7.0–8.3) | 25 (18–31) | |
| All patients (544 cases) | 544 | 317 (58) | 66 (10) | 9.0 (8.7–9.2) | 108 (90–127) | |
| Statistic value | P = 0.953 | P = 0.262 | P = 0.031 | P = 0.009 | P < 0.000 |
RA, subgroup of patients treated with radiotherapy alone; SR, subgroup of patients treated with surgery and radiotherapy. P values are for differences between patients who did and patients who did not agree to participate in the survey
The overall average response rate for all included patients was 84% with no significant difference in response rates between surgically treated (81%) and non‐surgically treated patients (85%). The overall average completion rate for the included patients was 72%. There was no significant difference in completion rate between surgically (73%) and non‐surgically treated (71%) patients. Throughout the study period there were no significant differences in response or completion rates (Tables 2, 3).
Table 2.
Response and completion rates for patients responding at individual time points (cases [%])
| Response at individual time points | Patients alive | Subgroup treated with surgery | Subgroup treated with radiotherapy alone | ||
|---|---|---|---|---|---|
| Response | Completion | Response | Completion | ||
| Baseline | 544 | 74 (86) | 59 (69) | 406 (89) | 311 (68) |
| 6 weeks | 380 | 49 (75) | 47 (72) | 261 (86) | 215 (70) |
| 12 weeks | 305 | 42 (78) | 41 (76) | 201 (84) | 182 (76) |
| 26 weeks | 200 | 32 (86) | 29 (78) | 127 (83) | 118 (77) |
| 52 weeks | 131 | 23 (77) | 23 (77) | 65 (70) | 64 (69) |
| Average rates | (81) | (73) | (85) | (71) | |
Table 3.
Response and completion rates for patients both responding at baseline and follow‐up (cases [%])
| Patients both responding at baseline and follow up | Patients alive | Subgroup treated with surgery | Subgroup treated with radiotherapy alone | ||
|---|---|---|---|---|---|
| Response | Completion | Response | Completion | ||
| Baseline & 6 weeks | 380 | 47 (72) | 41 (61) | 234 (61) | 171 (56) |
| Baseline &12 weeks | 305 | 41 (76) | 33 (61) | 180 (75) | 142 (59) |
| Baseline & 26 weeks | 200 | 30 (81) | 25 (67) | 115 (75) | 91 (59) |
| Baseline & 52 weeks | 131 | 21 (70) | 20 (67) | 56 (58) | 50 (54) |
| Average rates | (73) | (63) | (74) | (57) | |
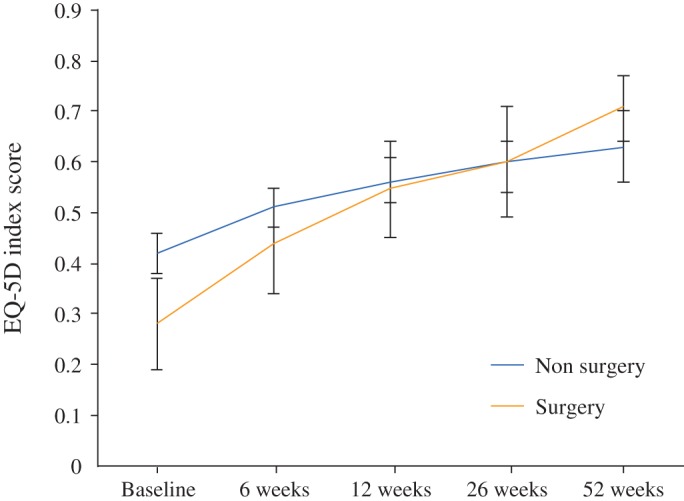
The mean EQ‐5D score increased at each follow‐up (Table 4). Patients who underwent surgery had significantly lower EQ‐5D scores at baseline (0.28; 95% CI, 0.19–0.36) than patients treated with radiotherapy alone (0.42; 95% CI, 0.38–0.46). However; on follow‐up patients who underwent surgery had a considerably higher EQ‐5D score (0.71; 95% CI, 0.64–0.71) than patients treated with radiotherapy alone (0.63; 95% CI, 0.56–0.70; Table 4 and Fig. 2).
Table 4.
Mean EQ‐5D scores at baseline and follow‐up with 95% confidence intervals
| Subgroups | Baseline | 6 weeks | 12 weeks | 26 weeks | 52 weeks |
|---|---|---|---|---|---|
| SR | 0.28 (0.19–0.36) | 0.44 (0.34–0.55) | 0.55 (0.45–0.64) | 0.60 (0.49–0.71) | 0.71 (0.64–0.77) |
| RA | 0.42 (0.38–0.46) | 0.51 (0.47–0.55) | 0.56 (0.52–0.64) | 0.60 (0.56–0.70) | 0.63 (0.56–0.70) |
| All patients | 0.40 (0.36–0.43) | 0.50 (0.46–0.53) | 0.56 (0.52–0.60) | 0.61 (0.55–0.64) | 0.65 (0.60–0.71) |
RA, subgroup of patients treated with radiotherapy alone; SR, subgroup of patients treated with surgery and radiotherapy
Figure 2.
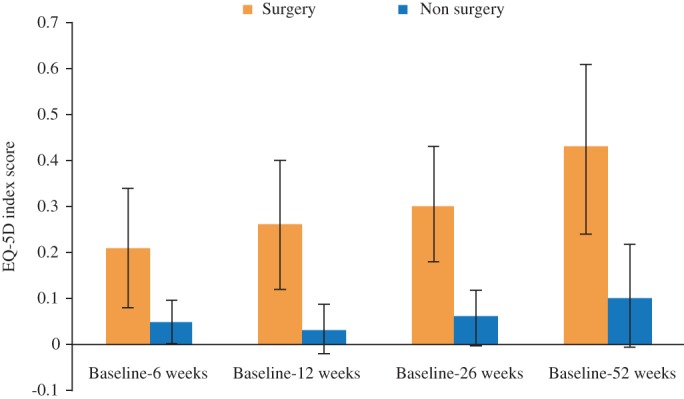
Mean EQ‐5D scores with 95% CIs for patient treated with surgery or non‐surgery at baseline and follow‐up.
Patients who underwent surgery were grouped according to survival. Those who survived at least 52 weeks had a lower mean EQ‐5D score at baseline (0.32; 95% CI, 0.20–0.44) than those with shorter survival, in whom the EQ‐5D was 0.71 (95% CI, 0.64–0.77) at the last follow‐up. Patients who underwent surgery and survived less than 6 months had the lowest mean EQ‐5D score at baseline (0.21; 95% CI, 0.08–0.35); however, the mean EQ‐5D score in this group declined at each follow‐up and was 0.12 (95% CI, −0.09 to 0.34) at 12 weeks follow‐up (Fig. 3).
Figure 3.
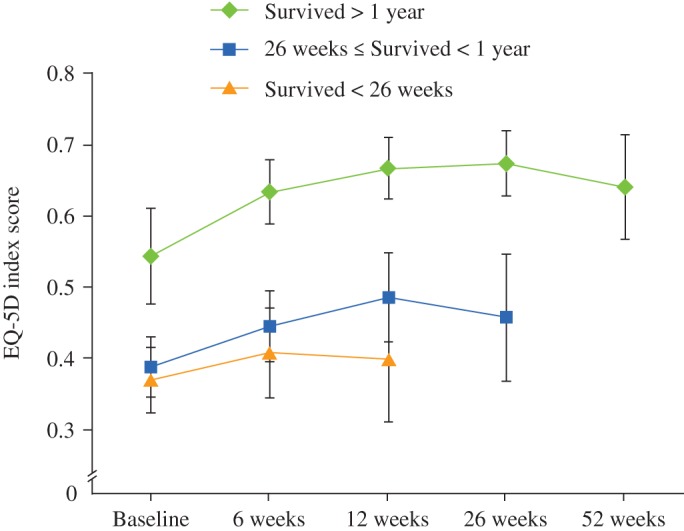
Mean EQ5D scores with 95% CIs for different survival strata of surgically treated patients.
Patients treated with radiotherapy alone were also grouped according to survival status. Those who survived more than 1 year had the highest mean EQ‐5D score at baseline (0.54; 95% CI, 0.48–0.61). The mean EQ‐5D score increased at 6, 12 and 26 weeks follow‐up and then decreased towards the end of follow‐up. At 52 weeks follow‐up the mean EQ‐5D score was 0.64 (95% CI, 0.56–0.71) for patients who were still alive (Fig. 4). Patients treated with radiotherapy alone who survived less than 26 weeks had a mean EQ‐5D score at baseline of 0.36 (95% CI, 0.32–0.41). The mean EQ‐5D score increased to 0.40 (95% CI, 0.32–0.49) at 12 weeks follow‐up; however, this difference was not statistically significant (Fig. 4).
Figure 4.
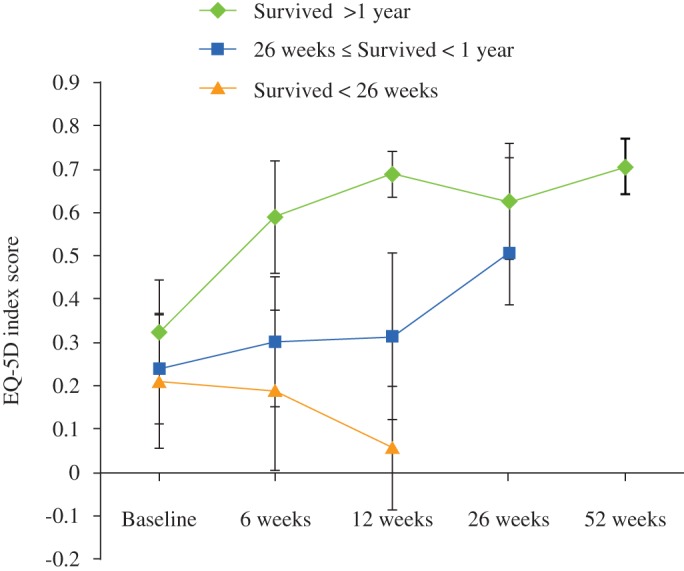
Mean EQ‐5D scores with 95% CIs for different survival strata of patients treated with radiotherapy alone.
The “gain” in mean EQ‐5D score from baseline to each follow‐up was statistically significant for patients who underwent surgery. Patients treated with radiotherapy alone showed no statistically significant “gain” in mean EQ‐5D score from baseline to each follow‐up (Fig. 5).
Figure 5.
Mean changes in EQ‐5D scores from baseline to follow‐up with 95% CIs.
Discussion
The present study, based on 544 consecutive patients admitted with MSCC, shows that it is feasible to routinely measure HRQoL using the EQ‐5D questionnaire. Furthermore, the study shows that both surgical and non‐surgical treatment of MSCC results in significant improvements in HRQoL. Patients who underwent surgery had a low mean EQ‐5D score at baseline, which increased at each follow‐up if they survived more than 6 months and decreased at each follow‐up if they survived less than 6 months. Patients treated with radiotherapy alone had a more moderate increase in mean EQ‐5D score for all survival states.
HRQoL in Patients with MSCC
It has been stated that HRQoL can be difficult to measure in this group of patients and that generic health profiles would be valuable4, 5, 12, 14, 15, 16, 24. The present study suggests that an assessment of HRQoL can be achieved with the EQ‐5D and that the response and completion rates are acceptable. In previous studies, the response rates have been between 65% and 79%, these percentages having been calculated as percentage participants among the patients who had already agreed to participate in the studies17, 18, 25. Given that both cancer treatment in general and surgical techniques are constantly improving, the present study contributes valuable data, being based on a large, consecutive, prospective, 1‐year cohort from a major spine center and accordingly reflecting current HRQoL in patients with acute symptoms of MSCC.
It is important to recognize certain issues when measuring utility scores and using them to calculate QALY's in terminal cancer patients26. One such issue is that a person's evaluation of their state of health tends to depend on its current level. A very slight improvement in mobility may seem negligible to a fully functioning and healthy individual, but may be very important and meaningful for a late‐stage cancer patient who is mostly bedridden27. There are also important differences in the ways in which patients adjust their activities to adapt to states of ill health over time and lessen the impact of their disability. Thus, some of the improvement in HRQoL reported by patients with the longest survival may have been influenced by the degree of adaptation28. However, this did not seem to have a great impact on our findings because the utility scores of patients treated with radiotherapy declined towards the end of follow‐up (Fig. 3).
Few other studies have reported the quality of life among patients with MSCC undergoing surgery15, 18. Falicov et al. showed that HRQoL improved after surgery at each follow‐up from 6 weeks to 1 year17. In the present study, HRQoL improved significantly at each follow up in patients undergoing surgery (Fig. 3). However, when we analyzed mean HRQoL at each follow up in the individual survival groups, their mean HRQoL only improved in patients who lived longer than 6 months after surgery (Fig. 4).
Clinical guidelines do generally not recommend surgery for patients with an expected survival of less than 3 months because the associated benefit is not always commensurate with the risk of complications8, 29. The results of the present study provide no evidence against treating survival as a crucial element when evaluating patients with spinal metastases. However, our results may indicate that clinical guidelines should specify that patients should an even longer expected survival before offering surgery: we identified no overall improvement in HRQoL in patients with observed survivals of less than six months treated surgically (Fig. 5).
The HRQoL, measured with the EQ‐5D questionnaire, has been assessed in a study comparing a single fraction versus multiple fractions of radiotherapy in patients with symptomatic spinal metastases18. In line with the results of the present study, those authors found that radiotherapy improves quality of life after treatment, but that the improvement subsequently declines18.
Limitations of the Present Study
A limitation of this study is the response rate at baseline and during follow‐up that was not caused by death. This means that at baseline the mean EQ‐5D scores would likely have been lower if all patients had completed the EQ‐5D questionnaires: patients treated with radiotherapy who were not willing to participate in the study had a significantly shorter survival and significantly lower Tokuhashi score than those who did participate (Table 1). In contrast, patients who underwent surgery had a significantly lower mean EQ‐5D score at baseline than patients treated with radiotherapy; however, the response rates did not differ significantly between these two groups (Tables 2, 3). It is thus unclear whether the patients' health state may have affected their overall response rates at baseline. We consider it likely that most of the loss to follow‐up was attributable to the mortality and morbidity associated with late‐stage cancer. However, we have no firm data to support the contention that the patients with the worst health state were the ones who were unwilling or unable to complete the questionnaire14, 16. This could have biased the results such that the EQ‐5D scores presented in this study are higher than they would have been if no patients had been lost to follow‐up. Furthermore, it should be noted that when patients with poor health die during a study, the average HRQoL of the remaining patients increases, other things being equal. Thus, the EQ‐5D scores gradually improve as the patients with the worst health die. We have tried to make this clear by dividing the patients into survival groups, but we cannot rule out bias due to different health states within the groups.
Conclusions
Despite these limitations, this study shows that it is feasible to routinely measure HRQoL with the EQ‐5D in patients with acute symptoms of MSCC admitted for evaluation. Further, HRQoL varies substantially over time among patients treated for acute symptoms of MSCC. We recommend that the utility scores from the present study be included in future health economic analysis on patients treated for MSCC.
Acknowledgements
The authors would like to thank the staff at the Trauma Centre at Rigshospitalet for their invaluable support.
Disclosure: This study was part of the CESpine project (www.cespine.org), which is supported by The Danish Strategic Research Council #2142–08‐0017. The authors have no conflicts of interest to declare.
References
- 1. Delank KS, Wendtner C, Eich HT, Eysel P. The treatment of spinal metastases. Dtsch Arztebl Int, 2011, 108: 71–79; quiz 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Morgen SS, Lund‐Andersen C, Larsen CF, Engelholm SA, Dahl B. Prognosis in patients with symptomatic metastatic spinal cord compression: survival in different cancer diagnosis in a cohort of 2321 patients. Spine (Phila Pa 1976), 2013, 38: 1362–1367. [DOI] [PubMed] [Google Scholar]
- 3. Shaw AT, Yeap BY, Solomon BJ, et al. Effect of crizotinib on overall survival in patients with advanced non‐small‐cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol, 2011, 12: 1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Choi D, Crockard A, Bunger C, et al. Review of metastatic spine tumour classification and indications for surgery: the consensus statement of the Global Spine Tumour Study Group. Eur Spine J, 2010, 19: 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Choi D, Morris S, Crockard A, et al. Assessment of quality of life after surgery for spinal metastases: position statement of the Global Spine Tumour Study Group. World Neurosurg, 2013, 80: e175–e179. [DOI] [PubMed] [Google Scholar]
- 6. Morgen SS, Nielsen DH, Larsen CF, Søgaard R, Engelholm SA, Dahl B. Moderate precision of prognostic scoring systems in a consecutive, prospective cohort of 544 patients with metastatic spinal cord compression. J Cancer Res Clin Oncol, 2014, 140: 2059–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ibrahim A, Crockard A, Antonietti P, et al. Does spinal surgery improve the quality of life for those with extradural (spinal) osseous metastases? An international multicenter prospective observational study of 223 patients. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2007. J Neurosurg Spine, 2008, 8: 271–278. [DOI] [PubMed] [Google Scholar]
- 8. Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet, 2005, 366: 643–648. [DOI] [PubMed] [Google Scholar]
- 9. Karnofsky DA, Burchenal J. The clinical evaluation of chemotherapeutic agents in cancer In: MacLeod CM, ed. Evaluation of Chemotherapeutic Agents. Columbia: Columbia University Press, 1949; 191–205. [Google Scholar]
- 10. Felce D, Perry J. Quality of life: its definition and measurement. Res Dev Disabil, 1995, 16: 51–74. [DOI] [PubMed] [Google Scholar]
- 11. Wittrup‐Jensen KU, Lauridsen J, Gudex C, Pedersen KM. Generation of a Danish TTO value set for EQ‐5D health state. Scand J Public Health, 2009, 37: 459–466. [DOI] [PubMed] [Google Scholar]
- 12. Matza LS, Chung K, Van Brunt K, et al. Health state utilities for skeletal‐related events secondary to bone metastases. Eur J Health Econ, 2014, 15: 7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mueller B, Carreon LY, Glassman SD. Comparison of the EuroQOL‐5D with the Oswestry disability index, back and leg pain scores in patients with degenerative lumbar spine pathology. Spine (Phila Pa 1976), 2013, 38: 757–761. [DOI] [PubMed] [Google Scholar]
- 14. Ballatori E. Unsolved problems in evaluating the quality of life of cancer patients. Ann Oncol, 2001, 12 (Suppl. 3): S11–S13. [DOI] [PubMed] [Google Scholar]
- 15. Furlan JC, Chan KK, Sandoval GA, et al. The combined use of surgery and radiotherapy to treat patients with epidural cord compression due to metastatic disease: a cost‐utility analysis. Neuro Oncol, 2012, 14: 631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bernhard J, Cella DF, Coates AS, et al. Missing quality of life data in cancer clinical trials: serious problems and challenges. Stat Med, 1998, 17: 517–532. [DOI] [PubMed] [Google Scholar]
- 17. Falicov A, Fisher CG, Sparkes J, Boyd MC, Wing PC, Dvorak MF. Impact of surgical intervention on quality of life in patients with spinal metastases. Spine (Phila Pa 1976), 2006, 31: 2849–2856. [DOI] [PubMed] [Google Scholar]
- 18. van den Hout WB, van der Linden YM, Steenland E, et al. Single‐ versus multiple‐fraction radiotherapy in patients with painful bone metastases: cost‐utility analysis based on a randomized trial. J Natl Cancer Inst, 2003, 95: 222–229. [DOI] [PubMed] [Google Scholar]
- 19. Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976), 2005, 30: 2186–2191. [DOI] [PubMed] [Google Scholar]
- 20. EuroQol Group . EuroQol‐‐a new facility for the measurement of health‐related quality of life. Health Policy, 1990, 16: 199–208. [DOI] [PubMed] [Google Scholar]
- 21. Sørensen J, Davidsen M, Gudex C, Pedersen KM, Brønnum‐Hansen H. Danish EQ‐5D population norms. Scand J Public Health, 2009, 37: 467–474. [DOI] [PubMed] [Google Scholar]
- 22. Datatilsynet . Introduction to the Danish Data Protection Agency Available from: www.datatilsynet.dk (accessed 10 May 2010).
- 23. EQ‐5D . Home page. Available from: http://www.euroqol.org/ (accessed 10 November 2010).
- 24. Kim S, Jo M, Kim H, Ahn J. Mapping EORTC QLQ‐C30 onto EQ‐5D for the assessment of cancer patients. Health Qual Life Outcomes, 2012, 10: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee CF, Luo N, Ng R, et al. Comparison of the measurement properties between a short and generic instrument, the 5‐level EuroQoL Group's 5‐dimension (EQ‐5D‐5L) questionnaire, and a longer and disease‐specific instrument, the Functional Assessment of Cancer Therapy‐Breast (FACT‐B), in Asian breast cancer patients. Qual Life Res, 2013, 22: 1745–1751. [DOI] [PubMed] [Google Scholar]
- 26. Garau M, Shah KK, Mason AR, Wang Q, Towse A, Drummond MF. Using QALYs in cancer: a review of the methodological limitations. Pharmacoeconomics, 2011, 29: 673–685. [DOI] [PubMed] [Google Scholar]
- 27. Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk. Econometrica, 1979, 47: 263–292. [Google Scholar]
- 28. Dolan P, Kahneman D. Interpretations of utility and their implications for the valuation of health. Econ J, 2007, 118: 215–234. [Google Scholar]
- 29. Sciubba DM, Gokaslan ZL. Diagnosis and management of metastatic spine disease. Surg Oncol, 2006, 15: 141–151. [DOI] [PubMed] [Google Scholar]


