Abstract
Objective
To investigate the six degrees of freedom (6DOF) kinematics of anterior cruciate ligament (ACL) deficient knees during gait and to explore the clinical significance of a novel knee joint stability assessment system (Opti_Knee, Innomotion, Shanghai, China) in comparison with imaging and arthroscopic examination.
Methods
Three subjects diagnosed with ACL deficient knees on the basis of preoperative MRI and CT findings were subjected to treadmill gait analysis. Motion of both knees in 6DOF was measured and analyzed with an optical joint kinematics measurement system. Arthroscopic examination, the gold standard, was performed to confirm the final diagnosis and the clinical diagnosis of ACL deficiency by imaging and motion marker techniques compared with this gold standard.
Results
Only two of the three subjects diagnosed with ACL deficiency by imaging techniques were later confirmed to have this condition by arthroscopic examination; the third was found to have an intact ACL. When the kinematics of their injured and contralateral knees were compared, abnormalities were found in the two subjects confirmed by arthroscopy to be ACL deficient However, no kinematic difference between the two knees was found in the ACL intact subject.
Conclusions
Opti_Knee (Innomotion) can detect abnormal kinematics in ACL deficient knees and thus provides an effective way of assisting the diagnosis of this condition and has potential for clinical application.
Keywords: Anterior cruciate ligament, Gait analysis, Kinematics, Knee
Introduction
Epidemiological studies have shown that anterior cruciate ligament (ACL) injuries total between 100,000 and 200,000 yearly in the USA, making it the most common ligamentous injury in that country.1 This injury typically leads in the short term to decreased activity; unsatisfactory knee function and poor knee‐related quality of life (because of joint instability).2 ACL injuries are also associated with an increased risk of osteoarthritis in the knee.3
There are many approaches to diagnosing ACL injuries and assessing the stability of knee joints. The Lachman test, which has been found to have a sensitivity of 85% and a specificity of 94% for ACL deficiency,4 is the most frequently used physical examination for establishing a diagnosis of ACL injury.5 The KT 1000/KT 2000 arthrometer (MEDmetric, San Diego, CA, USA) has also been used to quantitatively measure the anterior stability of the knee.6, 7 Its sensitivity reportedly ranges from 89% to 92% and its specificity is about 95%.8 At present, MR imaging is routinely performed to confirm a pre‐operative diagnosis of an ACL tear9; its sensitivity is 90%–96% and specificity 85%–99% according to various publications.9, 10 However, the accuracy in the diagnosis of ACL tear may be influenced by the imaging equipment, techniques used and radiologists’ experience and training.10, 11, 12 Moreover, in some cases, parts of ligaments are poorly imaged between consecutive MR slices, which can ultimately result in unnecessary surgery.13 Therefore, the decision to undertake ligament reconstruction should not rely entirely on imaging techniques.9 Thus, arthroscopic examination, which is invasive, still plays a crucial role in assessing ligamentous injuries in current clinical practice.
In this context and because ACL plays a critical role in the biomechanics of the knee, numerous researchers have used biomechanical techniques to investigate the kinetic and kinematic alterations associated with ACL deficiency. Cadaveric tests that have commonly been used to measure tibial rotation have limited capacity to assess the real biomechanics of knee joints during weight‐bearing activities in vivo.14 Radiostereometric analysis or 3‐D‐to‐2‐D registration is highly accurate for investigating knee joint kinematics in vivo.15, 16, 17 However, these techniques have the limitations of exposure to radiation, large space requirements and time‐consuming analysis.15 An alternative leverages the use of optical motion analysis systems with reflective skin markers to investigate in vivo dynamic joint motion. However, much time is required for marker placement and camera calibration, limiting the clinical use of this option.18
In this article, we introduce an improved optical motion analysis system for analyzing knee joint motion in vivo. We here report the clinical application of this device in three patients who were diagnosed as ACL deficient by MRI (one of these diagnoses was later found by arthroscopy to be incorrect) with the aim of establishing a new and convenient biomechanical process for evaluating the dynamic stability of knee joints. Additionally, we found this system to be more accurate than MRI for diagnosing ACL tears.
Materials and Methods
Patients
Three symptomatic subjects who had given written consent to the study procedure and to publication of their cases were recruited for this study. The design of this study was approved by our institution's review board. All three had experienced knee pain caused by trauma and/or instability syndrome manifested as “giving way”, buckling, and so on (Table 1).
Table 1.
Patient characteristics including symptoms
| Subject | Sex | Age (years) | Injured knee | Time since injury | Pain | Articular dyskinesia | Swelling | Giving way | Buckling |
|---|---|---|---|---|---|---|---|---|---|
| A | Male | 22 | Left | 1 year | + | + | + | + | − |
| B | Male | 38 | Right | 10 years | + | + | + | + | + |
| C | Male | 26 | Left | 2 years | + | + | + | + | − |
+, positive result and −, negative result.
After providing a clinical history, the three patients were subjected to physical examinations, including hyperextension (HE), hyperflexion (HF), the anterior drawer test (ADT), 30° internal rotation ADT, posterior drawer test (PDT), Lachman test; pivot shift test (PST), lateral stress test (LST) and McMurray test. The results of these tests on the injured knees are displayed in Table 2.
Table 2.
Results of physical examination of the injured knees
| Subject | HE | HF | ADT | 30° ADT | PDT | Lachman | PST | LST | McMurray |
|---|---|---|---|---|---|---|---|---|---|
| A | − | − | + | − | − | + | − | − | + (lateral) |
| B | − | − | + | − | − | + | − | − | + |
| C | − | − | − | − | − | + | − | − | − |
+, positive result; −, negative result; HE, hyperextension; HF, hyperflexion; ADT, anterior drawer test; PDT, posterior drawer test; PST, pivot shift test; LST, lateral stress test.
The pre‐operative diagnoses of three subjects were determined by 3.0T MR imaging (Magnetom Trio; Siemens Healthcare, Forchheim, Germany) and avulsion fracture ruled out by Siemens dual‐source 64‐slice spiral computed tomography (Definition CT; Siemens Healthcare). Parallel digital MR images of the injured knees with a thickness of 1.0 mm, without gaps and with a resolution of 512 × 512 pixels were obtained. The results of MRI of the three cases were as follows. Case A: an inwards concave posterior cruciate ligament (PCL) and relative thinner ACL were observed on T1‐weighted images (T1WI) of the injured knee. The posterior horn of a lateral meniscus tear was observed on T2 weighted images (T2WI). Case B: MR imaging demonstrated absence of the ACL. T1WI revealed an inwards concave PCL and T2WI revealed the caudomedial part of a medial meniscus tear. Case C: partial interruption of the ACL was observed on T1WI (Table 3, Fig. 1).
Table 3.
MRI findings in the three subjects
| Subject | MR imaging of injured knee |
|---|---|
| A | ACL tear, posterior horn of the lateral meniscus tear |
| B | ACL rupture, caudomedial part of medial meniscus tear |
| C | ACL tear |
ACL, anterior cruciate ligament.
Figure 1.
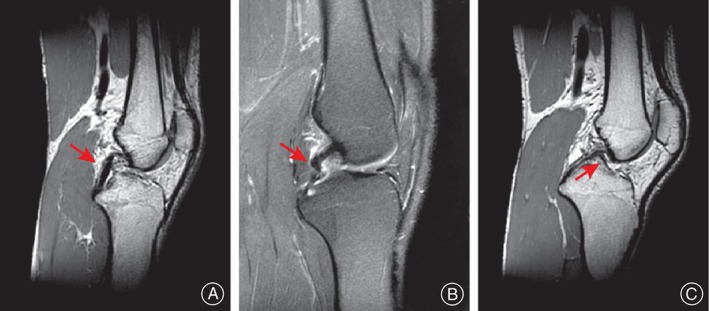
Sagittal MR images of three subjects. (A) An inwards concave posterior cruciate ligament (PCL) (arrow), indirect evidence of an ACL tear, can be observed in T1‐weighted images (arrow). (B) An inwards concave PCL can be observed in T2‐weighted images with fat suppression (arrow). (C) Partial interruption of the ACL can be observed in T1‐weighted images (arrow).
Instrumentation
Kinematic data of both knees in six‐degrees‐of‐freedom (6DOF, angle: flexion‐extension, internal‐external rotation, adduction‐abduction; translation: anteroposterior, proximo‐distal, medio‐lateral) was recorded and analyzed with a novel knee joint stability assessment system (Opti_Knee, Innomotion, Shanghai, China) (Fig. 2B). Each subject was asked to walk on a horizontal treadmill at a speed of 3 km/h before surgery. Two rigid plates, each composed of four infrared light‐reflecting markers (OK_Marquer; Innomotion), were attached to each subject's thighs and shanks with bandages. A digitizing pointer composed of four infrared light‐reflecting markers was also used to identify femoral and tibial landmarks (Fig. 2A). The 3‐D trajectories of the rigid plates during activities were tracked by an integrated two‐head stereo‐infrared camera at a frequency of 60 Hz with an accuracy of 0.3 mm root mean square.19 An integrated synchronous high‐speed camera was employed to divide the walking cycle and record video of the activity. A workstation computer with customized software performed supplementary real‐time calculations. The entire system is integrated in a mobile cart (1.1 m × 0.6 m × 0.4 m).
Figure 2.
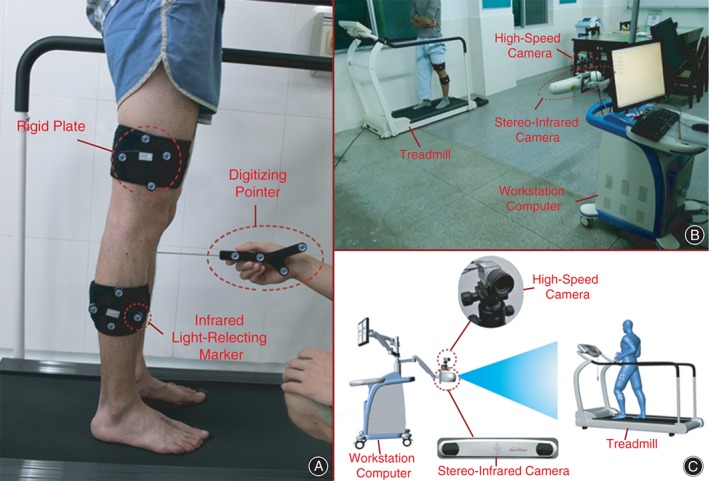
(A) Identification of surface markers. Two rigid plates, each composed of four infrared light‐reflecting markers, are attached to each subject's thighs and shanks with bandages. A digitizing pointer composed of four infrared light‐reflecting markers is used to identify femoral and tibial landmarks. (B) The instrument for knee kinematics analysis. The 3‐D trajectories of the rigid plates during activities are tracked by an integrated two‐head stereo‐infrared camera at a frequency of 60 Hz. An integrated synchronous high‐speed camera is used to divide the walking cycle and to record video of the activity. A workstation computer with customized software performs supplementary real‐time calculations. The entire system is integrated on a mobile cart (1.1 m × 0.6 m × 0.4 m). Each subject was asked to walk on a horizontal treadmill at a speed of 3 km/h. (C) Schematic representation of the instrument for knee kinematics analysis.
Data Calculation
The system recorded the 3‐D locations of the reflective markers at 60 Hz for 20 gait cycles. The 3‐D positions of the identified femoral and tibial anatomical landmarks were calculated in each frame of the gait based on their geometric relationship to the femoral or tibial rigid plates, respectively. Local femur and tibia coordinate systems were then established based on these landmarks.20 The 3‐D translation was quantified as the relative displacement between the origins of the femur in the tibial coordinate system. Similarly, the angular rotations were determined by the femoral coordinate system with respect to the tibial coordinate system.21 The 6DOF calculation was performed using Opti‐Knee software in real time.
Each gait cycle was analyzed by interpolating data to a 100 points to obtain an average gait cycle. The gait cycles were subsequently divided into the following two phases: (i) stance phase, from initial foot contact to toe‐off (TO, about 62% of the gait cycle); and (ii) swing phase, from toe‐off to next heel strike (HS).21
Motion data in flexion‐extension, internal‐external rotation and anteroposterior translation was calculated and compared between the knees of each subject.
Statistical Analysis
The knees of each subject were compared based on ANOVA for parametric variables and the Mann–Whitney test for non‐parametric variables. SPSS v13.0 (SPSS, Chicago, IL, USA) was used to perform the statistical analyses. The level of significance was set at P < 0.05.
Arthroscopic Examinations
After kinetic testing, arthroscopic examinations of the three subjects were performed by the same orthopedic surgeon to make a definitive diagnosis. During surgery, the status of each subject's ACL and menisci was documented and confirmed by at least two experienced surgeons with the same arthroscopic instruments. Meantime, other ligamentous and cartilaginous injuries of the knee that could potentially influence the knee kinematics were excluded. Reconstruction of the ACL was performed subsequently if necessary. The arthroscopic results were compared with MR imaging and gait analysis outcomes.
Results
The kinematics results in the three cases were as follows:
Subject A showed less knee flexion during the swing phase (between 69% and 82% of the gait cycle), greater femoral rotation between 21% and 64% of the gait cycle and higher posterior shift during the swing phase (between 72% and 87% of the gait cycle) in the injured than the uninjured knee (P < 0.05).
Subject B showed higher knee flexion during the swing phase (between 67% and 92% of the gait cycle) and lower peak extension angles during the mid‐stance (between 33% and 54% of the gait cycle) in the injured than the contralateral knee. Similarly, Subject B's injured knee was found to have greater femoral rotation between 62% and 90% of the gait cycle and posterior shift between 60% and 88%) than his contralateral knee (P < 0.05).
In contrast, no statistically significant differences between Subject C's knees in kinematic data were founds during the whole gait cycle (Fig. 3).
Figure 3.
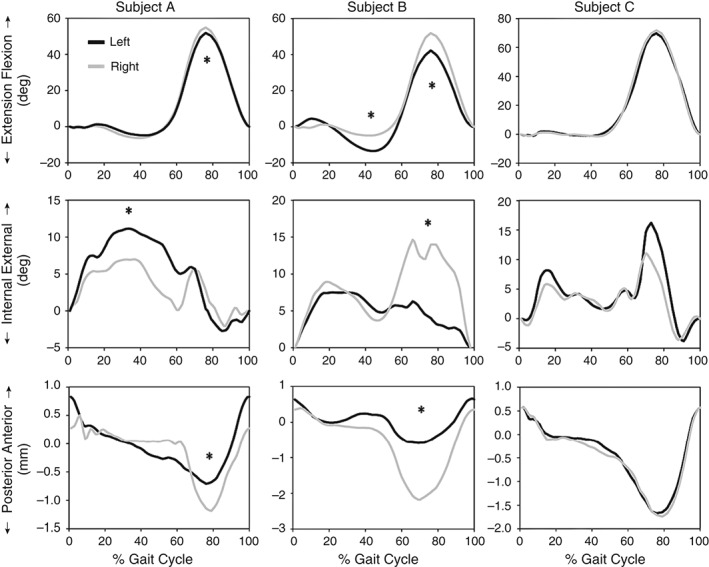
Tibiofemoral kinematics in flexion‐extension, internal‐external rotation and anterior‐posterior translation during treadmill gait. The findings are detailed in the text.
Arthroscopic examinations confirmed the diagnosis of ACL deficiency in Subjects A and B, but not in Subject C. The ACL of Subject C was found to be intact arthroscopically, these results being consistent with the results obtained by Opti_knee. Thus, this system could help identify false‐positive results of MR imaging and offer an effective way of assisting the diagnosis of ACL deficient knees.
Discussion
A novel, portable and non‐invasive gait analysis device was used to analyze 6DOF kinematics of both knees in three cases in whom ACL deficiency had been diagnosed by MRI. Significant differences in kinematics between the ACL deficient and contralateral knees were identified during treadmill walking in two of the subjects. However, the third subject showed no differences in kinematics between his knee and his symptomatic knee was later confirmed to have an intact ACL by arthroscopy. In other words, the novel kinematic analysis system correctly identified ACL deficiency in two subjects and a false positive by MRI in the third.
Some studies have identified the specific kinematic and kinetic adaptations of ACL deficient knees, called the “quadriceps avoidance” pattern22, 23: this term denotes increased knee extension during the stance phase and reduced flexion during the swing phase. During quadriceps avoidance gait, the anterior shear force applied to the tibia decreases in an ACL deficient knee, thus avoiding episodes of instability. In our study, one ACL deficient knee showed reduced flexion during the swing phase and increased flexion during stance phase. However, Roberts et al. concluded that quadriceps avoidance gait in ACL deficient patients is perhaps not as common as other studies have suggested.23 The absence of quadriceps avoidance in this study is likely attributable to the small sample size; additionally, one published study has reported the quadriceps avoidance phenomenon does not occur in subjects with chronic ACL deficiency.22 On the other hand, increased flexion during the stance phase in ACL deficient knees has been reported by several others.24
We found an increase of anterior tibial shift relative to the femur in both ACL deficient knees, similar to results reported by Chen et al. 25; however, those authors found the tibial shift pattern in the terminal stance phase of gait. Fuentes et al. considered gait patterns of ACL deficient knees are most frequently detected during the loading and the mid‐stance phases.26 However, the mechanism of this is that the force of the quadriceps femoris applied to the tibia and the tibia's inertia during swing lead to the greater anterior tibial shift.
In addition, we observed greater internal tibial rotation of ACL deficient knees during the swing or stance phase in our subjects, which is consistent with the observations of Gao and Zheng.24 In terms of anatomy and function of the ACL, we have concluded that the increased anterior tibial shift and internal tibial rotation relative to the femur during gait identified by associated kinematic changes suggest knee instability in subjects with ACL deficiency.
The diagnosis of ACL deficiency in Subjects A and B was documented by arthroscopic examination and they subsequently underwent ACL reconstruction. Arthroscopic examination of Subject C showed that his ACL was intact, yet MRI had identified an ACL tear in the injured knee. It should be noted that we identified no specific gait adaptation in Subject C's injured knee, this result being consistent with the arthroscopic findings, arthroscopy being regarded as the gold standard for diagnosing ACL deficiency. The failure of MRI to identify that the ACL was intact is likely attributable to the thickness of the slices.13 In addition, the imaging equipment, techniques and radiologists’ experience can also influence the accuracy of diagnosis of ACL.11, 12 From a clinical point of view, knee stability assessment could be employed as a supplementary tool for diagnosing ACL deficiency. We postulate that many unnecessary ACL reconstructions could be avoided if this suggestion was implemented.
The Opti‐knee system used in this study is technically superior to other available systems.18 It integrates stereo‐infrared and a high‐speed camera to provide both kinematic and visual data simultaneously (Fig. 2C). The accuracy with which the system tracks points in 3‐D is within 0.3 mm root mean square, which is comparable to that of conventional motion analysis systems.19 In this study, the preparation and test for each subject took on average of less than 15 minutes (both knees, including the time for gait training). In addition, the system is portable, and can be set up in a space of 4 m2. All these advantages make clinical application of this system possible. Besides the assistance with diagnosis, this system can be used to assess knee stability after reconstruction of the ACL in terms of biomechanics and thus to compare the outcomes of different surgical procedures. Moreover, analysis of knee kinematics may be helpful in avoiding inappropriate exercise during rehabilitation; alterations in kinematics can accelerate the knee degeneration.3 Therefore, improvement in treatment for ACL injuries and guidance for rehabilitation could be achieved with the help of this system in the future.
Of course, the results of this study should be interpreted in the context of its potential limitations. The universality of the findings may be questionable because the knee kinematics of only three subjects were assessed. Employing electromyography and stress plates may improve the accuracy of results, but would be time‐consuming.
In conclusion, this paper describes kinematic patterns of ACL deficient knees during gait identified by a novel portable gait analysis system that are similar to other reported findings. Gait analysis systems could help exclude false‐positive results of MRI. Combining MRI scans and knee stability assessment may be the best means of evaluating ACL deficient pre‐operatively in the future. We also conclude that treatments for ACL‐deficient knees can be optimized with the help of identification of kinematic alterations.
Disclosure: No funds were received in support of this work.
References
- 1. Levy BA. Is early reconstruction necessary for all anterior cruciate ligament tears? N Engl J Med, 2010, 363: 386–388. [DOI] [PubMed] [Google Scholar]
- 2. Spindler KP, Wright RW. Clinical practice. Anterior cruciate ligament tear. N Engl J Med, 2008, 359: 2135–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long‐term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med, 2007, 35: 1756–1769. [DOI] [PubMed] [Google Scholar]
- 4. Siegel L, Vandenakker‐Albanese C, Siegel D. Anterior cruciate ligament injuries: anatomy, physiology, biomechanics, and management. Clin J Sport Med, 2012, 22: 349–355. [DOI] [PubMed] [Google Scholar]
- 5. Benjaminse A, Gokeler A, van der Schans CP. Clinical diagnosis of an anterior cruciate ligament rupture: a meta‐analysis. J Orthop Sports Phys Ther, 2006, 36: 267–288. [DOI] [PubMed] [Google Scholar]
- 6. Anderson AF, Lipscomb AB. Preoperative instrumented testing of anterior and posterior knee laxity. Am J Sports Med, 1989, 17: 387–392. [DOI] [PubMed] [Google Scholar]
- 7. Hewett TE, Noyes FR, Lee MD. Diagnosis of complete and partial posterior cruciate ligament ruptures. Stress radiography compared with KT‐1000 arthrometer and posterior drawer testing. Am J Sports Med, 1997, 25: 648–655. [DOI] [PubMed] [Google Scholar]
- 8. Mulligan EP, Harwell JL, Robertson WJ. Reliability and diagnostic accuracy of the Lachman test performed in a prone position. J Orthop Sports Phys Ther, 2011, 41: 749–757. [DOI] [PubMed] [Google Scholar]
- 9. Boeree NR, Ackroyd CE. Magnetic resonance imaging of anterior cruciate ligament rupture. A new diagnostic sign. J Bone Joint Surg Br, 1992, 74: 614–616. [DOI] [PubMed] [Google Scholar]
- 10. Cotten A, Delfaut E, Demondion X, et al MR imaging of the knee at 0.2 and 1.5 T: correlation with surgery. AJR Am J Roentgenol, 2000, 174: 1093–1097. [DOI] [PubMed] [Google Scholar]
- 11. Krampla W, Roesel M, Svoboda K, Nachbagauer A, Gschwantler M, Hruby W. MRI of the knee: how do field strength and radiologist's experience influence diagnostic accuracy and interobserver correlation in assessing chondral and meniscal lesions and the integrity of the anterior cruciate ligament? Eur Radiol, 2009, 19: 1519–1528. [DOI] [PubMed] [Google Scholar]
- 12. White LM, Schweitzer ME, Deely DM, Morrison WB. The effect of training and experience on the magnetic resonance imaging interpretation of meniscal tears. Arthroscopy, 1997, 13: 224–228. [DOI] [PubMed] [Google Scholar]
- 13. Barry KP, Mesgarzadeh M, Triolo J, Moyer R, Tehranzadeh J, Bonakdarpour A. Accuracy of MRI patterns in evaluating anterior cruciate ligament tears. Skeletal Radiol, 1996, 25: 365–370. [DOI] [PubMed] [Google Scholar]
- 14. Lam MH, Fong DT, Yung PS, Chan KM. Biomechanical techniques to evaluate tibial rotation. A systematic review. Knee Surg Sports Traumatol Arthrosc, 2012, 20: 1720–1729. [DOI] [PubMed] [Google Scholar]
- 15. Mahfouz MR, Hoff WA, Komistek RD, Dennis DA. Effect of segmentation errors on 3D‐to‐2D registration of implant models in X‐ray images. J Biomech, 2005, 38: 229–239. [DOI] [PubMed] [Google Scholar]
- 16. Andriacchi TP, Dyrby CO. Interactions between kinematics and loading during walking for the normal and ACL deficient knee. J Biomech, 2005, 38: 293–298. [DOI] [PubMed] [Google Scholar]
- 17. Li G, Kozanek M, Hosseini A, Liu F, Van de Velde SK, Rubash HE. New fluoroscopic imaging technique for investigation of 6DOF knee kinematics during treadmill gait. J Orthop Surg Res, 2009, 4: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cappozzo A, Catani F, Leardini A, Benedetti MG, Croce UD. Position and orientation in space of bones during movement: experimental artefacts. Clin Biomech (Bristol, Avon), 1996, 11: 90–100. [DOI] [PubMed] [Google Scholar]
- 19. Güler Ö, Perwög M, Kral F, et al Quantitative error analysis for computer assisted navigation: a feasibility study. Med Phys, 2013, 40: 021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dyrby CO, Andriacchi TP. Secondary motions of the knee during weight bearing and non‐weight bearing activities. J Orthop Res, 2004, 22: 794–800. [DOI] [PubMed] [Google Scholar]
- 21. Zhang Y, Yao Z, Wang S, et al Motion analysis of Chinese normal knees during gait based on a novel portable system. Gait Posture, 2015, 41: 763–768. [DOI] [PubMed] [Google Scholar]
- 22. Knoll Z, Kiss RM, Kocsis L. Gait adaptation in ACL deficient patients before and after anterior cruciate ligament reconstruction surgery. J Electromyogr Kinesiol, 2004, 14: 287–294. [DOI] [PubMed] [Google Scholar]
- 23. Roberts CS, Rash GS, Honaker JT, Wachowiak MP, Shaw JC. A deficient anterior cruciate ligament does not lead to quadriceps avoidance gait. Gait Posture, 1999, 10: 189–199. [DOI] [PubMed] [Google Scholar]
- 24. Gao B, Zheng NN. Alterations in three‐dimensional joint kinematics of anterior cruciate ligament‐deficient and ‐reconstructed knees during walking. Clin Biomech (Bristol, Avon), 2010, 25: 222–229. [DOI] [PubMed] [Google Scholar]
- 25. Chen CH, Li JS, Hosseini A, Gadikota HR, Gill TJ, Li G. Anteroposterior stability of the knee during the stance phase of gait after anterior cruciate ligament deficiency. Gait Posture, 2012, 35: 467–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fuentes A, Hagemeister N, Ranger P, Heron T, de Guise JA. Gait adaptation in chronic anterior cruciate ligament‐deficient patients: Pivot‐shift avoidance gait. Clin Biomech (Bristol, Avon), 2011, 26: 181–187. [DOI] [PubMed] [Google Scholar]


