Abstract
The objective of this systematic review and meta‐analysis was to evaluate the efficacy and safety of i.v. tranexamic acid (TXA) in simultaneous bilateral total knee arthroplasty (TKA). Potentially relevant published reports were identified from the following electronic databases: Medline, PubMed, Embase, ScienceDirect and Cochrane Library. RevMan v5.3was used to pool data. Two randomized controlled trials and four case‐control studies met the inclusion criteria. The current meta‐analysis identified significant differences between TXA group and control groups in terms of postoperative hemoglobin concentration (P < 0.01), drainage volume (P < 0.01), transfusion rate (P < 0.01) and units transfused (P = 0.006). There were no significant differences in length of stay (P = 0.66), operation time (P = 0.81) or and incidence of adverse effects such as infection (P = 0.42), deep venous thrombosis (DVT) (P = 0.88) and pulmonary embolism (PE) (P = 0.11). Our results show that i.v. administration of TXA in simultaneous bilateral TKA reduces postoperative drops in hemoglobin concentration, drainage volume, and transfusion requirements and does not prolong length of stay or operation time. Moreover, no adverse effects, such as infection, DVT or PE, were associated with TXA.
Keywords: Blood loss, Meta analysis, Total knee arthroplasty, Tranexamic acid
Introduction
Total knee arthroplasty (TKA) is an effective means of relieving pain and maintaining motor function in patients with end‐stage osteoarthritis of the knee joint. However, because primary TKA requires extensive soft tissue dissection, long operative time and considerable cutting of bone, patients undergoing this procedure are particularly prone to considerable intra‐ and post‐operative blood loss. Substantial blood loss is potentially associated with numerous problems, which can result in unsatisfactory outcomes and systemic decline, especially in older individuals1, 2. Many techniques for addressing management of blood loss, including tourniquets, blood transfusion, administration of hemostatic agents and autologous donation have been tried3. Allogenic blood transfusion is associated with a risk of adverse effects such as viral infection, immunologically mediated disease and cardiovascular dysfunction and may thus cause increased financial burdens and potential ill‐health4.
Recently, tranexamic acid (TXA), administered via various routes, has been increasingly investigated as an adjunct to joint replacement. TXA, which is a synthetic analog of the amino acid lysine, inhibits dissolving of blood clots by plasminogen5. Several studies have reported that i.v. administration or topical application of TXA achieves the expected results of reducing perioperative blood loss and units transfused. Wong et al. assess the efficiency and safety of topical application of TXA in primary TKA in a randomized controlled trial (RCT) in 124 patients6. At the conclusion of TKA with cement, tranexamic acid was applied topically directly to the surgical wound; postoperative bleeding was reduced by 20%–25%, or 300–400 mL, resulting in 16%–17% higher postoperative hemoglobin concentrations compared with placebo, with no clinically important increase in complications being identified in the treatment group. Furthermore, a meta‐analysis of high quality RCTs indicated that TXA is effective and safe for management of blood loss in primary unilateral TKA7. Fu et al. performed a meta‐analysis to investigate the efficacy and safety of i.v. TXA in TKA by pooling 22 RCTs8. Thy found that i.v. TXA benefits patients undergoing TKA, significantly reducing total blood loss, postoperative blood loss, transfusion rate and volume transfused.
Patients who have bilateral knee arthritis may undergo simultaneous or staged bilateral TKAs; the outcomes of these two options have been widely debated. Simultaneous bilateral TKA has the advantage that there is only one operation, anesthetic, hospitalization, recovery period and analgesia regimen. Thus, the medical cost is lower. However, studies have shown a high incidence of cardiac and thrombotic complications, even death, in patients undergoing simultaneous bilateral TKAs9. On the contrary, some studies have reported opposite conclusions10. However, the 90‐day mortality of unilateral, simultaneous and staged bilateral TKAs is reportedly similar11. Generally, it is difficult to compare simultaneous with staged bilateral TKAs because a majority of patients who are disappointed with their first unilateral TKA, and those who die, do not undergo the planned contralateral TKA.
There is no doubt that simultaneous bilateral TKA is associated with greater declines in hemoglobin concentrations and higher transfusion requirements than unilateral TKA, together with a higher risk of complications such as deep vein thrombosis (DVT) and pulmonary embolism (PE). One study reported that an estimated 2.6 units of red blood cells are transfused per patient in bilateral TKA12. To our knowledge, i.v. administration of TXA in patients undergoing bilateral TKAs has rarely been reported. We therefore performed this systematic review and meta‐analysis to evaluate the efficiency and safety of i.v. TXA for management of blood loss in patients undergoing bilateral TKA.
Methods
Search Strategy
Potential relevant published reports were identified from electronic databases including Medline (1966 to November 2015), PubMed (1966 to November 2015), Embase (1980 to November 2015), ScienceDirect (1985 to March 2015) and Cochrane Library (inception to July 2016). Gray academic studies were also identified from the reference of included reports. There was no language restriction. The key words “Bilateral knee replacement OR arthroplasty” and “tranexamic acid,” ‘blood loss,” “blood transfusion” were used in combination with Boolean operators AND or OR. The search process was performed as presented in Fig. 1.
Figure 1.
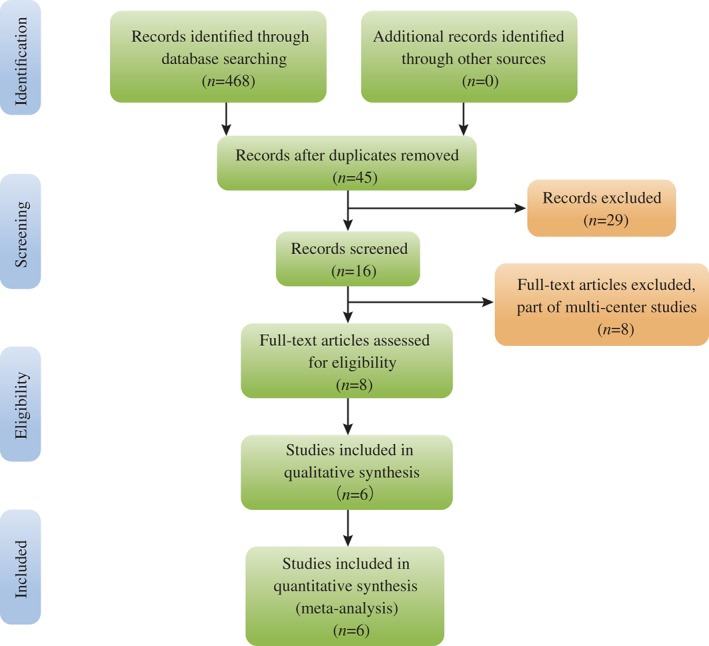
Search results and selection procedure.
Inclusion and Exclusion Criteria
Published reports were selected if they met the following criteria: (i) clinical trial (RCT or non‐RCT); (ii) patients with knee osteoarthritis undergoing simultaneous bilateral TKA, experiment group received intravenous TXA, control group received normal saline or nothing; (iii) reported surgical outcomes included hemoglobin decline or postoperative hemoglobin concentration, blood loss, drainage volume, transfusion requirements, length of stay, operation time and surgery‐related adverse effects such as wound infection, DVT and PE. Exclusion criteria comprised incomplete data, participants with a known allergy to TXA, severe cardiovascular dysfunction, history of thromboembolic event, renal failure or other contraindication to TXA.
Selection Criteria
The abstracts of the potential published reports were independently scanned by two reviewers (MJX and JX), after which full texts of the studies that met the inclusion criteria were screened and final decisions made. Disagreements were resolved by consulting a senior reviewer.
Data Extraction
Data were independently extracted from the included studies by two of the authors (MJX and JX). The corresponding authors were contacted to obtain missing data. The following data were extracted: first author names, published year, baseline for comparisons, intervention procedures, samples size, indications for transfusion, and outcome variables. Other relevant data were also extracted from individual articles.
Quality Assessment
Quality assessment of RCTs was based on the Cochrane Handbook13 and performed by one reviewer, who assessed the details of the randomization procedures, allocation concealment, blinding and follow‐up, each item being recorded as “Yes,” “No,” or “Unclear.” “Yes” indicated a low risk of bias and “No” a high risk of bias. “Unclear” indicated a lack of information or unknown risk of bias. For non‐RCTs, quality assessment was performed according to the Methodological Index for Non‐Randomized studies (MINORs)14, in which scores range from 0 to 24. Disagreement was resolved by consulting a third reviewer.
Data Analysis and Statistical Methods
Pooling of data was carried out with RevMan 5.3 (Cochrane Collaboration, Oxford, United Kingdom). Statistical heterogeneity was assessed based on the values of P and I 2 using a standard χ2 test. When I 2 > 50%, P < 0.1, indicating significant heterogeneity, meta‐analysis was performed using the random effects model. Otherwise, the fixed effects model was used. If possible, sensitivity analysis was conducted to determine the origins of any heterogeneity. Dichotomous outcomes are expressed as risk difference (RD) with 95% confidence intervals (CIs). For continuous outcomes, mean differences (MDs) and 95% CIs were calculated.
Results
Search Result
The initial search identified 468 studies. Scanning of the abstracts resulted in exclusion of 463 of these reports from this meta‐analysis. No gray references were included. Finally, six articles reporting two RCTs15, 16 and four case‐control studies (CCTs)17, 18, 19, 20 that had been published between 2011 and 2015 were selected for the present meta‐analysis. These studies contained a total of 274 participants in the experiment groups and 293 control patients.
Assessment of Risk of Bias
Relevant patient characteristics provided in the included articles are summarized in Table 1. The Cochrane Handbook for Systematic Review of Interventions was consulted to assess the quality of RCTs. Both RCTs provide clear inclusion and exclusion criteria and the methodology used for randomization; randomization was computer‐generated in one study15. None of them had concealed allocation by closed envelope or other techniques. Double blinding was implemented in both RCTs and one of them had attempted to blind assessors15. Intent–to‐treatment analysis was not performed in the included RCTs; thus, there was a potential risk of type II statistical error. There was no unclear bias attributable to incomplete outcome data or selective outcome reporting in the RCTs. The MINORS scale was applied for non‐RCTs. Quality assessment of methodologies is summarized in Tables 2 and 3.
Table 1.
Relevant study characteristics
| Studies | Year | Cases (T/C) | Mean age (T/C) | Female patient (T/C) | Prophylactic antithrombotic | Type of study indication for | Transfusion |
|---|---|---|---|---|---|---|---|
| Bagsby et al.17 | 2015 | 46/57 | 60.4/61.7 | 23/29 | LMWH, NS | CCT | Hb less than 8 g/L or anemia symptom |
| Kelley et al.19 | 2014 | 51/70 | 69.8/67.2 | 36/45 | Aspirin 325 mg, Bid | CCT | Hb less than 9 g/L or Hct less than 27% |
| Karam et al.18 | 2014 | 37/50 | 63.5/65.5 | 14/22 | Aspirin 325 mg, Bid | CCT | Hb less than 8 g/L or anemia symptom |
| Dhillon et al.20 | 2011 | 52/56 | 65.8/67.2 | 34/36 | LMWH, NS | CCT | Hb less than 9 g/L or Hct less than 27% |
| Karaaslan et al.15 | 2015 | 41/40 | 65.9/65.6 | 32/35 | LMWH, 40 mg | RCT | Hb less than 10 g/L |
| MacGillivray et al.16 | 2011 | 20/20 | 62/66 | 13/15 | Warfarin 2.5–5 mg | RCT | Hb less than 8 g/L |
C, control; Hct, hematocrit; LMWH, low molecular weight heparin; NS, not state; T, tranexamic acid.
Table 2.
Quality of methodology of the RCTs
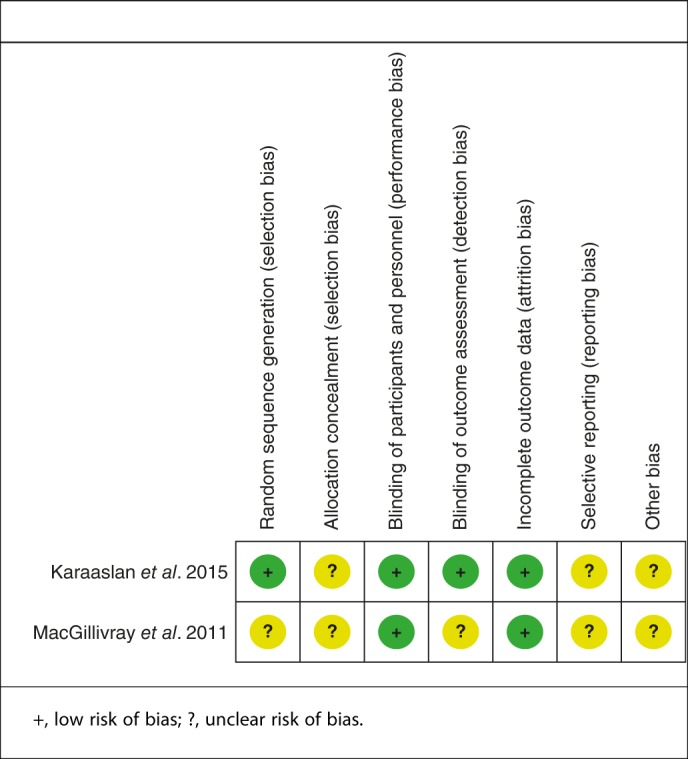
Table 3.
Quality assessment of non‐randomized trials
| Variable | Bagsby et al. 17 2015 | Kelley et al. 19 2014 | Karam et al. 18 2014 | Dhillon et al. 20 2011 |
|---|---|---|---|---|
| A clearly stated aim | 2 | 2 | 2 | 2 |
| Inclusion of consecutive patients | 2 | 2 | 2 | 2 |
| Prospective data collection | 2 | 2 | 2 | 2 |
| Endpoints appropriate to the aim of the study | 2 | 1 | 2 | 1 |
| Unbiased assessment of the study endpoint | 0 | 0 | 0 | 0 |
| A follow‐up period appropriate to the aims of study | 2 | 2 | 1 | 2 |
| Less than 5% loss to follow‐up | 2 | 2 | 2 | 2 |
| Prospective calculation of the sample size | 0 | 0 | 0 | 0 |
| An adequate control group | 2 | 2 | 2 | 2 |
| Contemporary groups | 1 | 1 | 1 | 1 |
| Baseline equivalence of groups | 2 | 2 | 2 | 2 |
| Adequate statistical analyses | 2 | 2 | 2 | 2 |
| Total score | 19 | 18 | 18 | 18 |
0, item not reported in the article evaluated; 1, reported but inadequately; 2, reported adequately.
Study Characteristics
The included studies had from 40 to 121 participants. Only studies of patients with sustained end‐stage knee arthritis were included in this meta‐analysis. Experimental groups had received i.v. TXA and control groups normal saline or nothing. Doses of TXA ranged from 10 g/L to 20 g/L. Only one study reported that bilateral TKA was performed by the same senior surgeon16. General anesthesia was used in three studies; the other studies did not provide this information. Tourniquets were routinely utilized in all studies15, 16, 17. In two studies a midline skin incision was followed by subvastus or medical parapatellar arthrotomy15, 17. All the included studies indicated that cemented prostheses were used except for the study by Bagsby et al. 17. A passive motion machine was used for early rehabilitation in two studies15, 17. Postoperative drainage was used in all patients. Details of antithrombotic therapy are presented in Table 1. All studies reported indications for transfusion in detail, these being based on postoperative hemoglobin, hematocrit or anemia symptoms. All studies provided outcomes for at least 95% of the patients.
Outcomes of Meta‐analysis
Postoperative Hemoglobin Concentration
Postoperative hemoglobin concentrations were supplied for five studies15, 16, 17, 18, 20. No significant heterogeneity having been found, the fixed effects model was applied (χ 2 = 3.42, df = 4, I 2 = 0, P = 0.49). The difference between the two groups was significant (MD = 1.17; 95% CI, 0.90–1.44, P < 0.01; Fig. 2).
Figure 2.
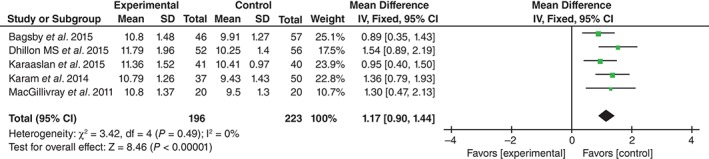
Forest plot diagram showing effect of TXA on postoperative hemoglobin concentration.
Transfusion Rate
Six studies reported the blood transfusion rate following bilateral TKA15, 16, 17, 18, 19, 20. There being no significant heterogeneity (χ 2 = 9.23, df = 5, I 2 = 46%, P = 0.10), the fixed effects model was used. Pooling results demonstrated that the transfusion rate was significantly higher in the control than in the TXA groups (RD = −0.35, 95% CI, −0.42 to −0.27, P < 0.01; Fig. 3).
Figure 3.
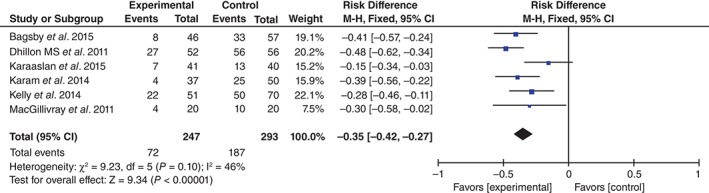
Forest plot diagram showing effect of TXA on transfusion rate.
Units Transfused
Units transfused were shown in four studies17, 18, 19, 20. Significant heterogeneity being found, the random effects model was applied (χ 2 = 68.13, df = 3, I 2 = 96%, P < 0.01). The difference between the two groups was statistically significance (MD = −1.17, 95% CI, −2.01 to −0.33, P = 0.006; Fig. 4).
Figure 4.
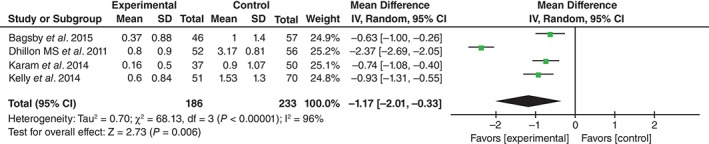
Forest plot diagram showing effect of TXA on units transfused.
Drainage Volume
Drainage volume was provided in two studies19, 20. No significant heterogeneity being found, the fixed effects model was used (χ 2 = 0.74, df = 1, I 2 = 0%, P = 0.39). Drainage volume was significantly higher the in control than in the TXA groups (MD = −547.82, 95% CI, −608.71 to −486.92, P < 0.01; Fig. 5).
Figure 5.
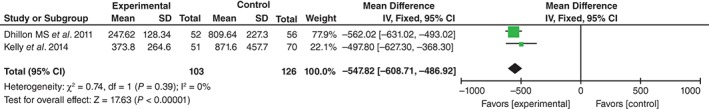
Forest plot diagram showing effect of TXA on drainage volume.
Operation Time
Operation time was reported in two studies17, 18. No significant heterogeneity being found, the fixed effects model was used (χ 2 = 0.22, df = 1, I 2 = 0%, P = 0.64). There was no significant heterogeneity between the two groups (MD = − 0.75, 95% CI, −6.82 to 5.31, P = 0.81; Fig. 6).
Figure 6.
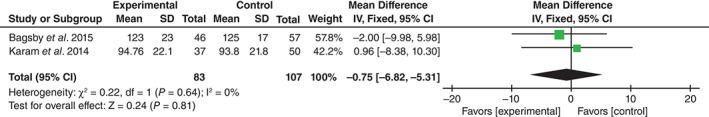
Forest plot diagram showing effect of TXA on operation time.
Length of Hospital Stay
Two studies reported the length of hospital stay according to group17, 18. Significant heterogeneity was shown for pooled results, thus the random effects model was used (χ 2 = 4.57, df = 1, I 2 = 78%, P = 0.03). The difference between the groups was not significant (MD = 0.11, 95% CI, −0.22 to 0.45, P = 0.66; Fig. 7).
Figure 7.
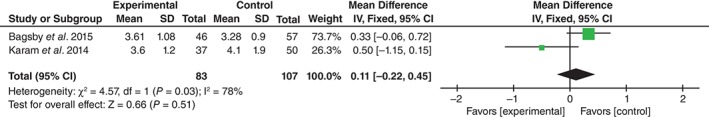
Forest plot diagram showing effect of TXA on length of stay.
Wound Infection
Wound infection was reported in four studies17, 18, 19, 20. No significant heterogeneity being found, the fixed effects model was used (χ 2 = 0.46, df = 3, I 2 = 0%, P = 0.93). The difference between the two groups was not significant (RD = 0.01, 95% CI, −0.02 to 0.05, P = 0.42; Fig. 8).
Figure 8.
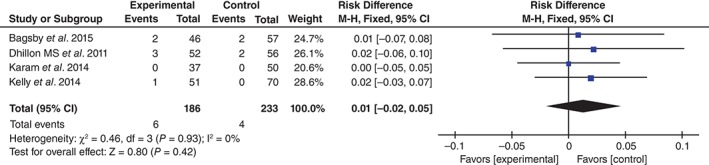
Forest plot diagram showing effect of TXA on risk of wound infection.
Deep Vein Thrombosis
Six articles reported the incidence of DVT following bilateral TKA15, 16, 17, 18, 19, 20. The fixed effects model was used because of the significantly low heterogeneity (χ 2 = 1.80, df = 5, I 2 = 0%, P = 0.88). No significant difference was found between groups (RD = 0.00, 95% CI, −0.02 to 0.03, P = 0.88; Fig. 9).
Figure 9.

Forest plot diagram showing effect of TXA on risk of DVT.
Pulmonary Embolism
PE was reported in six studies15, 16, 17, 18, 19, 20. No significant heterogeneity being found, the fixed effects model was used (χ 2 = 0.77, df = 5, I 2 = 0%, P = 0.98). There was no significant difference between the two groups (RD = 0.00, 95% CI, −0.02 to 0.02, P = 0.11; Fig. 10).
Figure 10.
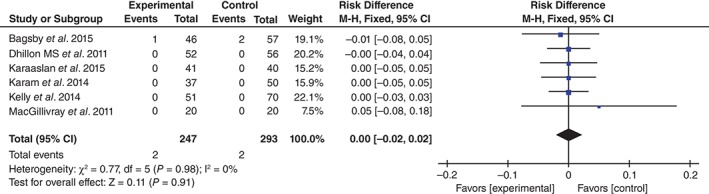
Forest plot diagram showing effect of TXA on risk of PE.
Discussion
New Discoveries in the Current Meta‐analysis
To our knowledge, this is the first systematic review and meta‐analysis evaluating efficiency and safety of i.v. tranexamic acid in bilateral TKA. The most important finding of this‐analysis is that i.v. tranexamic acid in bilateral TKA reduces postoperative hemoglobin decline, transfusion requirements and drainage volume with no identified increased risk of infection, DVT or PE. Furthermore, no TXA‐related adverse effects were identified.
Six studies were included in the current meta‐analysis, two of which were RCTs. However, both RCTs had methodological weaknesses that influenced the strength of point estimates. Both RCTs were of overall good methodologic quality. Randomization was reported in both RCTs; however, only one mentioned the randomization method15. Both provided a methodology for the blinding of participation and one of them attempted to blind the assessors. Intent‐to‐treat analysis was not performed in either RCT; thus, type II statistical error potentially influenced their results8. We also included non‐RCTs because there were so few published RCTs. This inclusion would, to some extent, have decreased the level of evidence of this meta‐analysis and should be taken into consideration when analyzing the pooling results. Though we searched the selected electronic databases systematically, language and publication bias may have resulted in omission of some report. In addition, all eligible studies were relatively small.
Controversy Concerning Simultaneous Bilateral TKA
The aging of the population has contributed to the increased incidence of knee osteoarthritis. Approximately 10% of patients undergo contralateral TKA in the years following a unilateral TKA11. Simultaneous bilateral TKA is considered superior to staged unilateral TKA in terms of lower surgical cost, shorter total length of stay, earlier rehabilitation, better knee function outcome and lower incidence of postoperative complications; it is thus frequently performed for bilateral knee osteoarthritis21. However, this procedure is characteristically associated with substantial blood loss, and consequently high transfusion requirements. Allogeneic blood transfusion carries infective and non‐infective risks22. Autologous blood transfusion is an alternative strategy; however, this is a high‐cost procedure with multiple difficulties for some medical centers. Blood loss management is still one of the foremost issues for surgeons, especially in major operations such as joint replacement.
Advances in Hemostatic Agents
Recently, hemostatic agents such as Floseal hemostatic matrix (Baxter International, Chicago, IL, USA), aprotinin and TXA have attracted our attention because of their high efficiency and cost‐effectiveness, as reported in several studies18, 23, 24. TXA, a known antifibrinolytic agent, has commonly been used in surgical procedures because of its low cost and allergenicity. Several high quality RCTs have confirmed better outcomes using TXA in unilateral TKA. However, blood loss is expected to be higher following bilateral TKA25, 26. Bleeding can flow into soft tissues around the knee joint, causing pain and stiffness and prolonging length of stay and duration of rehabilitation27. Furthermore, prolonged bed time increases the risk of thrombotic events. The current meta‐analysis indicates that i.v. TXA significantly reduces blood loss following simultaneous bilateral TKAs. Additionally, postoperative hemoglobin and hematocrit are significantly higher in TXA than control groups.
Clinical Effect of TXA in Bilateral TKA
Substantial previous reports have shown that estimated total blood loss in patient undergoing unilateral TKA without antifibrinolytics ranges from 761 to 1784 mL and 7.7%–18.93% of these patients require transfusion28, 29, 30, 31, 32, 33, 34, 35. Obviously, bilateral TKA is more commonly associated with perioperative blood loss and associated with higher blood transfusion rates and number of units transfused than is unilateral TKA. Transfusion is considered undesirable because of the associated risks of various adverse reactions. The current meta‐analysis shows that i.v. administration of TXA significantly reduces the transfusion rate and units transfused following simultaneous bilateral TKAs.
Prolonged confinement to bed and operation times increase operation costs. More importantly, adverse events such as hypostatic pneumonia, DVT and PE increase morbidity and mortality. Early weight bearing and rehabilitation has proven to contribute to better functional outcomes after TKA. The present meta‐analysis indicates that i.v. TXA does not prolong time confined to bed or operation time following simultaneous bilateral TKAs.
Wound infection occurs; however, it is disastrous for patients once it happens and may necessitate revision surgery. Moreover, sinus formation following inflammatory reactions leads to delayed union and poor joint function outcomes. The current meta‐analysis did not identify a significant difference in incidence of infection, which was 6/186 in the TXA groups and 4/233 in the controls. The overall incidence was 2.39%, which is in accordance with previous reports of 1%–3%36. However, there was a tendency toward higher risk of infection in the intervention group. Large sample size and high quality trails are required to further explore the correlation between infection and use of TXA.
DVT is a common complication of TKA and may lead to PE and even death. All participants in the assessed studies received routine prophylactic antithrombotic therapy. Previous studies have reported that there is a higher risk of developing DVT and PE when TXA is used, possibly because antifibrinolytic agents theoretically increase the risk of clotting37. However, in the present analysis, we found no significant differences in incidence of DVT or PE between groups.
Current Limitations of this Meta‐analysis
This meta‐analysis had several potential limitations that should be noted. (i) Only six studies were included, four of which were non‐RCTs and the sample sizes were relatively small. (ii) Some outcome variables such as total blood loss and range of motion were not fully described, preventing us from subjecting them to meta‐analysis. (iii) The small numbers of participants in the included studies prevented us from performing subgroup analysis; we therefore could not identify the source of heterogeneity. (iv) The short‐term follow‐ ups may have led to underestimation of complications. (v) Publication bias is an inherent weakness of all meta‐analysis.
TXA has been used in surgical field for more than 50 years. Recently, substantial studies have shown the efficiency of TXA in primary unilateral TKA. TXA in bilateral TKA has seldom been reported. Despite the above limitations, this is the first meta‐analysis to pool the results from controlled clinical trials to evaluate the efficiency and safety of i.v. TXA in simultaneous bilateral TKA. Well‐designed, long‐term follow up of RCTs is needed to explore the optimal dose and adverse effects.
Conclusions
Intravenous administration of TXA in simultaneous bilateral TKA could reduce postoperative hemoglobin decline, drainage volume and transfusion requirements and would not prolong the length of stay or operation time. Moreover, no adverse effects, such as infection, DVT or PE were associated with TXA. We believe that TXA demonstrates excellent clinical efficacy and safety in simultaneous bilateral TKAs.
Disclosure: No financial support was obtained for this work.
References
- 1. Sculco TP. Global blood management in orthopaedic surgery. Clin Orthop Relat Res, 1998, 357: 43–49. [DOI] [PubMed] [Google Scholar]
- 2. Mannucci PM, Levi M. Prevention and treatment of major blood loss. N Engl J Med, 2007, 356: 2301–2311. [DOI] [PubMed] [Google Scholar]
- 3. Conteduca F, Massai F, Iorio R, Zanzotto E, Luzon D, Ferretti A. Blood loss in computer‐assisted mobile bearing total knee arthroplasty. A comparison of computer‐assisted surgery with a conventional technique. Int Orthop, 2009, 33: 1609–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bilgili MG, Ercin E, Peker G, et al. Efficiency and cost analysis of cell saver auto transfusion system in total knee arthroplasty. Balkan Med J, 2014, 31: 149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benoni G, Lethagen S, Fredin H. The effect of tranexamic acid on local and plasma fibrinolysis during total knee arthroplasty. Thromb Res, 1997, 85: 195–206. [DOI] [PubMed] [Google Scholar]
- 6. Wong J, Abrishami A, El Beheiry H, et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am, 2010, 92: 2503–2513. [DOI] [PubMed] [Google Scholar]
- 7. Cid J, Lozano M. Tranexamic acid reduces allogeneic red cell transfusions in patients undergoing total knee arthroplasty: results of a meta‐analysis of randomized controlled trials. Transfusion, 2005, 45: 1302–1307. [DOI] [PubMed] [Google Scholar]
- 8. Fu DJ, Chen C, Guo L, Yang L. Use of intravenous tranexamic acid in total knee arthroplasty: a meta‐analysis of randomized controlled trials. Chin J Traumatol, 2013, 16: 67–76. [PubMed] [Google Scholar]
- 9. Restrepo C, Parvizi J, Dietrich T, Einhorn TA. Safety of simultaneous bilateral total knee arthroplasty. A meta‐analysis. J Bone Joint Surg Am, 2007, 89: 1220–1226. [DOI] [PubMed] [Google Scholar]
- 10. Memtsoudis SG, Ma Y, Gonzalez Della Valle A, et al. Perioperative outcomes after unilateral and bilateral total knee arthroplasty. Anesthesiology, 2009, 111: 1206–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walmsley P, Murray A, Brenkel IJ. The practice of bilateral, simultaneous total knee replacement in Scotland over the last decade. Data from the Scottish arthroplasty project. Knee, 2006, 13: 102–105. [DOI] [PubMed] [Google Scholar]
- 12. Bullock DP, Sporer SM, Shirreffs TG Jr. Comparison of simultaneous bilateral with unilateral total knee arthroplasty in terms of perioperative complications. J Bone Joint Surg Am, 2003, 85: 1981–1986. [DOI] [PubMed] [Google Scholar]
- 13. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions 5.1.0. The Cochrane Collaboration, 2011. Available from: www.handbook.cochrane.org (accessed 31 October 2015). [Google Scholar]
- 14. Slim K, Nini E, Forestier D, et al. Methodological index for non‐randomized studies (minors): development and validation of a new instrument. ANZ J Surg, 2003, 73: 712–716. [DOI] [PubMed] [Google Scholar]
- 15. Karaaslan F, Karaoglu S, Mermerkaya MU, Baktir A. Reducing blood loss in simultaneous bilateral total knee arthroplasty: combined intravenous‐intra‐articular tranexamic acid administration. A prospective randomized controlled trial. Knee, 2015, 22: 131–135. [DOI] [PubMed] [Google Scholar]
- 16. MacGillivray RG, Tarabichi SB, Hawari MF, Raoof NT. Tranexamic acid to reduce blood loss after bilateral total knee arthroplasty: a prospective, randomized double blind study. J Arthroplasty, 2011, 26: 24–28. [DOI] [PubMed] [Google Scholar]
- 17. Bagsby DT, Samujh CA, Vissing JL, Empson JA, Pomeroy DL, Malkani AL. Tranexamic acid decreases incidence of blood transfusion in simultaneous bilateral total knee arthroplasty. J Arthroplasty, 2015, 30: 2106–2109. [DOI] [PubMed] [Google Scholar]
- 18. Karam JA, Bloomfield MR, DiIorio TM, Irizarry AM, Sharkey PF. Evaluation of the efficacy and safety of tranexamic acid for reducing blood loss in bilateral total knee arthroplasty. J Arthroplasty, 2014, 29: 501–503. [DOI] [PubMed] [Google Scholar]
- 19. Kelley TC, Tucker KK, Adams MJ, Dalury DF. Use of tranexamic acid results in decreased blood loss and decreased transfusions in patients undergoing staged bilateral total knee arthroplasty. Transfusion, 2014, 54: 26–30. [DOI] [PubMed] [Google Scholar]
- 20. Dhillon MS, Bali K, Prabhakar S. Tranexamic acid for control of blood loss in bilateral total knee replacement in a single stage. Indian J Orthop, 2011, 45: 148–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brotherton SL, Roberson JR, de Andrade JR, Fleming LL. Staged versus simultaneous bilateral total knee replacement. J Arthroplasty, 1986, 1: 221–228. [DOI] [PubMed] [Google Scholar]
- 22. Sinclair KC, Clarke HD, Noble BN. Blood management in total knee arthroplasty: a comparison of techniques. Orthopedics, 2009, 32: 19. [DOI] [PubMed] [Google Scholar]
- 23. Wang C, Han Z, Zhang T, et al. The efficacy of a thrombin‐based hemostatic agent in primary total knee arthroplasty: a meta‐analysis. J Orthop Surg Res, 2014, 9: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kagoma YK, Crowther MA, Douketis J, Bhandari M, Eikelboom J, Lim W. Use of antifibrinolytic therapy to reduce transfusion in patients undergoing orthopedic surgery: a systematic review of randomized trials. Thromb Res, 2009, 123: 687–696. [DOI] [PubMed] [Google Scholar]
- 25. Bidolegui F, Arce G, Lugones A, Pereira S, Vindver G. Tranexamic acid reduces blood loss and transfusion in patients undergoing total knee arthroplasty without tourniquet: a prospective randomized controlled trial. Open Orthop J, 2014, 8: 250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang Z, Ma J, Shen B, Pei F. Combination of intravenous and topical application of tranexamic acid in primary total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty, 2014, 29: 2342–2346. [DOI] [PubMed] [Google Scholar]
- 27. Friedman RJ. Limit the bleeding, limit the pain in total hip and knee arthroplasty. Orthopedics, 2010, 33: 11–13. [DOI] [PubMed] [Google Scholar]
- 28. Hiippala S, Strid L, Wennerstrand M, et al. Tranexamic acid (Cyklokapron) reduces perioperative blood loss associated with total knee arthroplasty. Br J Anaesth, 1995, 74: 534–537. [DOI] [PubMed] [Google Scholar]
- 29. Benoni G, Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: a prospective, randomised, double‐blind study of 86 patients. J Bone Joint Surg Br, 1996, 78: 434–440. [PubMed] [Google Scholar]
- 30. Veien M, Sorensen JV, Madsen F, Juelsgaard P. Tranexamic acid given intraoperatively reduces blood loss after total knee replacement: a randomized, controlled study. Acta Anaesthesiol Scand, 2002, 46: 1206–1211. [DOI] [PubMed] [Google Scholar]
- 31. Camarasa MA, Olle G, Serra‐Prat M, et al. Efficacy of aminocaproic, tranexamic acids in the control of bleeding during total knee replacement: a randomized clinical trial. Br J Anaesth, 2006, 96: 576–582. [DOI] [PubMed] [Google Scholar]
- 32. Frisch NB, Wessell NM, Charters MA, Yu S, Jeffries JJ, Silverton CD. Predictors and complications of blood transfusion in total hip and knee arthroplasty. J Arthroplasty, 2014, 29: 189–192. [DOI] [PubMed] [Google Scholar]
- 33. Danninger T, Rasul R, Poeran J, et al. Blood transfusions in total hip and knee arthroplasty: an analysis of outcomes. Sci World J, 2014, 2014: 623460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Klika AK, Small TJ, Saleh A, Szubski CR, Chandran Pillai AL, Barsoum WK. Primary total knee arthroplasty allogenic transfusion trends, length of stay, and complications: nationwide inpatient sample 2000–2009. J Arthroplasty, 2014, 29: 2070–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yoshihara H, Yoneoka D. National trends in the utilization of blood transfusions in total hip and knee arthroplasty. J Arthroplasty, 2014, 29: 1932–1937. [DOI] [PubMed] [Google Scholar]
- 36. Soriano A, Bori G, Garcia‐Ramiro S, et al. Timing of antibiotic prophylaxis for primary total knee arthroplasty performed during ischemia. Clin Infect Dis, 2008, 46: 1009–1014. [DOI] [PubMed] [Google Scholar]
- 37. Onodera T, Majima T, Sawaguchi N, et al. Risk of deep venous thrombosis in drain clamping with tranexamic acid and carbazochrome sodium sulfonate hydrate in total knee arthroplasty. J Arthroplasty, 2012, 27: 105–108. [DOI] [PubMed] [Google Scholar]


