Abstract
Objective
Complex cobalt‐chromium alloys, bearing surfaces of the second‐generation metal‐on‐metal (MoM) hip prostheses, are subject to wear and generate cobalt nanoparticles (CoNPs). CoNPs could reduce cellular viability, activate the mitogen‐activated protein kinase (MAPK) pathway and increase cell apoptosis via reactive oxygen species (ROS). However, the detailed mechanisms of ROS functioning on CoNP‐mediated signaling molecules and cytotoxicity has not yet been fully demonstrated. The present study investigated the functional role of N‐acetylcysteine (NAC) in reversing the activation of ROS signaling pathways triggered by CoNPs in normal mice kidney cells (TCMK‐1 cells).
Methods
After being pretreated with NAC, TCMK‐1 cells were treated with 300–700 μmol/L CoNPs, then, CCK‐8 assay was used to verify the survival of TCMK‐1 cells. Annexin V/PI staining was performed to investigate the apoptosis of TCMK‐1 cells after NAC and different concentrations of CoNP treatments. In addition, western blot was performed to identify the cytokine (p‐ERK, p‐p38, and p‐JNK) expression of the ROS‐related MAPK signaling pathway.
Results
Apoptosis rate of TCMK‐1 cells was increased obviously after different concentrations of CoNP treatment. However, TCMK‐1 cells, pretreated with NAC, exhibited a significantly decreased apoptosis rate. In addition, p‐ERK, p‐p38, and p‐JNK expressions were increased with CoNP treatment, which indicated that CoNPs could activate the MAPK pathway. Interestingly, this entire stimulated phenomenon by CoNPs was reversed with NAC treatment.
Conclusions
These findings indicated that NAC could reverse CoNP‐induced cytotoxicity by inhibiting ROS‐induced cell death and cytokine expression. To our knowledge, this is the first report that describes how CoNP‐induced cytotoxicity in TCMK‐1 cells could be attenuated by anti‐oxidative agents (NAC), which may function through inhibition of cell death and ROS.
Keywords: Cobalt nanoparticle TCMK‐1 cells, Cytotoxicity, Metal‐on‐metal hip prostheses, N‐acetylcysteine
Introduction
Over the past ten years, there has been a rapid increase in the use of different nanomaterials owing to their unique physicochemical and bioreactive properties. Different metal oxide nanoparticles (NPs) are used in many products and industries, including sunscreen, food, paints, textiles, electronics, sports, biomedicine and imaging1, 2, 3, 4. The field of artificial joint replacement has been developing and improving over the past century. Total hip replacement is now used for the treatment of elderly patients with severe joint damage or femoral neck fractures. Total hip replacement is recognized as one of the most useful and cost‐effective surgical interventions for improving the life quality of patients5. Because of its function in reducing joint pain and joint deformity and improving the quality of life of patients, reconstruction of joint activity function has gradually been accepted by patients. Metal‐on‐metal (MoM) bearings, made of a cobalt‐chromium (CoCr) alloy, were first used in 19386. The high failure rate of polyethylene joints in those early years prompted the vast majority of studies paying attention to metal materials. At the end of the 1980s, as people gradually realized the high rate of bone loosening as a result of polyethylene wear, the study of MoM increased considerably. Improvement in materials greatly reduced the wear of metal prostheses; postoperative prosthesis loosening and bone dissolution were also reduced significantly7.
However, the surfaces of MOM bearings, made from CoCr alloys, could wear and generate CoCr nanoparticles. These nanoparticles can reduce cellular viability8, induce DNA damage9, 10, lead to chromosomal aberrations, and potentially increase metal hypersensitivity. These effects can be either local (soft‐tissue reactions) or systemic (arthroprosthetic cobaltism) responses10, 11.
Previous studies have demonstrated that nanoparticles can induce DNA damage, inflammatory reaction, and cell death, which can be mediated by reactive oxygen species (ROS12, 13). ROS can lead to the activation of several essential signaling pathways, including mitogen activated protein kinases (MAPKs), and the expression of corresponding downstream targets. MAPKs can regulate many cell biological changes, including aggravated inflammation and increased cell apoptosis rate. Cell death is a highly regulated process and a pivotal mechanism in the maintenance of tissue homeostasis in multicellular organisms. However, uncontrolled cell death can result in numerous pathophysiological conditions, such as cancer, neurodegenerative disorders, and inflammation. Apoptosis could be induced by cobalt nanoparticle accumulation. However, at present, there is no effective way to reduce cobalt nanoparticle accumulation and the cytotoxicity of cobalt ions.
There have been an increasing number of studies focusing on the in vitro cytotoxic effects of CoCr nanoparticles. MoM artificial joint has been used in a large number of joint surgeries, but its prognosis is affected by physical and chemical factors and the molecular and immune toxicity caused by CoCr nanoparticles. Cobalt nanoparticles, one of the degradation products of MoM that can cause cell toxicity, are receiving greater attention. Moreover, the detailed mechanisms deserve further research, which might help create a foundation for investigating new treatment strategies. N‐acetylcysteine (NAC), a sulfur alcohol compound, is an effective precursor of glutathione that has been used widely as an antioxidant. Lee et al. report that NAC can attenuate hexavalent chromium‐induced hypersensitivity through inhibition of cell death, ROS‐related signaling, and cytokine expression14. The sulphur structure of NAC can bind to the electrophilic group of ROS, and, thus, reduce cytotoxic effects. NAC can pass through the cell membrane and strip acetyl. In addition, the generated ammonia acid of NAC is a precursor of glutathione (GSH). It is well known that GSH, an antioxidant, plays an important role in preventing nanoparticles from damaging cells. The antioxidative activity of NAC can be attributed to its reactions with a variety of ROS species, such as superoxide, hydrogen‐peroxide, and peroxynitrite.
However, whether NAC can attenuate cobalt nanoparticle (CoNP)‐induced cytotoxic effects through inhibition of TCMK‐1 cells death; ROS‐related signaling and cytokine expression is unknown. The present study aims to illuminate: (i) the apoptosis rate of TCMK‐1 cells with CoNP treatment at different time points; (ii) whether NAC can attenuate CoNP‐induced cytotoxic effects in TCMK‐1 cells; and (iii) whether NAC can reverse CoNP‐induced cytotoxic effects mediated by the ROS‐related signaling pathway.
Materials and Methods
Chemicals and Antibodies
Dulbecco's modified Eagle's medium (DMEM), penicillin, and streptomycin were purchased from Cibco BRL (Paisley, Scotland, UK). DMSO was purchased from Sigma Chemical (Poole, Dorset, UK). CoNPs and N‐acetyl‐L‐cysteine (NAC) were obtained from Merck Chemical (Darmstadt, Germany). A Cell Counter Kit‐8 (CCK‐8) was purchased from Dojindo Lab (Kumamoto, Japan). An Annexin V Apoptosis Detection Kit was purchased from BD Biosciences Pharmingen (San Diego, CA, USA). Monoclonal anti‐ERK antibody, anti‐pERK antibody, anti‐JNK antibody, anti‐pJNK antibody, anti‐p38 antibody, and anti‐p‐p38 antibody were purchased from Cell Signaling Technology (Boston, MA, USA). Anti‐β‐actin antibody was purchased from Kangchen Bio‐tech (Shanghai, China). A Western Blot Detection Kit (ECL solution) was purchased from KeyGen Biotech (Nanjing, China).
TCMK‐1 Cells Culture
TCMK‐1 cells were a kind gift from the nephrology department (First Affiliated Hospital of Soochow University). The TCMK‐1 cells were cultured in DMEM medium (GIBCO, Grand Island, NY, USA) supplemented with 10% fetal calf serum (HyClone, South Logan, UT, USA), and 100 IU/mL penicillin and 100 mg/mL streptomycin (Life Technology, Grand Island, NY, USA). Cells were incubated at 37 °C in a humidified atmosphere containing 5% CO2. Exponentially growing cells were detached by 0.1% trypsin‐EDTA (GIBCO). For the treatment of NAC and CoNPs, 2 mmol/L stock solution was added to the culture medium in a concentrated form, gently mixed for 1 h. and then treated with different concentrations of CoNPs. The cells were then incubated for different periods of time.
CCK‐8 Cell Viability Assay
TCMK‐1 cells were incubated in 96‐well plates and then treated with NAC and different concentrations of CoNPs for 24 h. After that, 10 μL CCK‐8 was added per well. Subsequently, cells were incubated at 37 °C for another 4 h. For detection, the optical density was measured by an ELISA reader (Emax, Molecular Devices, Sunnyvale, CA, USA) at 570 nm and the amount of formazan generated was calculated.
Detection of Early and Late Apoptosis with Annexin V/PI Staining
Apoptosis was assessed by observing the translocation of phosphatidyl serine to the cell surface, which could be detected by Annexin V/PI, usually being used to distinguish early apoptotic and normal cells from late apoptotic and necrotic cells. PI, a nucleic acid dye, cannot pass through the normal cells or early apoptosis cells because of their intact cell membrane, but it can pass through the cell membrane of late apoptotic and necrotic cells and dye nuclei red. The Annexin V/PI Apoptosis Detection Kit (Calbiochem, San Diego, CA, USA) was used as described previously. In brief, cells were pretreated with NAC (2 mmol/L) and exposed to CoNPs in a concentrated manner for 24 h subsequently. Then, cells were trypsinized, washed with 2× PBS, and centrifuged at 500 g for 5 min. After that, cells were resuspended in 10 mL of 16 Annexin V‐binding buffer (10 mmol/L HEPES [pH 7.4], 0.14 mol/L NaCl, and 2.5 mmol/L CaCl2) that contained 5 mL Annexin V and 5 mL PI, and incubated at room temperature for 15 min. Finally, 1× binding buffer (500 mL) was added to stop the reaction, and the stained cells were collected for flow cytometry and fluorescent inverted microscope analyses.
Western Blot Analysis
Total cellular protein lysates were prepared by harvesting cells with protein extraction buffer (10 mmol/L Tris‐HCl, pH 7, 140 mmol/L sodium chloride, 3 mmol/L magnesium chloride, 0.5% [w/v] NP‐40, 2 mmol/L phenylmethylsulfonyl fluoride, 1% [w/v] aprotinin, and 5 mmol/L dithiothreitol) for 1 h at 4 °C. After that, proteins were loaded at 50 mg/lane on 12% (w/v) sodium dodecylsulfate‐polyacrylamide gels, subjected to electrophoresis, blotted, probed using antibodies and detected by a chemiluminescence (ECL) detection system (WBKLS0500, Millipore, Billerica, MA, USA). Then, proteins were used for the determination of apoptosis and the expression of cytokines in the MAPK pathway. β‐Actin was used as the protein loading control. The densities of the bands were quantified with a computer densitometer (AlphaImager 2200 System Alpha Innotech Corporation, San Leandro, CA, USA).
Statistical Analysis
All data represented the mean ± SD of at least three independent experiments. Experimental data were analyzed using Student's t‐test. Differences were considered statistically significant when P < 0.05. Images are representations of three or more experiments.
Results
Cytotoxicity Induced by Cobalt Nanoparticles in TCMK‐1 Cells
The toxicity of CoNPs on TCMK‐1 cell line was determined when the cells grew to a high degree of confluence (2 × 105 cells). Cells were treated with 300–700 μmol/L CoNPs and the effect of CoNPs on TCMK‐1 cell survival was determined by CCK‐8 assay 4 or 24 h later. The percentage of survived cells strikingly decreased with the increase of CoNP concentration (Fig. 1A,B). The IC50 value was 600 and 450 μmol/L 4 and 24 h later, respectively.
Figure 1.
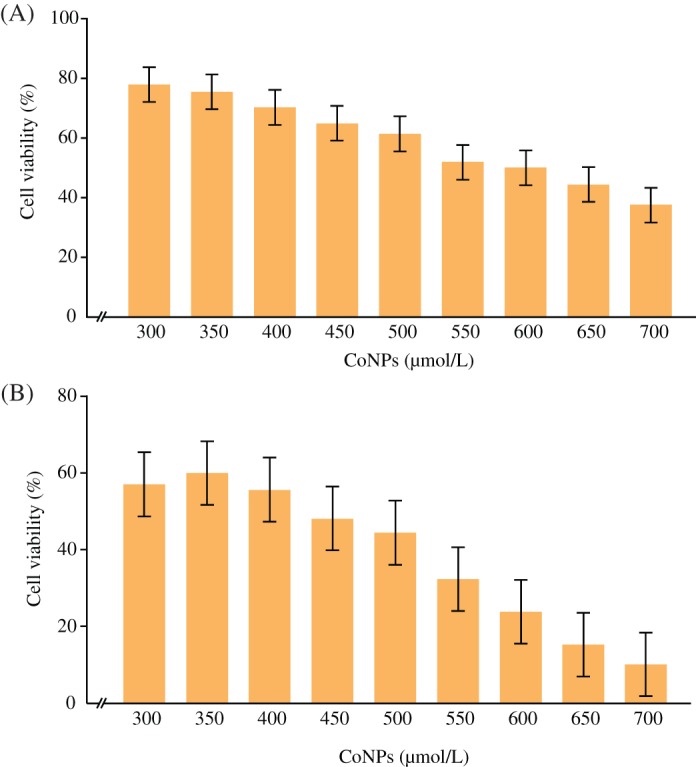
Cytotoxicity induced by cobalt nanoparticles (CoNPs). (A) Cells were incubated in the presence of varying concentrations of CoNPs for 4 h. Cell viability was determined by CCK‐8 assay. The data are presented as mean ± SD values of three independent experiments performed in triplicate. (*Significant vs CoNP‐treated group [300 μmol/L]; P < 0.05). (B) Cells were incubated in the presence of varying concentrations of CoNPs for 24 h. Cell viability was determined by CCK‐8 assay. The data are presented as mean ± SD values of three independent experiments performed in triplicate. (*Significant vs CoNP‐treated group [300 μmol/L]; P < 0.05).
Protective Effects of N‐acetylcysteine on Cobalt Nanoparticle‐induced Cytotoxicity
The viability of cultured cells was determined by CCK‐8 assay. Cells cultured in the medium with varying concentrations of NAC, but free from CoNPs, showed a dose‐dependent apoptosis rate. When the concentration of NAC increased, the viability of TCMK‐1 cells decreased. However, when cells were cultured in 450 μmol/L CoNPs and different concentrations of NAC, the viability of cells was significantly higher compared with the cells treated with CoNPs alone (Fig. 2).
Figure 2.
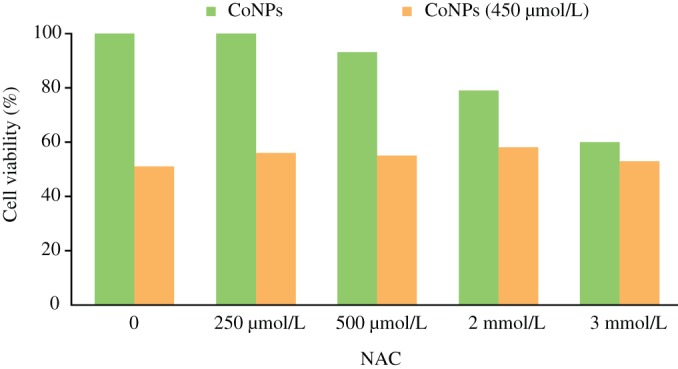
Effects of N‐acetylcysteine (NAC) on cell viability. Cells pretreated with different concentrations of NAC (0, 250, 500 μmol/L, 2, 3 mmol/L) were cultured for 1 h, and then incubated in the presence of 450 μmol/L cobalt nanoparticles (CoNPs) for another 24 h. Cell viability was determined by CCK‐8 assay. The data are presented as mean ± SD values of three independent experiments performed in triplicate. (*Significant vs no CoNP‐treated group; P < 0.05).
Nanoparticle‐attenuated Cr (VI)‐induced Apoptosis
To investigate whether there is a difference in the early apoptosis and late apoptosis induced by 450 μmol/L CoNPs and various concentrations of NAC, Annexin V/PI staining was carried out. Early apoptotic cells, stained by Annexin V, were detected by green fluorescence, while late apoptotic cells, stained by PI, were detected by red fluorescence. The Annexin V/PI apoptosis detection kit was used for fluorescent inverted microscope detection and flow cytometry assay. Incubation with 450 μmol/L CoNPs for 24 h significantly increased the cell apoptosis rate compared with the control group. When NAC was added as a pretreatment before CoNP exposure, CoNP‐induced apoptosis was attenuated (Fig. 3A,B). These results were further confirmed by the flow cytometry (Fig. 3C,D).
Figure 3.
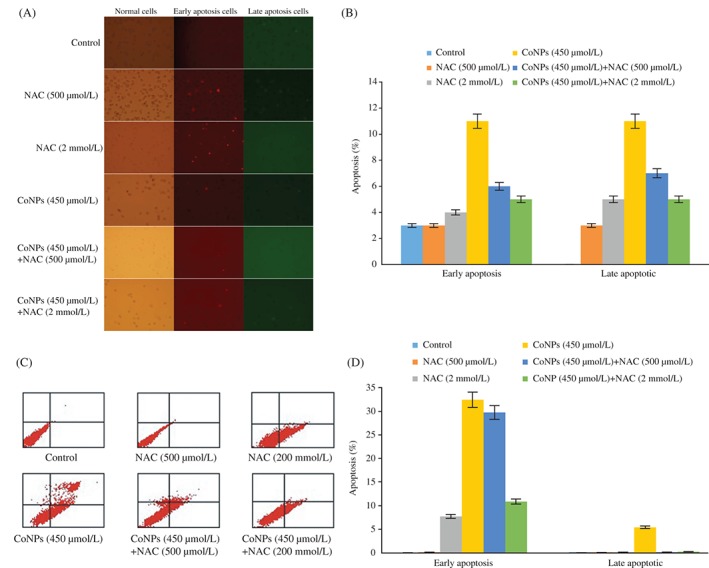
N‐acetylcysteine (NAC) attenuated Cr (VI)‐induced apoptosis. Cells were pretreated with different concentrations of NAC (500 μmol/L, 2 mmol/L) for 1 h and then incubated in the presence of cobalt nanoparticles (CoNPs) (450 μmol/L) for another 24 h. (A) Early apoptosis and late apoptosis were detected by fluorescent inverted microscope. (B) Percentage of apoptotic cells was calculated. The data are presented as mean ± SD values of three independent experiments performed in triplicate. (*Significant vs early apoptosis group, P < 0.05; *Significant vs late apoptosis group, P < 0.05). (C) Early apoptosis and late apoptosis were detected by flow cytometry. (D) Quantification of TCMK‐1 cells apoptosis. The data are presented as mean ± SD values of three independent experiments performed in triplicate. (*Significant vs early apoptosis group, P < 0.05).
Effects of N‐acetylcysteine on Cobalt Nanoparticle‐Mediated MAPK Pathway Activation
TCMK‐1 cells were treated with NAC alone, or CoNPs alone or in combination, then the activation of the MAPK pathway was investigated. As shown in Fig. 4, treatment with NAC (500 μmol/L or 2 mmol/L) effectively inhibited the activation of ERK (with 500 mmol/L NAC treatment), p38 and JNK (in 2 mmol/L NAC treatment). Previous studies showed that Cr (VI) could activate Akt, NFKB and MAPK pathways in certain cell types, especially in the lung15. In this study, we demonstrated that, in mice renal tubular epithelial cells, CoNPs could activate the MAPK pathway and that pretreatment with NAC could effectively reverse this process.
Figure 4.
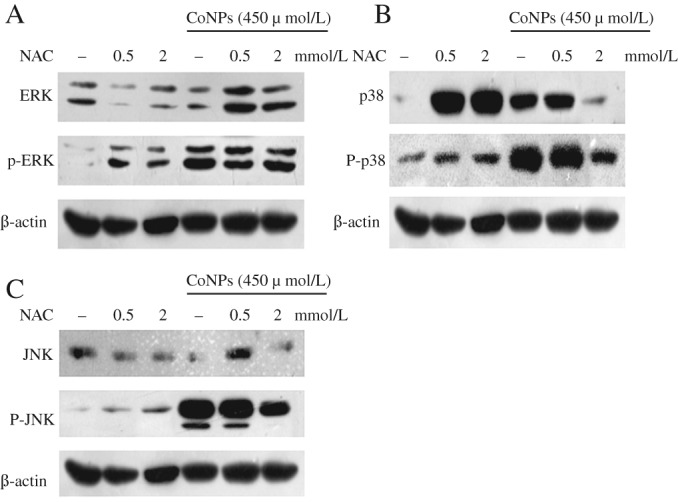
The expression of mitogen‐activated protein kinase (MAPK)‐related proteins (A, ERK, B, P38, C, JNK) was analyzed by western blot. In three independent experiments, TCMK‐1 cells were exposed to 450 μmol/L cobalt nanoparticles (CoNPs) for 4 h. CoNPs activated the MAPK pathway in TCMK‐1 cells. NAC (2 mmol/L) effectively inhibited the activation of p‐ERK, p‐p38, and p‐JNK.
Discussion
Cobalt toxicity is receiving more and more attention because of the adverse reaction of MoM joint prostheses. MoM prostheses could cause the generation of pseudotumors (significant series of soft tissue reactions, extensive tissue necrosis, and bone loss16), high blood metal ion level17, and early revision rate18. Investigation of periprosthetic tissue showed that the presence of metal wear debris was mainly in the form of cobalt–chromium nanoparticles, and cobalt was the most likely reactive agent19. However, the mechanisms of CoCr nanoparticle‐induced toxicity remain unknown. The present study focused on investigating the mechanisms of CoNP function, aiming to elucidate the role of cobalt in adverse reactions in vitro. It was shown that MAPK pathway played an essential role in CoNP‐induced toxicity in a mouse renal tubular epithelial cell model, TCMK‐1 cell line.
The functions of renal tubular cells include reabsorbing nutrients and excreting urine. Renal tubular epithelial cells are essential in renal function, and can absorb almost all the urine glucose and amino acids, and excrete all of the other non‐shedding nutrients into the urine. However, it is also very commonly involved in many congenital diseases, metabolic diseases, and immune diseases. Ischemia, infection and poison can induce renal tubular epithelial cell degeneration and necrosis, which can lead to kidney failure. Studies in recent years have shown that abnormal apoptosis of renal tubular epithelial cells may affect the occurrence and progression of acute kidney injury. CoNPs are extracted through the renal tubular.
The classical MTT assay is widely used for evaluating cell viability, but it may lead to false interpretations due to interferences of the nanoparticles with assay components. Thus, in the present study, the CCK‐8 cell viability assay was used to evaluate TCMK‐1 cell viability. We compared the viability of TCMK‐1 cells exposed to CoNPs and NAC and the results showed that exposure to CoNPs (4 or 24 h) decreased the viability of TCMK‐1 cells statistically (Fig. 1). When the concentration of NAC increased, the viability of TCMK‐1 cells decreased. However, when cells were cultured in 450 μmol/L CoNPs and different concentrations of NAC, cell viability was significantly increased relative to being treated with CoNPs alone (Fig. 2).
Cell death (apoptosis, necrosis, and autophagy) is a highly regulated process and a pivotal mechanism in the maintenance of tissue homeostasis in multicellular organisms. However, uncontrolled cell death can result in numerous pathophysiological conditions, including cancer, neurodegenerative disorders, and inflammation20, 21. Apoptosis, an orderly process, is a reaction of cells in response to physiological and pathological signals of the environment. The changes of apoptotic cell and tissue are obviously different from those of necrotic cells and tissues. Among the different cell death types, deregulation of epidermal keratinocyte apoptosis has been demonstrated as a pivotal pathological mechanism in cutaneous inflammatory diseases22. Apoptotic cells can carry important and complex information for the regulation of downstream immune response in a context‐dependent manner23. Apoptosis could be observed in renal tubular epithelial cell treated with CoNPs (Fig. 3). Apoptosis occurring at a late stage was detected by Annexin V/PI staining. Apoptosis involves two phases: early apoptosis and late apoptosis. Our study showed that the apoptosis of TCMK‐1 cells increased when the concentration of NAC increased. However, NAC could obviously reverse cell apoptosis, including early apoptosis and late apoptosis (Fig. 3).
At the molecular level, NAC has been shown to inhibit the activation of c‐Jun N‐terminal kinase, p38 MAP kinase, redox sensitive activating protein‐1 (AP‐1), and the NF‐kB transcriptional factors, thereby resulting in the suppression of numerous genes regulating the expression of many cytokines, such as TNF‐a and IL‐124, 25, 26, 27, 28. Some recent studies report that injection of CoNPs into guinea pigs (GP) models could induce skin hypersensitivity through a cytokines‐dependent increase in ROS production15. These results suggest that Cr (VI) might serve as both an antigen and a ROS producer to induce chromium hypersensitivity. Thus, antioxidative agents that can reduce the formation of ROS might provide be a potential approach to prevent CoNP‐induced apoptosis by interfering with the effects of ROS on cytokines (e.g. TNF‐a, IL‐1) by keratinocytes. In the present study, CoNPs were used to build the TCMK‐1 cells model. As expected, we found that CoNPs activated the MAPK pathway, accompanied with an increased expression of p‐ERK, p‐p38, and p‐JNK (Fig. 4). To explore new strategies to inhibit the CoNP‐caused cytotoxicity, NAC, one of the most common antioxidants, was used to investigate the effects of ROS in the pathogenesis of many oxidative stress‐related diseases26, 29, 30. Interestingly, the results revealed that NAC could significantly prevent the development of CoNP‐induced cytotoxicity by inhibiting ROS‐induced cell death and cytokine expression. In some cell lines, ROS‐regulated redox sensitive protein kinases and transcription factors, such as those involved in the Akt, NF‐kB, and MAPK pathways, might affect the release of cytokines, such as TNF‐a and IL‐125, 31, 32, 33. In normal mice kidney cells, CoNPs could increase the formation of ROS, activate the MAPK pathway, upregulate the expression of p‐ERK and p‐JNK and release of p‐p38 (Fig. 4). The antioxidative activity of NAC can be attributed to its reactions with a variety of ROS species, such as superoxide, hydrogen‐peroxide, and peroxynitrite34. This could explain why we observed broad spectrum effects of NAC in attenuating ROS generation and MAPK signaling.
In conclusion, the present study revealed that a concurrent activation of p‐ERK, p‐p38, and p‐JNK MAPK pathway is involved in CoNP‐induced cell death. NAC could protect TCMK‐1 cells against chemical hypoxia‐induced injuries through inhibiting ROS‐activated p‐ERK, p‐p38, and p‐JNK MAPK pathway. These results may have importantly scientific significance and practical significance and provide new evidence for clinical treatment after joint replacement.
Grant Sources: This study was funded by the Jiangsu Natural Science Foundation (BK20150399).
Disclosure: None.
Contributor Information
Fan Liu, Email: 15152888271@163.com, Email: liufan19575@aliyun.com.
Hui‐lin Yang, Email: yanghuilin914@163.com.
References
- 1. Nohynek GJ, Lademann J, Ribaud C, Roberts MS. Grey goo on the skin? Nanotechnology, cosmetic and sunscreen safety. Crit Rev Toxicol, 2007, 37: 251–277. [DOI] [PubMed] [Google Scholar]
- 2. Armstead AL, Arena CB, Li B. Exploring the potential role of tungsten carbide cobalt (WC‐Co) nanoparticle internalization in observed toxicity toward lung epithelial cells in vitro. Toxicol Appl Pharmacol, 2014, 278: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heim J, Felder E, Tahir MN, et al. Genotoxic effects of zinc oxide nanoparticles. Nanoscale, 2015, 7: 8931–8938. [DOI] [PubMed] [Google Scholar]
- 4. Pauksch L, Hartmann S, Szalay G, Alt V, Lips KS. In vitro assessment of nanosilver‐functionalized PMMA bone cement on primary human mesenchymal stem cells and osteoblasts. PLoS One, 2014, 9: e114740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet, 2007, 370: 1508–1519. [DOI] [PubMed] [Google Scholar]
- 6. Amstutz HC. Hip resurfacing arthroplasty. J Am Acad Orthop Surg, 2006, 14: 452–453. [DOI] [PubMed] [Google Scholar]
- 7. Macpherson GJ, Breusch SJ. Metal‐on‐metal hip resurfacing: a critical review. Arch Orthop Trauma Surg, 2011, 131: 101–110. [DOI] [PubMed] [Google Scholar]
- 8. Behl B, Papageorgiou I, Brown C, et al. Biological effects of cobalt‐chromium nanoparticles and ions on dural fibroblasts and dural epithelial cells. Biomaterials, 2013, 34: 3547–3558. [DOI] [PubMed] [Google Scholar]
- 9. Mittal S, Pandey AK. Cerium oxide nanoparticles induced toxicity in human lung cells: role of ROS mediated DNA damage and apoptosis. Biomed Res Int, 2014, 2014: 891934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paget V, Moche H, Kortulewski T, et al. Human cell line‐dependent WC‐Co nanoparticle cytotoxicity and genotoxicity: a key role of ROS production. Toxicol Sci, 2015, 143: 385–397. [DOI] [PubMed] [Google Scholar]
- 11. Horie M, Fujita K, Kato H, et al. Association of the physical and chemical properties and the cytotoxicity of metal oxide nanoparticles: metal ion release, adsorption ability and specific surface area. Metallomics, 2012, 4: 350–360. [DOI] [PubMed] [Google Scholar]
- 12. Landsiedel R, Kapp MD, Schulz M, Wiench K, Oesch F. Genotoxicity investigations on nanomaterials: methods, preparation and characterization of test material, potential artifacts and limitations‐‐many questions, some answers. Mutat Res, 2009, 681: 241–258. [DOI] [PubMed] [Google Scholar]
- 13. Karlsson HL. The comet assay in nanotoxicology research. Anal Bioanal Chem, 2010, 398: 651–666. [DOI] [PubMed] [Google Scholar]
- 14. Lee YH, Su SB, Huang CC, et al. N‐acetylcysteine attenuates hexavalent chromium‐induced hypersensitivity through inhibition of cell death, ROS‐related signaling and cytokine expression. PLoS One, 2014, 9: e108317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang BJ, Guo YL, Chang HY, et al. N‐acetylcysteine inhibits chromium hypersensitivity in coadjuvant chromium‐sensitized albino guinea pigs by suppressing the effects of reactive oxygen species. Exp Dermatol, 2010, 19: e191–e200. [DOI] [PubMed] [Google Scholar]
- 16. Pandit H, Glyn‐Jones S, McLardy‐Smith P, et al. Pseudotumours associated with metal‐on‐metal hip resurfacings. J Bone Joint Surg Br, 2008, 90: 847–851. [DOI] [PubMed] [Google Scholar]
- 17. Cobb AG, Schmalzreid TP. The clinical significance of metal ion release from cobalt‐chromium metal‐on‐metal hip joint arthroplasty. Proc Inst Mech Eng H, 2006, 220: 385–398. [DOI] [PubMed] [Google Scholar]
- 18. Huang DC, Tatman P, Mehle S, Gioe TJ. Cumulative revision rate is higher in metal‐on‐metal THA than metal‐on‐polyethylene THA: analysis of survival in a community registry. Clin Orthop Relat Res, 2013, 471: 1920–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hart AJ, Quinn PD, Lali F, et al. Cobalt from metal‐on‐metal hip replacements may be the clinically relevant active agent responsible for periprosthetic tissue reactions. Acta Biomater, 2012, 8: 3865–3873. [DOI] [PubMed] [Google Scholar]
- 20. Degterev A, Yuan J. Expansion and evolution of cell death programmes. Nat Rev Mol Cell Biol, 2008, 9: 378–390. [DOI] [PubMed] [Google Scholar]
- 21. Rock KL, Kono H. The inflammatory response to cell death. Annu Rev Pathol, 2008, 3: 99–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Raj D, Brash DE, Grossman D. Keratinocyte apoptosis in epidermal development and disease. J Invest Dermatol, 2006, 126: 243–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Poon IK, Lucas CD, Rossi AG, Ravichandran KS. Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol, 2014, 14: 166–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Faurschou A, Gniadecki R. TNF‐alpha stimulates Akt by a distinct aPKC‐dependent pathway in premalignant keratinocytes. Exp Dermatol, 2008, 17: 992–997. [DOI] [PubMed] [Google Scholar]
- 25. Gaestel M, Kotlyarov A, Kracht M. Targeting innate immunity protein kinase signalling in inflammation. Nat Rev Drug Discov, 2009, 8: 480–499. [DOI] [PubMed] [Google Scholar]
- 26. Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N‐acetylcysteine actions. Cell Mol Life Sci, 2003, 60: 6–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rao GN, Katki KA, Madamanchi NR, Wu Y, Birrer MJ. JunB forms the majority of the AP‐1 complex and is a target for redox regulation by receptor tyrosine kinase and G protein‐coupled receptor agonists in smooth muscle cells. J Biol Chem, 1999, 274: 6003–6010. [DOI] [PubMed] [Google Scholar]
- 28. Vlahopoulos S, Boldogh I, Casola A, Brasier AR. Nuclear factor‐kappaB‐dependent induction of interleukin‐8 gene expression by tumor necrosis factor alpha: evidence for an antioxidant sensitive activating pathway distinct from nuclear translocation. Blood, 1999, 94: 1878–1889. [PubMed] [Google Scholar]
- 29. Bickers DR, Athar M. Oxidative stress in the pathogenesis of skin disease. J Invest Dermatol, 2006, 126: 2565–2575. [DOI] [PubMed] [Google Scholar]
- 30. Luo J, Tsuji T, Yasuda H, Sun Y, Fujigaki Y, Hishida A. The molecular mechanisms of the attenuation of cisplatin‐induced acute renal failure by N‐acetylcysteine in rats. Nephrol Dial Transplant, 2008, 23: 2198–2205. [DOI] [PubMed] [Google Scholar]
- 31. Sen CK, Packer L. Antioxidant and redox regulation of gene transcription. FASEB J, 1996, 10: 709–720. [DOI] [PubMed] [Google Scholar]
- 32. Haddad JJ, Fahlman CS. Redox‐ and oxidant‐mediated regulation of interleukin‐10: an anti‐inflammatory, antioxidant cytokine? Biochem Biophys Res Commun, 2002, 297: 163–176. [DOI] [PubMed] [Google Scholar]
- 33. Winyard PG, Blake DR. Antioxidants, redox‐regulated transcription factors, and inflammation. Adv Pharmacol, 1997, 38: 403–421. [DOI] [PubMed] [Google Scholar]
- 34. Samuni Y, Goldstein S, Dean OM, Berk M. The chemistry and biological activities of N‐acetylcysteine. Biochim Biophys Acta, 2013, 1830: 4117–4129. [DOI] [PubMed] [Google Scholar]


