Abstract
Objective
To assess the clinical efficacy of mini‐invasive transforaminal lumbar interbody fusion (TLIF) through the Wiltse approach for treating lumbar spondylolytic spondylolisthesis.
Methods
In this retrospective controlled study, 69 cases with lumbar spondylolytic spondylolisthesis treated in Qilu hospital from April to November 2014 were randomly assigned to Wiltse approach (31 cases, 16 male, 15 female; mean age 45.1 years) and traditional approach groups (38 cases, 21 male, 17 female; 47.2 years. In the Wiltse approach group, the affected level was L4, 5 in 19 cases and L5S1 in 12, 9 of whom had low back pain (LBP) only and 21 both LBP and leg pain. There were 17 cases of I degree and 14 of II degree spondylolisthesis. Pre‐operative Japanese Orthopedic Association (JOA) score was 13.1 ± 2.6; visual analog scale (VAS) for LBP 7.4 ± 1.2; VAS for leg pain 6.1 ± 2.0 and Oswestry disability index (ODI) score 42.2% ± 1.2%. In the traditional approach group, the affected level was L4, 5 in 22 cases and L5S1 in 16, 11 of whom had LBP only and 27 both LBP and leg pain. There were 21 cases of I degree and 17 of II degree spondylolisthesis. Pre‐operative JOA score was 12.8 ± 1.2; VAS for LBP 6.9 ± 1.1; VAS for leg pain 7.1 ± 2.0 and ODI score 41.2% ± 2.0%. The JOA score, VAS for LBP and leg pain, ODI dynamic X‐rays, CT and/or MR were evaluated 3 and 6 months and 1 year postoperatively.
Results
There were no differences in sex, age, affected levels, spondylolisthesis degree, pre‐operative JOA score, VAS for LBP or leg pain and ODI score between the two groups (P > 0.05). The incision length, blood loss and time to achieving exposure were better in the Wiltse approach than the traditional approach group (P < 0.05). The VAS for LBP and muscle atrophy MRI scores were significantly lower in the Wiltse approach than the traditional approach group on Days 1 and 14 and at 1 year follow‐up (P < 0.05). The VAS for leg pain, JOA recovery rate and JOA and ODI scores tended to be lower in the Wiltse approach than the traditional approach group at 1 year follow‐up examinations (no differences statistically significant, P > 0.05). The interbody fusion rate was not significantly different between the groups (P > 0.05). There were no complications of internal fixation in either group.
Conclusion
TLIF via both approaches has satisfactory clinical efficacy. TLIF through the Wiltse approach significantly reduces the damage of multifidus and postoperative incidence of chronic LBP.
Keywords: Lumbar spondylolisthesis, Multifidus, Transforaminal lumbar interbody fusion, Wiltse approach
Introduction
The posterior erector spinae is mainly composed of the multifidus, longissimus dorsi and iliocostalis muscles. The multifidus originates from the mastoid and covers two to four segments in an oblique direction, attaching to the spinous process.1 The multifidus is controlled by the middle branch of the dorsal root2 and plays an important role in maintaining lumbar segmental stability.3 The traditional posterior lumbar approach requires stripping of paraspinal muscles on both sides of the spinous process to expose the lamina and facet joints. Operative thermal or mechanical injuries (electric knife stripping, caused by automatic retractors) can easily damage the multifidus muscle and its nerves, leading to postoperative paraspinal muscle atrophy. Stripping can damage the multifidus directly and bleeding can also result in fibrosis and scarring in this muscle.4 The middle branch of the dorsal root may be damaged when stripping the multifidus, resulting in denervation and accumulation of fatty tissue.5 Recent studies have shown that paraspinal muscle atrophy is an important cause of postoperative lumbar pain.6 In 1959, Watkins reported a surgical approach through the gap between the erector spinae and quadratus lumborum and used it to perform fusion of the lumbar and lumbosacral spine.7 In 1968, Wiltse et al. modified this surgical route to the gap between the multifidus and longissimus (paraspinal approach).8 In 1988, Wiltse and Spencer used the paraspinal approach to remove a far lateral disc, decompress a far‐out syndrome and insert pedicle screws.9 Kawaguchi et al. reported that creatine kinase concentrations can increase when the paraspinal muscle is extensively stripped.10 Clinical research has shown that postoperative low back pain (LBP) and functional disability, increases in MRI T2 signals and weakening of trunk strength are closely related to the duration of muscular traction.11, 12 Gejo et al. reported that paraspinal muscle damage can occur during surgical repair; thus, it is important to further analyze whether early damage can induce long‐term atrophy and accumulation of fatty tissue.13 Recently, the Wiltse approach has been widely used for surgical management of thoracic or lumbar vertebral fractures,14 lumbar disc herniation (lateral or far lateral disc herniation),15 lumbar spinal stenosis (lateral recess stenosis)16 and other spinal pathologies.17 Additionally, thoracic disc herniation, spinal tuberculosis18 and degenerative lumbar scoliosis have been managed via the Wiltse approach.19
In this prospective study, we compared preoperative Japanese Orthopedic Association (JOA) scores, visual analog scale (VAS) scores for LBP and leg pain, incision length, blood loss, exposure time, Oswestry disability index (ODI) and postoperative JOA scores, VAS for LBP and leg pain and ODI to assess the clinical efficacy of mini‐invasive transforaminal lumbar interbody fusion (TLIF) via the Wiltse approach for the treatment of lumbar spondylolytic spondylolisthesis. We also studied the importance of the paraspinal muscles in maintaining lumbar segmental stability and reducing postoperative LBP.
Materials and Methods
Patients
In this prospective controlled study, 69 patients with lumbar spondylolytic spondylolisthesis treated by TLIF in Qilu hospital from April to November 2014 were randomly allocated to a Wiltse (mini‐invasive; 31 cases, 16 male, 15 female; mean age 45.1 years) or traditional approach (38 cases, 21 males, 17 females; 47.2 years). Inclusion exclusive criteria were as follows: (i) single segment lumbar spondylolisthesis; (ii) lumbar spondylolisthesis degree I or II; (iii) absence of complications such as spinal stenosis, disc herniation and others; and (iv) spondylolisthesis caused by spondylolysis.
In the Wiltse approach group, the affected level was L4,5 in 19 cases and L5S1 in 12 of whom 9 cases had only LBP and 21 both LBP and leg pain. Seventeen cases had spondylolisthesis degree I and 14 cases degree II.
In the traditional approach group, the affected level was L4,5 in 22 cases and L5S1 in 16 of whom 11 cases had only LBP and 27 both LBP and leg pain. Twenty‐one cases had I degree spondylolisthesis and 17 II degree spondylolisthesis.
JOA scores, VAS scores for LBP and leg pain, ODI scores, dynamic X‐rays, CT and/or MRI were evaluated 3 months, 6 months and 1 year after surgery. Interbody fusion was achieved with autogenous iliac bone particles in 62 cases (29 in the Wiltse and 33 in the traditional approach group), whereas interbody fusion devices were used in 7 cases (2 in the Wiltse and 5 in the traditional approach group).
Surgical Approach
TLIF via the Wiltse Approach
Step 1: patients were placed in a prone position under general anesthesia. The spondylolisthesis segments were located by “C”‐shaped arm X‐rays. Step 2: a 5 cm incision was made in the middle of the low back skin, the skin and subcutaneous tissues cut open and the subcutaneous tissue separated from the surface of the fascia to both sides of the midline (approximately 2.5 cm). Next, a longitudinal incision through the thoracolumbar fascia was made from the side of the multifidus and longissimus dorsi to reveal the facet joints and transverse processes of the operative segments, after which a minimally invasive retractor was put in place (B. Braun Melsungen AG; Melsungen, Germany). Step 3: facet joints were removed to reveal the intervertebral disc and the disc tissue and cartilage gradually removed with an intervertebral reamer. The right intervertebral space was implanted with autologous iliac bone particles or a polyetherketoneketone cage filled with bone particles. Step 4: pedicle screws were placed in the left L4 and L5 under direct vision. A rod was inserted on the left side and rotated to distract and correct the spondylolisthesis. The screw and rod system were locked with slight pressure. The same method was used on the opposite side. Step 5: the inter fixations were located by “C”‐shaped arm X‐rays (Fig. 1).
Figure 1.
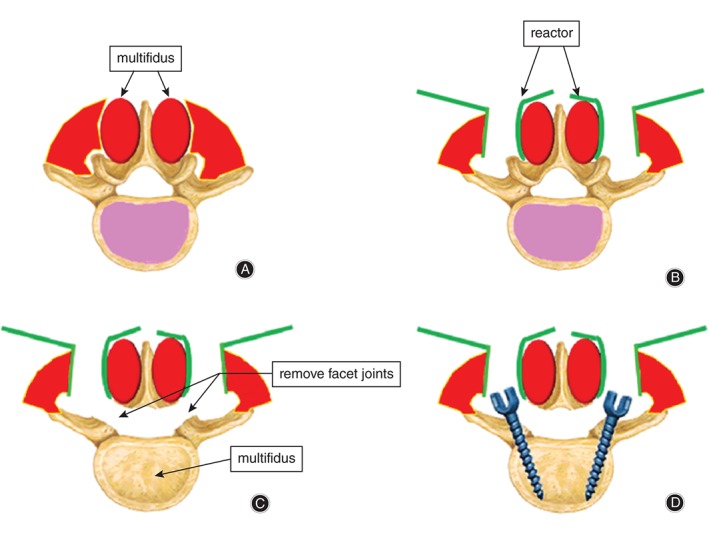
Schematic representation of the operative procedure via the Wiltse approach. (A) Incision of thoracolumbar fascia is made from the side of the multifidus and longissimus. (B) Placement of retractor between multifidus and longissimus muscle. (C) Facet joints and laminae are removed to reveal the intervertebral disc. (D) Fixation of the rod and screws.
TLIF via a Traditional Approach
Step 1: patients were placed in a prone position under general anesthesia. The segments of spondylolisthesis were located by “C”‐shaped arm X‐rays. Step 2: the skin and subcutaneous tissue were cut open in the middle line and the paraspinal muscles stripped off to reveal the facet joints and transverse processes of the diseased segments. Step 3: facet joints on both sides were removed to reveal the intervertebral disc, after which the disc tissue and cartilage were gradually removed with an intervertebral reamer. Intervertebral space was implanted with autologous iliac bone particles or polyetheretherketone cage filled with bone particles. Step 4: pedicle screws were placed in L4 and L5, after which a rod was inserted and rotated to distract and correct the spondylolisthesis. The screw and rod system were then locked with slight pressure. Step 5: the inter fixations were located by “C”‐shaped arm X‐rays.
Postoperative Treatment and Variables Assessed
Patients with good healing of the interbody according to appropriate examinations were allowed to walk while wearing a corset.
The JOA scoring system was used to evaluate LBP (maximum score: 29) and VAS scores as subjective and objective scoring criteria. Improvement was calculated according to the formula: improvement rate = (follow‐up score − preoperative score)/(29 − preoperative score) × 100%. VAS scores (0–10) were obtained for both LBP and leg pain, 0 indicating completely free of pain or numbness and 10 indicating severe painful or numbness. All VAS scoring was completed independently by the patients after the physician had briefly explained the required procedure. In patients with symptoms in both lower extremities, VAS scores for the side with more severe symptoms were used. Operative time, incision length, blood loss, JOA score (1 year after surgery), VAS score (1 year after surgery) and wound pain (3 days and 2 weeks after surgery) were compared between the two approaches. The ODI was used to evaluate quality of life (0–100%).20 The higher the ODI score, the worse the quality of life is.
Lateral and dynamic X‐rays, CT and MRI (1 year after surgery) were routinely performed. The surgery segments were scanned (Somatom Sensation 16 CT; Siemens, Erlangen, Germany) and 3‐D reconstruction of the lumbar spine obtained (layer thickness: 0.75 mm). A modified Brantigan scoring system (0–4)21 was used to evaluate intervertebral fusion as follows: (i) 4, complete fusion with good shape; (ii) 3, good fusion with faint transparent lines; (iii) 2, top and bottom parts connected (50%) with many transparent lines; (iv) 1, top and bottom parts unconnected, more bone present than had been implanted; and (v) 0, top and bottom parts unconnected, bones lost and implanted bone absorbed. Scores ≥3 were considered as fusion. Multifidus atrophy according to MRI was graded as normal, mild, moderate, and severe atrophy (3 to 0) as follows: (i) 3 (normal), no fiber or fatty tissue; (ii) 2 (mild), less than 10% fiber and fatty tissue; (iii) 1 (moderate), less than 50% fiber and fatty tissue; and (iv) 0 (severe atrophy), more than 50% fiber and fatty tissue.22 Paraspinal muscle atrophy was scored twice (0 and 15 days after scanning) by two radiologists. The final score was the average of the two scores. P < 0.05 was considered statistically significant.
Statistical Analysis
All statistical comparisons were performed using SPSS software version 12.0. The two‐group t‐test was used to compare incision length, operation time, exposure time, blood loss during exposure, total blood loss, incision pain (VAS) and 1‐year follow‐up examination scores (JOA, LBP and leg pain [VAS], muscle atrophy MRI) between the two groups. The χ2 test was used to compare the two groups’ interbody fusion rates. P < 0.05 was considered statistically significant.
Results
Pre‐operative Indexes for Wiltse and Traditional Approaches
For the Wiltse approach, the pre‐operative JOA score was 13.1 ± 2.6 (mean ± SD); VAS for LBP 7.4 ± 1.2; VAS for leg pain s 6.1 ± 2.0 and ODI score 42.2% ± 1.2%. For the traditional approach, the pre‐operative JOA score was 12.8 ± 1.2; VAS for LBP 6.9 ± 1.1; VAS for leg pain 7.1 ± 2.0 and ODI score 41.2% ± 2.0%. There were no differences in sex, age, affected levels, spondylolisthesis degree, pre‐operative JOA score, VAS for LBP or leg pain or ODI scores between the Wiltse and traditional approach groups (all P > 0.05). The VAS scores for incision pain on postoperative Days 3 and 14 days were 4.3 ± 0.2 and 2.1 ± 0.5, respectively in the Wiltse and 6.8 ± 0.3 and 3.0 ± 0.6, respectively, in the traditional approach group; these differences are statistically significant (P < 0.00 on Day 3; P < 0.05 on Day 14).
General Operative Indexes for the Wiltse and Traditional Approaches
For the Wiltse approach, the incision length was 4.9 ± 0.8 cm, operation time 160.0 ± 5.3 min, exposure time 17.3 ± 3.8 min, blood loss during exposure 26.1 ± 4.3 mL and total blood loss 154.43 ± 20.32 mL. For the traditional approach, the incision length was 7.3 ± 1.2 cm; operation time 158.0 ± 3.9 min; exposure time 31.0 ± 8.4 min, blood loss during exposures 72.0 ± 10.2 mL and total blood loss 249.89 ± 18.21 mL. The incision length, blood loss and exposure time were all significantly better in the Wiltse than the traditional approach group (all P < 0.05, Table 1). The exposure time in the Wiltse approach group was nearly half that of the traditional approach group.
Table 1.
General operative indexes for the Wiltse and traditional approaches (mean ± SD)
| Group | Incision length (cm) | Operation time (min) | Exposure time (min) | Blood loss during exposure (mL) | Total blood loss (mL) |
|---|---|---|---|---|---|
| Wiltse approach | 4.9 ± 0.8 | 160.0 ± 5.3 | 17.3 ± 3.8 | 26.1 ± 4.3 | 154.43 ± 20.32 |
| Traditional approach | 7.3 ± 1.2 | 158.0 ± 3.9 | 31.0 ± 8.4 | 72.0 ± 10.2 | 249.89 ± 18.21 |
| Statistical value | t = 2.67 | t = 0.45 | t = 2.89 | t = 3.96 | t = 2.92 |
| P < 0.01 | P > 0.05 | P < 0.01 | P < 0.00 | P < 0.01 |
Clinical Indexes for the Wiltse and Traditional Approaches
The average follow‐up times were 12.6 and 13.1 months for the Wiltse traditional approach groups, respectively. The post‐operative JOA scores, VAS for LBP and leg pain and ODI scores were significantly improved after surgery in both groups. The VAS for LBP and muscle atrophy MRI scores were lower in the Wiltse than the traditional approach group on Days 1 and 14 and at the 1 year follow‐up examinations; these differences are statistically significant (all P < 0.05). The VAS for leg pain and JOA and ODI scores were lower in the Wiltse than the traditional approach group at 1 year follow‐up; however, this difference was not statistically significant (P > 0.05). The JOA recovery rate was higher in the Wiltse than the traditional approach group at 1 year follow‐up; however, this difference was not statistically significant (P > 0.05). The interbody fusion rate did not differ significantly between the Wiltse and traditional approach groups (P > 0.05, Table 2).
Table 2.
Clinical efficacies of Wiltse and traditional approaches at 1 year follow‐up (mean ± SD)
| Group | JOA scores | JOA recovery rate (%) | LBP VAS | Leg pain VAS | Interbody fusion rate (%) | Muscle atrophy scores | ODI (%) |
|---|---|---|---|---|---|---|---|
| Wiltse approach | 24.7 ± 3.5 | 77.0 ± 3.1 | 1.0 ± 0.7 | 1.1 ± 0.3 | 71.0 ± 5.8 | 2.2 ± 0.2 | 11.1 ± 1.8 |
| Traditional approach | 24.3 ± 2.8 | 73.6 ± 6.4 | 3.1 ± 0.6 | 1.8 ± 0.5 | 76.3 ± 6.1 | 1.4 ± 0.1 | 17.8 ± 0.9 |
| Statistical value | t = 0.55 | U = 1.54 | t = 2.53 | t = 0.41 | χ2 = 0.39 | t = 3.77 | U = 1.22 |
| P > 0.05 | P > 0.05 | P < 0.00 | P > 0.05 | P > 0.05 | P < 0.00 | P > 0.05 |
JOA, Japanese Orthopedic Association; LBP, low back pain; ODI, Oswestry disability index.
There were no complications of internal fixation in either group (Fig. 2). Twenty‐two cases (71.0%) in the Wiltse and 29 (76.3%) in the traditional approach group had Brantigan scores ≥3.
Figure 2.
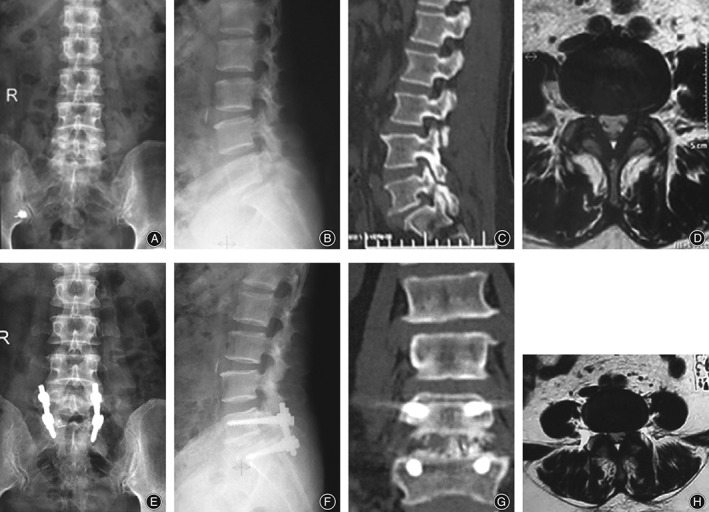
Spondylolytic spondylolisthesis (L5S1) in a 56‐year‐old woman (A, B) Pre‐operative X‐ray films showing spondylolytic spondylolisthesis (L5S1). (C) Pre‐operative CT image showing spondylolytic spondylolisthesis. (D) Pre‐operative T2 WI MR image showing mild multifidus atrophy. (E, F) One year postoperative X‐ray films showing excellent intervertebral fusion after TLIF via the Wiltse approach. (G) Three months postoperative CT image showing interbody fusion has been achieved. (H) One year postoperative T2 WI MR image showing the appearance of the multifidus does not differ significantly from its pre‐operative appearance.
Discussion
Advantages of Mini‐invasive TLIF via the Wiltse Approach
Our study showed that both the Wiltse and traditional approaches achieve satisfactory efficacy; however, the Wiltse approach has the following advantages.
Protection of Multifidus Muscle and Reduction in Incidence of LBP
The traditional approach requires extensive dissection of the multifidus, which damages its blood supply and nerves, leading to postoperative multifidus degeneration manifested as muscle atrophy, fibrosis and fat deposition.23 In addition, during the surgery, the paravertebral muscles need to be extensively distracted and the highest pressure occurs within the innermost multifidus, resulting in interruption to the local blood supply and occurrence of irreversible ischemic degeneration and necrosis.24 As a consequence, the physiological function of the multifidus is affected and the incidence of postoperative chronic LBP increased.25 In contrast, the Wiltse approach is via a natural gap between the multifidus and longissimus dorsi and does not require stripping or extensive distraction of the paraspinal muscles, thus avoiding denervation and subsequent degeneration of the multifidus and reducing any ischemic degeneration and necrosis as a result of interruption to the local intramuscular blood supply. Therefore, with this approach the multifidus better retains its physiological functions, the stability of the spine is maintained and the incidence of chronic LBP reduced. There was significantly less severe local wound pain in the Wiltse than the traditional approach group on postoperative Days 3 and 14 days. At the last follow‐up examination, the VAS score of LBP was only 1.0 ± 0.7, which is far less than that of the traditional approach group. Furthermore, the MRI score was significantly lower in the Wiltse than the traditional group at the last follow‐up examination. Therefore, the Wiltse approach causes less muscle damage and reduces the incidence of LBP.
Simplicity with Less Bleeding
In all cases of spondylolysis, the procedures of pedicle screw placement, facet joints resection and interbody bone implantation can easily be completed via the natural gap between the multifidus and longissimus dorsi without distraction of the paraspinal muscles. The Wiltse approach thus minimizes the extent and duration of muscle distraction. In addition, better surgical site exposure is achieved than with the traditional approach. Provided the muscle gap is accurately located and the facet joints and transverse processes carefully revealed, there is very little bleeding. In our study, there was significantly less blood loss than with the traditional approach. Blood loss with the Wiltse approach is mainly from bone surface bleeding after osteotomy and bleeding associated with rupture of the spinal venous plexus. Furthermore, the whole process via the Wiltse approach is under direct vision, making it easier to perform than an endoscopic procedure.
Combination with Minimally Invasive Retraction Further Reduces Retraction Injuries of the Multifidus
After revealing the facet joints by the Wiltse approach, a horns retractor is usually required to fully expose the surgical field. Because such retractors produce a large gap, the surgical incision does not have any advantages over the traditional approach. In addition, local distraction of the multifidus can still lead to muscle necrosis and fibrosis. Use of a minimally invasive retractor can avoid these problems. The blade of a minimally invasive retractor is wide and it retracts the muscles evenly in four directions, which reduces muscle pulling strength per unit.26 In this study, the incisions in the Wiltse approach were shorter than in the traditional approach, this being dependent on the horn‐like openings that allow even small incision to provide adequate exposure of the surgical segment.
Precautions and Problems with the Wiltse Approach
Identification of the Gap between Multifidus and Longissimus is the Key to the Wiltse Approach
Preoperatively, MR and CT images should be carefully examined and the distance from the outside of the multifidus to the spinous process measured to facilitate rapid identification of the gap.27 Because the muscle gap at the L5S1 segments is vague, we recommend identifying it from the top to the bottom.28 In general, after longitudinal incision of the thoracodorsal fascia dorsi, the muscle fibers of the longissimus usually cover the surface of multifidus in an arc‐shaped manner. The muscle fibers are carefully retracted to the lateral, allowing the muscle gap, which usually contains a thin layer of fat, to be felt with the fingers. Blunt dissection reveals a smooth myofascial gap. During separation, the vessels in the gap are carefully burned from the top to the bottom and the muscles attached to the facet joints and transverse processes removed with an electronic knife. If the muscle gap has not been identified, the screws should not be inserted into the muscles in order to avoid massive bleeding.
Choice of Incision
The double longitudinal incision over the spinous process and single median incision both have advantages and disadvantages. In this study, we found that a single median incision allows completion of the TLIF procedure on both sides. The required incision length is only 5 cm, which is shorter than the double longitudinal incision. However, the procedure has to be completed individually on each side. In contrast, a longitudinal incision allows the procedure to be finished on both sides at the same time. Therefore, the operation time is not significantly reduced with the single incision. However, considering the incision is so small, we still recommend it.
Choice of Retractors
Choosing appropriate retractors is very important.24, 29 In this study, we used retractors designed by Berenger. Use of such retractors for dual‐or multi‐segment surgery significantly reduces the distractive strength. Therefore, in this study, all the patients had single segment lesions. For cases with long segment operation, retractors designed for minimally invasive surgery are more appropriate.
Range of Spinal Decompression
All patients in this study had a single segment of lumbar spondylolysis (degree I or II). Therefore, surgery did not require a wide range of central spinal decompression. However, for patients with spondylolysis combined with severe lumbar spinal stenosis, the inside of the multifidus and facet joints need to be removed. Removal of the hypertrophied yellow ligament and extensive expansion of the spinal canal can achieve sufficient decompression.
Conclusions
TLIF through both Wiltse and traditional approaches has satisfactory clinical efficacy. Mini‐invasive TLIF via a Wiltse approach can significantly reduce damage to the multifidus and the postoperative incidence of chronic LBP.
Disclosure: The authors have no conflicts of interest to declare.
References
- 1. Macintosh JE, Bogduk N. Volvo award in basic science. The morphology of the lumbar erector spinae. Spine (Phila Pa 1976), 1987, 12: 658–668. [DOI] [PubMed] [Google Scholar]
- 2. Bogduk N. The lumber mamillo‐accessory ligament. Its anatomical and neurosurgical significance. Spine (Phila Pa 1976), 1981, 6: 162–167. [DOI] [PubMed] [Google Scholar]
- 3. Panjabi M. The stabilizing system of the spine. Part I. Function, dysfunction, adaptation, and enhancement. J Spinal Disord, 1992, 5: 383–389 discussion 397. [DOI] [PubMed] [Google Scholar]
- 4. Fan S, Hu Z, Zhao F, Zhao X, Huang Y, Fang X. Multifidus muscle changes and clinical effects of one‐level posterior lumbar interbody fusion: minimally invasive procedure versus conventional open approach. Eur Spine J, 2010, 19: 316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim DY, Lee SH, Chung SK, Lee HY. Comparison of multifidus muscle atrophy and trunk extension muscle strength: percutaneous versus open pedicle screw fixation. Spine (Phila Pa 1976), 2005, 30: 123–129. [PubMed] [Google Scholar]
- 6. Gille O, Jolivet E, Dousset V, et al Erector spinae muscle changes on magnetic resonance imaging following lumbar surgery through a posterior approach. Spine (Phila Pa 1976), 2007, 32: 1236–1241. [DOI] [PubMed] [Google Scholar]
- 7. Watkins MB. Posterolateral bone grafting for fusion of the lumbar and lumbarsacral spine. J Bone Joint Surg Am, 1959, 41: 388–396. [PubMed] [Google Scholar]
- 8. Wiltse LL, Bateman JG, Hutchinson RH. The paraspinal sacrospinalis‐splitting approach to the lumbar spine. J Bone Joint Surg Am, 1968, 50: 919–926. [PubMed] [Google Scholar]
- 9. Wiltse LL, Spencer CW. New uses and refinements of the paraspinal approach to the lumbar spine. Spine (Phila Pa 1976), 1988, 13: 696–706. [PubMed] [Google Scholar]
- 10. Kawaguchi Y, Matsui H, Tsuji H. Changes in serum creatine phosphokinase MM isoenzyme after lumbar spine surgery. Spine (Phila Pa 1976), 1997, 22: 1018–1023. [DOI] [PubMed] [Google Scholar]
- 11. Datta G, Gnanalingham KK, Peterson D, et al Back pain and disability after lumbar laminectomy: Is there a relationship to muscle retraction? Neurosurgery, 2004, 54: 1413–1420 discussion 1420. [DOI] [PubMed] [Google Scholar]
- 12. Gejo R, Matsui H, Kawaguchi Y, Ishihara H, Tsuji H. Serial changes in trunk muscle performance after posterior lumbar surgery. Spine (Phila Pa 1976), 1997, 24: 1023–1028. [DOI] [PubMed] [Google Scholar]
- 13. Gejo R, Kawaguchi Y, Kondoh T, et al Magnetic resonance imaging and histologic evidence of postoperative back muscle injury in rats. Spine (Phila Pa 1976), 2000, 25: 941–946. [DOI] [PubMed] [Google Scholar]
- 14. Li H, Yang L, Xie H, Yu L, Wei H, Cao X. Surgical outcomes of mini‐open Wiltse approach and conventional open approach in patients with single‐segment thoracolumbar fractures without neurologic injury. J Biomed Res, 2015, 29: 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li B, Wu ZG, Peng XZ, Zhuo XL. Lumbar discectomy via Wiltse approach. Orthop Surg, 2014, 6: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ulutaş M, Yaldız C, Seçer M, et al Comparison of Wiltse and classical methods in surgery of lumbar spinal stenosis and spondylolisthesis. Neurol Neurochir Pol, 2015, 49: 251–257. [DOI] [PubMed] [Google Scholar]
- 17. Ran B, Yan L, Cai L. Wiltse approach versus the conventional posterior midline approach for lumbar degenerative diseases: A metaanalysis. Zhong Nan Da Xue Xue Bao Yi Xue Ban, 2015, 40: 90–101(in Chinese). [DOI] [PubMed] [Google Scholar]
- 18. Zheng YP, Tian YH, Liu XY, et al Single‐stage posterior debridement, bone grafting and internal fixation in treatment of anterior spinal column thoracic tuberculosis through Wiltse approach. Ji Zhu Wai Ke Za Zhi, 2012, 10: 321–324(in Chinese). [Google Scholar]
- 19. Tuttle J, Shakir A, Choudhri HF. Paramedian approach for transforaminal lumbar interbody fusion with unilateral pedicle screw fixation. Technical note and preliminary report on 47 cases. Neurosurg Focus, 2006, 20: E5. [DOI] [PubMed] [Google Scholar]
- 20. Spiegel MA, Lafage R, Lafage V, et al Developing the total disability index based on an analysis of the interrelationships and limitations of ODI and NDI. Spine (Phila Pa 1976), 2016, 41: 74–81. [DOI] [PubMed] [Google Scholar]
- 21. Brantigan JW, Steffee AD, Lewis ML. Lumbar interbody fusion using the Brantigan I/F cage for posterior lumbar interbody fusion and the variable pedicle screw placement system: two‐year results from a Food and Drug Administration investigational device exemption clinical trial. Spine (Phila Pa 1976), 2000, 25: 1437–1446. [DOI] [PubMed] [Google Scholar]
- 22. Zhi‐Jun H, Wen‐Bin X, Shuai C, et al Accuracy of magnetic resonance imaging signal intensity ratio measurements in the evaluation of multifidus muscle injury and atrophy relative to that of histological examinations. Spine (Phila Pa 1976), 2014, 39: E623–E629. [DOI] [PubMed] [Google Scholar]
- 23. Kim KT, Lee SH, Suk KS, Bae SC. The quantitative analysis of tissue injury markers after mini‐open lumbar fusion. Spine (Phila Pa 1976), 2006, 31: 712–716. [DOI] [PubMed] [Google Scholar]
- 24. Schwender JD, Holly LT, Rouben DP, Foley KT. Minimally invasive transforaminal lumbar interbody fusion (TLIF): technical feasibility and initial results. J Spinal Disord Tech, 2005, 18 (Suppl): S1–S6. [DOI] [PubMed] [Google Scholar]
- 25. Haro H, Maekawa S, Hamada Y. Prospective analysis of clinical evaluation and self‐assessment by patients after decompression surgery for degenerative lumbar canal stenosis. Spine J, 2008, 8: 380–384. [DOI] [PubMed] [Google Scholar]
- 26. Stevens KJ, Spenciner DB, Griffiths KL, et al Comparison of minimally invasive and conventional open posterolateral lumbar fusion using magnetic resonance imaging and retraction pressure studies. J Spinal Disord Tech, 2006, 19: 77–86. [DOI] [PubMed] [Google Scholar]
- 27. Wiltse LL, Bateman JG, Hutchinson RH, Nelson WE. The paraspinal sacrospinalis‐splitting approach to the lumbar spine. J Bone Joint Surg Am, 1968, 50: 919–926. [PubMed] [Google Scholar]
- 28. Vialle R, Wicart P, Drain O, Dubousset J, Court C. The Wiltse paraspinal approach to the lumbar spine revisited: an anatomic study. Clin Orthop Relat Res, 2006, 445: 175–180. [DOI] [PubMed] [Google Scholar]
- 29. Foley KT, Holly LT, Schwender JD. Minimally invasive lumbar fusion. Spine (Phila Pa 1976), 2003, 28(Suppl. 15): S26–S35. [DOI] [PubMed] [Google Scholar]


