Abstract
Core body temperature (CBT) rhythm, locomotor activity, and actigraphy-sleep were evaluated in geriatric dogs with cognitive dysfunction. Dogs (n=33; 9–16 yrs) performed a spatial working memory task and divided into three memory groups: Low, Moderate, and High, with subsequent evaluation of learning and attention. Rectal CBT was recorded 6 times over a 17.5 h period and Actiwatch® activity monitoring system for 5 days while housed indoors with 12 h light/dark schedule. Rhythm of daily activity data was evaluated using the traditional cosinor analysis and generation of non-parametric measures of interdaily stability, intradaily variability, and relative amplitude. CBT differed with time (F (5, 130)=11.36, p<0.001), and was the highest at 19:00C. CBT at 19:00 was positively related (p<0.01) to memory (r(31)=0.50) and 3-domain cognitive performance index (memory, learning, attention; r(31)=0.39). Total daytime or night-time activity did not differ between memory groups, but hourly counts at 8:00 were positively related (p<0.05) to memory (r(31)=0.52), learning (r(31)=0.36), and 3-domain cognitive performance index (r(31)=0.53). There were no significant differences between age or memory groups for any circadian rhythm measures. Daytime naps were inversely related to memory accuracy (r(31)=−0.39; p<0.05) and BT at 15:00 (r(30)=−0.51; p<0.01). Lower peak BT and increased napping may predict some aspects of cognitive performance of working memory, learning, and/or attention processes in these geriatric dogs, but minimal diurnal rhythm disruption of locomotor activity is observed when these cognitive processes decline.
Keywords: Circadian rhythm, Spatial working memory, Cognition, Dog, Diurnal, Body temperature
Highlights
-
•
Diurnal rise in core body temperature present in old dogs (9–16 years) with peak at 19:00.
-
•
Lower peak body temperature in dogs with lowest spatial working memory ability.
-
•
Locomotor activity rhythm unchanged in dogs with impaired spatial working memory.
-
•
Impaired working memory related to more napping and lower afternoon body temperature.
1. Introduction
Many physiological processes oscillate with circadian rhythmicity and are regulated by the suprachiasmatic nucleus (SCN) of the hypothalamus. Circadian regulation can become altered with advanced age and in cognitive disorders or neurodegenerative diseases such as Alzheimer's disease (Harper et al., 2005, van Someren, 2000). Although SCN-mediated circadian timing provides temporal organization to most neurobehavioral, sleep/wake, physiological, and biochemical processes, multiple and divergent synaptic projections from the SCN differentially regulate these circadian rhythms (Fuller et al., 2006). Therefore, dysfunction in the regulation of some circadian processes will not necessarily predict dysfunction of others. Some of the processes that become altered with normal aging in dogs and people include locomotor activity, hormone secretion, thermoregulation, as well as sleep and food intake patterns. Changes in these processes can be a manifestation of many physical and neurological deficits that accumulate over the lifetime.
Dogs, like humans, show age-dependent cognitive decline with accompanying neuropathology, which raises the possibility that dogs may develop a similar syndrome of cognitive dysfunction to that seen in people with cognitive decline and/or dementia. (Head et al., 2008, Milgram et al., 2010). Furthermore, veterinarians can identify a cognitive dysfunction syndrome (CDS) in dogs through behavior-based impairment that becomes progressively more severe with increased age (Bain et al., 2001, Landsberg and Ruehl, 1997, Ruehl et al., 1995, Ruehl and Hart, 1998). Recently, we have demonstrated in aged dogs that several cognitive domains are functionally independent, and an age-related deficit in visuospatial working memory does not predict dysfunction in learning or selective attention processes (Zanghi et al., 2015). Canine cognition, like that of human cognition, subsumes several diverse domains (Milgram et al., 2010, Summers and Saunders, 2012), which include, but are not limited to, visuospatial function, learning, working memory and executive function. Yet, it is not clear how deficits in specific cognitive domains are related to dysfunction of various circadian or diurnal rhythms in aged dogs, as it is in humans with neurodegenerative diseases. Therefore, understanding age-related and diurnal changes in behavioral, physiological, and cognitive patterns associated with cognitive dysfunction may offer insight to the development of interventions to partially counteract both canine and human brain aging.
A link between circadian oscillation of core body temperature (CBT) and sleep/wake cycles exists in people, and decreased sleep quality appears to be partially related to age-related changes in thermoregulation (van Someren, 2000). Although circadian oscillation of CBT has been reported in healthy adult dogs (Refinetti and Piccione, 2003), the effect of advanced age and/or cognitive dysfunction on 24-h CBT oscillation has not been reported either independently, or in conjunction with behavioral sleep patterns or locomotor activity data in domestic dogs.
Previous work has established that healthy adult dogs exhibit diurnal locomotor activity/rest patterns (Nishino et al., 1997; Siwak et al., 2003; Tobler and Sigg, 1986; Zanghi et al., 2012) and also show a complex relationship between locomotor activity, age, cognition and housing environment (Siwak et al., 2003). Canine aging has been associated with changes in locomotor activity patterns (Siwak et al., 2002, Siwak et al., 2003, Zanghi et al., 2012) and sleep patterns (Takeuchi and Harada, 1986, Zanghi et al., 2013), but not phase shifts of early morning onset or delayed morning onset of locomotor activity (Zanghi et al., 2012). Siwak et al. (2003) observed that young dogs (1–4 yrs old) exhibit higher activity levels than aged dogs (9–14 yrs old), but also reported that cognitively impaired, aged dogs were more active and showed a delayed peak of daytime activity compared to unimpaired, aged dogs. This potential link may parallel age-related activity and cognitive changes observed to be exaggerated in dementias, such as AD (van Someren et al., 1996, Witting et al., 1990), and can be manifested as either hyper- or hypoactivity (Satlin et al., 1991).
In this study, we sought to determine whether cognitive impairment in the dog, using a test of working memory capacity, would be related to dysfunction of diurnal behavioral and physiological rhythms. Therefore, the objective of the study was to evaluate rhythms of locomotor activity, behavioral sleep, and CBT in aged dogs that displayed varying working memory proficiency, with subsequent evaluation of learning and attention performance. Working memory was assessed by performance on a variable delayed non-matching to position (vDNMP) paradigm.
2. Materials and methods
To briefly summarize the experimental design, CBT and locomotor activity were measured in dogs who were also being evaluated for neuropsychological test performance. This cognition data was previously published (Zanghi et al., 2015) and a subset of that neuropsychological test performance data is used here for further analysis of canine cognition and related physiological biomarkers.
2.1. Animals, housing, and feeding regimen
Aged Beagle dogs (Canis familiaris; N=33; 9–16 yrs) were in good health and adequate body condition, as assessed by a general health evaluation. The veterinary examination included verification of healthy haematology and blood-chemistry parameters. Selection of candidate dogs was also based on their responsiveness on cognitive tests and evidence of impairment during the baseline cognitive testing described below.
Prior to the study, the dogs were housed indoors with exposure to natural light cycles with daily outdoor access. The dogs were housed in groups of two to four per kennel-run (1.5 m wide×4.6 m in length) based on compatibility and sex. All dogs were housed in the same kennel-run location with ability to see other dogs in adjacent and opposing runs. Each dog had direct interaction with caretaker staff on daily basis and had access to toys (durable balls and breed-size appropriate chew toys). Two weeks prior to the start of this study, the dogs were switched to a 12-h light-dark cycle with lights on at 7:00 a.m. and off at 7:00 p.m. Dogs were maintained indoors during the study with 30-min of daily group play outside of kennel-run area with caretaker staff and pen-mates. The light-dark cycle was maintained until the completion of the study.
Before the start of the study, dogs were fed individually once daily (Purina ProPlan Adult Maintenance Chicken and Rice formula; Nestlé Purina PetCare, St. Louis, MO) between 8:00 and 10:00 a.m. within their normal housing area. Upon study initiation, dogs were switched to a prototypical complete and balanced dry extruded senior chicken and rice-based formula (Nestlé Purina PetCare, St. Louis, MO) over a 1 week period that contained 7.8% moisture, 28.5% crude protein, 14.3% crude fat, 1.3% crude fiber, and 6.7% ash. The food was formulated to meet or exceed the nutrient requirements outlined by the Association of American Feed Control Officials (AAFCO) guidelines. Food was provided twice daily at 9 a.m. (±30 min) and 6 p.m. (±30 min) with the total daily ration divided equally into two rations and water available ad libitum. Total food provided was regularly adjusted to permit the dogs to maintain their body weight over the duration of the study. The study was conducted in accordance with approved Animal Care and Use Committee protocols.
For CBT measurements, dogs were temporarily housed individually in metabolism kennels for 36 h to monitor body temperature, as reported by Refinetti and Piccione (2003) to observe the diurnal variation. Dogs were allowed free access to water and fed on the same schedule while in metabolism kennels. Dogs were allowed exercise with their kennel-run mates for environmental enrichment during the regularly scheduled times over this 36-h period.
2.2. Animals, cognitive test apparatus
The testing apparatus consisted of a wooden box that was approximately 0.609 m×1.15 m×1.08 m (Milgram et al., 1994) in size and was a modified version of the Wisconsin General Test Apparatus widely used in cognitive assessment of primates. The front contained three height-adjustable gates through which the dog responded. The experimenter was separated from the dog by a plastic partition containing a one-way mirror and a hinged door. The tray was made of Plexiglas and contained either one medial food well and two lateral food wells, or four equally spaced food wells, depending on the task. The food reward was the Pro Plan Adult Wet Dog Food Chicken & Rice Entree (Nestlé Purina Petcare, St. Louis, MO). Approximately, 1 g of the food constituted each reward, resulting in a maximum of 12 g of additional food, depending on the task.
2.3. Cognitive testing schedule
Initiation of a twice-daily feeding regimen was day 1 of the study. Cognitive testing for 60 dogs began 10 days after beginning the twice-daily feeding regimen (days 10–25). All dogs were first trained over 10 days on a spatial memory task using the vDNMP paradigm to assess baseline memory performance (Zanghi et al., 2015). The dogs were then trained over 30 days on a variable object oddity task to assess learning and selective attention.
To briefly summarize the stratification and group size, performance on the vDNMP task was used to stratify the dogs into three groups based on memory performance score (High memory performers [HMP; N=12], Moderate memory performers [MMP; N=24], and Low memory performers [LMP; N=24]). To establish group stratification, the 12 dogs with the best vDNMP cognitive performance were assigned to the HMP group based on highest percent accuracy for combined 20-s and 90-s scores. Lowest percent accuracy scores were used to select 24 LMP dogs, and then consequently establish MMP dogs out of the remaining pool of 24 dogs. Following grouping, both the MMP and LMP groups were equally sub-divided into two groups of 12, with one of the two groups randomly selected for further cognitive testing. Thus, a subset of dogs (N=36 out of N=60) was examined for cognitive performance on a variable object oddity task (Zanghi et al., 2015) over 30 days (day 38-67) to assess learning (2-object discrimination) and selective attention.
2.4. Cognition tests – variable delayed non-matching to position
All vDNMP testing consisted of delays of 20 or 90 s that occurred randomly within each daily test session. The 20 or 90 s delay was presented 6 times per daily test session, thus each delay resulting in 60 trials for each delay during baseline (6 trials/d/delay×10 d). To briefly describe the vDNMP task procedure, each trial began with a sample phase, in which an object covering a food well was presented at one of three positions: left, center, or right. After the dog displaced the object and obtained the reward, the tray was withdrawn. After a delay, the test phase started with the presentation of both the sample object and the non-match object (an identical object in one of the two other locations). The dog then had to displace the non-match object to obtain reward. If the dog responded to the sample object (incorrect response), then the tray was immediately withdrawn and an error was recorded. To prevent the dogs from using olfactory cues to solve the task, a quantity of food approximately equal to that associated with the non-match was stuck to the bottom of the incorrect stimulus. After a 60-s inter-trial interval, the sample phase of the next trial was initiated. The memory demands of this task were manipulated by varying the length of delay between the sample and test phase.
2.5. Cognition tests – variable object oddity task
The variable object oddity task was developed in order to assess selective attention processes (Christie et al. 2005). The task had two phases: acquisition phase and distractor phase. The attention task procedure was adapted from the two-choice object discrimination learning task, in which dogs are trained to select the odd object out of 2, 3, or 4 total objects where the other objects are all the same size and color. This task required the adoption of a specially modified test tray that contains four food wells. The preferred object of each animal was identified through a preference test at the outset of the acquisition phase, thus was the rewarded object for the remainder of the acquisition phase testing. During the acquisition phase, the dogs were initially trained on a two-choice object discrimination problem, which required them to learn that choosing a certain object was always associated with a food reward. The acquisition criterion was divided into 2 stages. The first stage required the dogs to perform above 90% accuracy on one test day, or above 80% accuracy over two consecutive test days, and the second stage required dogs to perform above 70% accuracy over three consecutive days subsequent to passing the first stage criterion. Each test session consisted of 10 trials. A maximum of 20 sessions was given (1 preference test session and 19 acquisition sessions). During the distractor phase (Attention test), the object that was rewarded during the acquisition phase was always rewarded during this'same distractor' phase. Dogs were tested with one positive stimulus and one, two, or three negative stimulus objects. Each test session consisted of 12 trials, with one session per day. The number of objects were varied pseudo-randomly and occurred equally over the 12 trials.
2.6. Locomotor activity recording and actigraphy-generated behavioral sleep
Twenty-four hour activity rhythms were assessed for 5 consecutive days using the Mini-Mitter® Actiwatch-16® activity monitoring system (Respironics Co., Inc., Bend, OR) in January. The Actiwatch® is an omni-directional accelerometer (Ancoli-Israel et al., 2003) placed inside a specially designed case and attached to a collar around the dog's neck, permitting normal rest, exercise, and feeding activity levels to be measured (Zanghi et al., 2013). The monitors recorded activity counts on a 30-sec epoch setting and actigraphy data generated as previously described (Zanghi et al., 2013) and previously validated to polysomnography (John et al., 2000).
2.7. Sleep/wake statistics
The Actiware® software was used to generate total number of minutes scored as wake during both the light phase and dark phase (wake minutes). In addition, we used the Actiware® software to generate the following parameters; sleep interval duration, sleep onset latency, morning activity onset time, sleep efficiency, light phase naps, and night-time wake bouts, as described previously (Zanghi et al., 2013).
2.8. Core body temperature
Rectal core body temperature (CBT) was measured (Accuflex Pro, Model 016-639, Physiologic, Montreal, QC, Canada) between days 16–31 after locomotor activity monitors were removed and following vDNMP testing. CBT recording times were at 08:00, 10:00, 13:00, 15:00, 19:00, and 01:30. Readings for all dogs were within 15 min of the target time.
2.9. Circadian rhythm analysis
A traditional cosinor analysis was performed as described by Nelson et al. (1979) to calculate the circadian rhythm variables; acrophase, and circadian quotient (mesor/amplitude ratio). Prior to performing the cosinor analysis, raw activity data recorded every 30 s during the first and all subsequent 60 min intervals was transformed to represent raw activity counts based on 60 min increments. Total activity counts per 60 min increment were transformed using natural logarithms of the counts +1.
The traditional cosine model used for circadian rhythm was:
where: M (mesor)=value about which oscillation occurs, A (amplitude)=half the difference between the highest and lowest values, ω (angular frequency)=degrees/unit time, with 360° representing a complete cycle (or 2π radians representing a complete cycle), t (acrophase)=timing of high point in radians.
The data were analyzed from day 1 at 7:00 a.m. through day 4 at 7:00 a.m. to represent five 24-h recording periods. Therefore, 7:00 a.m. was hour 0 for the calculation of the corresponding clock time of acrophase, as the cosinor model generates acrophase in radians.
R-squares values from fitting the 1 cycle per day cosine model were generated using the raw counts, the natural logarithms of the counts +1, and the square roots of the counts. In general, the R-squares values using the natural logarithms of the counts +1 transformation gave the best fit to the data, but only slightly better than the raw counts (data not shown). The natural log transformed data was used for the statistical analysis. R-squares values for the natural logarithms transformation ranged from 0.10 to 0.56 for all dogs.
Along with a traditional cosinor analysis, three non-parametric measures were also calculated as described by van Someren et al., 1999 to measure the rest-activity rhythm. The interdaily stability (IS) measures the strength of coupling of a rhythm between days and varies between 0 (Gaussian noise) and 1 (perfect stability). The intradaily variability (IV) measures the fragmentation of a rhythm, that is, the frequency and extent of rest-activity transitions. IV varies between 0 (perfect sine wave) and 2 or higher (Gaussian noise), and relative amplitude (RA) measures the amplitude of rest-activity transitions.
2.10. Statistical analysis
Ultimately, 3 dogs were removed after the completion of the study and not included in the statistical analyses, which included two LMP dogs for either loss of activity data (dog 1) or severe hyperactivity (dog 2). Statistical analyses were performed using R (R Core Team, 2015 version 3.2.1). Non-parametric activity measures (IS, IV, and RA) were calculated using the nparACT package (Blume et al., 2016). Locomotor activity, traditional cosinor statistics (mesor, amplitude, acrophase, and circadian quotient), non-parametric activity statistic, and sleep statistics were examined using 3 (vDNMP: LMP, MMP, and HMP)×2 (age: 9–11 yrs or 11–16 yrs) ANOVAs. For all ANOVAs, when significant main effects were found, Fisher's LSD post hoc test with Bonferroni corrections for the number of comparisons was used to examine group differences.
Body temperature data was analyzed using linear mixed effects models using the lme4 package (Bates et al., 2012). Since the data was not independent - the same dog's temperature was measured at multiple time points – a linear mixed effects model was used as it can account for this non-independence. Dog ID was entered as a random effect where the intercept was allowed to vary between dogs. Time, age, and cognitive classification, along with their interactions were entered in as fixed effects for both models. P-values were obtained using Satterthwaite approximations of degrees of freedom. When significant main effects were found, post hoc tests with Tukey corrections for the number of comparisons were conducted. All analyses were considered to be statistically significant at α=0.05.
3. Results
3.1. Effect of cognitive grouping and age on day/night locomotor activity
Daytime activity count (12-h; 07:00–19:00), nighttime activity counts (12-h; 19:00–07:00), and average hourly activity counts were used to characterize locomotor activity rhythms. To determine the relationship between cognitive performance and age on locomotor activity rhythms, the impact of age, vDNMP, and their interaction on each locomotor activity parameter was examined (Table 1).
Table 1.
Means (±SE) of locomotor activity, sleep/wake statistics during the 12-h light phase, and locomotor activity cosinor analysis for aged dogs that differ in spatial working memory ability and age groups.
| Variable | Spatial working memory cognition groups1 |
Cognition ANOVA p-value | Age groups |
Age ANOVA p-value | Age×Cog. interaction ANOVA p-value | |||
|---|---|---|---|---|---|---|---|---|
| HMP | MMP | LMP | 9 – 11 yrs | >11 – 16 yrs | ||||
| Activity counts2 | ||||||||
| Day time Activity counts | 22.01±4.01 | 22.07±2.71 | 14.45±2.06 | 0.17 | 23.89a ±3.32 | 16.29b ±1.56 | 0.04 | 0.32 |
| Night time activity counts | 4.99±0.60 | 3.68±0.60 | 5.00±0.98 | 0.22 | 4.63±0.70 | 4.41±0.46 | 0.15 | 0.01 |
| Cosinor analyses3 | ||||||||
| Mesor | 8.09±0.17 | 8.09±0.13 | 7.95±0.10 | 0.80 | 8.11±0.14 | 8.01±0.11 | 0.56 | 0.25 |
| Amplitude | 1.41±0.12 | 1.51±0.10 | 1.19±0.14 | 0.25 | 1.45±0.11 | 1.32±0.07 | 0.34 | 0.87 |
| Acrophase (radians)4 | −1.15±0.04 | −1.53±0.29 | −1.55±0.39 | 0.50 | −1.44±0.22 | −1.36±0.21 | 0.78 | 0.71 |
| Circadian quotient, A/M5 | 0.17±0.01 | 0.19±0.02 | 0.15±0.01 | 0.33 | 0.18±0.01 | 0.17±0.02 | 0.43 | 0.78 |
| Non-Parametric Analyses | ||||||||
| Interdaily Stability | 0.70±0.05 | 0.76±0.03 | 0.61±0.06 | 0.14 | 0.71±0.05 | 0.69±0.05 | 0.58 | 0.69 |
| Intradaily Variability | 1.15±0.05 | 1.11±0.05 | 1.27±0.08 | 0.17 | 1.11± 0.05 | 1.21±0.07 | 0.25 | 0.31 |
| Relative Amplitude | 0.89±0.04 | 0.91±0.02 | 0.88 ± 0.02 | 0.73 | 0.88±0.03 | 0.90±0.01 | 0.22 | 0.41 |
When significant main effects or interactions were found, post-hoc analyses were conducted using Fisher's LSD with Bonferroni corrections for the number of comparisons to examine group differences. Different letters indicate significantly different group means.
HMP: high memory performance, MMP: moderate memory performance, LMP: Low memory performance.
Expressed in X * 103 arbitrary units.
Data expressed as natural log.
Negative radian values reflect negative degrees, 0° to −360° (Nelson et al. 1979).
A/M: amplitude/mesor.
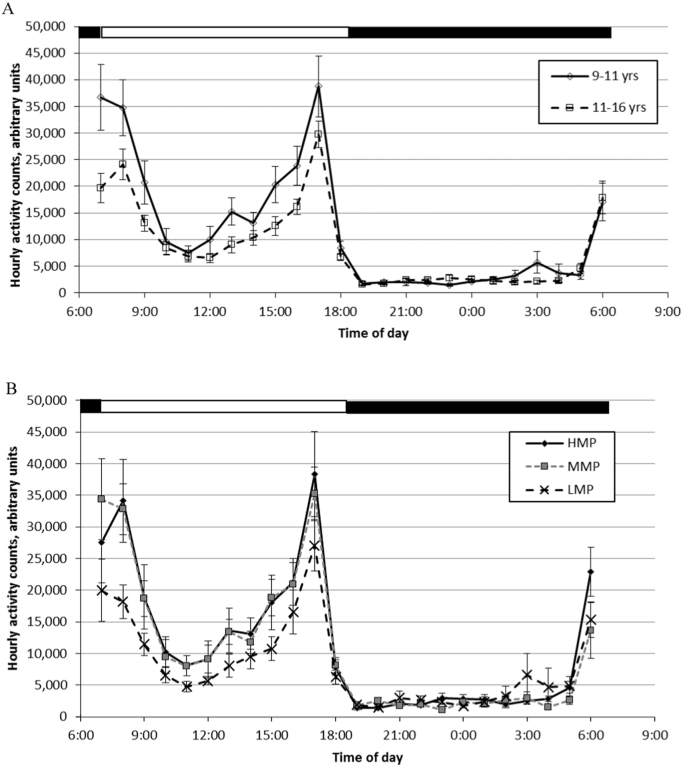
For daytime activity counts, there was a significant impact of age (F(1, 27)=4.58, p=0.04) such that dogs over 11 years of age had 29% lower total daytime activity counts (Table 1). Neither vDNMP (F(2, 27)=1.87, p=0.17) nor the interaction between age and vDNMP (F(2, 27)=1.20, p=0.32) were significantly related to daytime activity counts, although daytime activity counts were 34% lower for LMP dogs versus HMP and MMP dogs. For nighttime activity counts, there was a significant interaction between age and vDNMP (F(2, 27)=5.01, p=0.04). Night activity was significantly higher for LMP dogs over 11 years old (82,000±16,000 SE counts; N=3) compared to MMP dogs under 11 years old and LMP and MMP dogs over 11 years old. Compared to LMP dogs over 11 years old, all other age by vDNMP groups had similar total nighttime activity counts (range of group means: 32,000 to 53,000 counts). The average hourly activity counts are illustrated by age groups (Fig. 1A) and by vDNMP groups (Fig. 1B).
Fig. 1.
Mean (±SEM) hourly activity counts by cognition group based on performance on the variable Delay Non-Matching to Position (vDNMP).
3.2. Effect of cognitive grouping and age on rhythmicity of locomotor activity recordings
To assess the 24-hr oscillation of the locomotor activity count data, the traditional cosinor analysis was performed along with generation of non-parametric measures of interdaily stability, intradaily variability, and relative amplitude. The traditional cosinor analysis was used to calculate the acrophase and the circadian quotient (amplitude/mesor) based on 1 cycle per day. No significant differences in mesor, amplitude, acrophase, or circadian rhythm were found for age, vDNMP, or the interaction between age and vDNMP (Table 1). For the non-parametric measures of interdaily stability, intradaily variability, and relative amplitude, activity counts were grouped into 5 min increments1. There were no significant differences between the age or vDNMP groups for all three non-parametric measures (Table 1). Together, the cosinor and the non-parametric measures suggest that minimal rhythmic locomotor differences exist between the cognitive and age groups.
3.3. Effect of cognitive grouping and age on behavioral sleep statistics
The Actiware® software generated behavioral sleep statistics for the 12-h daytime (07:00–19:00) and 12-h nighttime (19:00–07:00) using the locomotor activity data. Along with daytime and nighttime periods, sleep interval periods were also calculated. Sleep interval was determined based on each dog's sleep onset and activity onset within the nighttime period. For all parameters within these three time periods, the impact of age, vDNMP, and the interaction of age with vDNMP was examined.
For parameters occurring during the day (sleep duration, number of nap bought, and nap duration), there were no significant differences (Table 2). For nap duration, there was a significant difference between ages with the older dogs (11–16 yrs) on average napping for shorter lengths of time than the younger dogs (9–11 yr; F(1, 27)=4.74, p=0.04). On average, the younger dogs had naps that were an average of 4.51 min long while the older dogs had naps that were an average of 3.84 min long. Neither vDNMP nor the interaction between age and vDNMP were significant for nap duration (F(2, 27)=0.43 vDNMP p=0.65; F(2, 27)=0.61 age*vDNMP p=0.55).
Table 2.
Means (±SE) of sleep and wake statistics during both the 12-h daytime (active interval) from 7:00 to 19:00, 12-h nightime (rest interval) from 19:00–07:00, and the sleep interval for aged dogs that differ in spatial working memory ability and age group.
| Variable | Spatial working memory cognition groups1 |
Cognition ANOVA p-value | Age groups |
Age ANOVA p-value | Age×Cog. interaction ANOVA p-value | |||
|---|---|---|---|---|---|---|---|---|
| HMP | MMP | LMP | 9 – 11 yrs | >11 – 16 yrs | ||||
| Daytime | ||||||||
| Total Sleep (min)2 | 232.96±27.19 | 568.08±21.28 | 282.22±15.93 | 0.30 | 253.49±21.42 | 243.32±17.05 | 0.49 | 0.20 |
| Number of Nap Bouts3 | 58.05±3.72 | 57.48±4.46 | 67.46±3.76 | 0.20 | 57.35±3.47 | 63.29±3.28 | 0.31 | 0.33 |
| Nap Duration (min)3 | 3.97±0.40 | 4.24±0.19 | 4.33 ± 0.20 | 0.65 | 4.51a,4±0.27 | 3.84b±0.18 | 0.04 | 0.55 |
| Nighttime | ||||||||
| Total sleep (min)5 | 554.33±10.16 | 580.38±10.59 | 553.08±17.05 | 0.20 | 577.62±9.66 | 550.23±9.89 | 0.24 | 0.30 |
| Sleep efficiency (%) | 76.99±1.41 | 80.61±1.47 | 76.82±2.37 | 0.20 | 80.22±1.34 | 76.41±1.36 | 0.24 | 0.30 |
| Sleep interval6 | ||||||||
| Sleep onset latency (min)7 | 12.18±2.56 | 11.46±2.39 | 9.93±2.62 | 0.84 | 11.43±2.08 | 11.18±2.01 | 0.93 | 0.51 |
| Sleep interval duration (min) | 648.54a±7.37 | 674.29b±6.75 | 664.62a,b±8.94 | 0.03 | 674.55±5.68 | 650.76±6.23 | 0.06 | 0.12 |
| Sleep efficiency (%) | 82.50±1.24 | 84.33±0.95 | 81.87±1.94 | 0.41 | 84.11±0.91 | 81.89±1.20 | 0.39 | 0.53 |
| Total Wake Time (min) | 112.18±7.14 | 105.34±5.80 | 119.88±11.66 | 0.49 | 106.71±5.71 | 116.58±7.03 | 0.53 | 0.62 |
| Wake Onset Latency (min)8 | −67.74a±7.52 | −40.93b±6.78 | −51.21a,b±9.66 | 0.04 | −38.83±4.99a | −67.28±6.64b | 0.02 | 0.44 |
| Number of wake bouts9 | 53.12±3.70 | 49.38±2.51 | 57.22±7.08 | 0.50 | 50.41±3.21 | 55.20±3.79 | 0.62 | 0.64 |
| Wake bout duration (min)9 | 2.29±0.19 | 2.19±0.08 | 2. 27±0.13 | 0.86 | 2.24±0.13 | 2.26±0.11 | 0.87 | 0.93 |
Different letters (a or b) indicate significantly different group means (p<0.05).
HMP: high memory performance, MMP: moderate memory performance, LMP: Low memory performance.
Total minutes classified as sleep during daytime (light phase) of 7:00–19:00.
Daytime nap bouts and nap duration were based on the light phase of 07:00–19:00.
Post-hoc analyses were conducted using Fisher's LSD with Bonferroni corrections for the number of comparisons to examine group differences. Different letters indicate significantly different group means.
Total minutes classified as sleep during rest interval (dark phase) of 19:00–07:00.
Determination of sleep interval was based each dog's sleep onset and activity onset time within the rest interval of 19:00–07:00.
Minutes after start of rest interval at 19:00.
Wake onset based on start time of last bout of at least 20 epochs of sleep. Negative activity onset data represents the number of wake minutes preceding lights on at 07:00.
Determination of night-time wake bouts and wake bout duration were determined only within each dog's sleep interval.
During the 12-h night-time period, there were no significant differences for age, vDNMP, or their interaction for sleep duration and sleep efficiency. During the sleep interval, on average all of the dogs were considered to be experiencing behavioral sleep within 15 min of the start of the 12-h nighttime. However, the ANOVA for sleep interval duration revealed a marginally significant main effect of age (F(1, 27)=3.86, p=0.06) and a significant main effect of vDNMP (F(2, 27)=2.32, p=0.03), in which the MMP dogs had the longest sleep interval, whereas the oldest dogs (11–16 yrs) had the shortest sleep interval. Similarly, the ANOVA for morning wake onset time relative to lights on at 07:00 also revealed a main effect of vDNMP (F(2, 27)=3.76, p=0.04) and age (F(1, 27)=6.09, p=0.02). HMP woke up at 5:52, MMP woke up at 6:19, and LMP dogs woke up at 6:11, such that MMP dogs woke up significantly later than HMP dogs. The oldest group of dogs (>11–16 yrs old) had an average morning wake time that started approximately 30 min earlier than the younger group of dogs (9–11 yrs. old). The oldest dogs had their last bout of sleep (inactivity greater than 10 min) at approximately 5:53, whereas average wake onset began about 28 min later at 06:21. There were no significant differences for sleep efficiency, total wake time, number of wake bouts, and wake duration (Table 2).
3.4. Effect of cognitive grouping and age on diurnal variation of core body temperature (CBT)
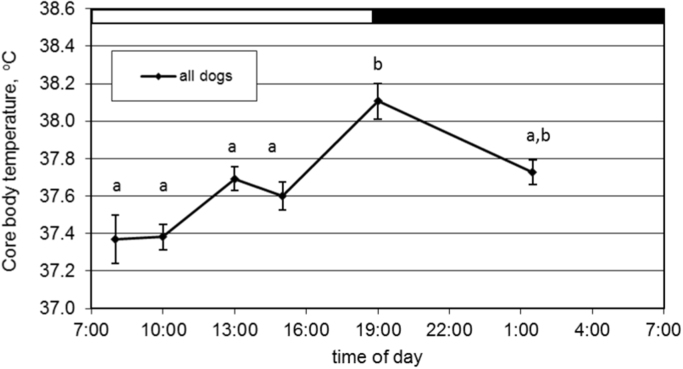
CBT was measured 6 times in each dog within a 17.5 h period and maintained on the same feeding schedule of twice daily. A linear mixed effect model was used to test the main effect of time-point, age, and vDNMP along with their interactions. Time was significant (F (5, 130)=11.36, p<0.001), but vDNMP, age, and their interactions were not significant. In all dogs, CBT oscillated with diurnal pattern and the temporal change is illustrated in Fig. 2A. Because CBT was not recorded to include a full 24 h cycle and data was collected over a single day for each dog, circadian rhythm analysis was not performed. Based on the time-points measured in this study, CBT was observed to rise through the daylight period to peak once during the 24-h cycle at 19:00. Mean CBT was lowest between 08:00 and 10:00, and significantly increased at 19:00, followed by a subsequently lower CBT in the night-time period at 1:30.
Fig. 2.
Mean (±SEM) hourly rectal core body temperature readings for all dogs Letters a or b indicate differences (p< 0.05) between specific times of day for Fig. 2A.
3.5. Correlation analysis of core body temperature, locomotor activity, behavioral sleep, and neuropsychological task performance
Post hoc correlation analyses were performed for all cognition data compared to hourly CBT data, locomotor activity data, and behavioral sleep statistics. A matrix of the resulting correlation data are compiled in Table 3, Table 4, and supplementary data tables.
Table 3.
Correlation matrix relating cognitive assessment measures based on percent accuracy performance and core body temperature measures in senior dogs (9–16 yrs).
| Variable | Age | vDNMP1 |
2-Choice | Attention | vDMNP+2-choice | vDMNP+2-choice + attention | Time of core body temperature measurement |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 s delay | 90 s delay | Combo | 8:00 | 10:00 | 13:00 | 15:00 | 19:00 | ||||||
| vDNMP 20s1 | 0.07 | ||||||||||||
| vDNMP 90s1 | 0.05 | 0.74*** | |||||||||||
| vDNMP Combo2 | 0.06 | 0.93*** | 0.94*** | ||||||||||
| 2-Choice3 | −0.53** | 0.05 | 0.15 | 0.11 | |||||||||
| Attention4 | −0.40* | 0.07 | 0.02 | 0.05 | 0.71*** | ||||||||
| vDMNP+2-choice5 | −0.25 | 0.74*** | 0.80*** | 0.83*** | 0.65*** | 0.44* | |||||||
| vDMNP+ 2-choice+ attention6 | −0.36* | 0.58*** | 0.61*** | 0.63*** | 0.78*** | 0.74*** | 0.93*** | ||||||
| Temp 8:00 | 0.26 | 0.09 | 0.09 | 0.1 | −0.31 | −0.42* | −0.09 | −0.24 | |||||
| Temp 10:00 | −0.09 | 0.01 | 0.21 | 0.12 | −0.04 | −0.24 | 0.07 | −0.05 | 0.36** | ||||
| Temp 13:00 | −0.18 | 0.29 | 0.25 | 0.29 | 0.12 | 0.12 | 0.29 | 0.26 | 0.19 | 0.37* | |||
| Temp 15:00 | 0.09 | 0.39* | 0.57*** | 0.52** | −0.12 | −0.20 | 0.34 | 0.17 | 0.44* | 0.62*** | 0.54*** | ||
| Temp 19:00 | 0.04 | 0.46** | 0.48** | 0.50** | 0.13 | 0.12 | 0.46** | 0.39* | 0.04 | 0.30* | 0.03 | 0.28 | |
| Temp 1:30 | 0.29 | 0.14 | 0.18 | 0.17 | −0.18 | −0.35 | 0.04 | −0.12 | 0.50** | 0.45** | 0.08 | 0.47** | 0.06 |
Correlations with asterisks are statistically significant with
p<0.05,
p<0.01,
p<0.001.
vDNMP: variable delay non-matching to position with a 20 s delay or 90 s delay.
vDNMP combo: average percent accuracy of 20 s delay and 90 s delay.
2-choice: two-choice object discrimination learning task conducted as the acquisition phase of the variable object oddity task (Zanghi et al., 2015).
Attention: object discrimination learning task with 1, 2, or 3 distracting objects conducted as the distractor phase of the variable object oddity task (Zanghi et al., 2015).
vDMNP+2-choice: data represented average of percent accuracy of both tasks.
vDMNP+2-choice+attention: data represented average of percent accuracy of all 3 tasks.
Table 4.
Correlations relating actigraphy-generated behavioral sleep statistics to cognitive assessment measures and core body temperature measures in senior dogs (9–16 yrs).
| Daytime |
Nighttime |
Sleep Interval |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sleep duration (min) | Number of nap bouts | Nap duration (min) | Sleep duration (min) | Sleep efficiency (%) | Sleep onset latency (min) | Sleep interval duration (min) | Sleep efficiency (%) | Total wake time (min) | Wake onset latency (min) | Number of wake bouts | Wake bout duration (min) | |
| Age | 0.09 | 0.2 | −0.11 | −0.15 | −0.15 | −0.07 | −0.25 | −0.11 | 0.07 | 0.30 | 0.10 | −0.03 |
| vDNMP 20 s | −0.18 | −0.22 | −0.09 | 0.12 | 0.12 | −0.12 | −0.08 | 0.19 | −0.22 | 0.07 | −0.26 | 0.01 |
| vDNMP 90 s | −0.38*,1 | −0.39* | −0.20 | −0.03 | −0.03 | 0.1 | −0.24 | 0.04 | −0.08 | 0.26 | −0.12 | 0.03 |
| vDNMP Combo | −0.30 | −0.33 | −0.15 | 0.05 | 0.05 | −0.01 | −0.17 | 0.12 | −0.16 | 0.17 | −0.2 | 0.02 |
| 2-Choice | −0.12 | 0.05 | −0.14 | −0.04 | −0.04 | 0.23 | −0.11 | −0.01 | 0.02 | 0.08 | 0.13 | −0.25 |
| Attention | −0.13 | −0.01 | −0.12 | −0.14 | −0.14 | 0.20 | −0.22 | −0.03 | 0.01 | 0.13 | 0.12 | −0.19 |
| vDMNP+ 2-choice | −0.29 | −0.22 | −0.19 | 0.01 | 0.01 | 0.12 | −0.19 | 0.09 | −0.11 | 0.18 | −0.09 | −0.13 |
| vDMNP+ 2-choice+ attention | −0.27 | −0.17 | −0.19 | −0.05 | −0.05 | 0.18 | −0.24 | 0.05 | −0.08 | 0.19 | −0.01 | −0.17 |
| Temp 8:00 | 0.08 | −0.13 | 0.21 | 0.03 | 0.03 | −0.24 | −0.05 | 0.07 | −0.03 | 0.17 | −0.30 | 0.30 |
| Temp 10:00 | −0.13 | −0.33 | 0.08 | 0.04 | 0.04 | −0.31 | 0.15 | −0.04 | −0.04 | 0.00 | −0.26 | 0.44* |
| Temp 13:00 | −0.31 | −0.38* | −0.09 | −0.13 | −0.13 | −0.16 | −0.14 | 0.00 | 0.13 | 0.05 | −0.19 | 0.14 |
| Temp 15:00 | −0.38* | −0.51** | −0.10 | −0.08 | −0.08 | −0.34 | −0.19 | 0.03 | 0.08 | 0.23 | −0.21 | 0.15 |
| Temp 19:00 | −0.30 | −0.28 | −0.20 | −0.05 | −0.05 | −0.04 | −0.14 | −0.06 | 0.05 | 0.17 | −0.11 | 0.09 |
| Temp 1:30 | 0.08 | −0.14 | 0.23 | −0.05 | −0.05 | −0.38* | −0.02 | −0.17 | 0.05 | 0.21 | −0.08 | 0.43* |
Correlations with asterisks are statistically significant with *p<0.05, **p<0.01.
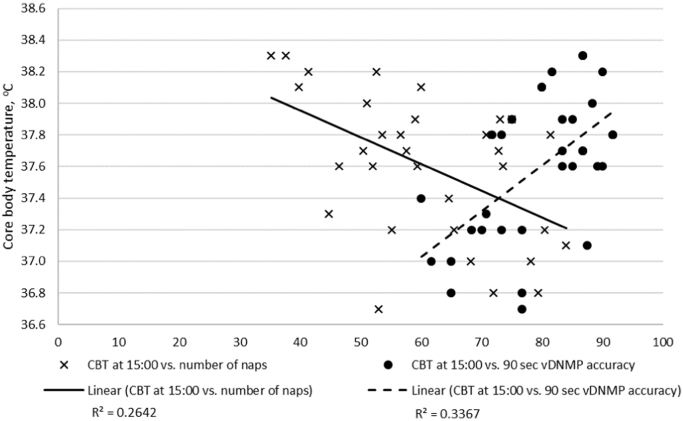
As anticipated, vDNMP at 20 s and 90 s delays were highly related with each other (r(31)=0.74; p<0.001). Similarly the 2-object discriminate test and the selective attention test were significantly related to each other (r(31)=0.71; p<0.001) and performance on these two tests significantly declined as the dogs aged (r(31)2-Object=−0.53, p 2-Object <0.001; r(31)Selective Attn=−0.40, pSelective Attn<0.05). The vDNMP measures were not significantly related with the 2-object discriminate and the selective attention tasks. There was considerable variation in the correlations between the 6 CBT time points. Correlations between the CBT time points ranged from r(30)=0.04 (p=n.s.) to r(30)=0.62 (p<0.001). The 20 s and 90 s delays for vDNMP were significantly related to CBT at 15:00 and 19:00 with correlations ranging from r(30)=0.39 (p<0.05) to r(30)=0.57 (p<0.001), however, they were not significantly related to any other time. CBT measures at 15:00 were plotted relative to percent accuracy with the vDNMP task at the 90 s delay to illustrate the positively related correlation (r(30)=0.57; p<0.001) between increasing CBT and improved accuracy (Fig. 3; Table 3). The 2-object discriminate test and the selective attention test were not significantly related to CBT at any measurement time (Table 3). Finally, peak CBT at 19:00 was the only time that was significantly related to 2-domain (r(30)=0.46; p<0.01) or 3-domain (r(30)=0.39; p<0.05) cognitive performance index.
Fig. 3.
Rectal core body temperature readings at 15:00 plotted relative to percent accuracy with the variable Delay Non-Matching to Position (vDNMP) task at the 90 s delay.
Correlational analyses also resulted in a significant inverse relationship (r(31)=−0.39; p<0.05) between 90-sec delay working memory and number of daytime nap bouts (Table 4). Similarly, an inverse relationship resulted between the number of daytime nap bouts and CBT at 13:00 (r(30)=−0.38; p<0.05) and 15:00 (r(30)=−0.51; p<0.01; Fig. 3; Table 4).
Finally, locomotor activity data had a low to marginal relationship to some aspects of cognition task performance (Supplementary Table 1) and (Supplementary Table 2). Non-parametric measures also did not significantly correlate with any individual cognition task or multi-domain performance index (data not shown). Data from the outlier dog that was observed to exhibit extreme hyperactive daytime counts were not included in the regression analysis.
4. Discussion
The objectives of this study were to 1) characterize the daily change of CBT, locomotor activity patterns, and behavioral sleep statistics in a population of aged domestic dogs with varying levels of cognitive performance on a working memory task, and 2) determine if age-independent differences in CBT, locomotor activity, and/or behavioral sleep are related to neuropsychological task performance. Performance on a single task, the vDNMP task, was used to stratify dogs into low performing, moderate performing, or high performing groups based on baseline spatial working memory at two delays. Subsequent cognitive testing evaluated performance on additional tasks related to discrimination learning and selective attention using the variable object oddity task. We initially reported that a portion of dogs from this same population of aged dogs uniquely demonstrated poor working memory performance, which did not dependently predict relative dysfunction for at least some aspects of learning and attention processes (Zanghi et al., 2015). These findings were unique because age-related dysfunction of attention, size discrimination learning, and working memory had been independently demonstrated in separate studies in the domestic dog (Milgram et al., 2010). Our previous work also demonstrated that performance on the vDNMP task remains relatively stable (Zanghi et al., 2015). Dogs that were characterized as high memory performers at baseline remained high performers when retested, and conversely, poor performing dogs at baseline remained poor performers when retested. We also previously demonstrated an age-related decline in day and night locomotor activity levels (Siwak et al., 2003, Zanghi et al., 2012) and behavioral sleep patterns (Zanghi et al., 2013), but the present data uniquely characterized behavioral sleep statistics, locomotor activity, and CBT relative to various cognitive domains.
4.1. Effect of age and cognitive performance on locomotor activity
All of the subjects used in this study were at least 9 years of age, and based on other research (Siwak et al., 2003, Zanghi et al., 2012), would be expected to show some degree of reduced locomotor activity counts. With both of these previous studies there was age variation within the ‘old aged’ population, with the age ranging from about 9 to 14 years, but only four dogs were over 13 yrs old. The data reported here further refines the understanding that daytime locomotor activity declines with advanced age beyond 11 yrs old, as ten dogs were 13 yrs through 16 yrs of age. Thus, this study provided a more robust age-separation of senior dogs, regardless of cognitive performance.
The first reported evidence of a link between canine aging, cognitive dysfunction, and locomotor activity was revealed with the categorization of cognitively impaired or non-impaired, aged dogs following a battery of neuropsychological tasks to establish impairment across multiple cognitive domains (discrimination learning, executive function, and spatial working memory; Siwak et al., 2003). The cognitively impaired dogs used by Siwak et al. (2003) were also observed to exhibit hyper-active locomotor activity compared to unimpaired, aged dogs. This initial observation was the basis to assess the relationship between not only the individual cognitive tasks in the current study, but also to generate a cognitive performance “index” that reflected the average performance accuracy across the multiple tasks. In contrast to the work by Siwak et al. (2003), when we examined the relationship between advanced age and only spatial working memory performance, a general daytime hypo-active pattern was observed in the poorest memory performing dogs. However, no statistically significant difference was observed between vDNMP groups and daytime activity. Linear regression analysis also revealed a moderate positive correlation (r=0.41–0.44; p<0.05) between daytime activity relative to the accuracy at the 90-s delay for vDNMP, 2-domain, and 3-domain cognitive performance index (Supplementary Table 1) indicating that daytime activity declined with declining cognitive performance. Therefore, our results are not consistent with the previous observations by Siwak et al. (2003). It is worth considering that the average cognitive task score used in current study did not include executive function performance, but did include an attention task. Therefore, daytime hyperactivity may have some link to declining executive function ability, as well as other undefined cognitive and/or physiological factors, including housing environment.
Finally, a clear diurnal oscillation of locomotor activity is observed in all dogs. However, the cosinor and the non-parametric measures suggest that minimal rhythmic dysregulation of locomotor activity exists between these dogs with varying spatial working memory ability or advanced geriatric age when fed twice daily. Because dogs were switched to a twice-daily feeding pattern at the onset of the study to accommodate other experimental design objectives related to PM-feeding intervention (Zanghi et al., 2014), the influence of advanced age and/or cognitive dysfunction may be masked by the twice-daily feeding pattern and 12-h light/dark cycle. Future research may reveal circadian rhythm dysregulation in old, cognitively impaired dogs based on a once-daily feeding regimen, as previous research has demonstrated that daily feeding frequency influences diurnal locomotor activity patterns in dogs (Zanghi et al., 2012).
4.2. Effect of age and cognitive performance on behavioral sleep statistics
Based on our previous research, healthy aged dogs would be expected to show some degree of increased number of daytime naps and fewer night-time awakenings (Zanghi et al., 2013). In general, there is awareness by dog owners that activity declines with advancing age (Houpt, 1998), and pet-owner survey data have captured some evidence of increased daytime sleep, but also increased night-time arousal of the owner by the pet (Bain et al., 2001, Neilson et al., 2001). Similarly, the data reported here in the current study further refines the understanding that daytime napping continues to increase with advanced age beyond 11 yrs old in dogs compared to 9–11 yrs old (Table 1), regardless of cognitive performance. The increased napping appears to be related to the new understanding that average napping duration per bout significantly decreased in the oldest dogs. This is a unique understanding for these very old dogs because napping duration was consistent (~5.0 min) between young, middle-aged, and senior dogs in our previous study (Zanghi et al., 2013), and the number of naps and nap duration observed for those “senior dogs” were similar to the data reported here in dogs 9–11 yrs old. However, advanced age in this population of dogs does not provide similar evidence for more frequent or longer night-time waking events (Table 2).
In contrast to the effect of advanced age on changes in behavioral sleep statistics, the relationship between cognitive performance using neuropsychological tasks and either daytime or night-time actigraphy-based sleep statistics has not been previously studied in dogs. Sleep disorders are frequently observed in people with AD, including nocturnal sleep fragmentation, decreased nocturnal sleep duration, increased daytime napping or sleepiness, and temporal shifts in sleep cycle (Peter-Derex et al., 2015). Somewhat similarly, sleep disruptions and altered sleep cycles are also reported by petowners and is a component behavioral sign of the DISHA assessment for diagnosing canine CDS (Landsberg et al., 2011, Landsberg et al.,). Therefore, it was unexpected to see very few sleep statistics differ between the 3 defined vDNMP groups, such as with daytime naps (Table 1) and/or night-time awakenings (Table 2).
However, linear regression analysis revealed relationships between some sleep statistics and cognitive dysfunction within and across various cognitive domains. Although a change in daytime napping was not apparent between the vDNMP groups, a significant inverse relationship (r=−0.40; p<0.05) was observed between increased accuracy at the 90 s-delay on the spatial working memory task and fewer daytime naps (Table 4). Accuracy at the 20 s delay, or on the multi-domain cognitive index, was not related (r<−0.24; p=n.s.) to napping. This potentially explains why LMP dogs had on average 10 more naps per day compared to MMP and HMP dogs, but did not result in a statistically significant difference (Table 1), because the vDNMP groups were stratified based on the combined accuracy score at both the 20 and 90 s delay. This suggests that while increased daytime napping may partially predict a loss of working memory ability, it does not predict a more severe cognitive decline in which cognitive dysfunction is present across multiple cognitive domains. Although a working memory task was not included in the battery of neuropsychological tests for people considered to be “at risk” of dementia, actigraphy-based sleep analysis also revealed that increased napping or longer naps per day was significantly correlated with higher rates of mild cognitive impairment, as measured by verbal memory, processing speed, verbal fluency, and/or mental flexibility (Cross et al., 2015). Even though domestic dogs and people have diurnal sleep cycles, it is important to consider that napping patterns between dogs and people are significantly different, as daily actigraphy-generated ‘behavioral naps’ in dogs are significantly more frequent and shorter (Zanghi et al., 2013).
Differences in night-time awakenings or increases in duration of awakenings were not observed between the two age-groups of dogs in the current study. Interestingly, a significant correlation was observed with declining attention performance or multiple cognitive domain dysfunction relative to a later onset of early morning activity. This suggests that a phase delay in the onset of morning activity rhythm may predict cognitive decline. This observation is consistent to the study by Siwak et al. (2003) in which peak daytime locomotor activity was delayed in cognitively impaired senior dogs compared to non-impaired senior dogs.
Alternatively, it is also reasonable to assert that the population of senior dogs with lower combined cognitive scores evaluated in this study were not representative of dogs typically diagnosed with CDS. At present, the relationship between performance levels on specific, or a combination of, neuropsychological tests and CDS severity in dogs has yet to be established (Head et al., 2008). Because sleep disturbances in AD are believed to be multifactorial (Peter-Derex et al., 2015), it is likely that sleep dysregulation and cognitive dysfunction in senior dogs is also multifactorial.
4.3. Effect of age and cognitive performance on core body temperature rhythm
The present study demonstrates cognition-dependent differences of spatial working memory, but not advancing age, in core body temperature of aged dogs. Specifically, this is the first study we are aware of reporting differences of CBT at certain times of the day in a population of dogs of varying cognitive status or in association with altered activity/sleep patterns. CBT rhythm is also generally accepted as yielding the best estimate of endogenous circadian amplitude and endogenous circadian phase in human subjects (Czeisler et al., 1992). CBT has been shown to decline with advanced age in healthy people over 60 years of age compared to young adults (Blatteis, 2012, Harper et al., 2005). In AD patients, a phase delay in CBT rhythm is also observed compared to healthy, aged people, as well as a desynchronization of the CBT rhythm and locomotor activity/rest rhythm (Harper et al., 2005, Satlin et al., 1995).
Preliminary data from our lab indicated that kennel-housed, older Labrador Retriever dogs (10–14 yrs old) have a lower CBT by approximately 0.5°F across multiple time-points during the day versus young (3–7 yrs old) Labrador Retriever dogs (Zanghi, unpublished data). This is consistent with an age-related decrease (0.7°F) reported in people (Blatteis, 2012, Harper et al., 2005). The current study indicated that cognitive status rather than age was primarily associated with changes in CBT. Therefore, it is plausible that although changes in relative amplitude of CBT may manifest with advanced age between young and old dogs, as it does in other species, further dysregulation of diurnal CBT changes in old dogs may be more affected by cognitive changes associated with neurodegenerative processes. Particularly since dysregulation of circadian CBT is also observed in people with AD (Harper et al., 2005; Satlin et al., 1995) and transgenic rodent-models (Knight et al., 2013) of AD.
This data uniquely demonstrates that dogs with low spatial working memory function have significantly reduced afternoon, peak CBT at 19:00 (Fig. 2B). Linear regression analysis further indicated that lower afternoon CBT at 15:00 and 19:00 was moderately related not only to spatial working memory impairments, but also impairments in selective attention, and therefore, multi-domain cognitive performance (Table 3). Because the cognitive performance index on these tasks was not related to age in this population of senior dogs, significant reductions of afternoon or peak daytime CBT may be a better predictor of cognitive dysfunction in attention, memory, and learning impairments.
Since the dogs were temporarily and individually housed in metabolism kennels for the 36 h of the CBT recording period, rectal body temperature reflects a resting metabolic heat production that varies with diurnal fluctuation, and is independent of kennel-run-related or exercise-related activity. This observation is consistent with previous work by Refinetti and Piccione (2003) in which they specifically demonstrated that the circadian CBT rhythm becomes apparent only when adult dogs have limited locomotor activity by having them temporarily housed in metabolism kennels during the CBT recording period.
Because the LMP group demonstrated lower CBT measures amongst the 3 vDNMP groups (Fig. 2B), one might conclude that reduced CBT rhythm is a function of reduced locomotor activity. However, this is likely not accurate for the following reasons. First, locomotor activity was recorded while the dogs were group housed in their respective kennels several days before or after, and not while, they were in the metabolism cages for CBT measurement. Therefore, lower CBT was not a consequence of simultaneously recorded reduced locomotor activity. Second, a correlation matrix was generated from linear regression analysis of hourly CBT measures and hourly locomotor activity data, which resulted in very few significant correlations at similar time-points; rather the significant correlations were observed between activity levels from 07:00 to 13:00 and the 15:00 CBT measure. Therefore, high activity levels were temporally separate from the highest CBT recording.
Because circadian regulation of various physiological processes are controlled by differing neuronal projections within the hypothalamus, this data may aid in examining anatomical changes or loss of neurons through use of imaging technologies. Mapping of regional brain functions have determined that locomotor activity, sleep-wake cycles, corticosteroid secretion, and feeding are controlled by projections via the ventral subparaventricular zone (SVZ) to the dorsomedial hypothalamic nucleus (DMH) (Chou et al., 2003, Lu et al., 2001), whereas, circadian regulation of body temperature is controlled via the dorsal SVZ (Lu et al., 2001). These also are separate from direct innervation of the paraventricular hypothalamic nucleus that regulates circadian melatonin release (Saper et al., 2005). Therefore this study provides some initial evidence to suggest that neurodegeneration or loss of neurons of the dorsal SVZ for CBT regulation likely precedes degeneration within the ventral SVZ for locomotor activity, and may implicate these regions in early cognitive dysfunction in aged canines, possibly preceding the progression to CDS.
In conclusion, this study uniquely demonstrated that lower diurnal CBT peak and/or increased daytime napping, but not night-time locomotor activity, were most significantly associated with some aspects of cognitive impairment. More specifically, spatial working memory performance compared to learning or selective attention performance, and is independent of later advancing age. Furthermore, lower afternoon peak CBT, measured as resting metabolic heat production, may be a biomarker that predicts multiple-domain cognitive dysfunction. Additional work to assess the longitudinal changes of these multiple cognitive domains relative to further changes in behavioral and physiological circadian or diurnal rhythms may aid in distinguishing the difference between normal cognitive aging and canine CDS. It is also noteworthy that most of the dogs performing in the study did not exhibit many of the typical signs of CDS, which supports the hypothesis that cognitive impairments assessed with neuropsychological tests may precede behavioral changes associated with CDS.
Conflict of interest
The authors would like to indicate that funding was solely provided by Nestle Purina Research through a contract to CanCog Technologies to complete the collaboratively designed study. No other conflict of interests exists.
Acknowledgment
The authors would like to thank Wendell Kerr and Xuemei Si for their statistical analysis assistance.
Footnotes
10, 15, 30, and 60 min increments were also examined. No significant differences were found between the increment levels, therefore, only the results for the 5 min increment are shown.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.nbscr.2016.07.001.
Appendix A. Supplementary material
Supplementary material
Supplementary material
References
- Ancoli-Israel S., Cole R., Alessi C., Chambers M., Moorcroft W., Pollak C. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- Bain M.J., Hart B.L., Cliff K.D., Ruehl W.W. Predicting behavioral changes associated with age-related cognitive impairment in dogs. J. Am. Vet. Med. Assoc. 2001;218:1792–1795. doi: 10.2460/javma.2001.218.1792. [DOI] [PubMed] [Google Scholar]
- Bates, D.M., Maechler, M., Bolker, B. 2012. lme4: Linear mixed-effects models using S4 classes. R package version 0.999999-0.
- Blatteis C. Age-dependent changes in temperature regulation – a mini review. Gerontology. 2012;58:289–295. doi: 10.1159/000333148. [DOI] [PubMed] [Google Scholar]
- Blume, C., Santhi, N., Schabus, M., 2016. nparACT: Non-parametric measures of actigraphy data. R package version 0.4. [DOI] [PMC free article] [PubMed]
- Chou T.C., Scammell T., Gooley J., Gaus S., Saper C., Lu J. Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. J. Neurosci. 2003;23:10691–10702. doi: 10.1523/JNEUROSCI.23-33-10691.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie L.A., Studzinski C.M., Araujo J.A., Leung C., Ikeda-Douglas C., Head E., Cotman C., Milgram N.W. A comparison of egocentric and allocentric age-dependent spatial learning in the beagle dog. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29:361–369. doi: 10.1016/j.pnpbp.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Cross N., Terpening Z., Rogers N.L., Duffy S.L., Hickie I.B., Lewis S.J., Naismith S.L. Napping in older people ‘at risk’ of dementia: relationships with depression, cognition, medical burden and sleep quality. J. Sleep Res. 2015;24:494–502. doi: 10.1111/jsr.12313. [DOI] [PubMed] [Google Scholar]
- Czeisler C.A., Dumont M., Duffy J.F., Steinberg J.D., Richardson G.S., Brown E.N., Sánchez R., Ríos C.D., Ronda J.M. Association of sleep-wake habits in older people with changes in output of circadian pacemaker. Lancet. 1992;340:933–936. doi: 10.1016/0140-6736(92)92817-y. [DOI] [PubMed] [Google Scholar]
- Fuller P.M., Gooley J.J., Saper C.B. Neurobiology of the sleep-wake cycle: sleep architecture, circadian regulation, and regulatory feedback. J. Biol. Rhythm. 2006;21:482–493. doi: 10.1177/0748730406294627. [DOI] [PubMed] [Google Scholar]
- Harper D.G., Volicer L., Stopa E.G., McKee A.C., Nitta M., Satlin A. Disturbance of endogenous circadian rhythm in aging and Alzheimer disease. Am. J. Geriatr. Psychiatry. 2005;13:359–368. doi: 10.1176/appi.ajgp.13.5.359. [DOI] [PubMed] [Google Scholar]
- Head E., Rofina J., Zicker S. Oxidative stress, aging and CNS disease in the canine model of human brain aging. Vet. Clin. North Am. Small Anim. Pract. 2008;38:167–177. doi: 10.1016/j.cvsm.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houpt K.A. 3rd ed. Iowa State University Press; Ames: 1998. Domestic animal behavior for veterinarians and animal scientists. [Google Scholar]
- Knight E.M., Brown T.M., Gümüsgöz S., Smith J.C., Waters E.J., Allan S.M., Lawrence C.B. Age-related changes in core body temperature and activity in triple-transgenic Alzheimer’s disease (3xTgAD) mice. Dis. Model Mech. 2013;6:160–170. doi: 10.1242/dmm.010173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John J., Wu M.F., Siegel J.M. Systemic administration of hypocretin-1 reduces cataplexy and normalizes sleep and waking durations in narcoleptic dogs. Sleep Res. 2000;3:23–28. 〈http://www.sro.org/2000/John/23/〉 [PMC free article] [PubMed] [Google Scholar]
- Landsberg G.M., Deporter T., Araujo J.A. Clinical signs and management of anxiety, sleeplessness, and cognitive dysfunction in the senior pet. Vet. Clin. North Am. Small Anim. Pract. 2011;41:565–590. doi: 10.1016/j.cvsm.2011.03.017. [DOI] [PubMed] [Google Scholar]
- Landsberg, G.M., Nichol, J., Araujo, J.A., 2012.Cognitive dysfunction syndrome: a disease of canine and feline brain aging. Vet. Clin. North Am. Small Anim. Pract. 42, 749–768. [DOI] [PubMed]
- Landsberg G.M., Ruehl W. Geriatric behavioral problems. Vet. Clin. North Am. Small Anim. Pract. 1997;27:1537–1559. doi: 10.1016/s0195-5616(97)50138-0. [DOI] [PubMed] [Google Scholar]
- Lu J., Zhang Y., Chou T., Gaus S., Elmquist J., Shiromani P., Saper C. Contrasting effects of ibotenate lesions of the paraventricular nucleus and subparaventricular zone on sleep-wake and temperature regulation. J. Neurosci. 2001;21:4864–4874. doi: 10.1523/JNEUROSCI.21-13-04864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgram N.W., Head E., Weiner E., Thomas E. Cognitive function and aging in the dog: acquisition of non-spatial visual tasks. Behav. Neurosci. 1994;108:57–68. doi: 10.1037//0735-7044.108.1.57. [DOI] [PubMed] [Google Scholar]
- Milgram, N.W., de Rivera, C., Zanghi, B., Pan, Y., Mongillo, P., Cotman, C.W., Araujo, J., 2010. Modeling human cognitive aging in the beagle dog. In: Proc. Nestlé Purina Companion Animal Nutrition Summit. pp. 81–93.
- Neilson J., Hart B., Cliff K., Ruehl W.W. Prevalence of behavioral changes associated with age-related cognitive impairment in dogs. J. Am. Vet. Med. Assoc. 2001;218:1787–1791. doi: 10.2460/javma.2001.218.1787. [DOI] [PubMed] [Google Scholar]
- Nelson W., Tong Y., Lee J., Halberg F. Methods for cosinor-rhythmometry. Chronobiologia. 1979;6:305–323. [PubMed] [Google Scholar]
- Nishino, S., Tafti, M., Sampathkumaran, R., Dement, W.C., Mignot, E., 1997. Circadian distribution of rest/activity in narcoleptic and control dogs: assessment with ambulatory activity monitoring. J. Sleep Res. 6, pp. 120–127. [PubMed]
- Peter-Derex L., Yammine P., Bastuji H., Croisile B. Sleep and Alzheimer’s disease. Sleep Med. Rev. 2015;19:29–38. doi: 10.1016/j.smrv.2014.03.007. [DOI] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2015. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Refinetti R., Piccione G. Daily rhythmicity of body temperature in the dog. J. Vet. Med. Sci. 2003;65:935–937. doi: 10.1292/jvms.65.935. [DOI] [PubMed] [Google Scholar]
- Ruehl W.W., Bruyette D.S., DePaoli A., Cotman C., Head E., Milgram N.W., Cummings B.J. Canine cognitive dysfunction as a model for human age-related cognitive decline, dementia and Alzheimer's disease: clinical presentation, cognitive testing, pathology and response to l-deprenyl therapy. Prog. Brain Res. 1995;106:217–225. doi: 10.1016/s0079-6123(08)61218-2. [DOI] [PubMed] [Google Scholar]
- Ruehl W.W., Hart B.L. Canine cognitive dysfunction. In: Dodman N.H., Schuster L., editors. Psychopharmacology of Animal Behavior Disorders. Blackwell Science Inc; Mass: 1998. pp. 283–304. [Google Scholar]
- Saper C., Lu J., Chou T., Gooley J. The hypothalamic integrator for circadian rhythms. Trends Neurosci. 2005;28:152–157. doi: 10.1016/j.tins.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Satlin A., Teicher M.H., Lieberman H.R., Baldessarini R.J., Volicer L., Rheaume Y. Circadian locomotor activity rhythms in Alzheimer's disease. Neuropsychopharmacology. 1991;5:115–126. [PubMed] [Google Scholar]
- Satlin A., Volicer L., Stopa E.G., Harper D. Circadian locomotor activity and core-body temperature rhythms in Alzheimer’s disease. Neurobiol. Aging. 1995;16:765–771. doi: 10.1016/0197-4580(95)00059-n. [DOI] [PubMed] [Google Scholar]
- Siwak C.T., Murphey H.L., Muggenburg B.A., Milgram N.W. Age-dependent decline in locomotor activity in dogs is environment specific. Physiol. Behav. 2002;75:65–70. doi: 10.1016/s0031-9384(01)00632-1. [DOI] [PubMed] [Google Scholar]
- Siwak C.T., Tapp P.D., Zicker S.C., Murphey H.L., Muggenburg B.A., Head E., Cotman C.W., Milgram N.W. Circadian activity rhythms in dogs vary with age and cognitive status. Behav. Neurosci. 2003;117:813–824. doi: 10.1037/0735-7044.117.4.813. [DOI] [PubMed] [Google Scholar]
- Summers M.J., Saunders N.L. Neuropsychological measures predict decline to Alzheimer's dementia from mild cognitive impairment. Neuropsychology. 2012;26:498–508. doi: 10.1037/a0028576. [DOI] [PubMed] [Google Scholar]
- Takeuchi T., Harada E. Age-related changes in sleep-wake rhythm in dog. Behav. Brain Res. 1986;136:193–199. doi: 10.1016/s0166-4328(02)00123-7. [DOI] [PubMed] [Google Scholar]
- Tobler I., Sigg H. Long-term motor activity recording of dogs and the effect of sleep deprivation. Experientia. 1986;42:987–991. doi: 10.1007/BF01940702. [DOI] [PubMed] [Google Scholar]
- van Someren E.J. Circadian and sleep disturbances in the elderly. Exp. Gerontol. 2000;24:1229–1237. doi: 10.1016/s0531-5565(00)00191-1. [DOI] [PubMed] [Google Scholar]
- van Someren E.J., Colenda C.C., McCall W.V., Rosenquist P.B. Bright light therapy: Improved sensitivity to its effects on rest-activity rhythms in Alzheimer patients by application of nonparametric methods. Chronobiol. Intern. 1999;16:505–518. doi: 10.3109/07420529908998724. [DOI] [PubMed] [Google Scholar]
- van Someren E.J., Hagebeuk E.E., Lijzenga C., Scheltens P., de Rooij S.E., Jonker C., Pot A.M., Mirmiran M., Swaab D.F. Circadian rest-activity rhythm disturbances in Alzheimer’s disease. Biol. Psychiatry. 1996;40:259–270. doi: 10.1016/0006-3223(95)00370-3. [DOI] [PubMed] [Google Scholar]
- Witting W., Kwa I.H., Eikelenboom P., Mirmiran M., Swaab D.F. Alterations in the circadian rest-activity rhythm in aging and Alzheimer's disease. Biol. Psychiatry. 1990;27:563–572. doi: 10.1016/0006-3223(90)90523-5. [DOI] [PubMed] [Google Scholar]
- Zanghi B.M., Araujo J., Milgram N.W. College of Veterinary Internal Medicine Forum; Nashville, Tennessee, USA: 2014. PM-Supplementation With Melatonin, Zinc, and Haematococcus pluvialis Selectively Improves Attention and Motor Learning in Aged, Memory-impaired Dogs. Abstract NM-6. [Google Scholar]
- Zanghi B.M., Araujo J., Milgram N.W. Cognitive domains in the dog: independence of working memory from object learning, selective attention, and motor learning. Anim. Cogn. 2015;18:789–800. doi: 10.1007/s10071-015-0847-3. [DOI] [PubMed] [Google Scholar]
- Zanghi B.M., Kerr W., deRivera C., Araujo J., Milgram N.W. Characterization of diurnal rest/activity rhythms in adult dogs of various cages fed once or twice daily. J. Vet. Behav.: Clin. Appl. Res. 2012;7:339–347. [Google Scholar]
- Zanghi B.M., Kerr W., Gierer J., deRivera C., Araujo J., Milgram N.W. Characterizing behavioral sleep using actigraphy in adult dogs of various ages fed once or twice daily. J. Vet. Behav.: Clin. Appl. Res. 2013;8:195–203. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material