Abstract
Targeted therapy for osteosarcoma includes organ, cell and molecular biological targeting; of these, organ targeting is the most mature. Bone‐targeted drug delivery systems are used to concentrate chemotherapeutic drugs in bone tissues, thus potentially resolving the problem of reaching the desired foci and minimizing the toxicity and adverse effects of neoadjuvant chemotherapy. Some progress has been made in bone‐targeted drug delivery systems for treatment of osteosarcoma; however, most are still at an experimental stage and there is a long transitional period to clinical application. Therefore, determining how to combine new, polymolecular and multi‐pathway targets is an important research aspect of designing new bone‐targeted drug delivery systems in future studies. The purpose of this article was to review the status of research on targeted therapy for osteosarcoma and to summarize the progress made thus far in developing bone‐targeted drug delivery systems for neoadjuvant chemotherapy for osteosarcoma with the aim of providing new ideas for highly effective therapeutic protocols with low toxicity for patients with osteosarcoma.
Keywords: Cell‐targeted therapy, Drug delivery system, Molecular biological targets, Organ‐targeted therapy, Osteosarcoma
Introduction
Osteosarcoma, the most common malignant tumor in the skeletal system, originates from mesenchymal tissue, is characterized by spindle‐shaped stromal cells that are capable of generating bone‐like tissues and accounts for 20% of primary malignant bone tumors. The annual incidence is 1–3 cases per 1,000,000 people, 70%–80% of patients being aged 10–25 years1. Osteosarcoma has a propensity to metastasize and recur because it often occurs in the metaphyses of long tubular bones such as the distal femur, proximal tibia and proximal humerus, which have rich blood supplies. Thus, hematogenous metastasis tends to occur early and progress rapidly2.
Before the 1970s, treatment for osteosarcoma was restricted to simple surgical excision, amputation being required for most patients. The 1‐year survival rate was therefore very low, about 80% of patients dying of pulmonary metastases3, 4. This low cure rate prompted intensive study of possible means of treating osteosarcoma. With progress in molecular biology, treatments for osteosarcoma initially developed from simple surgical excision to individualized limb‐salvage surgery and adjuvant chemotherapy and now include immunotherapy, gene therapy, and targeted therapy5, 6, 7. Limb‐salvage surgery is currently applicable for stage IIB osteosarcomas, osteosarcomas in children and pathological fracture associated with osteosarcoma; the its indications for it continue to expand. However, the major difficulty with successful treatment lies not in tumor resection but in reconstruction surgery. Because limb osteosarcoma often grow near joints, wide excision is often necessary. Additionally, it is difficult to find adequate bone for use in autogenous bone grafting, which hinders reconstruction. Tumor inactivation and in situ replantation weakens the bone, resulting in a high incidence of subsequent fracture; it is also not appropriate for patients in whom a large proportion of the affected bone has been damaged by the tumor. Allogeneic bone or articular transplantation has the disadvantage of a high incidence of rejection reactions. Rotationplasty is only applicable for osteosarcomas in the lower‐middle section of the femur and the patient still requires an artificial limb. Replacement with artificial prostheses has good short‐term outcomes, with a short healing period, but the prostheses are susceptible to looseness and infection. Adding chemotherapy to treatment regimens for osteosarcoma, especially since neoadjuvant chemotherapy has been implemented, has resulted to a large extent in replacement of conventional amputation by limb‐salvage surgery. However, there is no consensus on whether preoperative chemotherapy improves the long‐term prognosis of patients and the overall response rate to chemotherapy for patients with osteosarcoma is still about 60%. Immunotherapy for osteosarcoma includes nonspecific immune therapy, specific immunotherapy, adoptive immunotherapy and targeting therapy. Presently, interleukin‐2 has been used in postoperative chemotherapy for osteosarcoma. This agent stimulates generation of natural killer cells and lymphocyte activated killer cells; however, its therapeutic effect is still uncertain. Gene therapy for osteosarcoma is still at the research stage and cannot yet be used in the clinic. In other words, these therapies either fall short of expectations for improving patients’ prognoses or they are still at an exploratory stage with is a long way to go before being available for clinical application. The purpose of this article was to review the status of research on targeted therapy for osteosarcoma and to summarize the progress made thus far in developing bone‐targeted drug delivery systems for neoadjuvant chemotherapy for osteosarcoma with the aim of providing new ideas for highly effective therapeutic protocols with low toxicity for patients with osteosarcoma.
Pertinent published reports were collected by computer searching of PubMed with the following key words and subject terms: osteosarcoma, bone targeting, targeted therapy, hydroxyapatite (HA). In all, 457 articles were retrieved. After 85 non‐English articles had been excluded, the abstracts of the remaining articles English (372) were browsed and preliminarily assessed. This resulted in exclusion of 298 articles that were not in conformity with the subject of this study. The remaining 74 full texts were then browsed to identify any duplicate or similar studies. Finally, 67 articles that had recently been published in authoritative journals were comprehensively screened to determine whether the quality of their research designs was acceptable, their research methods standard, they had randomized their subjects and used scientifically acceptable statistical methods and whether the results research were consistent with theoretical expectations. Further, to enrich the material included in this review, articles related to treatment of osteosarcoma and bone targeting were also assessed; finally, 51 articles were selected for inclusion. This process is depicted as a flow chart in Fig. 1.
Figure 1.
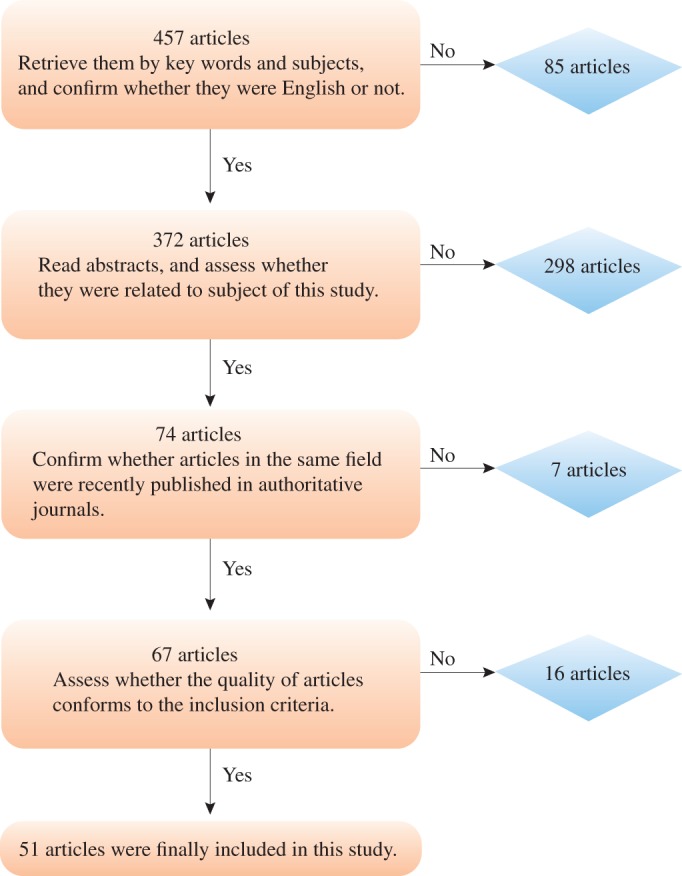
Flow chart showing the process of selecting articles for this review.
Targeted Therapy for Osteosarcoma
Targeted therapy is defined as the process of drugs being selectively concentrated and delivered to targeted organs, tissues, cells and specific intracellular structures by special guiding mechanisms with the aim of achieving the local treatment of lesions. These agents may be administered locally drug, orally or intravenously. Targeted therapy can be classified on the basis of the targets into three categories: organ‐targeted, cell‐targeted and molecular‐targeted therapy. Organ‐targeted therapy involves guiding substances to accumulate in high concentrations in the targeted organs or tissues. Cell‐targeted therapy involves delivering specific molecules (such as proteins or nucleic acids) to within cells or using particular techniques to impair the biological activity of specific cells. It can be achieved by utilizing the different affinities of particular proteins for the cell surface, elements that regulate gene expression or viruses specific to certain cells. Molecular‐targeted therapy involves delivering protein molecules, nucleotide fragments or gene products to targeted areas by cell fusion and phagocytosis. A flow chart depicting targeted therapy for osteosarcoma is shown in Fig. 2.
Figure 2.
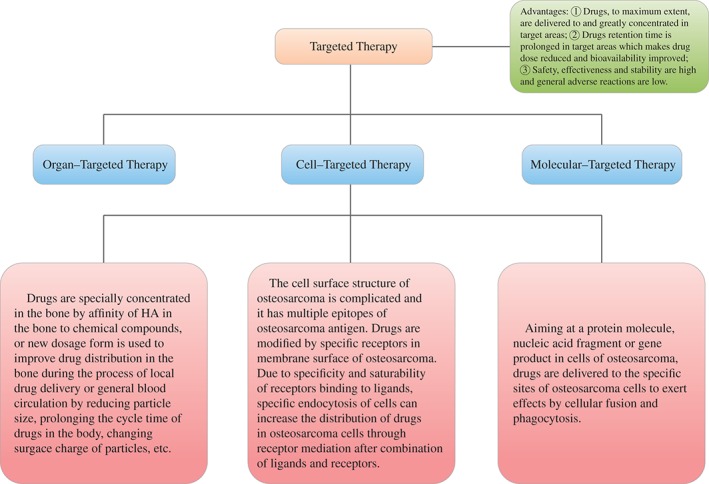
Flow chart of osteosarcoma targeted therapy.
Organ‐targeted Therapy for Osteosarcoma
Bone consists of bone cells, collagen fibers and bone matrix, the last being composed mainly of HA. It has been estimated that 99% of all calcium in the human body is in the form of HA in bones, only 1% being distributed through soft tissues and extracellular fluid in forms other than HA8. On this basis, in 1987 Pierce and Waite were the first to propose the concept of bone‐targeting by compound molecules being deposited in bone and permeating into HA. They synthesized WP‐1, the first pharmaceutical molecule with a bone‐targeting role and minimal effect on non‐bone tissues, thus providing a theoretical and laboratory basis for research in this field9. Thompson et al. and Saari et al. subsequently put forward a consistent concept of bone seeking based on their laboratory experiments in 1989 and in 1992 on targeting bone by exploiting a specific affinity for HA10, 11. Since then, great progress has been made in research on bone‐targeted drugs.
With further in‐depth research, the definition of bone tissue targeting has gradually been extended. In addition to specifically binding to HA, drugs can act directly on bone to increase their concentrations in bone tissues. Organ‐targeted therapy for osteosarcoma mainly comprises the following three drug‐carrying modes: (i) coupling drug‐carrying mode: bone‐seeking substances bind with antineoplastic drugs directly or via intermediate substances; (ii) scion grafting drug‐carrying mode: bone‐seeking substances and antineoplastic drugs are chemically grafted onto polymer in specific proportions; and (iii) nano‐drug carrier: drugs are coated in nanoparticles, the surfaces of which are modified or not by bone‐seeking substances.
Cell‐targeted Therapy for Osteosarcoma
At present, aptamer‐based tumor‐targeted therapy has become a hot topic of research in China and elsewhere. Aptamers, also termed “bait” or “chemical antibodies”, are short single‐stranded DNA and RNA oligonucleotides or polypeptide fragments that exist in the body in the form of three‐dimensional structures and are capable of closely connecting with targeted proteins with high affinity12. Generally, aptamers act directly on extracellular targets rather than by entering cells by signal transduction. Hence, cell‐targeted therapy uses aptamers combined with anti‐tumor drugs to act on tumor cell surfaces. In addition, aptamers are capable of recognizing different tumor cells and accurately distinguishing the targeted tumors by having amplified sequence fragments bound to them; however, different types of tumor do have some characteristics in common13. It is anticipated that in the future more aptamers that can specifically recognize and bind with osteosarcoma will be identified for the diagnosis and treatment of osteosarcoma in clinical research and practice.
Molecular‐targeted Therapy for Osteosarcoma
Molecular‐targeted therapy, the most specific of targeted therapies, is based on organ‐targeted and cell‐targeted therapies. It targets sites such as protein molecules or gene segments in tumor cells that are specific to certain cancers, the therapeutic substances having specific affinity to selectively bind to these sites, thus leading specifically to the death of tumor cells, which is the key point of molecular‐targeted therapy. It is anticipated that molecular‐targeted drugs will be developed in the future for a variety of new targets (such as cellular receptors, key genes, control molecules and kinase of osteosarcoma); however at present, the targets for osteosarcoma are mainly angiogenesis and pulmonary metastasis14.
Some progress has been made in cell‐ and molecular‐targeted therapies for osteosarcoma; however, most are still at the experimental stage and there is a long transitional period to availability for clinical application. How to identify highly specific targets, develop targeted drugs and control toxicity and adverse effects is therefore yet to be explored15. However, organ‐targeted therapy is expected to become an effective form of treatment for osteosarcoma in the clinic because of its clear therapeutic mechanism, strong target, and predictable in‐vivo process.
Use of Bone‐targeted Drug Delivery Systems in Neoadjuvant Chemotherapy for Osteosarcoma
In recent years, neoadjuvant chemotherapy has become a common means of treating osteosarcoma. Neoadjuvant chemotherapy combined with excision currently preserves the limbs of over 75% of patients and has increased the 5‐year survival rate from less that 20% to 65%–70%16, 17, 18. However, because bone tissue is characterized by hardness, poor permeability and low blood flow, it is difficult to deliver chemotherapeutic drugs into tumor sites via conventional modes of administration. Because there is a direct relationship between drug concentration and extent of necrosis, it is difficult to achieve an effective dose in tumors, which limits the curative effect of chemotherapy. Thus, the dosage and therefore serum concentrations of chemotherapeutic must be increased to increase the concentration of chemotherapeutic agents within tumors; however, high‐dose chemotherapy is associated with severe toxicity. Although local drug delivery (such as by regional arterial chemotherapy, hyperthermic isolated limb perfusion and so on) reduces the overall toxicity of chemotherapeutic drugs and improves the response rate of tumors to chemotherapy, long‐term follow‐up has shown that regional arterial chemotherapy and hyperthermic isolated limb perfusion do not have superior effects on prognosis compared with systemic intravenous chemotherapy. Therefore, it is necessary and urgent to seek a new types of chemotherapy or drug delivery systems that selectively act on bone tissues and thus reduce toxicity and improve efficacy19. Research on organ‐targeted therapy for osteosarcoma may provide feasible solutions to the above dilemmas because bone‐targeted drug delivery systems can concentrate chemotherapeutic agents in bone tissue and specifically act on osteosarcomas. Bone‐targeted drug delivery systems can be categorized according to their mechanisms as employing active, passive and multiple targeting.
Active Targeting
Molecules that bind specifically to HA can act as the guides or carriers of bone‐targeted drugs, enabling those drugs to act selectively on bone tissue. Presently, the guides or carriers used for bone‐targeting drugs include tetracyclines, diphosphonic acid, propylene acid, heterocyclic small molecule and oligopeptides20, 21, 22, 23.
Diphosphonic acid compound is a bone resorption inhibitor that can inhibit the release of bone‐matrix growth factor and cancer cell adhesion to bone matrix and decrease the occurrence of osseous metastases in individuals with cancer. It is also the preferred carrier for bone‐targeted drugs because it has a significant affinity to HA24. In 1996, Hosain et al. used amido bonds to link tetraethyl methylene diphosphonate and methotrexate and observed the distribution of the resultant conjugates in blood, skin, muscle, liver, lung, and bone of mice after technetium 99 m labeling. They reported that the conjugate was eliminated from the blood after intravenous injection, 20% of it being deposited in bone and 55% being excreted in urine25. After the conjugate had been administered to New Zealand rabbits intravenously, radionuclide imaging indicated that it was mainly distributed in bone and joints 1.5 hours after administration. However, no further research was performed on tetraethyl methylene diphosphonate‐methotrexate conjugate until 2014, when Yang et al. prepared a methotrexate–diphosphonic acid conjugate that acted on osteosarcoma MG‐63 cells with a concentration gradient of 2–3000 μg/mL. They found that MG‐63 cells showed the typical characteristics of apoptosis and cell viability declined significantly when a concentration of over 2000 μg/mL for 24–96 hours was achieved26. Yang et al. also reported that the apoptotic effect of a methotrexate–diphosphonic acid conjugate on MG‐63 cells is time‐ and dose‐dependent, which indicates that binding to diphosphonic acid only changes the pharmacokinetics and distribution of methotrexate but does not reduce its lethality for tumors26.
Besides diphosphonic acid compounds, tetracycline, which is strongly bone seeking, is also capable of inhibiting collagenase activity and bone resorption and promoting fibroblast adhesion27, 28. Tetracycline fluoresces under ultraviolet light, which facilitates analyzing and detecting conjugates29. Oligopeptides with certain bone‐seeking properties can target various sites in the bone depending on their amino acid sequences. This is because HA in sites of bone formation is in a minimally crystalline form, whereas HA in sites of bone absorption is in a highly crystalline form. Repeated amino acid sequence in oligopeptides can bind to HA, different amino acid sequences having different affinities for different crystal forms of HA30, 31. For example, the octapeptide of aspartic acid has a strong affinity for the highly crystalline HA in sites of bone absorption, whereas (AspSerSer)6, which comprises six repeats of the aspartic acid‐serine‐ serine sequence, has a strong affinity for the minimally crystalline HA in sites of bone formation32. Zhang et al. used (AspSerSer)6 to achieve targeted delivery of small nucleic acids to osteoblasts33. However, diphosphonic acid compounds are mainly regarded as carriers for bone‐targeting drugs in the treatment of osteosarcoma; there are few reports of other carrier‐associated agents.
Passive Targeting
In passive targeting, drug‐carrier particles are taken in by the mononuclear phagocyte system in vivo and concentrated into bone tissues through normal physiological processes in that myeloid tissues contain specific macrophages that produce particles that enable small circulating cells to enter the bone marrow34. Therefore, given the phagocytic ability of bone marrow macrophages, the drug delivery system would be concentrated into bone by specific phagocytosis by bone marrow macrophages. Thus it is clear that the main obstacle to achieving passive bone‐targeted drug delivery lies in the marrow–blood barrier and the strong phagocytic ability of liver and spleen cells. Brusa et al. found that when particles of 0.1–0.2 μm entered the body, they were removed rapidly from the blood by macrophages in the reticuloendothelial system, eventually reaching lysosomes in Kupffer cells35. Additionally, particle systems of 50–100 nm can enter parenchymal hepatic cells and particle systems of less than 50 nm can enter the spleen and bone by penetrating through the endothelial cells of the liver or through lymph. Hence, reducing the size of particles is a key factor in inhibiting intake by the liver and increasing distribution of the particles in the bone. Moreover, research has shown that modifying the particle surface can increase the cycle time of particles in vivo, inhibiting intake by the spleen and increasing their content in bone36.
Sou et al. prepared L‐glutamate, N‐[3‐carboxyl‐1‐oxygen propyl]‐1 and 5–26 alkyl ester (SA)‐coated liposomes and demonstrated bone targeting in rabbits37. They found that, 24 hour after intravenous injection, SA‐Ve is distributed in the bone marrow and liver, whereas uncoated liposomes are mainly distributed in the spleen and liver. Liposomes that have been further coated with polyethylene glycol (PEG) reportedly inhibit intake by the liver of SA‐Ve. The amount of SA‐Ve in the bone marrow is maximal when the amount of PEG is 0.6%, which indicates that SA‐Ve targets bone marrow. However, this process is in mutual competition with the intake of SA‐Ve by the liver. Therefore, appropriate modification by PEG of the SA‐Ve surface inhibits the intake of SA‐Ve by the liver, resulting in SA‐Ve targeting bone marrow. Based on these data, Sou et al. performed another feasible study of SA‐Ve bone targeting in primate macaques, and found that 70% of SA‐Ve is taken in by bone marrow macrophages after intravenous injection38. Wang et al. used PEG‐coated polylactide‐co‐glycolide acid nanoparticles as carriers to prolong the cycle time of paclitaxel and etoposide in the blood, consequently utilizing a combination of the two different mechanisms of these chemotherapeutic agents for osteosarcoma39. Hu et al. prepared PEG‐coated pH‐sensitive doxorubicin nanoparticles, thus prolonging the cycle time of doxorubicin40. When it reaches the tumor sites, the nanoparticles release the doxorubicin quickly, completing the directed delivery of doxorubicin to the tumor. Thus, nanoparticles are potential components of bone‐targeted drug delivery systems; anti‐drugs can be coated in them and delivered into bone to treat osteosarcoma.
Multiple Targeting
Multiple targeting strengthens drug targeting and selectivity by simultaneously utilizing multiple targeting mechanisms. Dhule et al. coated the antineoplastic agents curcumin and C6 ceramide in a lipid bilayer of liposomes and found that the half‐life and cycling time of liposomes modified by PEG were prolonged. Additionally, liposomes modified by folic acid actively targeted over‐expressed folate receptor α on the surface of osteosarcoma, indicating that curcumin–C6 ceramide liposomes have an inhibiting effect on osteosarcoma in vivo and in vitro 41. Wu and Wan performed a similar experiment in which doxorubicin was sealed in a lipid bilayer of liposomes and diphosphonic acid and found that this system had less systemic toxicity and stronger selectivity for osteosarcoma than a bone‐target drug delivery system with a single mechanism42. Low et al. used aspartic acid as a bone‐targeting guidance molecule to coat acid‐sensitive doxorubicin and found that doxorubicin was not released under physiological conditions before reaching to tumor site, where the pH dropped to 5.5, resulting in the release of doxorubicin, enabling it to exert an anti‐tumor effect22. Furthermore, Morton et al. and Rudnick‐Glick et al. showed that diphosphonic acid‐coated nanoparticle has good bone targeting and antitumor activity22, 43. Therefore, combining multiple targeting mechanisms in one targeted drug delivery system increases targeting and reduces the toxic effects of drugs. Thus, multiple targeting is a potential means of treating osteosarcoma44, 45.
Conclusion
In recent years, great progress has been made in research on bone‐target drug delivery systems, but there are still exists some limitations. Firstly, carriers are the key factors in passive targeting and their distribution in the body is influenced by many factors. Of these, changes in particle size and modification of the surface can reportedly prolong the cycle time and enhance bone targeting by liposomes; however, the influence of surface charge and structure on bone targeting has not yet reported46, 47, 48, 49. Secondly, specific affinity for HA on bone surface is currently mostly used for active targeting. Additionally, combining bone marrow macrophage receptors and osteosarcoma surface receptors is another means of enhancing bone targeting. In a word, exploring the combined use of new, polymolecular and multi‐pathway targets is an important aspect of research into designing new bone‐targeted drug delivery systems.
Acknowledgments
The work on methotrexate‐diphosphonic acid conjugate and the inhibiting effect of its bone targeting on osteosarcoma was supported by the Natural Science Foundation General Project of Jiangsu Province‐Synthesis (No. BK20151373).
Disclosure: All authors of this article declare they have no conflicts of interest.
References
- 1. Ritter J, Bielack SS. Osteosarcoma. Ann Oncol, 2010, 21: vii320–vii325. [DOI] [PubMed] [Google Scholar]
- 2. Denduluri SK, Wang Z, Yan Z, et al Molecular pathogenesis and therapeutic strategies of human osteosarcoma. J Biomed Res, 2015, 30, doi: 10.7555/JBR.29.20150075. [DOI] [PubMed] [Google Scholar]
- 3. Wycislo KL, Fan TM. The immunotherapy of canine osteosarcoma: a historical and systematic review. J Vet Intern Med, 2015, 29: 759–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li C, Cong Y, Liu X, et al The progress of molecular diagnostics of osteosarcoma. Front Biosci (Landmark Ed), 2016, 21: 20–30. [DOI] [PubMed] [Google Scholar]
- 5. Zhou W, Hao M, Du X, Chen K, Wang G, Yang J. Advances in targeted therapy for osteosarcoma. Discov Med, 2014, 17: 301–307. [PubMed] [Google Scholar]
- 6. Wang T, Xu Z, Wang K, Wang N. Network analysis of microRNAs and genes in human osteosarcoma. Exp Ther Med, 2015, 10: 1507–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ning J, Guo X, Wang N, Xue L. Construction and analysis of three networks of genes and microRNAs in adenocarcinoma. Oncol Lett, 2015, 10: 3243–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ignjatović N, Wu V, Ajduković Z, Mihajilov‐Krstev T, Uskoković V, Uskoković D. Chitosan‐PLGA polymer blends as coatings for hydroxyapatite nanoparticles and their effect on antimicrobial properties, osteoconductivity and regeneration of osseous tissues. Mater Sci Eng C Mater Biol Appl, 2016, 60: 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pierce WM Jr, Waite LC. Bone‐targeted carbonic anhydrase inhibitors: effect of a proinhibitor on bone resorption in vitro . Proc Soc Exp Biol Med, 1987, 186: 96–102. [DOI] [PubMed] [Google Scholar]
- 10. Wang Y, Metcalf CA 3rd, Shakespeare WC, et al Bone‐targeted 2, 6, 9‐trisubstituted purines: novel inhibitors of Src tyrosine kinase for the treatment of bone diseases. Bioorg Med Chem Lett, 2003, 13: 3067–3070. [DOI] [PubMed] [Google Scholar]
- 11. Saari WS, Rodan GA, Fisher TE, Anderson PS. Novel bone acting agents. European Patent Application, EP0496520[P], 1992.
- 12. Gilboa E, Berezhnoy A, Schrand B. Reducing toxicity of immune therapy using aptamer‐targeted drug delivery. Cancer Immunol Res, 2015, 3: 1195–1200. [DOI] [PubMed] [Google Scholar]
- 13. Wu X, Chen J, Wu M, Zhao JX. Aptamers: active targeting ligands for cancer diagnosis and therapy. Therapie, 2015, 5: 322–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Paige M, Kosturko G, Bulut G, et al Design, synthesis and biological evaluation of ezrin inhibitors targeting metastatic osteosarcoma. Bioorg Med Chem, 2014, 22: 478–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sampson VB, Yoo S, Kumar A, Vetter NS, Kolb EA. MicroRNAs and potential targets in osteosarcoma: review. Front Pediatr, 2015, 3: 69 (E‐pub). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: current treatment and a collaborative pathway to success. J Clin Oncol, 2015, 33: 3029–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boro A, Arlt MJ, Lengnick H, et al Prognostic value and in vitro biological relevance of neuropilin 1 and neuropilin 2 in osteosarcoma. Am J Transl Res, 2015, 7: 640–653. [PMC free article] [PubMed] [Google Scholar]
- 18. Ferrari S, Meazza C, Palmerini E, et al Nonmetastatic osteosarcoma of the extremity. Neoadjuvant chemotherapy with methotrexate, cisplatin, doxorubicin and ifosfamide. An Italian Sarcoma Group study (ISG/OS‐Oss). Tumori, 2014, 100: 612–619. [DOI] [PubMed] [Google Scholar]
- 19. Yamamoto N, Tsuchiya H. Chemotherapy for osteosarcoma–where does it come from? What is it? Where is it going? Expert Opin Pharmacother, 2013, 14: 2183–2193. [DOI] [PubMed] [Google Scholar]
- 20. Cheng W, Yue Y, Fan W, et al Effects of tetracyclines on bones: an ambiguous question needs to be clarified. Pharmazie, 2012, 67: 457–459. [PubMed] [Google Scholar]
- 21. Choi SW, Kim JH. Design of surface‐modified poly (D, L‐lactide‐co‐glycolide) nanoparticles for targeted drug delivery to bone. J Control Release, 2007, 122: 24–30. [DOI] [PubMed] [Google Scholar]
- 22. Low SA, Yang J, Kopeček J. Bone‐targeted acid‐sensitive doxorubicin conjugate micelles as potential osteosarcoma therapeutics. Bioconjug Chem, 2014, 25: 2012–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Salerno M, Cenni E, Fotia C, et al Bone‐targeted doxorubicin‐loaded nanoparticles as a tool for the treatment of skeletal metastases. Curr Cancer Drug Targets, 2010, 10: 649–659. [DOI] [PubMed] [Google Scholar]
- 24. Debiais F. Bone targeting agents: bisphosphonates. Bull Cancer, 2013, 100: 1199–1206. [DOI] [PubMed] [Google Scholar]
- 25. Hosain F, Spencer RP, Couthon HM, Sturtz GL. Targeted delivery of antineoplastic agent to bone: biodistribution studies of technetium‐99m‐labeled gem‐bisphosphonate conjugate of methotrexate. J Nucl Med, 1996, 37: 105–107. [PubMed] [Google Scholar]
- 26. Yang XN, Zeng JC, Song YC, Zhang H, Pei FX. Targeted antiosteosarcoma methotrexate‐bisphosphonate conjugate induces apoptosis of osteosarcoma cells in vitro . Eur Rev Med Pharmacol Sci, 2014, 18: 2116–2123. [PubMed] [Google Scholar]
- 27. Cheong JM, Gunaratna NS, McCabe GP, Jackson GS, Kempa‐Steczko A, Weaver CM. Bone‐seeking labels as markers for bone turnover: validation of urinary excretion in rats. Osteoporos Int, 2011, 22: 153–157. [DOI] [PubMed] [Google Scholar]
- 28. Li Z, Lu WW, Deng L, Chiu PK, et al The morphology and lattice structure of bone crystal after strontium treatment in goats. J Bone Miner Metab, 2010, 28: 25–34. [DOI] [PubMed] [Google Scholar]
- 29. Wang H, Liu J, Tao S, et al Tetracycline‐grafted PLGA nanoparticles as bone‐targeting drug delivery system. Int J Nanomedicine, 2015, 10: 5671–5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Posner AS, Betts F. Synthetic amorphous calcium phosphate and its relation to bone mineral structure. Acc Chem Res, 1975, 8: 273–281. [Google Scholar]
- 31. Wang D, Miller SC, Shlyakhtenko LS, et al Osteotropic peptide that differentiates functional domains of the skeleton. Bioconjug Chem, 2007, 18: 1375–1378. [DOI] [PubMed] [Google Scholar]
- 32. Yarbrough DK, Hagerman E, Eckert R, et al Specific binding and mineralization of calcified surfaces by small peptides. Calcif Tissue Int, 2010, 86: 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang G, Guo B, Wu H, et al A delivery system targeting bone formation surfaces to facilitate RNAi‐based anabolic therapy. Nat Med, 2012, 18: 307–314. [DOI] [PubMed] [Google Scholar]
- 34. Gu X, Ding J, Zhang Z, Li Q, Zhuang X, Chen X. Polymeric nanocarriers for drug delivery in osteosarcoma treatment. Curr Pharm Des, 2015, 21: 5187–5197. [DOI] [PubMed] [Google Scholar]
- 35. Brusa P, Dosio F, Pacchioni D, et al Pharmacokinetics of an antibody‐ricin conjugate administered intraperitoneally to mice. J Pharm Sci, 1994, 83: 514–519. [DOI] [PubMed] [Google Scholar]
- 36. Jokerst JV, Lobovkina T, Zare RN, Gambhir SS. Nanoparticle PEGylation for imaging and therapy. Nanomedicine, 2011, 6: 715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sou K, Goins B, Takeoka S, Tsuchida E, Phillips WT. Selective uptake of surface‐modified phospholipid vesicles by bone marrow macrophages in vivo . Biomaterials, 2007, 28: 2655–2666. [DOI] [PubMed] [Google Scholar]
- 38. Sou K, Goins B, Leland MM, Tsuchida E, Phillips WT. Bone marrow‐targeted liposomal carriers: a feasibility study in nonhuman primates. Nanomedicine (Lond), 2010, 5: 41–49. [DOI] [PubMed] [Google Scholar]
- 39. Wang B, Yu XC, Xu SF, Xu M. Paclitaxel and etoposide co‐loaded polymeric nanoparticles for the effective combination therapy against human osteosarcoma. J Nanobiotechnology, 2015, 13: 22, doi: 10.1186/s12951-015-0086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hu FQ, Zhang YY, You J, Yuan H, Du YZ. pH triggered doxorubicin delivery of PEGylated glycolipid conjugate micelles for tumor targeting therapy. Mol Pharm, 2012, 9: 2469–2478. [DOI] [PubMed] [Google Scholar]
- 41. Dhule SS, Penfornis P, He J, et al The combined effect of encapsulating curcumin and C6 ceramide in liposomal nanoparticles against osteosarcoma. Mol Pharm, 2014, 11: 417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wu D, Wan M. Methylene diphosphonate‐conjugated adriamycin liposomes: preparation, characteristics, and targeted therapy for osteosarcomas in vitro and in vivo . Biomed Microdevices, 2012, 14: 497–510. [DOI] [PubMed] [Google Scholar]
- 43. Rudnick‐Glick S, Corem‐Salkmon E, Grinberg I, Gluz E, Margel S. Biodegradable bisphosphonate nanoparticles for imaging and therapeutic applications in osteosarcoma. Proceedings of SPIE 9550, Biosensing and Nanomedicine VIII, 9 August 2015, 2015; San Diego, CA, doi: 10.1117/12.2186765. [DOI]
- 44. Liao Z, Nan G, Yan Z, et al The anthelmintic drug niclosamide inhibits the proliferative activity of human osteosarcoma cells by targeting multiple signal pathways. Curr Cancer Drug Targets, 2015, 15: 726–738. [DOI] [PubMed] [Google Scholar]
- 45. Li H, Guo L, Huang A, et al Nanoparticle‐conjugated aptamer targeting hnRNP A2/B1 can recognize multiple tumor cells and inhibit their proliferation. Biomaterials, 2015, 63: 168–176. [DOI] [PubMed] [Google Scholar]
- 46. Yang DH, Lee DW, Kwon YD, et al Surface modification of titanium with hydroxyapatite‐heparin‐BMP‐2 enhances the efficacy of bone formation and osseointegration in vitro and in vivo . J Tissue Eng Regen Med, 2015, 9: 1067–1077. [DOI] [PubMed] [Google Scholar]
- 47. Palaiologou A, Stoute D, Fan Y, Lallier TE. Altered cell motility and attachment with titanium surface modifications. J Periodontol, 2012, 83: 90–100. [DOI] [PubMed] [Google Scholar]
- 48. Guan HP, Sun JZ, Feng XL, et al Effects of RNA interference‐mediated knockdown of livin and survivin using monomethoxypolyethylene glycol‐chitosan nanoparticles in MG‐63 osteosarcoma cells. Mol Med Rep, 2016, 13: 1821–1826. [DOI] [PubMed] [Google Scholar]
- 49. Altındal DÇ, Gümüşderelioğlu M. Melatonin releasing PLGA micro/nanoparticles and their effect on osteosarcoma cells. J Microencapsul, 2016, 33: 53–63. [DOI] [PubMed] [Google Scholar]


