Abstract
Objective
Few studies have concentrated on the sagittal alignment of lumbar disc herniation (LDH), especially the parameters of the pelvis, and controversy exists about whether pelvic morphology is involved in the pathogenesis of LDH. The present study analyzed the characteristics of the sagittal alignment in young Chinese LDH patients and explored the impact of pelvic morphology on the pathogenesis of LDH.
Methods
A retrospective analysis was conducted on 100 young patients with LDH (69 men and 31 women, aged 18–35 years), and the clinical and imaging findings met the criteria for the diagnosis of LDH. The control group included 100 asymptomatic volunteers with matching age and gender. Coronal and sagittal parameters were measured on the anteroposterior and lateral radiographs of the whole spine, including lumbar lordosis (LL), pelvic incidence (PI), sacral slope (SS), pelvic tilt (PT), thoracic kyphosis (TK), and sagittal balance (SVA). The cases were classified into four types by the apex position of lumbar lordosis (type I, L 5 or the L 4–5 intervertebral space; type II, bottom or middle of L 4; type III, upper part of L 4 or the intervertebral space between L 3 and L 4; type IV, L 3 or more high level), and divided into three groups by PI; namely, a low PI group (PI < 40°), a medium PI group (40° ≤ PI < 50°), and a high PI group (PI ≥ 50°). The sagittal parameters, especially PI, were compared between the LDH group and the control group. Correlations between the parameters in the LDH group were analyzed.
Results
The PI value of the LDH group was not different from that of the control group (46.1° ± 10.0° vs 47.2° ± 8.8°, P > 0.05). The LDH group showed lower average LL, SS, and TK (P < 0.01), as well as higher PT and SVA compared with the control group (P < 0.01). The LL (34.4° ± 15.3° vs 50.8° ± 10.2°) and SVA (21.6 ± 53.6 mm vs − 18.4 ± 32.8 mm) showed a significant difference (P < 0.01); LL was correlated with PI, SS, PT, TK and SVA (P < 0.01); and SVA was also correlated with the parameters above (P < 0.01) except PI (P > 0.05), and the lordosis apex tended to be higher. The distributions of PI groups between the LDH group and the control group were not different. Pairwise correlations were found among LL, PI, SS, and PT. In addition, TK and SVA were correlated with LL, SS, and PT.
Conclusions
There is no difference in PI between young Chinese patients with lumbar disc herniation and the normal population. Young LDH patients may present flat lumbar and thoracic curves, as well as lower sacral slope. The anteversion sagittal imbalance is regulated by both the spine and the pelvis.
Keywords: Lumbar disc herniation, Pelvic incidence, Sagittal alignment, Young patients
Introduction
Research has shown that spino‐pelvic sagittal alignment is closely related with lumbar spondylolisthesis1, adult idiopathic scoliosis2, 3, thoracic angular posterior convex4, and many other spinal diseases. In addition, it has been proved to be associated with the clinical symptoms and outcomes of these diseases5, 6. The spino‐pelvic alignment in these degenerative diseases is often different from the normal population (e.g. a lower lumbar lordosis was reported in degenerative lumbar spondylolisthesis patients)7, 8, and to restore the sagittal balance and obtain a better outcome, appropriate correction of LL is emphasized9, 10, 11, 12. The most common method for LL reconstruction is the equation lumbar lordosis (LL) = pelvic incidence (PI) ± 9° proposed by Schwab et al. 5, 13. Therefore, research into sagittal alignment will be helpful in exploring the mechanism of LDH and surgical treatments.
Lumbar disc herniation (LDH) is one of the main degenerative diseases of the lumbar spine, which often occurs in young adults. However, presently, few studies have concentrated on the sagittal alignment of LDH, especially the parameters of the pelvis. PI was first proposed by Duval‐Beaupère et al. for describing the pelvic morphology14. The surgical strategy for and clinical prognosis of spinal deformity are also affected by pelvic morphology5, 15, 16, 17. Recently, PI has been found to be associated with low back pain18. Studies have found that PI in lumbar degenerative diseases may be lower19, 20, and have inferred that a low PI may result in disc degeneration. Nonetheless, whether PI participates in the pathogenesis of LDH remains controversial. Some studies point out the difference in PI between LDH patients and the normal population19, 20, while some other studies didn't found such difference21, 22, 23. These studies range widely in regards to patients’ age, but, as we know, young LDH patients tend to have less lumbar degeneration, while elder patients often have spinal stenosis resulting from lumbar degeneration, so it was difficult to exclude the factors age and degeneration, and large‐sample studies on the LDH patients of young age focusing on the relationship between PI and LDH are needed.
In contrast, lumbar lordosis is correlated with PI, and it may influence the process of disc degeneration24, 25. It has been reported that the lordosis apex may be associated with low back pain, but there has been little research on this and it remains controversial. Gautier et al. determined that there was no relationship between low back pain and the number of vertebrae in the proximal or distal direction to the apex24. However, Jackson and McManus et al. found that the patients with low back pain tended to have more proximal lordosis, which was a compensatory mechanism for sagittal balance by stretching the hip joint and setting the sacrum upright25. In our previous study on 139 asymptomatic young adults (with a mean age of 23.2 years)26, we found that sagittal alignment was related to the lumbar lordosis apex, and classified spino‐pelvic alignment into four types according to the apex position of the total lumbar lordosis. In type I, the apex was located in L5 or the L4–5 intervertebral space, with a lower PI and a lower sacral slope (SS) than other types, and lordosis was composed of the vertebrae above the apex. In type II, the apex was located in the bottom or middle of L4, and in type III the apex was located in the upper part of L4 or the intervertebral space between L3 and L4; the PI and SS had no difference between the two types and were higher than type I, and there were more vertebrae below the apex. In type IV, the apex was located at L3 or a higher level, with significantly increased PI and SS, and more vertebrae below the apex. Compared with other typing methods introduced by Roussouly et al. 27 and Lee et al. 28, this classification further described the characteristics of sagittal alignment when the lordosis apex was in different positions of L4. In addition, it can be used in exploring the relationship between the lordosis apex and LDH.
In normal adults and patients with other spinal diseases such as isthmic spondylolisthesis1 and adult idiopathic scoliosis8, cross‐correlations were found among the sagittal parameters. Lumbar lordosis was closely related to PI, SS, pelvic tilt (PT), and SVA, which indicates that the lumbar spine was able to regulate the sagittal balance. In addition, the pelvis can adjust posture by rotating and tilting backward, with PT increasing and SS decreasing, so as to compensate for the sagittal imbalance. Although young LDH patients may have less degeneration and the sagittal balance is less of an issue, this regulating system may still exist and help explain the sagittal morphology of the spine in LDH patients.
To further clarify the sagittal features of LDH patients and reduce the interference caused by age‐related spinal degeneration, this study analyzed the spino‐pelvic parameters in young LDH patients (18–35 years). The study aimed to analyze the characteristics of the sagittal alignment in young Chinese LDH patients and to explore the impact of pelvic morphology on the pathogenesis of LDH.
Materials and Methods
Inclusion and Exclusion Criteria
This was a retrospective study approved by the Regional Ethics Committee of our hospital. For the present study, 100 young LDH patients were enrolled randomly in our hospital during the period from January 2012 to April 2015. A preliminary sample selection was done by a senior investigator according to the patients’ age and diagnosis without knowing other information. All the patients were 18–35 years old. The clinical and imaging findings met the criteria for the diagnosis of LDH. Comorbidities included: arachnoid cyst (1 case), posterior bony edges separation of lumbar vertebrae without compression (2 cases), and vertebral hemangioma (1 case).
The exclusion criteria involved complicated adolescent idiopathic scoliosis, spinal tumors, vertebral fractures, spondylolisthesis and other symptomatic spinal diseases, or lower extremity disease and pelvic fractures, lumbar operation history, and obviously forced posture caused by pain.
At the same time, 100 asymptomatic volunteers (aged 18–35 years) were included as the control group, whose age and gender matched the LDH group. The subjects in the control group with low back pain, leg pain, lumbar spondylolisthesis, scoliosis, and other spinal disorders were excluded through history taking and imaging examination.
Among the subjects, there were 62 patients for whom we already had the data for their preoperative functional scoring. The visual analog scale (VAS) was adopted for low back pain and lower limb pain; the Oswestry Disability Index (ODI) was used to quantify the disability.
Imaging Measurement Method
Anteroposterior and lateral radiographs of the entire spine and pelvis (standing position, full extension of hip and knee, with elbow flexion and hand on the clavicle, including bilateral femoral head) were taken for all the subjects. The images were reviewed by an experienced senior investigator, and unclear images were also excluded. As there may be differences in measurement between different observers, all the measurement was done by a single researcher, who took the average value of two measurements. The osteophyma was not added into the calculation when assigning the point in measurement. The picture archiving and communication system (PACS System, GE, USA) was used to measure the following parameters (Fig. 1):
Figure 1.
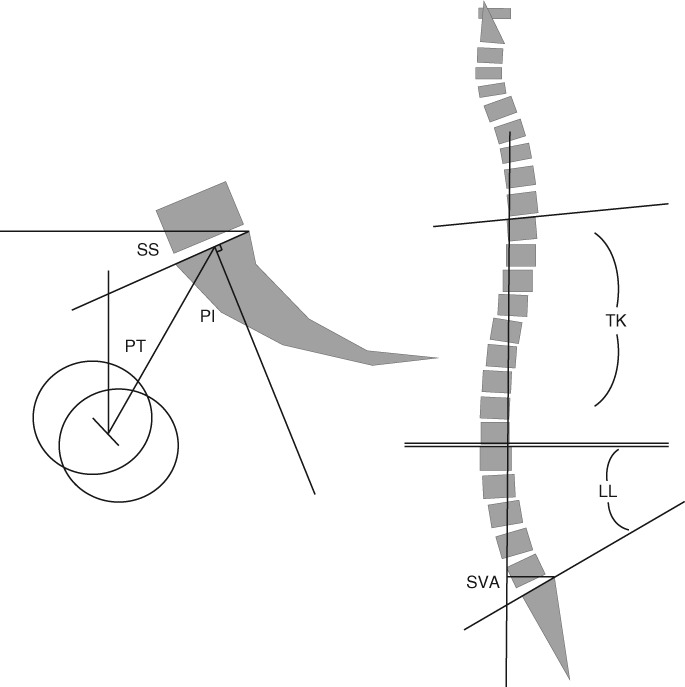
Diagrammatic sketch of lumbar lordosis (LL), pelvic incidence (PI), sacral slope (SS), pelvic tilt (PT), thoracic kyphosis (TK), and sagittal balance (SVA).
Spinal parameters included: lumbar lordosis (LL), the angle between the upper endplate of L1 and the upper endplate of S1; thoracic kyphosis (TK), the angle between the upper endplate of the T4 and the lower endplate of the T12; and sagittal balance (sagittal vertical axis, SVA), the perpendicular distance between the C7 sagittal plumb line (C7PL) and the posterior superior corner of the sacrum, which is negative when C7PL lies posterior to the posterior superior corner of the sacrum.
Pelvic parameters included: pelvic incidence (PI), the angle between the line perpendicular to the sacral plate at its midpoint and the line connecting this point to the femoral head axis (midway between the centers of the femoral heads); sacral slope (SS), the angle between the sacral endplate and the horizontal line; and pelvic tilt (PT), the angle between the vertical line and the line joining the middle of the sacral plate and the hip axis, which is positive when the hip axis lies in front of the middle of the sacral plate.
Grouping
In this study, all the subjects were divided into four groups according to the classification made by Peking University Third Hospital9. They were also grouped by PI.
The cases were grouped according to PI in this study. Because the normal PI value had been reported to be approximately 45° in the previous studies26, 29, three groups were set up: namely a low PI group (PI < 40°), a middle PI group (40° ≤ PI < 50°), and a high PI group (PI ≥ 50°).
The spino‐pelvic alignment was classified into four types according to the apex position of the total lumbar lordosis, which has been mentioned earlier.
Statistical Analysis
All the data were analyzed using SPSS 20.0 statistical software (SPSS, Chicago, IL, USA). An independent‐sample Student’s t test was used to compare the parameters, especially PI, between the LDH group and the control group, a Pearson correlation analysis was used to analyze the correlation between the parameters in the LDH group, and a χ2‐test was used to analyze the frequency components of each group. The statistical level of significance was set at 0.05.
Results
General Data
The LDH group included 69 males and 31 females, with a mean age of 27.2 ± 5.1 years, and the control group included 64 males and 36 females, with a mean age of 26.1 ± 3.8 years. The mean VAS score of the 62 cases with complete data was 4.8 ± 2.2 for low back pain and 5.5 ± 2.1 for leg pain, and the mean ODI was 19.5% ± 9.2%. Based on the independent sample t‐test and χ2‐test, there was no significant difference in age and gender distribution between the LDH group and the control group (P > 0.05), which indicated that these two groups matched well in terms of age and gender.
Radiographic Outcomes
The measurement and comparison results of the parameters are listed in Table 1, and the correlation among the parameters in the young LDH group is shown in Table 2. The independent sample t‐test results showed that there was no prominent difference in PI between the LDH group and the control group (46.1° ± 10.0° vs 47.2° ± 8.8°, P > 0.05). The LL (34.4° ± 15.3° vs 50.8° ± 10.2°, P < 0.01), SS (29.7° ± 9.1° vs 37.5° ± 7.1°, P < 0.01), and TK (22.3° ± 12.6° vs 28.4° ± 8.2°) of the LDH group were lower, while its PT (16.4° ± 8.4° vs 9.6° ± 5.9°, P < 0.01) and SVA (21.6 ± 53.6 mm vs −18.4 ± 32.8 mm, P < 0.01) were higher than those of the control group. The LL and SVA, which have been proved to be related to surgical effect previously5, 6, showed a significant difference (P < 0.01), LL was correlated with PI, SS, PT, TK, and SVA (P < 0.01), and SVA was also correlated with the parameters above (P < 0.01) except PI (P > 0.05).
Table 1.
Parameters in the lumbar disc herniation (LDH) group and the control group (mean ± standard deviation)
| Groups | LL (°) | PI (°) | PT (°) | SS (°) | TK (°) | SVA (mm) |
|---|---|---|---|---|---|---|
| LDH (100 cases) | 34.4 ± 15.3* | 46.1 ± 10.0 | 16.4 ± 8.4* | 29.7 ± 9.1* | 22.3 ± 12.6* | 21.6 ± 53.6* |
| Control (100 cases) | 50.8 ± 10.2 | 47.2 ± 8.8 | 9.6 ± 5.9 | 37.5 ± 7.1 | 28.4 ± 8.2 | −18.4 ± 32.8 |
LL, lumbar lordosis; PI, pelvic incidence; PT, pelvic tilt; SS, sacral slope; SVA, sagittal vertical axis; TK, thoracic kyphosis
P < 0.05.
Table 2.
The correlation between the parameters in the young lumbar disc herniation (LDH) group (n = 100, correlation efficient listed)
| Parameters | LL | PI | SS | PT | TK | SVA |
|---|---|---|---|---|---|---|
| LL | — | 0.401* | 0.819* | −0.409* | 0.550* | −0.622* |
| PI | — | — | 0.622* | 0.527* | −0.027 | 0.051 |
| SS | — | — | — | −0.337* | 0.258* | −0.298* |
| PT | — | — | — | — | −0.320* | 0.376* |
| TK | — | — | — | — | — | −0.272* |
LL, lumbar lordosis; PI, pelvic incidence; PT, pelvic tilt; SS, sacral slope; SVA, sagittal vertical axis; TK, thoracic kyphosis
P < 0.05.
For the four types of cases classified by Peking University Third Hospital (Table 3), the χ2‐test showed that there were fewer II cases and more IV cases in the LDH group (P < 0.001). As for the cases grouped by PI (Table 4), the χ2‐test suggested that there was no difference among the three groups in the distribution of PI (P > 0.05).
Table 3.
Classification of the lumbar disc herniation (LDH) group and the control group (cases [%])
| Groups | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| LDH (100 cases) | 26 (26) | 12 (12) | 24 (24) | 38 (38) |
| Control (100 cases) | 28 (28) | 34 (34) | 32 (32) | 6 (6) |
Table 4.
Distribution of pelvic incidence (PI) groups (cases [%])
| Groups | 1 | 2 | 3 |
|---|---|---|---|
| LDH (100 cases) | 30 (30) | 36 (36) | 34 (34) |
| Control (100 cases) | 20 (20) | 43 (43) | 37 (37) |
Discussion
Pelvic Incidence in Lumbar Disc Herniation Patients
Pelvic incidence was found to be associated with low back pain18. Yang et al. discovered that the PI of the 80 patients with lumbar degeneration (with a mean age of 36.5 ± 7.4 years) was lower19. They assumed that a low PI would lead to more vertical pressure on the disc and cause degeneration. They also assumed that the low PI of the patients with asymptomatic degeneration might reduce the average PI in the control group, so they made a more rigorous comparison excluding the cases with disc degeneration in the control group (20%–28% of the total) according to their MR image, but, finally, the mean PI of the two groups differed a lot (40.0° vs 48.7°). Barrey et al. analyzed 25 young patients (aged below 45 years) with degenerative disc disease, and found a lower PI (48.3° vs 52°). They presumed that the backward rotation of the pelvis might compensate for the loss of lumbar lordosis and the sagittal imbalance, presenting a higher PT and a lower SS. Furthermore, a low PI might affect this compensate mechanism because PI = SS + PT, which would result in disc degeneration. However, these studies have investigated elder patients aged 30–60 years, and the age‐related spinal degeneration and the limitation of the sample size may cause deviation.
This study compared 100 young LDH patients (aged 18–35 years) with asymptomatic volunteers. The results show that there is no difference in PI between them. This indicates that PI may be not be involved in the pathogenesis of young LDH. However, the etiology of LDH may be complicated, which could also be interfered by factors such as inheritance, chronic injury, and weight. Therefore, it cannot simply be explained by the difference in the anatomical structure of the pelvis. As a result, we infer that it will be hard to predict LDH according to one’s PI and pelvic morphology.
Pelvic incidence has great individual differences; as a consequence, this study shall not simply deny the difference in distribution pattern even though no remarkable difference has been found in the average value. The coexistence of low PI and high PI may neutralize the average PI. On the basis of previous researches, a PI too high or too low would be prone to degenerate lumbar spine30. The possible mechanism may be that a lower PI with a lower LL may lead to greater pressure on the intervertebral disc in the vertical direction, while a high PI with a higher LL may increase the stress on the rear of the annulus fibrosus. For this reason, the constituent ratios of the three groups were compared in the present study. In accordance with the comparison results, there is no statistical difference among them. Using this method helps prevent the influence of PI polarization on the experimental results, and the findings further confirm that PI is not so important in the pathogenesis of young LDH.
Our findings are consistent with some previous published studies. Kenji Endo et al. found no difference in the pelvic morphology of 61 LDH patients (with a mean age of 32.7 years)21. A similar result was obtained by Jiang et al. 22. However, their study was aimed at adolescent LDH patients (with a mean age of 16.9 years), whose PI was still changing before skeletal maturation. Given this, their conclusion may not be applicable to adults. Rajnics et al. also found that there was no difference in PI between the LDH group (50 patients) and the control group, but the ages of the two groups were different (47.7 years vs 34.3 years), and patients of older age were affected by age‐related degeneration23. With a larger sample size, our research, which was aimed at young LDH cases, reduced the impact of age and degeneration on the sagittal alignment and parameter measurement. On account of this, the research results can further support the conclusions above.
Other Sagittal Spinal Parameters
Previous studies have demonstrated the correlation among the spino‐pelvic sagittal parameters (especially LL) in normal adults and some spinal diseases, such as isthmic spondylolisthesis1, and adult idiopathic scoliosis31. The present study has uncovered the pairwise correlation among LL, PI, SS, and PT in young LDH patients, and indicated that the sagittal alignment in young LDH patients is also regulated by pelvis and lumbar lordosis. The result that young LDH patients show lower LL, SS, and TK as well as higher PT, and SVA is consistent with previous views, suggesting that there is a loss of lumbar lordosis and a consequential sagittal imbalance in young LDH patients. The compensation mechanism includes the decrease of TK. Moreover, the higher PT and lower SS reflect the backward rotation of the pelvis to compensate for the sagittal imbalance. As the LL and SVA have been proved to be related to symptoms and surgical effect5, 8, 32, 33, 34, the LL and SVA should be restored during the surgical treatment for LDH. However, in this study, loss of lordosis in the young LDH patients may have resulted from pain‐related forced posture, and lordosis may be restored automatically after using muscle relaxants and muscle removal.
However, as the lordosis is correlated with PI and disc degeneration, this study also explored the pathogenesis of LDH by analyzing the apex of lumbar lordosis using the classification of our hospital. According to the results of this study, the number of Type II cases decreases significantly in young LDH patients, which makes it the rarest type, while the number of Type IV cases increases, which indicates that young LDH patients tend to present a higher lordosis apex, and the stress change on the disc resulting from a higher apex may be related to LDH. However, degeneration‐related intervertebral space collapse may cause a decrease of LL, sagittal imbalance, and a straighter lordosis, leading to apex position changes, so the cause and effect relationship between LDH and apex changes is difficult to define. Moreover, although we excluded the patients who had difficultly naturally standing straight because of low back pain, mildly forced posture resulting from self‐protect mechanism may influence the result.
Limitations
If a relationship between PI and LDH really exists, we can predict the LDH in a normal population beforehand and take measures to prevent it. Unfortunately, studies supporting the relationship have many limitations, and there are other studies that dispute the existence of a relationship, including ours, which is also accompanied by limitations. Mildly forced posture resulting from a self‐protecting mechanism may influence the results. Having a larger sample may help reduce the deviation, and using MRI to exclude the cases with asymptomatic disc degeneration would be useful. In contrast, a low PI accompanied by lower SS and LL may increase the vertical pressure on the disc, but a long period of time is needed for the abnormal anatomic structure to influence the pathogenesis of LDH. In our research, young LDH patients were studied and there may not be enough time to cause degeneration and LDH because the change of pressure kept a shorter duration. In this context, whether pelvic morphology would affect the intervertebral disc remains unclear, and more studies on this topic are necessary. It will be a long time before PI can be utilized as a predictor for LDH.
Disclosure: This work was supported by grants from the 2013 AOSpine China Research Award (Project no.: AOSCN(R) 2014‐22), the Beijing Municipal Science and Technology Commission (Grant No. Z131100006813038), and the National Natural Science Foundation of China (Grant No. 81450025). All authors listed meet the authorship criteria according to the latest guidelines of the International Committee of Medical Journal Editors and are in agreement with the manuscript. This study has received no financial support, and there is no conflict of interest.
References
- 1. Marty C, Boisaubert B, Descamps H, et al The sagittal anatomy of the sacrum among young adults, infants, and spondylolisthesis patients. Eur Spine J, 2002, 11: 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li WS, Li G, Chen ZQ, Wood KB. Sagittal plane analysis of the spine and pelvis in adult idiopathic scoliosis. Chin Med J (Engl), 2010, 123: 2978–2982. [PubMed] [Google Scholar]
- 3. Mac‐Thiong JM, Labelle H, Charlebois M, Huot MP, de Guise JA. Sagittal plane analysis of the spine and pelvis in adolescent idiopathic scoliosis according to the coronal curve type. Spine (Phila Pa 1976), 2003, 28: 1404–1409. [DOI] [PubMed] [Google Scholar]
- 4. Li W, Sun Z, Guo Z, et al Analysis of spinopelvic sagittal alignment in patients with thoracic and thoracolumbar angular kyphosis. Spine (Phila Pa 1976), 2013, 38: E813–E818. [DOI] [PubMed] [Google Scholar]
- 5. Schwab FJ, Blondel B, Bess S, et al Radiographical spinopelvic parameters and disability in the setting of adult spinal deformity: a prospective multicenter analysis. Spine (Phila Pa 1976), 2013, 38: E803–E812. [DOI] [PubMed] [Google Scholar]
- 6. Tsai TH, Huang TY, Lieu AS, et al Functional outcome analysis: instrumented posterior lumbar interbody fusion for degenerative lumbar scoliosis. Acta Neurochir (Wien), 2011, 153: 547–555. [DOI] [PubMed] [Google Scholar]
- 7. Jimbo S, Kobayashi T, Aono K, Atsuta Y, Matsuno T. Epidemiology of degenerative lumbar scoliosis: a community‐based cohort study. Spine (Phila Pa 1976), 2012, 37: 1763–1770. [DOI] [PubMed] [Google Scholar]
- 8. Schwab FJ, Smith VA, Biserni M, Gamez L, Farcy JP, Pagala M. Adult scoliosis: a quantitative radiographic and clinical analysis. Spine (Phila Pa 1976), 2002, 27: 387–392. [DOI] [PubMed] [Google Scholar]
- 9. Cho KJ, Suk SI, Park SR, et al Short fusion versus long fusion for degenerative lumbar scoliosis. Eur Spine J, 2008, 17: 650–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marchesi DG, Aebi M. Pedicle fixation devices in the treatment of adult lumbar scoliosis. Spine (Phila Pa 1976), 1992, 17 (8 Suppl): S304–S309. [DOI] [PubMed] [Google Scholar]
- 11. Zurbriggen C, Markwalder TM, Wyss S. Long‐term results in patients treated with posterior instrumentation and fusion for degenerative scoliosis of the lumbar spine. Acta Neurochir, 1999, 141: 21–26. [DOI] [PubMed] [Google Scholar]
- 12. Xu JQ, Chen WS. Advanced progress on adult degenerative scoliosis. China J Orthop, 2005, 18: 574–575. [Google Scholar]
- 13. Schwab F, Patel A, Ungar B, Farcy J, Lafage V. Adult spinal deformity‐postoperative standing imbalance: how much can you tolerate? An overview of key parameters in assessing alignment and planning corrective surgery. Spine (Phila Pa 1976), 2010, 35: 2224–2231. [DOI] [PubMed] [Google Scholar]
- 14. Duval‐Beaupère G, Schmidt C, Cosson P. A Barycentremetric study of the sagittal shape of spine and pelvis: the conditions required for an economic standing position. Ann Biomed Eng, 1992, 20: 451–462. [DOI] [PubMed] [Google Scholar]
- 15. Roussouly P, Pinheiro‐Franco JL. Biomechanical analysis of the spino‐pelvic organization and adaptation in pathology. Eur Spine J, 2011, 20: 609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Silva FE, Lenke LG. Adult degenerative scoliosis: evaluation and management. Neurosurg Focus, 2010, 28: E1. [DOI] [PubMed] [Google Scholar]
- 17. Cho KJ, Suk SI, Park SR, et al Risk factors of sagittal decompensation after long posterior instrumentation and fusion for degenerative lumbar scoliosis. Spine (Phila Pa 1976), 2010, 35: 1595–1601. [DOI] [PubMed] [Google Scholar]
- 18. Chaléat‐Valayer E, Mac‐Thiong JM, Paquet J, Berthonnaud E, Siani F, Roussouly P. Sagittal spino‐pelvic alignment in chronic low back pain. Eur Spine J, 2011, 20 (Suppl. 5): 634–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang X, Kong Q, Song Y, Liu L, Zeng J, Xing R. The characteristics of spinopelvic sagittal alignment in patients with lumbar disc degenerative diseases. Eur Spine J, 2014, 23: 569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barrey C, Jund J, Noseda O, Roussouly P. Sagittal balance of the pelvis‐spine complex and lumbar degenerative diseases. A comparative study about 85 cases. Eur Spine J, 2007, 16: 1459–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Endo K, Suzuki H, Tanaka H, Kang Y, Yamamoto K. Sagittal spinal alignment in patients with lumbar disc herniation. Eur Spine J, 2010, 19: 435–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jiang L, Zhu Z, Qiu Y, Liu Z, Qian B, Wu T. Sagittal spino‐pelvic alignment in adolecent patients with lumbar disc herniation. Zhongguo Ji Ju Ji Sui Za Zhi, 2013, 23: 140–144 (in Chinese). [Google Scholar]
- 23. Rajnics P, Templier A, Skalli W, Lavaste F, Illes T. The importance of spinopelvic parameters in patients with lumbar disc lesions. Int Orthop, 2002, 26: 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gautier J, Morillon P, Marcelli C. Does spinal morphology influence the occurrence of low back pain? A retrospective clinical, anthropometric, and radiological study. Rev Rhum Engl Ed, 1999, 66: 29–34. [PubMed] [Google Scholar]
- 25. Jackson RP, McManus AC. Radiographic analysis of sagittal plane alignment and balance in standing volunteers and patients with low back pain matched for age, sex, and size. A prospective controlled clinical study. Spine (Phila Pa 1976), 1994, 19: 1611–1618. [DOI] [PubMed] [Google Scholar]
- 26. Li WS, Sun ZR, Chen ZQ. Radiographic analysis of spino‐pelvic alignment in asymptomatic Chinese adults. Zhonghua Gu Ke Za Zhi, 2013, 33: 447–453 (in Chinese). [Google Scholar]
- 27. Roussouly P, Gollogly S, Berthonnaud E, Dimnet J. Classification of the normal variation in the sagittal alignment of the human lumbar spine and pelvis in the standing position. Spine (Phila Pa 1976), 2005, 30: 346–353. [DOI] [PubMed] [Google Scholar]
- 28. Lee CS, Chung SS, Kang KC, Park SJ. Normal patterns of sagittal alignment of the spine in young adults radiological analysis. Spine (Phila Pa 1976), 2011, 36: 1648–1654. [DOI] [PubMed] [Google Scholar]
- 29. Zhu Z, Xu L, Zhu F, et al Sagittal alignment of spine and pelvis in asymptomatic adults: norms in chinese populations. Spine (Phila Pa 1976), 2014, 39: E1–E6. [DOI] [PubMed] [Google Scholar]
- 30. Liu H, Shrivastava SR, Zheng ZM, et al Correlation of lumbar disc degeneration and spinal‐pelvic sagittal balance. Zhonghua Yi Xue Za Zhi, 2013, 93: 1123–1128. [PubMed] [Google Scholar]
- 31. Gu XM, Jia LS, Chen XS. Correlation among lumbar curvature, sacral angle and degenerative lumbar scoliosis and its clinical significance. Zhongguo Gu Yu Guanjie Sun Shang Za Zhi, 2007, 22: 969–971. [Google Scholar]
- 32. Lord M, Small J, Dinsay J, Watkins R. Lumbar lordosis: effects of sitting and standing. Spine (Phila Pa 1976), 1997, 22: 2571–2574. [DOI] [PubMed] [Google Scholar]
- 33. Glassman SD, Bridwell K, Dimar JR, Horton W, Berven S, Schwab F. The impact of positive sagittal balance in adult spinal deformity. Spine (Phila Pa 1976), 2005, 30: 2024–2029. [DOI] [PubMed] [Google Scholar]
- 34. Glassman SD, Berven S, Bridwell K, Horton W, Dimar JR. Correlation of radiographic parameters and clinical symptoms in adult scoliosis. Spine (Phila Pa 1976), 2005, 30: 682–688. [DOI] [PubMed] [Google Scholar]


