Abstract
ACL injury rates are greater in female athletes than their male counterparts. As female athletes are at increased risk, it is important to understand the underlying mechanics that contribute to this sex bias. The purpose of this investigation was to employ a robotic manipulator to simulate male and female kinematics from athletic tasks on cadaveric specimens and identify sex-based mechanical differences relative to the ACL loading. It was hypothesized that simulations of female motion would generate the higher loads and ligament strains associated with in vivo ACL injury risk than simulations of male motion. A 6-degree-of-freedom robotic manipulator articulated cadaveric lower extremity specimens from 12 donors through simulations of in vivo kinematics recorded from male and female athletic tasks. Simulation of female kinematics exhibited lower peak lateral joint force during the drop vertical jump and lower peak anterior and lateral joint force and external joint torque during the sidestep cut (P<0.05). Peak ACL strain during a drop vertical jump was 6.27% and 6.61% for the female and male kinematic simulations, respectively (P = 0.86). Peak ACL strain during a sidestep cut was 4.33% and 7.57% for female and male kinematic simulations respectively (P = 0.21). For the tasks simulated, the sex-based loading and strain differences identified were unlikely to have a significant bearing on the increased rate of ACL injures observed in female athletes. Additional perturbation may be necessary to invoke the mechanisms that lead to higher rates of ACL injury in female populations.
Keywords: Anterior cruciate ligament strain, Cadaveric simulation, Robotic manipulator, Gender sex bias, Knee joint loading
1. Introduction
Over 127,000 anterior cruciate ligament (ACL) reconstructions are performed annually in the United States (Kim et al., 2011). ACL failures are debilitating knee injuries that incur extensive surgical and rehabilitation costs, can be devastating to athletic careers, and provide a long term prospectus of osteoarthritis and decreased quality of life (Hewett et al., 1999a; Lohmander et al., 2004; von Porat et al., 2004). These injuries exhibit a sex bias, as 1 in 50–70 female athletes sustain a traumatic knee injury each year (Myer et al., 2006), and females are 4–8 times more likely to experience an ACL tear than their male counterparts (Arendt et al., 1999; Boden et al., 2000; Toth and Cordasco, 2001).
The sex bias exhibited in ACL injuries may stem from reduced neuromuscular control with up to 70% of ACL ruptures occurring in non-contact situations (Boden et al., 2000; Hewett et al., 2005; McNair et al., 1990). Rather than originating from a direct blow to the knee, these injuries appear to occur as the joint is geometrically manipulated into positions that place abnormally large forces and torques on the ACL (Griffin et al., 2000; McLean et al., 2005). The ACL may be particularly susceptible to these loads as it provides greater mechanical restraint to the knee during simulated athletic tasks than do other ligamentous structures such as the medial collateral ligament (Bates et al., 2015d). Female athletes tend to exhibit reduced neuromuscular control compared to their male counterparts, which has been associated with increased ACL injury risk (Ford et al., 2003, 2007a, 2006, 2010b; Hewett et al., 2006, 1999b; Malinzak et al., 2001). Specifically, poor neuromuscular control during athletic tasks involving rapid deceleration and/or change of direction can lead to knee abduction coupled with internal rotation of the tibia, otherwise known as knee valgus (Ford et al., 2003, 2010a, 2010c). Valgus torque has been identified as a precursor to ACL rupture and is used to predict an athlete’s relative injury risk (Hewett et al., 2005; Myer et al., 2010a, 2010b, 2010c, 2011).
It follows that female athletes demonstrate greater risk of ACL injury because they exhibit increased knee valgus compared to male counterparts during equivalent athletic tasks (Ford et al., 2003, 2010a, 2010b; Hughes et al., 2008; Kernozek et al., 2005). A multitude of investigations have demonstrated that the application of knee valgus increases the biomechanical load on the ACL (Levine et al., 2013; Shin et al., 2011; Withrow et al., 2006). Computerized simulations of the human knee have identified that peak ACL strain shows a non-linear increase with increasing valgus moments (Shin et al., 2008). Similarly, the addition of knee abduction rotation to a mechanical simulation of jump landing in cadaveric specimens also increased peak ACL strain (Withrow et al., 2006). Further, robotic manipulators have simulated clinical tests on cadaveric knees and documented that the combined abduction and internal tibial rotations experienced during knee valgus increase ACL load and anterior tibial translation more than either rotation individually (Bates et al., 2015b). All of these scenarios indicate that knee valgus increases mechanical load on the ACL and is a sex-specific mechanism that may make the ligament more susceptible to injury. Ligaments and intra-articular surfaces are passive restraints to joint loading; and therefore, are mechanically loaded by the positional changes of each limb segment relative to one another. Therefore, robotically-driven models of physiologic joint motion capable of positional control over corresponding limb segments could be valuable to the assessment of how in vivo recorded, sex-based differences influence intra- articular mechanics during athletic tasks (Bates et al., 2015c).
As female athletes are at increased risk of ACL injury compared to males, it is important to understand the underlying mechanics that contribute to this sex bias. The purpose of this investigation was to use a robotic manipulator to simulate male and female kinematics from athletic tasks on cadaveric specimens and identify sex-based mechanical differences relative to the ACL loads. It was hypothesized that simulations of female kinematics would generate higher loads and strains associated with ACL injury risk than simulations of male motion.
2. Method
2.1. Experimental design
22 Human cadaveric lower extremity limbs from 14 unique specimens were obtained from an anatomical donations program (Anatomical Gifts Registry, Hanover, MD) and tested in this study. Three specimens and two unique donors were excluded due to specimen failure and non-functional ACLs, which left 19 specimens from 12 unique donors included in the analysis (9 males; 3 females, age=47.9 ± 7.0 years; mass=84.8 ± 19.4 kg). Cadaveric specimens were limited to under 55 years of age, but were not anatomically matched to the in vivo subjects tested. Previous literature had demonstrated that kinematics are not correlated to stature and do not require normalization to specimen anthropometrics, which indicates that in vivo kinematics should be applicable to unmatched anatomical specimens (Bates et al., 2015a). The limbs were tested using a six-degree-of-freedom (6-DOF) robotic manipulator (KR210; KUKA Robotics Corp., Clinton Township, MI) mounted with a six-axis load cell (Theta Model; ATI Industrial Automation, Apex, NC). The robot simulated 10 cycles of motion for each sex-specific drop vertical jump (DVJ) and sidestep cutting task that was derived from in vivo recorded kinematics while the force sensor recorded corresponding joint forces and torques as previously documented (Bates et al., 2015c). Specimens were simulated in intact and ACL-isolated conditions to quantify whole joint mechanics and ACL specific contributions, respectively.
2.2. Kinematic model
To develop 6-DOF input kinematics for robotic simulation, a model to represent sex-specific, three-dimensional (3D), in vivo motion of athletic tasks has been previously described (Bates et al., 2015c). Briefly, a matched male (age=24 years; height= 175 cm; mass=675 N) and female (age=25; height = 170 cm; mass=632 N) subject performed three trials each of a drop vertical jump (DVJ) and sidestep cutting task while a 10-camera motion analysis system (Eagle Cameras, Motion Analysis Corp, Santa Rosa, CA) recorded the 3D positions of retroreflective markers placed at anatomical landmarks on the skin. 3D kinematics were processed from the positional data using custom code in MATLAB (version 2012b, The MathWorks, Inc., Nantick, MA) and an established biomechanical model in Visual3D (version 4.0, C-Motion, Inc., Germantown, MD) (Ford et al., 2007b). Marker trajectories were filtered through a fourth-order, low-pass, digital filter with a cutoff frequency of 6 Hz and the resultant kinematics from all three trials of each task were averaged into a subject mean for each motion task. Literature-based scale factors were then used to individually convert each DOF into kinematic input for a position-controlled robotic manipulator while reducing the confounding influence of skin-artifact errors. The kinematic profiles generated from the described methodology have previously been reported in the literature (Bates et al., 2015c). It should be noted that the male subject was computationally identified as “low-risk” for ACL injury, while the female was identified as “high-risk” based on their peak knee abduction moments calculated in Visual3D (Hewett et al., 2005; Myer et al., 2007).
2.3. Specimen preparation
Specimen criteria were defined as no previous history of knee trauma, knee surgery, bone cancer, or ankle or shin implants. The limbs were kept frozen at –20 °C and allowed to thaw 24 h prior to testing. Thawed specimens were resected of all soft tissue down to the joint capsule, leaving the collateral and cruciate ligaments and menisci intact. Based on the tibial joint coordinate system (Grood and Suntay, 1983), the tibia was secured into custom fixtures then aligned with and rigidly affixed to the load cell and robot end effector axes. A coordinate measuring machine (Faro Digitizer F04L2, FARO Technologies Inc., Lake Mary, FL) digitized the tibial joint center point and all rotations and translations were applied while forces and torques were recorded about this point. The limb was articulated to a minimally loaded condition where custom-barbed, 3 mm microminiature differential variable resistance transducers (DVRT, LORD MicroStrain Inc., Williston, VT) were implanted on the anteromedial bundle of the ACL following previously described protocols (Beynnon et al., 1992; Levine et al., 2013). 45° of flexion was selected for DVRT insertion, as previous human gait simulations have exhibited that the ACL is unloaded in this position (Renstrom et al., 1986). DVRTs were also implanted on the MCL at midsubstance across the joint space, proximal to the tibial insertion, and distal to the femoral insertion. For each task simulation, the knee was placed in a position that matched the initial contact orientation recorded in vivo to within 0.5° for all three rotations. From this position the limb was cycled several times to account for viscoelastic effects and to establish and maintain the initial load necessary to reach 1.5–2.5 bodyweights of peak vertical force previously reported from in vivo recordings of athletic tasks (Bates et al., 2013). A more explicit account of how the specimens were prepared and oriented for testing can be found in previous literature (Bates et al., 2015c; Herfat et al., 2012).
2.4. Robotic simulation
All tests were performed at room temperature and the joint was consistently hydrated with saline. Four motions conditions (male DVJ, male sidestep cut, female DVJ, female sidestep cut) were applied to each limb in a randomized order regardless of specimen gender. To minimize viscoelastic effects, an initial set of 10 cycles was applied to precondition the joint, followed by another 10 cycles to record forces, torques, and strain for the intact knee. After the intact simulations were completed, the joint was resected of all soft tissues along with the distal portions of the femoral condyles to achieve the ACL-isolated condition, where only the ligament was transmitting force across the joint. The isolated ACL joint was returned to the initial contact orientation, compressed to relieve any tension, and slowly distracted again to identify the neutral strain position of the ACL. With the ACL as the only intact load-bearing structure, neutral strain was identified when the force sensors first registered a constant distraction force. The ACL was then resected and simulations were repeated with the tibia rotating freely in space.
2.5. Data analysis
The forces and torques recorded for the disarticulated joint (to account for gravity and robot inertial effects) and were subtracted from the corresponding intact joint and isolated ACL simulations. All translational forces were smoothed through a Fourier transform with a 12 Hz frequency and normalized to percent bodyweight. ACL strain was calculated relative to the neutral strain position rather than relative to the position where the DVRTs were implanted, which is standard procedure (Beynnon et al., 1992; Levine et al., 2013). The identification of the neutral strain position allowed for the reporting of absolute ACL strain rather than relative strain changes. The 8th and 9th cycles of each 10 cycle test were used for analysis to eliminate cycle effects. All data was time normalized to percent landing phase for each motion, beginning with initial contact and ending at peak flexion, the minimum center of gravity (Bates et al., 2013). Forces and moments were analyzed in the tibial reference frame based on the knee joint coordinate system (Grood and Suntay, 1983). Pairwise comparisons with a Bonferroni correction were used to evaluate for statistical significance in force and torque outcomes generated from simulations of between-sex in vivo kinematics for like athletic tasks. Kolmogorov-Smirnov tests were used to examine data normality with a 5% significance level and found that all data was normally distributed with the exception of the DVJ values for peak internal torque, peak internal torque within 50 ms of initial contact, and internal torque at initial contact. Levene tests with a probability of < 0.05 were used to examine homogeneity among variances and found that the assumption of homoscedasticity was met for all variables except frontal plane torque within the first 50 ms of initial contact. Chi squared tests with a probability of < 0.05 were used to test for independence and found that all variable groups (male, female, DVJ, cut) were independent of one another. Statistical analyses were performed in MATLAB using built-in functions and verified in SPSS (version 21, IBM Corp, Armonk, NY). Statistical significance was established at a < 0.05. To prevent confounding factors, data from contralateral pairs were averaged before they were included in statistical analysis.
3. Results
3.1. Sidestep cut
During the full landing phase, the simulation of female kinematics expressed smaller peak forces than the male in both the anterior (8.1% bodyweight vs. 16.3% bodyweight, P< 0.01) and lateral (4.2% bodyweight vs. 13.9% bodyweight, P< 0.01) directions (Table 1; Fig. 1). The simulation of female kinematics also demonstrated reduced external torque relative to the male (1.9 N*m vs. 4.6 N*m, P=0.01). In the 50 ms following initial contact, the simulation of female kinematics expressed greater peak compressive force than the simulation of male kinematics (82.0% bodyweight vs. 39.2% bodyweight, P < 0.01). At initial contact the simulation of female kinematics expressed greater compressive force (70.9% bodyweight vs. 23.4% bodyweight, P < 0.01) than the male and reduced anterior force compared to the male (1.2% bodyweight vs. 2.4% bodyweight, P< 0.05). No torsional differences were observed between sexes at initial contact (P > 0.23). At maximum knee flexion, the simulation of female kinematics had less anterior force (4.3% bodyweight vs. 14.7% bodyweight, P < 0.01), lateral force (0.0% bodyweight vs. 8.0% bodyweight, P=0.02), compressive force (68.4% bodyweight vs. 143.3% bodyweight, P = 0.02), external torque (1.0 N*m vs. 4.3 N*m, P< 0.01), and trended toward lower flexion torque (5.0 N*m vs. 24.8 N*m, P=0.06) than the simulation of male kinematics. The only strain difference observed between sexes was at maximum knee flexion, where the ACL was 4.0% less in the simulation of female kinematics (P= 0.05; Fig. 2).
Table 1.
Peak forces and torques recorded at the tibiofemoral joint during robotically simulated sidestep cutting tasks. Peak values were reported for both directions of loading within each degree of freedom.
| Landing phase |
First 50 ms |
Initial contact |
Maximum flexion |
|||||
|---|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | Female | Male | |
| Force (%BW) | ||||||||
| Posterior | 5.6 | 5.5 | 0.1 | −0.1 | −1.2a | −2.4a | −4.3a | −14.7a |
| Anterior | 8.1a | 16.3a | 1.9 | 2.6 | - | - | - | - |
| Lateral | 4.2a | 13.9a | 1.4 | 1.2 | 0.4 | −0.2 | 0.0a | 8.0a |
| Medial | 4.9 | 2.2 | −0.2 | 0.4 | - | - | - | - |
| Distraction | −43.6a | −13.7a | −69.2a | −23.2a | −70.9a | −23.4a | −68.4a | −143.3a |
| Compression | 180.4 | 183.1 | 82.0a | 39.2a | - | - | - | - |
| Torque (N*m) | ||||||||
| External | 1.9a | 4.6a | 0.2 | − 0.5 | 0.0 | −0.6 | 1.0a | 4.3a |
| Internal | 0.6 | 0.9 | 0.1 | 0.7 | - | - | - | - |
| Extension | 15.4 | 19.9 | 6.3 | 7.3 | 4.6 | 3.1 | − 5.0a | −24.8a |
| Flexion | 12.5 | 28.4 | −3.2 | − 2.9 | - | - | - | - |
| Abduction | 2.6 | 3.0 | 2.4 | 0.7 | 2.1 | 0.7 | −11.5 | −7.1 |
| Adduction | 21.1 | 23.6 | 0.4 | 1.9 | - | - | - | - |
Indicates significant difference (P< 0.05) between values generated during simulation of male and female kinematics.
Fig. 1.
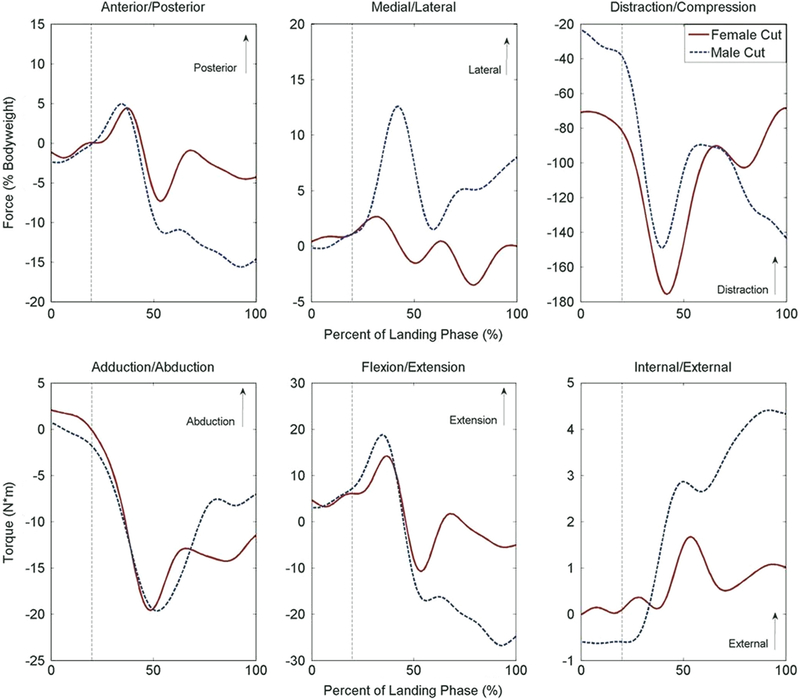
Displays the population average force (top row) and torque (bottom row) measured at the tibiofemoral joint in response to simulated male and female sidestep cut articulations. Landing phase begins at initial contact (0%) and concludes at maximum flexion (100%) (Bates et al., 2013). The vertical, dashed line represents the approximate cutoff for the first 50 ms after initial contact, where ACL injuries are most likely to occur (Krosshaug et al., 2007).
Fig. 2.
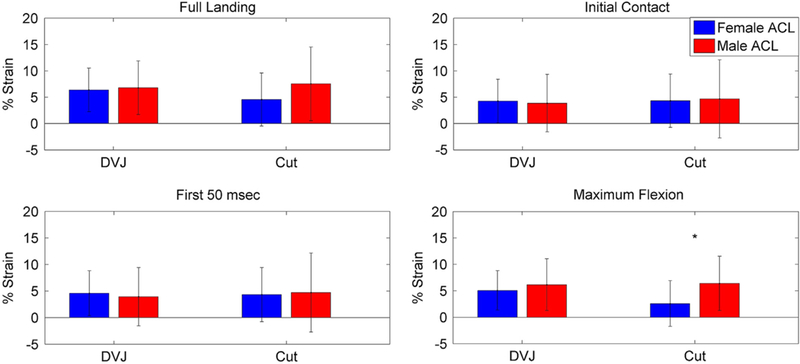
Displays peak values for ACL strain in the male and female DVJ and sidestep cut simulations at four different time periods within landing phase. ‘Indicates a significant strain difference between genders within ligament.
3.2. Drop vertical jump
A limited number of significant differences between sexes were noted for the DVJ task (P < 0.05; Table 2; Fig. 3). During the full landing phase, the simulation of female kinematics model expressed an additional 7.5% bodyweight greater peak lateral (P= 0.02) force than the simulation of male kinematics. In the 50 ms following initial contact, there were no statistically significant force differences between sexes (P > 0.07). At initial contact and maximum knee flexion, there were also no statistically significant loading differences between genders (P > 0.07). There were no significant gender differences in peak torque or ACL strain for any period of the DVJ simulations (P > 0.09; Fig. 2).
Table 2.
Peak forces and torques recorded at the tibiofemoral joint during robotically simulated DVJ tasks. Peak values were reported for both directions of loading within each degree of freedom.
| Landing phase |
First 50 ms |
Initial contact |
Maximum flexion |
|||||
|---|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | Female | Male | |
| Force (%BW) | ||||||||
| Posterior | 12.9 | 9.2 | −1.4 | 0.5 | −2.2 | −0.6 | −15.8 | −12.5 |
| Anterior | 17.2 | 15.3 | 2.5 | 0.7 | − | − | − | − |
| Lateral | 20.2a | 13.7a | 1.6 | 2.2 | −0.5 | 0.3 | −0.3 | −1.4 |
| Medial | 3.6 | 5.2 | 0.6 | 0.0 | − | − | − | − |
| Distraction | −17.7 | −29.5 | −38.7 | −54.4 | −39.1 | −54.7 | −79.9 | −106.7 |
| Compression | 187.9 | 207.1 | 56.5 | 71.6 | − | − | − | - |
| Torque (N*m) | ||||||||
| External | 4.2 | 3.2 | 0.3 | 0.5 | −0.1 | 0.1 | 2.8 | 2.0 |
| Internal | 0.2 | 0.4 | 0.1 | 0.0 | − | − | − | − |
| Extension | 29.9 | 22.0 | 5.3 | 7.7 | 3.8 | 5.8 | −24.9 | −22.9 |
| Flexion | 27.7 | 28.2 | −3.1 | −5.4 | − | − | − | − |
| Abduction | 3.2 | 2.9 | 0.3 | 1.2 | 0.3 | 0.4 | −14.1 | −20.4 |
| Adduction | 20.1 | 25.6 | 3.2 | 0.6 | − | − | − | − |
Indicates significant difference (P < 0.05) between values generated during simulation of male and female kinematics.
Fig. 3.
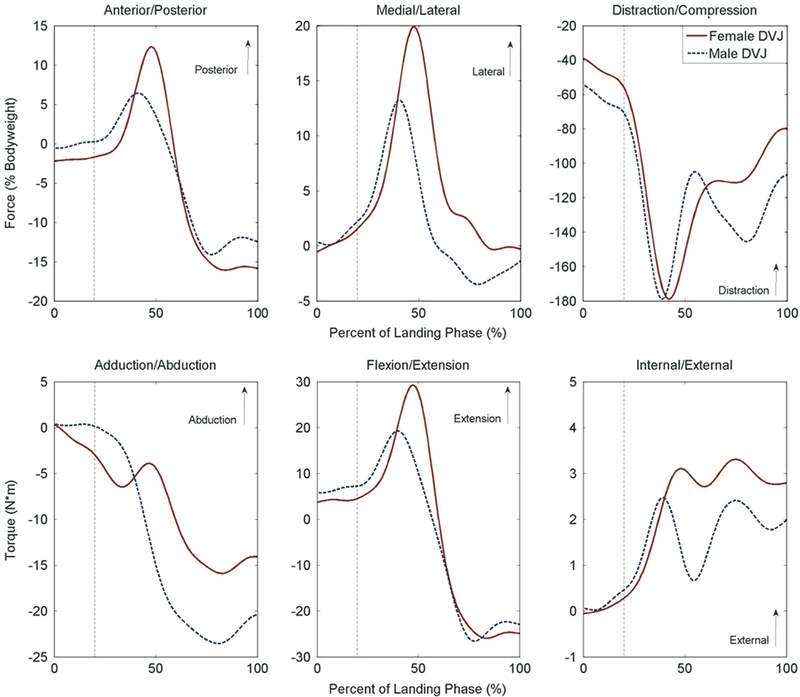
Displays the population average force (top row) and torque (bottom row) measured at the tibiofemoral joint in response to simulated male and female DVJ articulations. Landing phase begins at initial contact (0%) and concludes at maximum flexion (100%) (Bates et al., 2013). The vertical, dashed line represents the approximate cutoff for the first 50 ms after initial contact, where ACL injuries are most likely to occur (Krosshaug et al., 2007).
4. Discussion
Biomechanical disparities between sexes during athletic performance have been well documented. Females demonstrate greater knee abduction (Ford et al., 2006, 2005b; Garrison et al., 2005; Hughes et al., 2008), greater increase in knee abduction during puberty (Ford et al., 2010b), greater peak internal tibial rotation (Garrison et al., 2005; Kiriyama et al., 2009), decreased knee flexion at contact (Huston et al., 2001), different muscle activation patterns (Gehring et al., 2009), decreased hamstring to quadriceps strength ratios (Hewett et al., 2008), and greater ground reaction forces than males during athletic tasks (Kernozek et al., 2005). However, as many of these sex differences can be small in magnitude, their clinical significance on ligament injury has been questioned (Benjaminse et al., 2011). The purpose of this investigation was to use a robotic manipulator to simulate male and female kinematics from athletic tasks on cadaveric specimens and identify sex-based mechanical differences relative to ACL loading. Female athletes are 4–8 times more likely to tear their ACL than male counterparts (Arendt et al., 1999; Boden et al., 2000; Toth and Cordasco, 2001). As such, it is important to identify and understand the underlying mechanical differences that lead to this gender disparity in injury rates. Improved understanding of underlying mechanics could be used to design more efficacious methods of ACL injury prevention.
We found minimal difference between ACL strain in the simulated male and female tasks. ACL strain was only different between sexes at peak knee flexion during sidestep cutting. The sidestep cut simulations exhibit peak flexion angles of approximately 62° and 50° for the male and female, respectively. At these angles, under passive knee flexion, with and without hamstrings and quadriceps activation, the ACL has demonstrated that it was unstrained (Renstrom et al., 1986). This indicated that the only significant gender differences in ACL strain occurred at a position where the ligament is likely unloaded or minimally loaded. That combined with the knowledge that most ACL injuries occur within 50 ms of initial contact (Krosshaug et al., 2007), indicated that the noted strain difference, while statistically significant, has little bearing on real world injury scenarios.
The female motion did not generate higher joint loads compared to the male motion. Increased knee abduction torque has been prospectively associated with increased ACL injury risk (Hewett et al., 2005), and the ACL has been identified as a secondary restraint to both knee abduction and internal tibial torque (Boguszewski, 2012; Meyer and Haut, 2008; Oh et al., 2012; Shin et al., 2008, 2011). However, in the present study neither of these degrees of freedom exhibited gender differences. The ACL is also the primary restraint against anterior tibial translation and a secondary restraint against medial translation (Butler et al., 1980; Nesbitt et al., 2014). Again, there were no between-sex differences in medial joint loading for either simulated task. There were significant differences in anterior loading for both tasks, but the simulation of female kinematics actually exhibited lower peak forces. Combined, this absence of observation of significant gender differences in each degree of freedom related to ACL loading and ACL injury indicate that, in the controlled athletic tasks simulated, the simulation of female kinematics placed the ACL at no more risk for injury than the simulation of male kinematics.
Approximately 70% of ACL injuries occur in non-contact scenarios, the product of poor neuromuscular control and rapid deceleration and/or change of direction generating abnormal loading at the knee (Boden et al., 2000; McNair et al., 1990). As the magnitude of perturbation incurred during these activities increases, so does the respective kinematic excursion and kinetic loading at the knee (Ford et al., 2011; Levine et al., 2013). Computational and mechanical models confirm that increased joint excursion and loading correspond to increased ACL loading (Levine et al., 2013; Shin et al., 2008, 2011). In the present study, the simulations performed were relative to controlled athletic tasks that were performed with minimal perturbation to the athlete, resulting in no injury. In this controlled-task setting, the simulation of male and female kinematics exhibited minimal mechanical differences. In vivo, increased intensity in an athletic task has been shown to increase the prevalence of certain ACL-injury-risk biomechanics in female athletes (Ford et al., 2011). Similarly, the addition of perturbation to an in vivo athletic task has also been shown to exacerbate observable sex-based biomechanical differences (Ford et al., 2005a). Accordingly, the incorporation of kinematics from a more intense or more perturbed athletic task into our present model has the potential to similarly enhance the observable intra-articular differences between sexes.
The peak ligament strains reported during the activities simulated in the present study did not approach previously reported failure levels. Depending on the applied methodology, mechanical impact testing has indicated ACL failure occurs at an average of 18.7% strain (Levine et al., 2013). Uniaxial testing performed on the ACL has indicated failure at an average strain of 28.0% (Chandrashekar et al., 2006; Quapp and Weiss, 1998). The average peak values observed were well below these respective failure levels. This presence of a sizeable safety factor between the functional and failure strains indicated that structural failures would be highly unlikely to occur during a typical athletic task that is properly constrained through neuromuscular control.
A limitation of the current study was the gender bias in the tested specimen population. There were three times as many male specimens as female specimens. Ideally, equivalent numbers of male and female specimens would be articulated and gender differences would be examined not only between simulation models, but also between cadaveric specimens. However, our criteria to procure cadaveric limbs (good physical condition below 50 years of age) limited our ability to obtain a sufficient number of female specimens. In addition, female ACLs in vitro have exhibited decreased mechanical strength than their male counterparts (Chandrashekar et al., 2006). As such, though the sex-specific task simulations in the present study exposed knee ligaments to comparable loading, it is possible that the reduced mechanical properties of the female ACL make it more susceptible to injury than the male ACL during equivalent loading. With that consideration, none of the loads in the current investigation approached previously reported thresholds of failure (Chandrashekar et al., 2006). A second limitation, was the lack of muscle attachments and therefore muscle forces. Muscle contractions do not act directly on the joint or ligaments, but on bones. The robot manipulates limb segments with high precision and positions them relative to their in vivo orientations that were driven by muscle contractions. Therefore, the intra-articular loading conditions should still be mechanically representative of the in vivo physiology and neuromuscular control mechanisms of the 3D motion analysis subject. The current model only accounts for the neuromuscular activation differences present between the representative male and female subjects from which the input kinematics were originally recorded. Further differences in neuromuscular activation effects could be studied with this model, but would first require 3D motion analysis to be performed on subjects who demonstrate the neuromuscular pathways targeted for investigation. In this manner, the in vivo kinematics from those subjects could be represented in the current model and used to investigate the intra-articular mechanical differences that result from their specific neuromuscular pathways.
An additional limitation of the current model as documented in previous literature, was that kinematic translations input for each motion task were universal, with differences stemming only from rotations. In reality, each subject and task has their own unique translations, but current in vivo skin marker systems do not accurately record these kinematics. Therefore, to avoid confounding effects of arbitrary translational input, universal values were applied within each type of athletic task. As the ACL is primarily a restraint against anterior tibial translation (Boguszewski, 2012; Butler et al., 1980; Nesbitt et al., 2014), it is possible that sex-specific translations would have a greater influence on ligament strain than the sex-specific rotations presently simulated. Further, the input kinematics used in this model were gathered from a single male and single female subject. While these subjects were representative in that their kinematic profiles fell within the midrange of their gender, they are still subject-specific. Subject-specific profiles were selected as they provide the greatest degree of physiologic accuracy relative to actual motion pathways. Future investigations should assess the intra-articular biomechanical differences that result from the simulation of average population kinematics vs. the subject-specific kinematics currently used.
5. Conclusion
For the controlled DVJ and sidestep cutting activities, there were no sex-based loading or strain differences that would indicate an increased risk of ACL injures observed in female athletes. As ACL strain and joint loading were comparable between genders during controlled athletic tasks, the controlled activities prescribed for ACL injury prevention and rehabilitation should provide similar mechanical stimuli regardless of the patient gender. Further work is needed to differentiate the sex-specific mechanisms that incite ACL ruptures at a more frequent rate in females than their male counterparts.
Acknowledgments
This work was supported by the National Institutes of Health/ NIAMS Grants #R01-AR049735, #R01-AR055563, #R01-AR056660 and #R01-AR056259 (TEH). The authors would also like to acknowledge the support of the staff at the Sports Health and Performance Institute at the Ohio State University and the Sports Medicine Biodynamics Laboratory at Cincinnati Children’s Hospital.
Footnotes
Conflict of interest
We declare that we have no conflicts of interest in the authorship or publication of this contribution.
References
- Arendt EA, Agel J, Dick R, 1999. Anterior cruciate ligament injury patterns among collegiate men and women. J. Athl. Train. 34, 86–92. [PMC free article] [PubMed] [Google Scholar]
- Bates NA, Ford KR, Myer GD, Hewett TE, 2013. Impact differences in ground reaction force and center of mass between the first and second landing phases of a drop vertical jump and their implications for injury risk assessment. J. Biomech. 46, 1237–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates NA, Myer GD, Hewett TE, 2015a. Prediction of kinematic and kinetic performance in a drop vertical jump with individual anthropometric factors in adolescent female athletes: implications for cadaveric investigations. Ann. Biomed. Eng. 43, 929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates NA, Myer GD, Shearn JT, Hewett TE, 2015b. Anterior cruciate ligament biomechanics during robotic and mechanical simulations of physiologic and clinical motion tasks: a systematic review and meta-analysis. Clin. Biomech. 30, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates NA, Nesbitt RJ, Shearn JT, Myer GD, Hewett TE, 2015c. A novel methodology for the simulation of athletic tasks on cadaveric knee joints with respect to in vivo kinematics. Ann. Biomed. Eng. 43, 2456–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates NA, Nesbitt RJ, Shearn JT, Myer GD, Hewett TE, 2015d. Relative strain in anterior cruciate ligament and medial collateral ligament during simulated jump landing and sidestep cutting tasks implications for injury risk. Am. J. Sport Med. 43, 2259–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjaminse A, Gokeler A, Fleisig GS, Sell TC, Otten B, 2011. What is the true evidence for gender-related differences during plant and cut maneuvers? A systematic review. Knee Surg. Sports Traumatol. Arthrosc. 19, 42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beynnon B, Howe JG, Pope MH, Johnson RJ, Fleming BC, 1992. The measurement of anterior cruciate ligament strain in vivo. Int. Orthop. 16, 1–12. [DOI] [PubMed] [Google Scholar]
- Boden BP, Dean GS, Feagin JA, Garrett WE, 2000. Mechanisms of anterior cruciate ligament injury. Orthopedics 23, 573–578. [DOI] [PubMed] [Google Scholar]
- Boguszewski DV, 2012. Characterizing the Porcine Knee as a Biomechanical Surrogate Model of the Human Knee to Study the Anterior Cruciate Ligament. University of Cincinnati, Cincinnati, OH, USA. [Google Scholar]
- Butler DL, Noyes FR, Grood ES, 1980. Ligamentous restraints to anteriorposterior drawer in the human knee. A biomechanical study. J. Bone Jt. Surg. Am. 62, 259–270. [PubMed] [Google Scholar]
- Chandrashekar N, Mansouri H, Slauterbeck J, Hashemi J, 2006. Sex-based differences in the tensile properties of the human anterior cruciate ligament. J. Biomech. 39, 2943–2950. [DOI] [PubMed] [Google Scholar]
- Ford KR, Myer GD, Hewett TE, 2003. Valgus knee motion during landing in high school female and male basketball players. Med. Sci. Sports Exerc. 35, 1745–1750. [DOI] [PubMed] [Google Scholar]
- Ford KR, Myer GD, Hewett TE, 2007a. Increased trunk motion in female athletes compared to males during single leg landing. Med. Sci. Sports Exerc. 9, S70. [Google Scholar]
- Ford KR, Myer GD, Hewett TE, 2007b. Reliability of landing 3D motion analysis: implications for longitudinal analyses. Med. Sci. Sports Exerc. 39, 2021–2028. [DOI] [PubMed] [Google Scholar]
- Ford KR, Shapiro R, Myer GD, Van Den Bogart AJ, Hewett TE, 2010a. Longitudinal sex differences during landing in knee abduction in young athletes. Med. Sci. Sports Exerc. 42, 1923–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford KR, Myer GD, Hewett TE, 2010b. Longitudinal effects of maturation on lower extremity joint stiffness in adolescent young athletes. Am. J. Sports Med. 38, 1829–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford KR, Myer GD, Schmitt LC, Uhl TL, Hewett TE, 2011. Preferential quadriceps activation in female athletes with incremental increases in landing intensity. J. Appl. Biomech. 27, 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford KR, Myer GD, Smith RL, Byrnes RN, Dopirak SE, Hewett TE, 2005a.. Use of an overhead goal alters vertical jump performance and biomechanics. J. Strength Cond. Res. 19, 394–399. [DOI] [PubMed] [Google Scholar]
- Ford KR, Myer GD, Smith RL, Vianello RM, Seiwert SL, Hewett TE, 2006. A comparison of dynamic coronal plane excursion between matched male and female athletes when performing single leg landings. Clin. Biomech. 21, 33–40. [DOI] [PubMed] [Google Scholar]
- Ford KR, Myer GD, Toms HE, Hewett TE, 2005b. Gender differences in the kinematics of unanticipated cutting in young athletes. Med. Sci. Sports Exerc. 37, 124–129. [PubMed] [Google Scholar]
- Ford KR, Shapiro R, Myer GD, van den Bogert AJ, Hewett TE, 2010c. Longitudinal sex differences during landing in knee abduction in young athletes. Med. Sci. Sports Exerc, 1923–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison JC, Hart JM, Palmieri RM, Kerrigan DC, Ingersoll CD, 2005. Comparison of knee-joint moments in male and female college soccer players during a single-leg landing. J. Sport Rehabil. 14, 332. [Google Scholar]
- Gehring D, Melnyk M, Gollhofer A, 2009. Gender and fatigue have influence on knee joint control strategies during landing. Clin. Biomech. 24, 82–87. [DOI] [PubMed] [Google Scholar]
- Griffin LY, Agel J, Albohm MJ, Arendt EA, Dick RW, Garrett WE, Garrick JG, Hewett TE, Huston L, Ireland ML, Johnson RJ, Kibler WB, Lephart S, Lewis JL, Lindenfeld TN, Mandelbaum BR, Marchak P, Teitz CC, Wojtys EM, 2000. Noncontact anterior cruciate ligament injuries: risk factors and prevention strategies. J. Am. Acad. Orthop. Surg. 8, 141–150. [DOI] [PubMed] [Google Scholar]
- Grood ES, Suntay WJ, 1983. A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J. Biomech. Eng. 105, 136–144. [DOI] [PubMed] [Google Scholar]
- Herfat ST, Boguszewski DV, Shearn JT, 2012. Applying simulated in vivo motions to measure human knee and ACL kinetics. Ann. Biomed. Eng. 40, 1545–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewett TE, Ford KR, Myer GD, Wanstrath K, Scheper M, 2006. Gender differences in hip adduction motion and torque during a single-leg agility maneuver. J. Orthop. Res. 24, 416–421. [DOI] [PubMed] [Google Scholar]
- Hewett TE, Lindenfeld TN, Riccobene JV, Noyes FR, 1999a. The effect of neuromuscular training on the incidence of knee injury in female athletes. A prospective study. Am. J. Sports Med. 27, 699–706. [DOI] [PubMed] [Google Scholar]
- Hewett TE, Myer GD, Ford KR, Heidt RS Jr., Colosimo AJ, McLean SG, van den Bogert AJ, Paterno MV, Succop P, 2005. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am. J. Sports Med. 33, 492–501. [DOI] [PubMed] [Google Scholar]
- Hewett TE, Myer GD, Zazulak BT, 2008. Hamstrings to quadriceps peak torque ratios diverge between sexes with increasing isokinetic angular velocity. J. Sci. Med. Sport 11, 452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewett TE, Paterno MV, Noyes FR, 1999b. Differences in single leg balance on an unstable platform between female and male normal, ACL-deficient and ACL-reconstructed knees In: Lephardt S, Fu FH (Eds.), Proprioception and Neuromuscular Control in Joint Stability. Human Kinetics, United States, pp. 77–88. [Google Scholar]
- Hughes G, Watkins J, Owen N, 2008. Gender differences in lower limb frontal plane kinematics during landing. Sports Biomech. 7, 333–341. [DOI] [PubMed] [Google Scholar]
- Huston LJ, Vibert B, Ashton-Miller JA, Wojtys EM, 2001. Gender differences in knee angle when landing from a drop-jump. Am. J. Knee Surg. 14, 219–220 (discussion 215–219). [PubMed] [Google Scholar]
- Kernozek TW, Torry MR, H., V.H., Cowley H, Tanner S, 2005. Gender differences in frontal and sagittal plane biomechanics during drop landings. Med. Sci. Sports Exerc. 37, 1003–1012 (discussion 1013). [PubMed] [Google Scholar]
- Kim S, Bosque J, Meehan JP, Jamali A, Marder R, 2011. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J. Bone Jt. Surg. Am. 93, 994–1000. [DOI] [PubMed] [Google Scholar]
- Kiriyama S, Sato H, Takahira N, 2009. Gender differences in rotation of the shank during single-legged drop landing and its relation to rotational muscle strength of the knee. Am. J. Sports Med. 37, 168–174. [DOI] [PubMed] [Google Scholar]
- Krosshaug T, Nakamae A, Boden BP, Engebretsen L, Smith G, Slauterbeck JR, Hewett TE, Bahr R, 2007. Mechanisms of anterior cruciate ligament injury in basketball: video analysis of 39 cases. Am. J. Sports Med. 35, 359–367. [DOI] [PubMed] [Google Scholar]
- Levine JW, Kiapour AM, Quatman CE, Wordeman SC, Goel VK, Hewett TE, Demetropoulos CK, 2013. Clinically relevant injury patterns after an anterior cruciate ligament injury provide insight into injury mechanisms. Am. J. Sports Med. 41, 385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmander LS, Ostenberg A, Englund M, Roos H, 2004. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 50, 3145–3152. [DOI] [PubMed] [Google Scholar]
- Malinzak RA, Colby SM, Kirkendall DT, Yu B, Garrett WE, 2001. A comparison of knee joint motion patterns between men and women in selected athletic tasks. Clin. Biomech. 16, 438–445. [DOI] [PubMed] [Google Scholar]
- McLean SG, Huang X, van den Bogert AJ, 2005. Association between lower extremity posture at contact and peak knee valgus moment during sidestepping: implications for ACL injury. Clin. Biomech. 20, 863–870. [DOI] [PubMed] [Google Scholar]
- McNair PJ., Marshall RN, Matheson JA, 1990. Important features associated with acute anterior cruciate ligament injury. N. Z. Med. J. 103, 537–539. [PubMed] [Google Scholar]
- Meyer EG, Haut RC, 2008. Anterior cruciate ligament injury induced by internal tibial torsion or tibiofemoral compression. J. Biomech. 41, 3377–3383. [DOI] [PubMed] [Google Scholar]
- Myer GD, Ford KR, Brent JL, Hewett TE, 2007. Differential neuromuscular training effects on ACL injury risk factors in “high-risk” vs. “low-risk” athletes. BMC Musculoskelet. Disord. 8, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer GD, Ford KR, hewett tE., 2006. Preventing ACL injuries in women. J. Musculoskel. Med. 23, 12–38. [Google Scholar]
- Myer GD, Ford KR, Khoury J, Succop P, Hewett TE, 2010a. Biomechanics laboratory-based prediction algorithm to identify female athletes with high knee loads that increase risk of ACL injury. Br. J. Sports Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer GD, Ford KR, Khoury J, Succop P, Hewett TE, 2010b. Clinical correlates to laboratory measures for use in non-contact anterior cruciate ligament injury risk prediction algorithm. Clin. Biomech. 25, 693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer GD, Ford KR, Khoury J, Succop P, Hewett TE, 2010c. Development and validation of a clinic-based prediction tool to identify female athletes at high risk for anterior cruciate ligament injury. Am. J. Sports Med. 38, 2025–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer GD, Ford KR, Khoury J, Succop P, Hewett TE, 2011. Biomechanics laboratory-based prediction algorithm to identify female athletes with high knee loads that increase risk of ACL injury. Br. J. Sports Med. 45, 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbitt RJ, Herfat ST, Boguszewski DV, Engel AJ, Galloway MT, Shearn JT, 2014. Primary and secondary restraints of human and ovine knees for simulated in vivo gait kinematics. J. Biomech. 47, 2022–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh YK, Lipps DB, Ashton-Miller JA, Wojtys EM, 2012. What strains the anterior cruciate ligament during a pivot landing? Am. J. Sports Med. 40, 574–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quapp KM, Weiss JA, 1998. Material characterization ofhuman medial collateral ligament. J. Biomech. Eng. 120, 757–763. [DOI] [PubMed] [Google Scholar]
- Renstrom P, Arms SW, Stanwyck TS, Johnson RJ, Pope MH, 1986. Strain within the anterior cruciate ligament during hamstring and quadriceps activity. Am. J. Sports Med. 14, 83–87. [DOI] [PubMed] [Google Scholar]
- Shin CS, Chaudhari AM, Andriacchi TP, 2008. The effect of isolated valgus moments on ACL strain during single-leg landing: a simulation study. J. Bio- mech. 42, 280–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin CS, Chaudhari AM, Andriacchi TP, 2011. Valgus plus internal rotation moments increase ACL strain more than either alone. Med. Sci. Sports Exerc. [DOI] [PubMed] [Google Scholar]
- Toth AP, Cordasco FA, 2001. Anterior cruciate ligament injuries in the female athlete. J. Gend. Specif. Med. 4, 25–34. [PubMed] [Google Scholar]
- von Porat A, Roos EM, Roos H, 2004. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann. Rheum. Dis. 63, 269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withrow TJ, Huston LJ, Wojtys EM, Ashton-Miller JA, 2006. The effect of an impulsive knee valgus moment on in vitro relative ACL strain during a simulated jump landing. Clin. Biomech. 21, 977–983. [DOI] [PubMed] [Google Scholar]


