Abstract
Objective
Melatonin, a neurohormone secreted by the pineal gland, controls circadian rhythmicity, modulates sleep and plays a role in glucose metabolism. Low secretion of nocturnal urinary 6-sulfatoxymelatonin (aMT6S) was associated with incident diabetes. Sleep disturbances have also been shown to be risk factors for diabetes. In this study, we explored the relationship between nocturnal urinary aMT6s and markers of glucose metabolism in prediabetes patients, considering sleep related factors.
Methods
Sixty two non-shift working patients with prediabetes [hemoglobin A1c (HbA1c) 5.7–6.49%] who were not on beta-blockers participated. Sleep duration and efficiency was recorded using 7-day actigraphy. Obstructive sleep apnea was evaluated using an overnight in-home monitoring device. Nocturnal urinary aMT6s/creatinine ratio was measured from an overnight urine sample. Oral glucose tolerance test (OGTT, 75-grams glucose) was performed, with measurements of insulin and glucose levels.
Results
Mean (SD) age was 55.3 (8.2) years and mean HbA1c level was 6.01 (0.2)%. Mean (SD) sleep duration 6.0 (0.9) h, sleep efficiency was 83.4 (6.6)% and a median (interquartile rage) apnea hypopnea index was 10.3 (3.6, 16.4). Median nocturnal urinary aMT6s was 17.4 (9.4, 28.2) ng/mg creatinine. Higher nocturnal urinary aMT6s significantly correlated with lower fasting insulin (p = 0.004), lower insulin response to OGTT (p = 0.027), and lower fasting and whole body insulin resistance as indicated by lower HOMA-IR and higher Matsuda insulin sensitivity index (p = 0.006 and p = 0.011, respectively), but it was not correlated with fasting glucose, glucose response to OGTT, or HbA1c. Sleep duration inversely correlated with HbA1c but no other correlations were found between other sleep variables and markers of glucose metabolism or nocturnal urinary aMT6s. After adjusting for body mass index, higher nocturnal urinary aMT6s significantly correlated with lower HOMA-IR (p = 0.025) and fasting insulin levels (p = 0.014).
Conclusion
Nocturnal urinary aMT6s inversely correlated with fasting insulin resistance and insulin levels in patients with prediabetes. These results support the role of melatonin in glucose metabolism.
Keywords: Melatonin, Glucose metabolism, Insulin resistance, Insulin, Prediabetes
Highlights
-
•
This study was performed in prediabetes patients
-
•
Higher nocturnal urinary 6-sulfatoxymelatonin was related to lower fasting insulin.
-
•
Higher nocturnal urinary 6-sulfatoxymelatonin was related to lower HOMA-IR.
-
•
These results support the role of melatonin in glucose metabolism.
1. Introduction
The circadian system, controlled by the master circadian clock located in the suprachiasmatic nucleus (SCN) in the hypothalamus, plays a major role in regulating daily rhythms of sleep/wake cycle, central and peripheral tissue metabolism, and hormonal secretions (Huang et al., 2011). The central clock is entrained by the light-dark cycle and other environmental factors, and relays the information via various pathways to the peripheral organs, leading to coordinated rhythms. In humans, it was estimated that ∼ 15% of all identified metabolites in plasma and saliva were under circadian control, especially plasma fatty acids and salivary amino acids (Dallmann et al., 2012). Growing evidence has confirmed the detrimental effects of the disruption of circadian organization on metabolism. Experimental circadian misalignment in healthy human volunteers led to increased glucose levels, reduced insulin sensitivity, increased mean arterial blood pressure, reversal of daily cortisol rhythm, impaired autonomic function and elevated inflammatory markers (i.e. interleukin-10, interleukin-6, tumor necrosis factor-α, C-reactive protein) (Buxton et al., 2012, Leproult et al., 2014, Scheer et al., 2009, Wright et al., 2015, Morris et al., 2015, Morris et al., 2016). Cohort studies have also confirmed an association between shift work, usually associated with circadian misalignment, to an increase risk of incident diabetes (Morikawa et al., 2005, Suwazono et al., 2006), with longer duration of shift work being associated with higher risk (Pan et al., 2011).
Melatonin is a neurohormone secreted by a pineal gland during the biological night under the control of the central clock, and inhibited by light exposure (Brzezinski, 1997). Two G-protein coupled receptors, MT1 and MT2 (found in the SCN, brain tissues and peripheral tissues including pancreatic β-cells), mediate the effects of melatonin (Peschke and Muhlbauer, 2010). The 24 h rhythm of melatonin acts as an internal synchronizer of the circadian system (Morris et al., 2012). In addition, melatonin has potent anti-oxidant and anti-inflammatory effects (Mauriz et al., 2013). Data suggested that melatonin plays a role in glucose regulation. In a nested case control study within the Nurses’ Health Study Cohort, women with the lowest tertile of nocturnal urinary 6-sulfatoxymelatonin (aMT6s, a major urine melatonin metabolite) to creatinine ratio had a significantly increased risk of diabetes development compared to those in the highest tertile (odds ratio 2.17) (McMullan et al., 2013b). A cross sectional study of 513 elderly Japanese also found that high nocturnal urinary aMT6S (75th percentile vs 25th percentile) was associated with a 32% decrease in prevalent diabetes (Obayashi et al., 2014). In addition, variants in the melatonin receptor type 1B (MTNR1B) have been shown to be associated with impaired fasting glucose and type 2 diabetes (Sparso et al., 2009, Bonnefond et al., 2012). Moreover, 8–10 weeks of melatonin administration in diabetic rats resulted in reduction in glucose levels along with an improvement in insulin resistance (Agil et al., 2012, de Oliveira et al., 2012). These data support the possible beneficial effect of melatonin on glucose metabolism. However, administrations of immediate release melatonin in healthy women, both in the morning and evening, resulted in an increase in glucose response following glucose challenge, as compared to placebo (Rubio-Sastre et al., 2014). This was related to decreased insulin secretion in the morning, and decreased insulin sensitivity in the evening. In another study of 14 postmenopausal women, 1 mg melatonin administered at 0800 h resulted in reduced glucose tolerance and insulin sensitivity as assessed by oral glucose challenge 45 min later (Cagnacci et al., 2001). These conflicting data highlight further research needed to clarify the role of melatonin in human’s glucose metabolism.
The prevalence of diabetes is increasing in the U.S. and worldwide. Alarming statistics revealed that additional significant number of population have prediabetes, a state of abnormal glucose levels not yet meeting the criteria for diabetes (Centers for Disease Control and Prevention, 2014). These individuals, estimated at 86 million adults in the U.S. in 2012, are at high risk of developing diabetes (Centers for Disease Control and Prevention, 2014). Whether melatonin secretion is related to glucose metabolism in this population has not been studied. Besides traditional risk factors for diabetes, sleep disturbances [i.e. insufficient sleep duration, poor sleep quality and obstructive sleep apnea (OSA)] have been shown to be associated with metabolic dysfunction and increased risk of incident diabetes (Anothaisintawee et al., 2015). Some of these sleep disturbances were also found to be related to lower nocturnal aMT6S levels (Saksvik-Lehouillier et al., 2015). Therefore, this study was conducted with the aim to explore the relationship between nocturnal aMT6s and markers of glucose metabolism in patients with prediabetes, taken into consideration sleep related factors.
2. Material and methods
A cross-sectional study was conducted in non-shift working adults attending the outpatient department at the Faculty of Medicine Ramathibodi Hospital with a previous diagnosis of prediabetes defined as hemoglobin A1c (HbA1c) ≥ 5.70 to < 6.50% (American Diabetes Association: Standars of Medical Care in Diabetes, 2017). Exclusion criteria included beta blocker use (known to affect melatonin secretion), being currently pregnant, a history of congestive heart failure or low ejection fraction, chronic obstructive pulmonary disease, end stage renal disease or severe chronic liver disease such as cirrhosis, stroke, permanent pacemaker placement, and use of certain medications (opioids/ narcotics, alpha adrenergic blockers, clonidine, methyldopa, nitroglycerin). The data utilized in this study were a part of those obtained from a larger cross-sectional study exploring the role of sleep duration and markers of glucose metabolism (clinicaltrials.gov, NCT02108197). All participants gave written informed consent. The protocol was approved by the Committee on Human Rights Related to Research Involving Human Subjects, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand.
After obtaining informed consent, weight and height were measured. Body mass index (BMI) was calculated as weight (kg)/height2 (m)2. The participants then underwent the following assessments, all performed within a two-week period.
2.1. Sleep duration and efficiency measurement
Participants wore an Actiwatch 2 wrist activity monitor (Philips Respironics, Bend, Oregon) for 7 days. These monitors use highly sensitive omnidirectional accelerometers to count the number of wrist movements in 30-s epochs. The software scores each 30-s epoch as sleep or wake based on a threshold of activity counts that is estimated using activity within the epoch being scored as well as the epochs 2 min before and after that epoch. The participants were instructed to press the event marker button upon going to bed and awakening. Bedtime and wake time are set by the researcher using the event markers, a sleep log data as well as an in-person review of sleep timing with the participants upon return of the watch. Sleep duration was defined as the amount of actual sleep obtained at night, and sleep efficiency (a measure of sleep quality) was defined as percentage of time in bed spent sleeping. These two variables were calculated using Actiware 6.0 software, supplied by the manufacturer. Mid sleep time was defined as a midpoint between sleep start and sleep end. For each participant, the mean across all available nights was used. For 95% of participants in these analyses, at least 6 days of actigraphy recording were available and the remaining 5% had 5 days of actigraphy recording.
2.2. Assessment of OSA
OSA was diagnosed using an FDA-approved portable diagnostic device, WatchPAT 200 (Itamar Medical, Israel), which has been validated against PSG in populations with and without diabetes (Zou et al., 2006, Yuceege et al., 2013). This non-invasive device is shaped similar to a large watch, worn on the non-dominant wrist immediately before bedtime and removed upon awakening in the morning. The device has two probes connecting to the participants’ fingers to measure changes in peripheral arterial tone (PAT) and oxygen saturation, along with a built-in actigraphy to measure sleep time. The severity of OSA is assessed by PAT Apnea Hypopnea Index (AHI) which is automatically generated by the software, using changes in the peripheral arterial tonometry. OSA is considered present if AHI ≥ 5. OSA is considered mild if AHI ≥ 5 but < 15, moderate if AHI ≥15- 30, and severe if AHI > 30.
Because this device relies on changes in peripheral arterial tone, use of certain medications including alpha-blockers and short acting nitrates, as well as permanent pacemaker, was not allowed according to the device’s operation manual. In addition, it cannot differentiate obstructive from central apnea events. Therefore, we also excluded patients with certain conditions which may pose a higher risk for central apnea. These were described in the exclusion criteria.
2.3. Nocturnal urinary excretion of 6-sulfatoxymelatonin assessment
The participants were instructed to discard the last void prior to bedtime and collect each subsequent void until the first next morning void. The samples were stored in a dark bottle at room temperature, the total volume measured, and then stored at -20C until assay. Urinary 6-sulfatoxymelatonin (aMT6s), a major melatonin metabolite, was measured by a competitive enzyme-linked immunosorbent assay (Bühlmann Laboratories AG, Schönenbuch, Switzerland). Urine creatinine was analyzed on an automated biochemical analyzer (Dimension RxL; Dade Behring, Newark, DE, USA). Urinary aMT6s/creatinine ratios were calculated by dividing urinary aMT6s levels by urine creatinine concentration, expressed as ng/mg, as previously described (McMullan et al., 2013b, McMullan et al., 2013a). These values were referred to as nocturnal urinary aMT6s/Cr ratio. In addition, total urinary aMT6s was calculated from the concentration of aMT6s and urine volume.
2.4. Glucose, HbA1c and insulin measurements
After an overnight fast, 75-gram glucose was given orally. Blood samples were obtained at 0 minutes for HbA1c, and at 0, 30, 60, 90, 120 min for glucose and insulin. Glucose and HbA1c were assayed in the clinical laboratory of Ramathibodi Hospital using hexokinase enzymatic method and turbid metric Inhibition Immunoassay, respectively. Serum insulin was measured by electrochemiluminescence immunoassay on a Cobas e 411 analyzer (Roche Diagnostics GmbH, Mannheim, Germany). The assays have intra-and inter-assay precision of 2.0% and 2.8%, respectively.
In addition, the following markers of glucose metabolism were calculated. Area under the curve (AUC) of glucose and insulin response to glucose challenge was calculated using the trapezoid rule. HOMA-IR, an index of fasting insulin resistance, was calculated using the formula [fasting glucose (mg/dl)x fasting insulin (μU/ml)]/405 (Matthews et al., 1985). The Insulinogenic Index, an estimate of early insulin secretion, was calculated by dividing increment in insulin during the first 30 min by the increment in glucose over the same period (ΔI30/ΔG30) (Seltzer et al., 1967). The Matsuda index, an index of whole body insulin sensitivity, was calculated from the glucose and insulin levels during the glucose challenge as previously described (Matsuda and DeFronzo, 1999). Finally, the disposition index, an indicator of β-cell function adjusted for insulin sensitivity, was calculated as a product of the insulinogenic index and the Matsuda index (Weiss et al., 2007).
2.5. Statistical analysis
Data are expressed as mean (SD) or median (Interquartile range) or frequencies/percentages. Spearman’s correlations were used to explore the associations between demographic, sleep variables, nocturnal urinary aMT6s/Cr ratio, and total nocturnal urinary aMT6s with markers of glucose metabolism. Multiple linear regression analyses were used to determine independent predictors of markers of glucose metabolism. Nocturnal urinary aMT6s/Cr ratio, total nocturnal urinary aMT6s, fasting insulin, AUC insulin, HOMA IR, Matsuda index, insulinogenic index and disposition index were not normally distributed, and therefore the natural logarithm (Ln) transformation of these variables was used in the regression analyses. All analyses were performed using SPSS 18.0 (Chicago, IL).
3. Results
A total of 62 participants underwent the assessments. Their demographic, HbA1c levels, metabolic parameters in response to glucose challenge, sleep characteristics and nocturnal urinary aMT6s are shown in Table 1. A majority (75%) of the participants were female with a mean age of 55 years and mean HbA1c level of 6.01%. OSA assessment was completed in 60 participants. An overall median AHI was 10.3 with 41 participants (69.4%) found to have OSA (40.3% with mild, 22.6% moderate, and 3.2% severe OSA, respectively). Mean sleep duration was 6.0 h/night.
Table 1.
Demographic, glycemic, sleep and nocturnal urinary aMT6s in all participants.
| Results (n = 62) | |
|---|---|
| Demographic | |
| Age (years) | 55.3 (8.2) |
| Female (n)(%) | 47 (75.8%) |
| BMI (kg/m2) | 26.7 (3.8) |
| Glycemic characteristics | |
| HbA1c (%) | 6.01 (0.20) |
| Fasting glucose (mg/dl) | 101.8 (7.9) |
| AUC glucose (mg/dl h) | 362 (59) |
| Fasting insulin (μU/ml) | 11.6 (9.0, 17.8) |
| AUC insulin (μU/ml h) | 215 (148, 294) |
| HOMA IR | 2.86 (2.24, 4.95) |
| Matsuda index | 2.04 (1.48, 2.92) |
| Insulinogenic index | 0.90 (0.49, 1.25) |
| Disposition index | 2.04 (1.48, 2.92) |
| Sleep and urinary aMT6s | |
| Nocturnal urinary aMT6s/ Cr ratio (ng/mg) | 17.4 (9.4, 28.2) |
| Total nocturnal urinary aMT6s (ng) | 6256.5 (2878.6, 9546.2) |
| AHIa | 10.3 (3.6, 16.4) |
| Sleep duration (min) | 359 (53) |
| Sleep efficiency (%) | 83.4 (6.6) |
| Mid sleep time (h) | 2:23 (0:47) |
Data are expressed as mean (SD) or median (interquartile range).
n = 60.
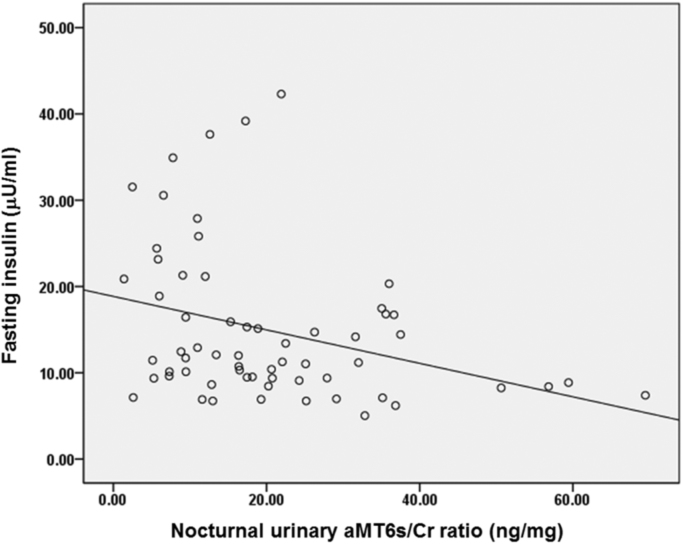
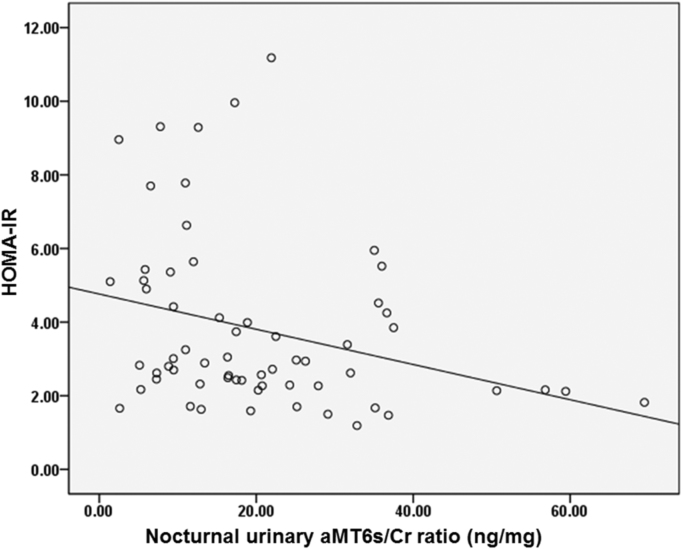
Table 2 showed correlations between demographic, sleep, nocturnal urinary aMT6s/Cr ratio, total nocturnal urinary aMT6s and markers of glucose metabolism. Age and sex were not related to any glucose metabolism markers (p ≥ 0.05) while higher BMI significantly correlated with higher fasting insulin, AUC glucose and AUC insulin in response to glucose challenge, higher fasting insulin resistance (HOMA-IR) and higher whole body insulin resistance as indicated by lower Matsuda index. Shorter habitual sleep duration significantly correlated with higher HbA1c levels while none of the other sleep variables significantly correlated with any markers of glucose metabolism. Higher nocturnal urinary aMT6s/Cr ratio significantly correlated with lower fasting insulin (p = 0.004, Fig. 1) and lower AUC insulin response to glucose challenge (p = 0.027) but it was not correlated to fasting glucose, HbA1c or AUC glucose. In addition, higher nocturnal urinary aMT6s/Cr ratio significantly correlated with lower fasting and whole body insulin resistance as indicated by lower HOMA-IR (p = 0.006, Fig. 2) and higher Matsuda index (p = 0.011). None of the sleep variables (sleep duration, sleep efficiency and AHI), however, correlated with nocturnal urinary aMT6s/Cr ratio.
Table 2.
Correlations between demographic, sleep, nocturnal urinary aMT6s and markers of glucose metabolism.
| HbA1c | Fasting glucose | Fasting insulin | AUC glucose | AUC insulin | HOMA IR | Matsuda index | Insulinogenic index | Disposition index | |
|---|---|---|---|---|---|---|---|---|---|
| Age | .064 | .028 | .149 | .116 | .152 | .135 | –.178 | .045 | – .092 |
| BMI | .178 | .082 | .462*** | .257* | .404** | .443*** | –.477*** | .121 | – .197 |
| Urinary aMT6s/Cr ratio | – .108 | .000 | – .362** | – .071 | – .280* | – .347** | .319* | − .163 | .106 |
| Total urinary aMT6s | – .072 | .036 | – .288* | – .046 | – .243 | – .263* | .257* | – .092 | .135 |
| AHI | .159 | .055 | .204 | .122 | .139 | .211 | – .207 | .067 | – .104 |
| Sleep duration | – .366** | .114 | .168 | – .160 | .112 | .157 | – .127 | .193 | .088 |
| Sleep efficiency | – .169 | − .034 | – .131 | – .049 | .014 | – .130 | .037 | –.019 | .053 |
| Mid sleep time | – .048 | – .007 | .221 | – .065 | .180 | .205 | – .166 | .094 | – .087 |
p < 0.05.
p < 0.01.
p < 0.001.
Fig. 1.
Correlation between nocturnal urinary aMT6s/Cr ratio and fasting insulin levels.
Fig. 2.
Correlation between nocturnal urinary aMT6s/Cr ratio and HOMA-IR.
For total nocturnal urinary aMT6s, the relationships with markers of glucose metabolism were similar to those of nocturnal urinary aMT6s/Cr ratio. Higher total nocturnal urinary aMT6s was associated with lower fasting insulin (p = 0.023) and tended to be associated with lower AUC insulin response to glucose challenge (p = 0.057). In addition, higher total nocturnal urinary aMT6s significantly correlated with lower fasting and whole body insulin resistance (p = 0.039 and p = 0.044, respectively). Of note, nocturnal urinary aMT6s/Cr ratio was highly correlated with total nocturnal urinary aMT6s (correlation coefficient = 0.83, p < 0.001).
Regression analyses to determine if nocturnal urinary aMT6s/Cr ratio independently predicts insulin levels or insulin resistance are shown in Table 3. After adjusting for BMI, higher nocturnal urinary aMT6s was significantly associated with lower fasting insulin levels (B = − 0.180, p = 0.014) and lower fasting insulin resistance (HOMA-IR) (B = − 0.176, p = 0.025), while the relationship with AUC insulin and Matusda index was attenuated.
Table 3.
Multiple regression analyses between nocturnal urinary aMT6s/Cr ratio and markers of glucose metabolism.
|
Fasting insulin |
AUC insulin |
HOMA IR |
Matsuda index |
|||||
|---|---|---|---|---|---|---|---|---|
| B | P value | B | P value | B | P value | B | P value | |
| BMI | .060 | < 0.001 | .048 | 0.002 | .060 | < 0.001 | – .060 | < 0.001 |
| Urinary aMT6s/Cr ratio | – .180 | 0.014 | – .114 | 0.117 | – .176 | 0.025 | .134 | 0.069 |
B = unstandardized coefficient.
Table 4 demonstrated regression analyses to determine if total nocturnal urinary aMT6s was independently associated with markers of glucose metabolism. The results were similar to those of nocturnal urinary aMT6s/Cr ratio. After adjusting for BMI, higher total nocturnal urinary aMT6s significantly correlated with lower fasting insulin levels (B = −0.133, p = 0.034), and borderline correlated with lower HOMA-IR (B = − 0.127, p = 0.057). We further explored by categorizing total nocturnal urinary aMT6s in to quartiles. After adjusting for BMI, those in the highest quartile of total nocturnal urinary aMT6s had significantly lower fasting insulin (B = − 0.139, p = 0.013) and lower HOMA-IR (B = − 0.130, p = 0.031) than those in the lowest quartile.
Table 4.
Multiple regression analyses between total nocturnal urinary aMT6s and markers of glucose metabolism.
|
Fasting insulin |
AUC insulin |
HOMA IR |
Matsuda index |
|||||
|---|---|---|---|---|---|---|---|---|
| B | P value | B | P value | B | P value | B | P value | |
| BMI | .063 | 0.015 | .049 | 0.002 | .063 | < 0.001 | – .062 | < 0.001 |
| Total nocturnal Urinary aMT6s | – .133 | 0.034 | – .110 | 0.075 | – .127 | 0.059 | 1.783 | 0.079 |
4. Discussion
In this study of patients with prediabetes, we demonstrated that lower nocturnal secretion of aMT6s/Cr ratio, a major melatonin metabolite, was associated with higher degree of insulin resistance, as measured by HOMA-IR. Higher nocturnal urinary aMT6s/Cr ratio was associated with lower fasting insulin level but without any adverse effects on glucose levels. Similar results were demonstrated using total nocturnal urinary aMT6s. These data suggested that while nighttime melatonin may inhibit insulin secretion, it is associated with improved morning insulin sensitivity. The results support and further extend the knowledge regarding the role of melatonin in glucose metabolism.
The finding of the relationship between nocturnal urinary aMT6s level and insulin extends the findings in animal models of the antagonism between the two factors (Peschke et al., 2013). Both MT1 and MT2 are present in pancreatic islet. The inhibitory action of melatonin on insulin secretion is thought to be transmitted via a Gi protein signaling cascade, either involving cyclic adenosine monophosphate (cAMP) pathway (MT1 signaling), or cyclic guanosine monophosphate (cGMP) pathway (MT2 signaling) (Peschke et al., 2013). Thus, its action on insulin secretion may help maintain glucose levels overnight. In our population with prediabetes, despite being associated with lower insulin levels, higher nocturnal urinary aMT6s/Cr ratio was not associated with higher glucose levels, likely due to decreased insulin resistance as indicated by HOMA-IR. Our findings are in agreement with a study in 1,075 US women without diabetes which revealed that high nocturnal urinary aMT6S secretion was inversely associated with fasting insulin levels and insulin resistance (McMullan et al., 2013a). Although both hepatic and peripheral insulin sensitivity contribute to HOMA-IR and Matsuda insulin sensitivity index, hepatic insulin sensitivity is thought to be an important determinant of HOMA-IR, especially in those with impaired glucose tolerance (Tripathy et al., 2004), while Matsuda index represents whole-body insulin sensitivity (Singh and Saxena, 2010). As nocturnal urinary aMT6s in our study only borderline correlated with Matsuda insulin sensitivity index, this suggests that the relationship between urinary aMT6s and insulin sensitivity is more dependent upon hepatic insulin sensitivity. This is supported by several animal studies in which melatonin administration was associated with a reduction in HOMA-IR. Eight weeks of melatonin treatment in diabetic rats reduced hyperglycemia and insulin resistance as measured by HOMA-IR, as well as improved glucose uptake into adipocytes (de Oliveira et al., 2012). Another study in rats revealed that daily intraperitoneal administration of melatonin ameliorated insulin resistance associated with high fructose intake (Kitagawa et al., 2012). This could partly be due to reduction in hepatic gluconeogenesis and increased hypothalamic Akt phosphorylation as demonstrated by an intraventricular injection of melatonin in rats (Faria et al., 2013). The authors suggested that melatonin activates hypothalamus-liver communication that may contribute to circadian adjustments of gluconeogenesis (Faria et al., 2013). In addition, melatonin has been shown to regulate insulin sensitivity in adipocytes by enhancing the function of IRS-1 (She et al., 2014), increase glycogen synthesis in hepatic cells (Shieh et al., 2009), and prevent mitochondrial dysfunction and insulin resistance in rat skeletal muscle (Teodoro et al., 2014). Another potential mechanism in the association between melatonin and HOMA-IR could be related to the high oxidative state and free radicals generation seen in insulin resistance and hyperglycemia (Zephy and Ahmad, 2015). Melatonin has antioxidant properties and may act as a scavenger to change the redox state of the cells (Zephy and Ahmad, 2015). Collectively, these data support the role of melatonin in regulating glucose homeostasis. However, more human data are needed as the data derived from nocturnal animals that produce melatonin during their active phase while also consuming calories may not be completely applicable to the diurnal nature of humans.
Our data raise the question if melatonin administration may improve glucose metabolism in patients with prediabetes, a population at risk of developing diabetes. Diet, exercise, and metformin have been proven efficacious in diabetes prevention (Knowler et al., 2002). Interestingly, in a rat model of circadian disruption and diet-induced obesity, treatment with melatonin improved circadian activity rhythms, attenuated induction of β-cell failure, and enhanced glucose tolerance while treatment with metformin alone improve insulin sensitivity and glucose tolerance but had no effects on circadian activity (Thomas et al., 2016). Melatonin and metformin, in combination, worked synergistically and resulted in improved adiposity, circadian activity, and reduced metabolic dysfunction (Thomas et al., 2016). A few studies have also explored the benefits of melatonin in humans. In a study of 30 patients with metabolic syndrome who did not respond to 3-month lifestyle intervention, 5 mg of melatonin two hour before bedtime for two months resulted in significantly improved blood pressure, reduced low density lipoprotein and increased antioxidative defense, while glucose levels did not change (Kozirog et al., 2011). In another study, 44 obese women were randomized to receive 6 mg of melatonin two hour before bedtime or placebo for 40 days. Melatonin was associated with a significant reduction in inflammatory markers, including TNF-α and IL-6, compared to placebo, although glucose levels were not assessed (Mesri et al., 2015). Melatonin is also known to affect sleep, which in turn can affect glucose metabolism. In a randomized, double-blind, crossover study of 36 patients with type 2 diabetes and insomnia, three weeks of prolonged-release melatonin was associated with improved sleep but no changes in glucose levels were observed (Garfinkel et al., 2011). HbA1c levels, however, decreased significantly during the non-randomized extension phase but the reduction was not predicted by sleep improvements. Not all studies demonstrated benefits of melatonin. Acute melatonin administration (1 mg in the morning) in postmenopausal women resulted in reduced glucose tolerance and insulin sensitivity, compared to placebo, as assessed by an oral glucose tolerance test (OGTT) performed 45 minutes after melatonin ingestion (Cagnacci et al., 2001). Similarly, immediate release melatonin (5 mg) administration in healthy volunteer resulted in an increase in glucose response following an OGTT performed 15 min after the ingestion, as compared to placebo, both in the morning and the evening (Rubio-Sastre et al., 2014). Timing of melatonin administration, formulary (immediate vs prolonged release) and duration of usage may play a role in different responses seen from various studies. Moreover, polymorphism in MTNR1B may play a role in the response to melatonin. In a study of 17 normoglycemic women, 5 mg of melatonin administered in the morning resulted in increased AUC glucose in response to OGTT, with the effects seen six times as large in those carrying MTNR1B genetic variant rs10830963 (known to be associated with type 2 diabetes risk) than in non-carriers (Garaulet et al., 2015). In another study of 45 nondiabetic individuals, 23 with the risk allele (GG) of rs10830963 and 22 with non-risk allele (CC), 4 mg of melatonin before bedtime for 3 months resulted in a decrease in first-phase insulin release and an increase in glucose levels following an OGTT (Tuomi et al., 2016). However, the changes were more pronounced in those with GG allele. Interestingly, insulin sensitivity also improved which may have contributed to the lowering of insulin secretion (Tuomi et al., 2016). Therefore, melatonin action may be genotype specific. Future research in larger number of participants, especially those at risk of or with abnormal glucose metabolism, is needed to evaluate the effects of melatonin, possibly as an adjunct to lifestyle modifications, on glucose homeostasis and future diabetes risk.
To our knowledge, this is the first study exploring the relationship between nocturnal secretion of urinary aMT6s and markers of glucose metabolism in patients with prediabetes, along with comprehensive objective sleep assessments. However, there are limitations. We do not have the melatonin level at the time of the OGTT start. This could have influenced the results as melatonin was shown to acutely affect glucose tolerance (Rubio-Sastre et al., 2014, Cagnacci et al., 2001), and that insulin sensitivity worsened the longer melatonin levels remained high after wake time (Eckel et al., 2015). The cross sectional design did not allow us to determine the direction of causality in the associations found. Despite being associated with higher HOMA-IR, lower nocturnal urinary aMT6s was not associated with higher glucose levels. This was in contrast to a previous small study which found that lower urinary aMT6s was related to higher HbA1c levels in patients with type 2 diabetes (Reutrakul et al., 2017). This difference could possibly be due to the narrower range of glucose levels in this patient group, compare to those with type 2 diabetes, and the ability of the β-cell to compensate for insulin resistance as their disease is in its early stage. In contrast to the previous study, we did not find the relationship between sleep variables (sleep duration and efficiency) and nocturnal urinary aMT6s (Saksvik-Lehouillier et al., 2015). The reason for this is unclear but the relatively small number of participants in the current study may play a role. Lastly, sleep variables were obtained using actigraphy and WatchPAT instead of the gold standard polysomnography in this study.
In conclusion, lower nocturnal secretion of urinary aMT6s inversely associated with insulin resistance and insulin levels in patients with prediabetes. These results support the role of melatonin in glucose metabolism.
Conflicts of interest
Authors RS, SS, SC, and TA have nothing to declare. SR reports investigator-initiated grants from Merck Sharp and Dohme, Research equipment support from ResMed, Thailand; and speaker/educational consultant fees from Sanofi Aventis; Medtronic; and Novo Nordisk.
Acknowledgement
Funding: This study was supported in part by a research Grant from Investigator-Initiated Studies Program of Merck Sharp & Dohme Corp, MSIP 0000-349. The opinion expressed in this paper are those of the authors and do not necessarily represent those of Merck Sharp & Dohme Corp.
S.R. researched and analyzed the data, wrote manuscript, contributed to discussion, reviewed/edited manuscript and is the guarantor of this work and, as such, had full access to the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; R.S., S.S., S.C., L.C. and A.T. researched data, reviewed/ edit manuscript.
S.R. receives grant support from Merck Sharp and Dohme, and speaker honoraria from Sanofi, Novo Nordisk and Medtronic. All other authors reported no conflict of interest.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.nbscr.2017.06.001.
Appendix A. Supplementary material
Supplementary material
References
- Agil A., Rosado I., Ruiz R., Figueroa A., Zen N., Fernandez-Vazquez G. Melatonin improves glucose homeostasis in young Zucker diabetic fatty rats. J. Pineal Res. 2012;52:203–210. doi: 10.1111/j.1600-079X.2011.00928.x. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association: Standards of Medical Care in Diabetes, 2017. Diabetes Care 40:S1-S142, 2017. [DOI] [PubMed]
- Anothaisintawee T., Reutrakul S., Van Cauter E., Thakkinstian A. Sleep disturbances compared to traditional risk factors for diabetes development: systematic review and meta-analysis. Sleep Med. Rev. 2015;30:11–24. doi: 10.1016/j.smrv.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Bonnefond A., Clement N., Fawcett K., Yengo L., Vaillant E., Guillaume J.L., Dechaume A., Payne F., Roussel R., Czernichow S., Hercberg S., Hadjadj S., Balkau B., Marre M., Lantieri O., Langenberg C., Bouatia-Naji N., Charpentier G., Vaxillaire M., Rocheleau G., Wareham N.J., Sladek R., McCarthy M.I., Dina C., Barroso I., Jockers R., Froguel P. Rare MTNR1B variants impairing melatonin receptor 1B function contribute to type 2 diabetes. Nat. Genet. 2012;44:297–301. doi: 10.1038/ng.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzezinski A. Melatonin in humans. N. Engl. J. Med. 1997;336:186–195. doi: 10.1056/NEJM199701163360306. [DOI] [PubMed] [Google Scholar]
- Buxton O.M., Cain S.W., O’Connor S.P., Porter J.H., Duffy J.F., Wang W., Czeisler C.A., Shea S.A. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci. Transl. Med. 2012;4:129ra43. doi: 10.1126/scitranslmed.3003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagnacci A., Arangino S., Renzi A., Paoletti A.M., Melis G.B., Cagnacci P., Volpe A. Influence of melatonin administration on glucose tolerance and insulin sensitivity of postmenopausal women. Clin. Endocrinol. 2001;54:339–346. doi: 10.1046/j.1365-2265.2001.01232.x. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Diabetes Report Card, 2014.available at http://www.cdc.gov/diabetes/pdfs/library/diabetesreportcard2014.pdf, accessed 2/1/2017, last updated 7-23-2015.
- Dallmann R., Viola A.U., Tarokh L., Cajochen C., Brown S.A. The human circadian metabolome. Proc. Natl. Acad. Sci USA. 2012;109:2625–2629. doi: 10.1073/pnas.1114410109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel R.H., Depner C.M., Perreault L., Markwald R.R., Smith M.R., McHill A.W., Higgins J., Melanson E.L., Wright K.P., Jr Morning circadian misalignment during short sleep duration impacts insulin sensitivity. Curr. Biol. 2015;25:3004–3010. doi: 10.1016/j.cub.2015.10.011. [DOI] [PubMed] [Google Scholar]
- Faria J.A., Kinote A., Ignacio-Souza L.M., de Araujo T.M., Razolli D.S., Doneda D.L., Paschoal L.B., Lellis-Santos C., Bertolini G.L., Velloso L.A., Bordin S., Anhe G.F. Melatonin acts through MT1/MT2 receptors to activate hypothalamic Akt and suppress hepatic gluconeogenesis in rats. Am. J Physiol. Endocrinol. Metab. 2013;305:E230–E242. doi: 10.1152/ajpendo.00094.2013. [DOI] [PubMed] [Google Scholar]
- Garaulet M., Gomez-Abellan P., Rubio-Sastre P., Madrid J.A., Saxena R., Scheer F.A. Common type 2 diabetes risk variant in MTNR1B worsens the deleterious effect of melatonin on glucose tolerance in humans. Metabolism. 2015;64:1650–1657. doi: 10.1016/j.metabol.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel D., Zorin M., Wainstein J., Matas Z., Laudon M., Zisapel N. Efficacy and safety of prolonged-release melatonin in insomnia patients with diabetes: a randomized, double-blind, crossover study. Diabetes Metab. Syndr. Obes. 2011;4:307–313. doi: 10.2147/DMSO.S23904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Ramsey K.M., Marcheva B., Bass J. Circadian rhythms, sleep, and metabolism. J. Clin. Investig. 2011;121:2133–2141. doi: 10.1172/JCI46043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa A., Ohta Y., Ohashi K. Melatonin improves metabolic syndrome induced by high fructose intake in rats. J. Pineal Res. 2012;52:403–413. doi: 10.1111/j.1600-079X.2011.00955.x. [DOI] [PubMed] [Google Scholar]
- Knowler W.C., Barrett-Connor E., Fowler S.E., Hamman R.F., Lachin J.M., Walker E.A., Nathan D.M. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozirog M., Poliwczak A.R., Duchnowicz P., Koter-Michalak M., Sikora J., Broncel M. Melatonin treatment improves blood pressure, lipid profile, and parameters of oxidative stress in patients with metabolic syndrome. J. Pineal Res. 2011;50:261–266. doi: 10.1111/j.1600-079X.2010.00835.x. [DOI] [PubMed] [Google Scholar]
- Leproult R., Holmback U., Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63:1860–1869. doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M., DeFronzo R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Mauriz J.L., Collado P.S., Veneroso C., Reiter R.J., Gonzalez-Gallego J. A review of the molecular aspects of melatonin’s anti-inflammatory actions: recent insights and new perspectives. J. Pineal Res. 2013;54:1–14. doi: 10.1111/j.1600-079X.2012.01014.x. [DOI] [PubMed] [Google Scholar]
- McMullan C.J., Curhan G.C., Schernhammer E.S., Forman J.P. Association of nocturnal melatonin secretion with insulin resistance in nondiabetic young women. Am. J. Epidemiol. 2013;178:231–238. doi: 10.1093/aje/kws470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullan C.J., Schernhammer E.S., Rimm E.B., Hu F.B., Forman J.P. Melatonin secretion and the incidence of type 2 diabetes. JAMA. 2013;309:1388–1396. doi: 10.1001/jama.2013.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesri A.N., Mahdavi R., Roshanravan N., Lotfi Y.N., Ostadrahimi A.R., Faramarzi E. A double-blind, placebo-controlled trial related to the effects of melatonin on oxidative stress and inflammatory parameters of obese women. Horm. Metab. Res. 2015;47:504–508. doi: 10.1055/s-0034-1384587. [DOI] [PubMed] [Google Scholar]
- Morikawa Y., Nakagawa H., Miura K., Soyama Y., Ishizaki M., Kido T., Naruse Y., Suwazono Y., Nogawa K. Shift work and the risk of diabetes mellitus among Japanese male factory workers. Scand. J. Work Environ. Health. 2005;31:179–183. doi: 10.5271/sjweh.867. [DOI] [PubMed] [Google Scholar]
- Morris C.J., Aeschbach D., Scheer F.A. Circadian system, sleep and endocrinology. Mol. Cell Endocrinol. 2012;349:91–104. doi: 10.1016/j.mce.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C.J., Yang J.N., Garcia J.I., Myers S., Bozzi I., Wang W., Buxton O.M., Shea S.A., Scheer F.A. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc. Natl. Acad. Sci. USA. 2015;112:E2225–E2234. doi: 10.1073/pnas.1418955112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C.J., Purvis T.E., Hu K., Scheer F.A. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc. Natl. Acad. Sci. USA. 2016;113:E1402–E1411. doi: 10.1073/pnas.1516953113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obayashi K., Saeki K., Iwamoto J., Ikada Y., Kurumatani N. Independent associations of exposure to evening light and nocturnal urinary melatonin excretion with diabetes in the elderly. Chronobiol. Int. 2014;31:394–400. doi: 10.3109/07420528.2013.864299. [DOI] [PubMed] [Google Scholar]
- de Oliveira A.C., Andreotti S., Farias T.S., Torres-Leal F.L., de Proenca A.R., Campana A.B., de Souza A.H., Sertie R.A., Carpinelli A.R., Cipolla-Neto J., Lima F.B. Metabolic disorders and adipose tissue insulin responsiveness in neonatally STZ-induced diabetic rats are improved by long-term melatonin treatment. Endocrinology. 2012;153:2178–2188. doi: 10.1210/en.2011-1675. [DOI] [PubMed] [Google Scholar]
- Pan A., Schernhammer E.S., Sun Q., Hu F.B. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med. 2011;8:e1001141. doi: 10.1371/journal.pmed.1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschke E., Muhlbauer E. New evidence for a role of melatonin in glucose regulation. Best Pract. Res. Clin. Endocrinol. Metab. 2010;24:829–841. doi: 10.1016/j.beem.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Peschke E., Bahr I., Muhlbauer E. Melatonin and pancreatic islets: interrelationships between melatonin, insulin and glucagon. Int. J. Mol. Sci. 2013;14:6981–7015. doi: 10.3390/ijms14046981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reutrakul S., Siwasaranond N., Nimitphong H., Saetung S., Chirakalwasan N., Chailurkit L.O., Srijaruskul K., Ongphiphadhanakul B., Thakkinstian A. Associations between nocturnal urinary 6-sulfatoxymelatonin, obstructive sleep apnea severity and glycemic control in type 2 diabetes. Chronobiol. Int. 2017;34:393–402. doi: 10.1080/07420528.2016.1278382. [DOI] [PubMed] [Google Scholar]
- Rubio-Sastre P., Scheer F.A., Gomez-Abellan P., Madrid J.A., Garaulet M. Acute melatonin administration in humans impairs glucose tolerance in both the morning and evening. Sleep. 2014;37:1715–1719. doi: 10.5665/sleep.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksvik-Lehouillier I., Harrison S.L., Marshall L.M., Tranah G.J., Ensrud K., Ancoli-Israel S., Clemons A., Redline S., Stone K.L., Schernhammer E.S. Association of urinary 6-sulfatoxymelatonin (aMT6s) levels and objective and subjective sleep measures in Older men: the MrOS sleep study. J. Gerontol. A Biol. Sci. Med. Sci. 2015;70:1569–1577. doi: 10.1093/gerona/glv088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer F.A., Hilton M.F., Mantzoros C.S., Shea S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci USA. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer H.S., Allen E.W., Herron A.L., Jr., Brennan M.T. Insulin secretion in response to glycemic stimulus: relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J. Clin. Investig. 1967;46:323–335. doi: 10.1172/JCI105534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She M., Hou H., Wang Z., Zhang C., Laudon M., Yin W. Melatonin rescues 3T3-L1 adipocytes from FFA-induced insulin resistance by inhibiting phosphorylation of IRS-1 on Ser307. Biochimie. 2014;103:126–130. doi: 10.1016/j.biochi.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Shieh J.M., Wu H.T., Cheng K.C., Cheng J.T. Melatonin ameliorates high fat diet-induced diabetes and stimulates glycogen synthesis via a PKCzeta-Akt-GSK3beta pathway in hepatic cells. J. Pineal Res. 2009;47:339–344. doi: 10.1111/j.1600-079X.2009.00720.x. [DOI] [PubMed] [Google Scholar]
- Singh B., Saxena A. Surrogate markers of insulin resistance: a review. World J. Diabetes. 2010;1:36–47. doi: 10.4239/wjd.v1.i2.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparso T., Bonnefond A., Andersson E., Bouatia-Naji N., Holmkvist J., Wegner L., Grarup N., Gjesing A.P., Banasik K., Cavalcanti-Proenca C., Marchand M., Vaxillaire M., Charpentier G., Jarvelin M.R., Tichet J., Balkau B., Marre M., Levy-Marchal C., Faerch K., Borch-Johnsen K., Jorgensen T., Madsbad S., Poulsen P., Vaag A., Dina C., Hansen T., Pedersen O., Froguel P. G-allele of intronic rs10830963 in MTNR1B confers increased risk of impaired fasting glycemia and type 2 diabetes through an impaired glucose-stimulated insulin release: studies involving 19,605 Europeans. Diabetes. 2009;58:1450–1456. doi: 10.2337/db08-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwazono Y., Sakata K., Okubo Y., Harada H., Oishi M., Kobayashi E., Uetani M., Kido T., Nogawa K. Long-term longitudinal study on the relationship between alternating shift work and the onset of diabetes mellitus in male Japanese workers. J. Occup. Environ. Med. 2006;48:455–461. doi: 10.1097/01.jom.0000214355.69182.fa. [DOI] [PubMed] [Google Scholar]
- Teodoro B.G., Baraldi F.G., Sampaio I.H., Bomfim L.H., Queiroz A.L., Passos M.A., Carneiro E.M., Alberici L.C., Gomis R., Amaral F.G., Cipolla-Neto J., Araujo M.B., Lima T., Akira U.S., Silveira L.R., Vieira E. Melatonin prevents mitochondrial dysfunction and insulin resistance in rat skeletal muscle. J. Pineal Res. 2014;57:155–167. doi: 10.1111/jpi.12157. [DOI] [PubMed] [Google Scholar]
- Thomas A.P., Hoang J., Vongbunyong K., Nguyen A., Rakshit K., Matveyenko A.V. Administration of melatonin and metformin prevents deleterious effects of circadian disruption and obesity in male rats. Endocrinology. 2016;157:4720–4731. doi: 10.1210/en.2016-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy D., Almgren P., Tuomi T., Groop L. Contribution of insulin-stimulated glucose uptake and basal hepatic insulin sensitivity to surrogate measures of insulin sensitivity. Diabetes Care. 2004;27:2204–2210. doi: 10.2337/diacare.27.9.2204. [DOI] [PubMed] [Google Scholar]
- Tuomi T., Nagorny C.L., Singh P., Bennet H., Yu Q., Alenkvist I., Isomaa B., Ostman B., Soderstrom J., Pesonen A.K., Martikainen S., Raikkonen K., Forsen T., Hakaste L., Almgren P., Storm P., Asplund O., Shcherbina L., Fex M., Fadista J., Tengholm A., Wierup N., Groop L., Mulder H. Increased melatonin signaling is a risk factor for type 2 diabetes. Cell Metab. 2016;23:1067–1077. doi: 10.1016/j.cmet.2016.04.009. [DOI] [PubMed] [Google Scholar]
- Weiss R., Cali A.M., Dziura J., Burgert T.S., Tamborlane W.V., Caprio S. Degree of obesity and glucose allostasis are major effectors of glucose tolerance dynamics in obese youth. Diabetes Care. 2007;30:1845–1850. doi: 10.2337/dc07-0325. [DOI] [PubMed] [Google Scholar]
- Wright K.P., Jr, Drake A.L., Frey D.J., Fleshner M., Desouza C.A., Gronfier C., Czeisler C.A. Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain Behav. Immun. 2015;47:24–34. doi: 10.1016/j.bbi.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuceege M., Firat H., Demir A., Ardic S. Reliability of the Watch-PAT 200 in detecting sleep apnea in highway bus drivers. J. Clin. Sleep Med. 2013;9:339–344. doi: 10.5664/jcsm.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zephy D., Ahmad J. Type 2 diabetes mellitus: role of melatonin and oxidative stress. Diabetes Metab. Syndr. 2015;9:127–131. doi: 10.1016/j.dsx.2014.09.018. [DOI] [PubMed] [Google Scholar]
- Zou D., Grote L., Peker Y., Lindblad U., Hedner J. Validation a portable monitoring device for sleep apnea diagnosis in a population based cohort using synchronized home polysomnography. Sleep. 2006;29:367–374. doi: 10.1093/sleep/29.3.367. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material