Abstract
Improving children’s oral health is a long-standing area of priority and sustained efforts by many stakeholders. Despite these efforts, dental caries, particularly early childhood caries (ECC), persists as a clinical and dental public health problem with multi-level consequences. Despite recent successes in the non-restorative management of dental caries, remarkably little has been done in the domain of ECC prevention. There is promise and expectation that meaningful improvements in early childhood oral health and ECC prevention can be made via the advent of precision medicine in the oral health domain. We posit that precision dentistry, including genomic influences, may be best examined in the context of well-characterized communities (versus convenience clinical samples) and the impact of contextual factors including geography and social disadvantage may be explainable via mechanistic (i.e., biological) research. This notion is aligned with the population approach in precision medicine, which calls for the latter to be predictive, preventive, personalized and participatory. The paper highlights research directions that must be developed for precision dentistry and precision dental public health to be realized. In this context, we describe the rationale, activities and early insights gained from the ZOE 2.0 study—a large-scale, community-based, genetic epidemiologic study of early childhood oral health. We anticipate that this long-term research program will illuminate foundational domains for the advancement of precision dentistry and precision dental public health. Ultimately, this new knowledge can help catalyze the development of effective preventive and therapeutic modalities via actions at the policy, community, family and person level.
Keywords: dental caries, precision medicine, children, genomics
Improving children’s oral health via the advancement of precision health
Improving children’s oral health is a long-standing area of priority and sustained efforts by many stakeholders (1). Despite these efforts, dental caries, particularly early childhood caries (ECC), persists as a clinical and dental public health problem with multi-level consequences (2,3). Important advances have been recently made in the non-restorative management of dental caries; however, remarkably little has been done in the domain of ECC prevention. ECC is best understood as a supragingival biofilm-mediated dysbiotic disease, whereas social, environmental and behavioral determinants, as well as innate susceptibility are known influences on its incidence (4,5). There is promise and expectation that meaningful improvements in early childhood oral health, both in context of public health and clinical practice, can be made via the advent of precision medicine in the oral health domain (6,7).
The cornerstone of precision health is the evolving ability of tailoring care according to people’s lifestyle, environment, and genomic information (8–10). Arguably, to be effective, precision medicine (and dentistry) must be predictive, preventive, personalized and participatory (11). While a comprehensive understanding of the full spectrum of factors underlying oral health and disease at the individual level is key, the consideration of upstream agents and social determinants is warranted (12). In fact, accounting of and operationalizing social and contextual factors, along the lines of the precision public health paradigm (11), is considered a complementary, synergistic approach (13). Although a complete enumeration of all possible, multi-level influences on early childhood oral health and ECC is impractical and unattainable, the overarching principle is clear: precision dentistry, including genomic influences, may be best examined in the context of well-characterized communities (versus convenience clinical samples, or in isolation) and the impact of contextual factors such as geography and social disadvantage may be explainable via mechanistic (i.e., biological) research.
The building blocks of precision oral health in early childhood
The development of precision medicine in pediatric oral health is an ambitious, long-term goal. Arguably, a major limitation in the field is the unavailability of a solid evidence base and high-quality data structures (e.g., sufficiently large, population-based cohorts, with quality phenotype and high-density genotype data, and additional biological and contextual information layers). Without high fidelity and large-scale information, aside from theoretical and conceptual contributions, little progress can be made. We propose that 6 major building blocks are needed to support the development and realization of precision dentistry and precision dental public health in the early childhood oral health domain (Table): phenotype definition and measurement (including the identification of biologically-informed and clinically relatable ECC subtypes); accounting for and operationalization of proximal (e.g., salivary properties and supragingival microbiome) and distal (e.g., environment and community characteristics) influences; leveraging big data; development and utilization of health and disease biomarkers; training of precision health-minded oral health professionals; and advocacy for investments in science, technology, education and policy change.
Table.
Proposed building blocks for the advancement and realization of precision dentistry and precision dental public health in the early childhood oral health domain
| Domain | Details |
|---|---|
| 1. Phenotype definition and measurement | Ideally, dental traits/phenotypes (e.g., early childhood caries) should be measured in a valid and reproducible manner, and robust to misclassification. Case definitions for use in clinical, public health, and research settings may differ, but should be valid representations of the underlying disease processes. Biologically-informed disease definitions and disease subtypes are likely to emerge. |
| 2. Accounting for and operationalization of distal and proximal influences | Social determinants of health and other ‘distal’ influences should be explicitly considered and measured rigorously—similar to important biological mediators and proximal influences. For example, supragingival biofilm features (e.g., transcriptome or metabolome) may provide promising and readily-measurable endophenotypes for dental caries. |
| 3. Leveraging of big data | There is enormous potential and novel insights to be gained from leveraging large, administrative datasets or biobanks combining phenotype or proxy-oral health information and genotype data. Although not benefitting in terms of precise measurement, these data structures can be used for the generation of hypotheses or combined with mechanistic or biologically-informed studies for replication or validation of findings. |
| 4. Discovery, validation and application of health and disease biomarkers | The generation of multiple omics data structures and candidate biomarkers is commonplace—the integration of different omics data structures (e.g., metagenomics, metatranscriptomics, metabolomics, proteomics) and the prospective validation (i.e., incident or progressive disease) of such biomarkers, is a tall order but also the logical next step. Ability to measure them in non-invasive and continuous (e.g., live) manners would be desirable. |
| 5. Training of precision-minded oral health professionals | Oral health professionals could be a limiting factor for the paradigm shifts that need to take place for the realization of precision health care, unless they adopt evidence-based dentistry principles and develop an agility for the adoption of new practice principles and philosophies. |
| 6. Advocacy for investments in science, technology, education and policy change | Considerable investments at all levels (e.g., support of the best science and technology, development of new investigators, training/education of the next generations, and development of advocacy for policy change) are necessary for discovery, translation and implementation of precision dentistry and precision dental public health. |
The ZOE 2.0 study
Overview and data domains collected
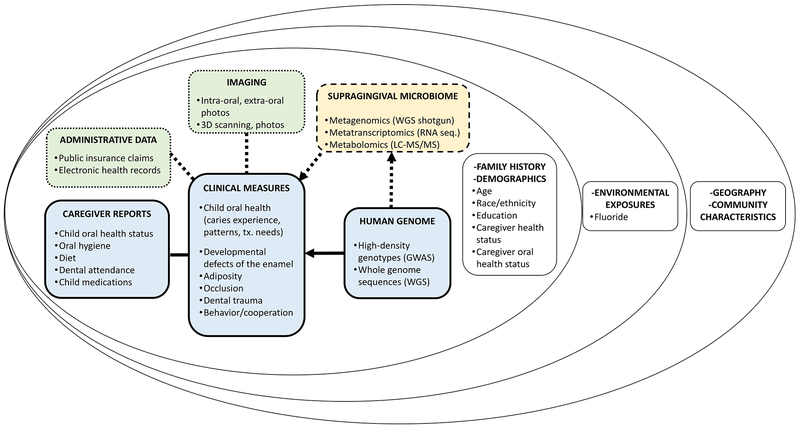
ZOE 2.0 is an NIH-funded genetic epidemiologic study of early childhood oral health, conducted among a large community-based sample of preschool-age children enrolled in Head Start centers in North Carolina. The study has been approved by the Institutional Review Board of UNC-Chapel Hill (#14–1992). It is designed as a precision oral health cohort and includes the collection of a wide array of information domains (Figure 1) ranging from the most proximal (e.g., human genome and microbiome) to the most distal (e.g., geographical location and community characteristics). The primary goal of the current phase of the study is the conduct of a trans-ethnic genome-wide association study (GWAS) of ECC. Sampling across all Head Start programs in North Carolina is based upon a probability proportional to size scheme. A pilot GWAS of ECC, demonstrating approximately 40% heritability in the measured trait was recently reported by Ballantine and colleagues (4).
Figure 1.
Overview of multi-level data domains and information collection in the ZOE 2.0 study, including the person and family level (inner circle) and the community level (middle and outer circles). Boxes with solid outline represent currently collected data, dash outline patterns represent bio-banked (not currently analyzed) biological samples (e.g., supragingival microbiome) and dotted outline patterns represent envisaged future additions to the protocol (e.g., administrative data/insurance claims and intra-oral imaging).
The central operational vehicle for ZOE 2.0 is the conduct of comprehensive clinical-dental characterizations of participating children (mainly ages 3 and 4, few age 5), including surface-level dental caries experience measurement using ICDAS criteria by trained and calibrated dental examiners (14). Additional clinical measures collected include tooth-level developmental defects of the enamel, occlusal characteristics, dental trauma, behavior/cooperation during the dental examination, and adiposity. Saliva is collected for human DNA extraction and subsequent genomic analyses (15). Two supragingival biofilm plaques are collected and stored for future metagenomic, metatranscriptomic and metabolomics investigations (16). Additional, caregiver-reported data are collected via questionnaires on perceived (oral) health statuses, oral hygiene, diet, dental attendance (e.g., dental visit frequency and reasons), receipt of medications, as well as demographic information and caregivers’ educational attainment.
Administrative data (e.g., public insurance, Medicaid claims) is one domain examined for possible inclusion in future protocol amendments, as this information can usually illuminate the ‘lifetime’ of oral health care received, including the timing and type of specific procedures (e.g., fluoride varnish applications, dental restorations, visits at the emergency room, etc.). Intraoral and extraoral imaging is an additional possible future addition to the study protocol—we posit such images or scans (two- or three-dimensional) may help create additional traits or phenotypes of interest and can serve as the basis for the validation of tele-dentistry applications (e.g., remote oral health screening) among this age group.
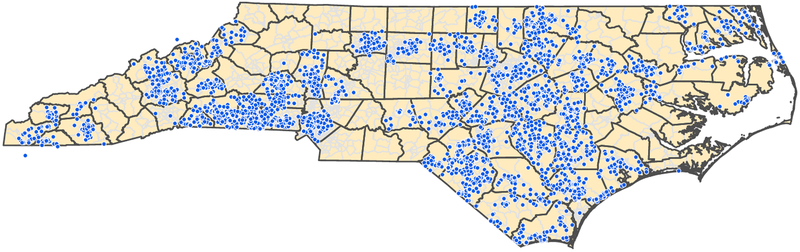
Participating families are invited to provide a home water sample that the team, in collaboration with the North Carolina State Laboratory of Public Health, is using to measure fluoride concentration. Additional contextual and community-level information is gained via geographic information systems (GIS; geocoding) of participants’ residential addresses (Figure 2). There is a wealth of census tract and county-level information (e.g., social vulnerability, area-level resources) that can be overlaid onto the person-level study outcomes. We support that this ecological perspective on early childhood oral health, and an explicit consideration of ‘place’ (17), places research findings, inference and messaging one step closer to ‘real life’ and people—frequently, health and disease cluster within communities—de-emphasizing individual ‘risk factor’ narratives. Moreover, ecological approaches can provide actionable leads about areas, communities and groups that should be prioritized for resource allocation and interventions.
Figure 2.
Geographical distribution of enrolled participants in the ZOE 2.0 study in North Carolina. The study is ongoing--as of October 2018, approximately 7,000 participants (children ages 3 and 4, and few age 5) had been enrolled and 4,600 had completed the study’s clinical examination and biospecimen collection protocol.
Early outcome insights and lessons learned
It is anticipated that data collection in ZOE 2.0 will continue through early spring of 2019 and for this reason, primary and secondary outcomes will be reported between 2019 and 2020. During 2020, we anticipate obtaining human genome data (i.e., high-density genotype data from a ‘multi-ethnic’ content array for the entire cohort and likely whole genome sequence data on 10% of the cohort, representing the most severe ECC cases) and conducting genetic association analyses. In the meantime, we have identified that over 50% of participants are ECC cases, when defined at the ICDAS≥3 threshold. Approximately 20% of participants are classified as overweight or obese based on their body mass index percentile (adjusted for age and sex) and parental education attainment appears to be a strong collate of children’s adiposity. Most children report having a dental home, and such reports are associated with favorable oral health behaviors (e.g., oral hygiene). Our pilot studies have demonstrated that the study’s clinical protocol and field operations generate high-fidelity human genome and supragingival microbiome data, that can be carried forward to high-throughput analyses.
The first major lesson learned from the ZOE line of research is the upfront time investment required to design, build and fine-tune all operations and logistics of a large-scale, community-based, clinical and genetic-epidemiologic investigation. ZOE 2.0 evolved from a smaller scale community-based oral epidemiologic study (ZOE; Principal Investigator Dr. Gary Rozier) conducted among Early Head Start programs in NC (18). Nevertheless, we estimate that it took approximately 5 years to fine-tune the clinical protocol and manual of operations, recruit and train all study staff and go through inevitable learning curves. The second major lesson learned is related to the development of meaningful, mutually beneficial partnerships with community stakeholders (e.g., Head Start Programs/Centers and other community agents). We support that considerable time and effort investments are necessary to appreciate the real life conditions, challenges and needs of each early childhood education institution that our team has partnered and collaborated with. Building lasting relationships and listening closely have helped inform our research agenda and likely increase our team’s ability to give back in a meaningful way to participants, their families and their educators. Finally, we have been made aware of the immediate positive benefits of providing preschool-age children with a positive ‘dental experience’ via the study’s research examinations. Our community partners have expressed their appreciation for the facts that the team visits the children “in their own environment”, creates a “safe and comfortable” environment, works “with their schedule”, families received “incentives and dental supplies”, had staff members that “spoke to children in their language”.
Anticipated impact and future directions
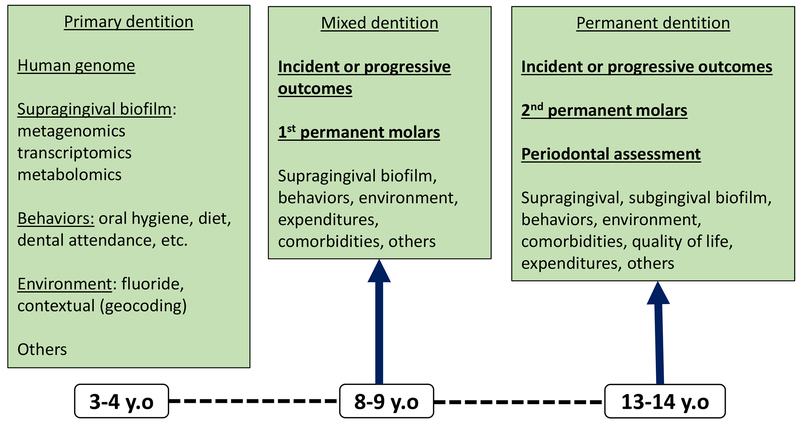
We anticipate that the current research program will offer novel molecular, clinical, behavioral and contextual insights into early childhood oral health and ECC. We expect that this new knowledge will help inform the development of precision oral health and care, including precision dental public health. It is envisioned that prospective investigations building upon the ZOE 2.0 cohort (Figure 3) will generate valuable new information on the incidence and progression of dental caries and other clinical and non-clinical endpoints in childhood and adolescence. Moreover, such longitudinal study designs offer opportunities to validate biomarkers collected at baseline (e.g., oral microbiome features) and identify distinct patterns and longitudinal trajectories of dental disease. Taken together, this rich information can aid in the identification and characterization of biologically-informed disease sub-types that will aid in caries risk assessment, outcome prediction and ultimately better oral health and care.
Figure 3.
Data domains targeted for collection in envisioned prospective investigations (e.g., follow-up) building upon the ZOE 2.0 cohort, focusing on the primary (ZOE 2.0; mainly ages 3–4), mixed (ages 8–9) and permanent (ages 13–14) dentitions.
Acknowledgments:
Research supported by grant from the National Institute of Dental and Craniofacial Research NIDCR Grant #U01DE025046
Footnotes
Disclaimers: The views expressed in the article are those of the authors and do not necessarily reflect the views of the National Institute of Dental and Craniofacial Research or the University of North Carolina at Chapel Hill
Disclosures: None of the authors has any financial interest related to the article.
References
- 1.Casamassimo PS, Lee JY, Marazita ML, Milgrom P, Chi DL, Divaris K. Improving children’s oral health: an interdisciplinary research framework. J Dent Res. 2014;93(10):938–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleming E, Afful J. Prevalence of Total and Untreated Dental Caries Among Youth: United States, 2015–2016. NCHS Data Brief. 2018;(307):1–8. [PubMed] [Google Scholar]
- 3.Casamassimo PS, Thikkurissy S, Edelstein BL, Maiorini E. Beyond the dmft: the human and economic cost of early childhood caries. J Am Dent Assoc. 2009;140(6):650–7. [DOI] [PubMed] [Google Scholar]
- 4.Ballantine J, Carlson JC, Zandona A, Agler CS, Zeldin LP, Rozier G, Roberts MW, Basta PV, Luo J, Antonio-Obese ME, McNeil D, Weyant R, Crout RJ, Slayton R, Levy S, Shaffer JR, Marazita ML, North KE, Divaris K. Exploring the genomic basis of early childhood caries: a pilot study. Int J Paed Dentistry. 2018;28(2):217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Divaris K Predicting caries outcomes among children: a “risky” concept. J Dent Res. 2016;95(3):248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Divaris K Precision dentistry in early childhood: the central role of genomics. Dent Clin North Am. 2017;61(3):619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Divaris K Fundamentals of precision medicine. Compend Contin Educ Dent. 2017;38(Spec Iss 2):31–33. [PMC free article] [PubMed] [Google Scholar]
- 8.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372(9):793–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dzau VJ, Ginsburg GS. Realizing the Full Potential of Precision Medicine in Health and Health Care. JAMA. 2016;316(16):1659–60. [DOI] [PubMed] [Google Scholar]
- 10.Ashley EA. Towards precision medicine. Nat Rev Genet. 2016;17(9):507–52. [DOI] [PubMed] [Google Scholar]
- 11.Khoury MJ, Gwinn ML, Glasgow RE, Kramer BS. A population approach to precision medicine. Am J Prev Med. 2012;42(6):639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JY, Divaris K. The ethical imperative of addressing oral health disparities: a unifying framework. J Dent Res. 2014;93(3):224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor-Robinson D, Kee F. Precision public health-the Emperor’s new clothes. Int J Epidemiol. 2018. Sep 12. doi: 10.1093/ije/dyy184. Forthcoming [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginnis J, Ferreira Zandoná AG, Slade GD, Cantrell J, Antonio ME, Pahel BT, Meyer BD, Divaris K. Measurement of early childhood oral health for research purposes: dental caries experience and developmental defects of the enamel in the primary dentition. Methods Mol Biol. Forthcoming [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agler CS, Shungin D, Ferreira Zandoná AG, Basta PV, Luo J, Cantrell J, Pahel TD Jr., Meyer BD, Shaffer JR, Schäefer AS, North KE, Divaris K. Protocols, methods and tools for genome-wide association studies (GWAS) of dental traits. Methods Mol Biol. Forthcoming [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Divaris K, Shungin D, Basta PV, Rodriguez-Cortes A, Roach J, Wu D, Cho H, Ferreira Zandoná AG, Ginnis J, Ramamoorthy S, Kwintkiewicz J, Butz N, Ribeiro AA, Azcarate-Peril MA. The supragingival biofilm in early childhood caries: clinical and laboratory protocols and bioinformatics pipelines supporting oral metagenomics, metatranscriptomics and metabolomics studies of the oral microbiome. Methods Mol Biol. Forthcoming [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cummins S, Curtis S, Diez-Roux AV, Macintyre S. Understanding and representing ‘place’ in health research: a relational approach. Soc Sci Med. 2007;65(9):1825–38. [DOI] [PubMed] [Google Scholar]
- 18.Born CD, Divaris K, Zeldin LP, Rozier RG. Caregiver- and child-level influences on young children’s oral health-related quality of life. J Public Health Dent. 2016;76(4):276–86. [DOI] [PubMed] [Google Scholar]