Abstract
Background
Despite the most recent surgical aids and tools, surgical removal of infiltrating brain tumors remains a challenge. Unclear margins, edematous areas, and infiltrative behavior are the main causes for failing gross total removals. Also, excessive resection of peri-tumoral tissue often carries risks of damaging the nearby functioning cortical and subcortical structures with an unacceptable decrease in patient's quality of life and postoperative functional status, and the risk of making patients not eligible to adjuvant treatments. Awake surgery and intraoperative magnetic resonance imaging (ioMRI) are among the most effective aids in preventing damage to functional brain while maximizing the extent of resection.
Methods
We present our series of 46 patients operated on at Southmead Hospital (North Bristol NHS Trust) in between July 2014 and February 2017 using ioMRI plus or minus awake surgery. Setting, patient features, indications, type and size of tumors, surgical times, extent of resection, morbidity, and survival are analyzed and discussed.
Results
Overall, ioMRI check led to a +43% resections in Group 1 and +58% in Group 2. In grade 2 tumors, GTR was 46% in Group 1 and 55% in Group 2 (41% in control group). In grade 3 tumors, GTR was 57% in Group 1 and 66% in Group 2 (30% in control group). In Grade 4 tumors, GTR was 63% in Group 1, 66% in Group 2 (36% in control group). In terms of theatre occupation, the use of ioMRI added 1/2 operative session; the addition of awake surgery implied the use of another 1/2 operative session. Morbidity did not differ among the groups, with low incidence of permanent post-operative deficits (<5%). Group 2 OS was statistically longer when compared to the control group.
Conclusions
Using ioMRI together with awake surgery is demanding for the anesthetic team, staff nurses, and for the patient. Nevertheless, low morbidity, greater total resections rates, and longer survival suggest its use is effective in making more approachable gliomas of all grades that we would consider “complex” due to their intrinsic features or locations.
Key words: Awake surgery, Glioma, ioMRI, Neuro-oncology, Survival, Volumetric analysis
Abbreviations and Acronyms: 5-ALA, 5-Aminolevulinic acid; EOR, Extent of resection; FLAIR, Fluid-attenuated inversion recovery; GBM, Glioblastoma multiforme; GTR, Gross total resection; HGG, High-grade glioma; ioMRI, Intraoperative magnetic resonance imaging; LGG, Low-grade glioma; MAC, Monitored anesthesia care; OS, Overall survival; PFS, Progression-free survival; PR, Partial resection; PS, Performance Status; WHO, World Health Organization
Introduction
Gliomas greater than World Health Organization (WHO) grade 1 are still incurable. Nevertheless, improvements in progression-free survival (PFS) and overall survival (OS) have been achieved over the last 20 years thanks to a combination of more precise diagnoses, individualized oncologic management, and the use of cutting-edge technologies for assisting resections.1, 2, 3, 4, 5, 6 Despite individual, histologic, and genetic variables, surgery is still the first independent factor for OS and PFS in patients with both high-grade gliomas (HGGs) and low-grade gliomas (LGGs)4, 6, 7, 8, 9, 10: in fact, only patients with >90% resections will have significantly longer survivals.2
The obvious risk with resections in the brain is the potential damage to functioning nervous tissue, leading to neurologic morbidity. This is particularly true when tumors are near so-called “eloquent areas” defined as “brain areas whose damage/removal will result in loss of any of sensory processing, linguistic ability, or motor function.”2, 4 Progress in the understanding of how our brain works has replaced the classical brain locationist theory with a more dynamic web and hub-based concept of connectome.11, 12, 13, 14 The idea of eloquent areas becomes very loose in this context, stretching the boundaries of dangerous zones to pretty much the whole brain. Nevertheless, it is an unalterable and permanent fact that certain areas are more critical than others for specific functions: that's what we strictly call eloquent tissue. These are the portions of brain we can currently monitor during surgery because their dysfunction will cause significant changes in patient's functions and behavior.
Ideally, margin detection ability should go beyond our senses (mainly vision and touch) through a hypothetical real-time, high-definition, easy, cheap, and fast tool. In parallel, we'd need a similarly perfect method to monitor for any early failure of brain function. To make the issue even more complex, we still don't have enough data to fully understand the potential of recovery of many higher cognitive functions and to know whether and how much brain plasticity will compensate for them.4, 13, 14, 15 Nowadays, although far from the ideal scenario, we have very effective aids for morphologic tumor assessment (segmentation tools, neuronavigation, intraoperative magnetic resonance imaging [ioMRI], ultrasonography, dye-guided resections, the experimental confocal microscopy) and for functional assessment (neurophysiology and awake surgery).
Multiple series from the literature show that ioMRI is the most accurate tool for margin assessment, allowing total resections in about two-thirds of cases both in high- and low-grade tumor.16, 17 Neurophysiological monitoring on its own has proven vital in reducing postoperative morbidity while allowing resections as high as 75%.16 The use of awake surgery alone has enabled surgeons to achieve total resections in selected low-grade tumors.4
Backed by pioneering experiences from other institutions,18, 19, 20 we used a combination of 2 of the most effective tools available, ioMRI and awake surgery, in addition to the standard image-guided surgery and neurophysiology monitoring. We report the results of our series of 46 patients.
Materials and Methods
This study was undertaken at Southmead Hospital North Bristol NHS Trust, which is a tertiary care center in Bristol, United Kingdom, catering to a neurosurgical population of roughly 3 million adults and undertaking around 200 glioma surgeries a year. The hospital is equipped with a 2-room solution BrainSUITE (Brainlab AG, Munich, Germany) and an Ingenia 1.5T MRI machine (Philips, Andover, Massachusetts, USA). Forty-six patients underwent surgery for gliomas assisted by ioMRI between July 2014 and February 2017. These were retrospectively separated into 2 groups. Group 1 received surgery under general anesthesia. Neurophysiology monitoring also was used in 11 of these tumors, due to vicinity to motor areas. Group 2 went through awake surgery. Sixteen patients had tumors nearby/in the broadly defined language networks.21 In 10 patients, neurophysiology monitoring also was used because of the concomitant involvement/vicinity to motor areas.
A control arm (Group 3) of 46 patients was selected from the larger pool of all patients with gliomas who were operated on in the same period, using standard neuronavigation in all cases and neurophysiology for tumors nearby motor areas (23 patients). The control group was matched to Group 1 and 2 for WHO grade, location, and tumor volume; the same number of patients with similar history of recurrence also were selected to balance the recurrent cases treated in Group 1 and 2. Exclusion criteria for control group were American Society of Anesthesiologists physical class (anesthetic physical status assessment) greater or equal to grade III.
Irregular shape/diffuse behavior, patchy contrast, or noncontrast-enhancing lesions with cloudy T2/fluid-attenuated inversion recovery (FLAIR) margins were the main inclusion criteria for the use of ioMRI. Tumor location nearby eloquent areas, minimal dysphasia, body mass index <35, motivated patient with good tolerance to pain, and surgeon's preferences were the most important inclusion criteria for using the awake surgery in combination with ioMRI.
Exclusion criteria for the awake surgery were patients with body mass index >35, obstructive sleep apnea, preoperative assessment of difficult intubation, active acute or chronic cough, heart failure and orthopnea, symptomatic arrhythmias, poorly controlled seizures despite antiepileptic drugs, significant dural involvement (painful), altered mental status or severe dysphasia, language barrier; patients going into the prone position; not able to tolerate the standard preoperative MRI investigations; or with an unfavorable psychological profile (anxiety and phobias, agitation, unmotivated, etc.).
Pre- and postoperative MRI scan were performed either with an Ingenia 1.5T or Ingenia Elition 3.0T MRI machine (Philips). The preoperative scans were obtained within 1 week from surgery and the postoperative scan within 72 hours.
The MRI used for the intraoperative scan (Philips Ingenia 1.5T) is dedicated to neurosurgery but is not restricted to intraoperative use only. The room is accessible through a double door from the theater. A special surgical bed enables us to slide the patient to an MRI-compatible trolley and from there to the MRI bed. All ioMRIs were performed when the surgeon assumed a total resection was achieved or when doubts existed about the nature of a tissue in contact with eloquent or deep areas, as navigation or cortical/subcortical stimulation for language or motor could have shown.
Patient MRIs were retrospectively collected and independently analyzed by 2 senior neurosurgeons and a consultant neuroradiologist. Contrast-enhanced volume T1 were used to calculate the tumor volumes in HGGs, whereas abnormalities in the volume FLAIR sequences were considered for the volumetric assessment of LGGs. Volumetric analysis has been performed using pre- and postcontrast volume T1 and T2/FLAIR MRI and highlighting the pathologic tissue on the 3 orthogonal planes using the Fujitsu 3D Synapse Advanced Visualisation Software (Fujifilm, Valhalla, New York, USA), which enabled us to obtain 3-dimensional objects of the tumors with their volume automatically calculated in milliliters. Pre-, intra-, and postoperative tumor volumes were analyzed and the extent of resections (EORs) calculated accordingly.
Some controversies exist regarding the definition of EOR.2, 6, 22 For HGG, we considered gross total resection (GTR) as a 98% reduction of original tumor volume on T1-MRI with contrast; subtotal resection as removal between 90% and 98% resection; and anything less than 90% was considered partial resection (PR); for LGG, we considered the same thresholds using a FLAIR-MRI sequence. In case of residuals, we also reported their absolute volume values. The absolute residual volume has been described as an equally powerful prognostic predictor, with 5 mL being considered the minimum surgical goal to benefit from longer OS and PFS in HGG2 and 15 mL for LGG.23
The Pearson χ2 test was used for univariate in-between analyzing significance in age, tumor volume, and remnant. The histology of patients operated on before 2016 was revised to update the diagnoses according to the most recent WHO classification24: this was easily done because of our neuropathologists' habit to perform genetic/molecular assessment before 2016.
Operative procedure and anesthetic times were recorded from theater logs and plotted graphically. We define “1 session” as a 4-hour time of theater occupancy, which is the UK standard. Rather than only the skin-to-skin time, we also considered the total in-theater stay, which better reflects the burden of the whole procedure on the departmental finances, also considering that preanesthesia starts directly in theater, in our Department. We extracted the net operative time removing the time spent for the ioMRI preparation, acquisition, and transfers from the skin-to-skin time: this represent the actual time spent for operating. The Student t test was used for assessing the differences in operative times.
Preoperative, immediate postoperative, and follow-up WHO performance status (PS) were recorded from the 3 groups of patients. We defined “permanent postoperative deficits” as those that were still present at 3-month follow-up.
A recurrence was defined as the interval between the date of surgery and the first evidence of radiologic progression (increase bulk on T2/FLAIR or increase/new contrast enhancement on T1). PFS and OS were projected as Kaplan–Meyer curves: considering the 3 groups are balanced and matched for WHO grade, we only compared the groups and not the grades, to make the sample bigger and give more power to the statistics. We also excluded all patients initially presenting with a recurrent tumor, for a more accurate analysis: 4 patients from Group 1, 2 from Group 2, and 6 from the control group. The log-rank test has been used for statistical significance.
Details of the Awake Surgery Technique
Both asleep–awake–asleep and monitored anesthesia care (MAC) are used, according to co-morbidities or patient's will and psychological profile. Patients are counseled about expectations at each stage of the procedure by a speech and language therapist. Induction starts in the operative theater. A first venous line is inserted and antiemetics and dexamethasone are given. A light sedation also is achieved with propofol/remifentanil or dexmedetomidine, titratable, and readily reversible agents. The surgeon performs local blocks on the facial and scalp sensory nerves and on the point selected for the Mayfield insertion. A second line is started, a Foley catheter is inserted, antibiotics and seizure prophylaxis are given, and neurophysiology monitoring setup is completed. Patient's sedation is weaned off for positioning on bed. Possible airway-related complications can be managed by optimizing the position at the beginning of the operation. Lateral positioning with the head on the same axis of the body allows optimal access to airways, while maintaining a good visual interaction during the intraoperative verbal/motor tasks. Patients are kept fully awake during the positioning to get feedback on how comfortable they are, in particular regarding the extension of the head, as nausea can be triggered by the throat/pharynx being in awkward/angled positions and minimal changes in head position can often make the difference.
A vacuum mattress (Vacuform 2.0 surgical mattress; B.u.W. Schmidt GmbH, Garbsen, Germany) is used for optimal positioning and body support without excessive and intricate strapping techniques and consequent greater comfort: the mattress is particularly useful for lateral or semilateral positions. Intraoperative seizures are generally well managed by immediately pouring ice-cold water on the brain, by using the “seizure disruption function” on our cortical stimulator if the seizure happens during the cortical mapping, and/or by anesthetic actions. The major part of psychological contraindication to the awake surgery can be partly spotted in the preoperative psychological assessment: distress, agitation, anxiety, terror, and lack of compliance are also detrimental to the assessment, as they compromise the quality of the mapping or can lead to a wrong interpretation of changes in patient's performances. Patient are sedated until the dura is open: this allows a faster exposure, prevents patient's exhaustion, and enhance his/her compliance.
Reinforcement of local anesthesia with topic Bupivacaine 0.25% is also performed before manipulating deep allogenic structures (temporal muscle, pericranium, dura). The awakening process is actually started during the craniotomy. Cotton strips soaked with Bupivacaine 0.25% are applied for 5 minutes on the dura, which is then opened. After the mapping is complete, patients are kept awake during the resection, with the surgeon trying as much as possible to remove the tumor nearby the language networks first, to re-sedate the patient as soon as the monitoring is no longer needed.
Results
Details of pre-, intra-, and postoperative tumor volumes and EOR are summarized in Table 1. Table 2 contains more detailed information regarding EOR, PFS, and OS of patients where GTR was not achieved. Overall, further resections after the ioMRI check led to a +43% resection in Group 1 and +58% in Group 2.
Table 1.
Patient Demographics, Volumetric Data, EOR, and Operating Times
| Demographics | Histology (No. Patients, %) | Average V (SD) | Average ioV(SD) | Patients with Residual at ioMRI | Average rV and % of Further Resection | Final EOR (%) | |
|---|---|---|---|---|---|---|---|
| ioMRI in GA Group 1 |
26 patients: 15 M, 11 F Avg. age 44 years (SD 16 years) Frontal 10, Temporal 3, Parietal 5, Insular 4, Occipital 2, Cerebellar 2 |
Grade 2 glioma (11/26, 42%) | 45.3 mL (34 mL) | 14.9 mL (20.5 mL) | 73% | 9.8 mL (15 mL) (+34.3%) |
GTR 46%, STR 18% PR 36% |
| Grade 3 glioma (7/26, 27%) | 40.3 mL (32 mL) | 3 mL (3 mL) | 71% | 0.8 mL (1 ml) (+73.3%) |
GTR 57% STR 43% PR 0% |
||
| Grade 4 glioma (8/26, 31%) | 26 mL (26 mL) | 1.7 mL (3 mL) | 38% | 1.2 mL (2 mL) (+29.5%) |
GTR 63% STR 37% PR 0% |
||
| ioMRI awake surgery Group 2 |
20 patients: 14 M, 6 F Avg. age 46 years (SD 14 years) Frontal 11, Temporal 5, Insular 4, (Language areas 10, motor cortex 4, both systems 6) |
Grade 2 glioma (11/20, 55%) | 55.8 mL (51 mL) | 9.6 mL (14 mL) | 91% | 5.3 mL (5 mL) (+44.8%) |
GTR 55% STR 0% PR 45% |
| Grade 3 glioma (3/20, 15%) | 63.2 mL (34 mL) | 7.5 mL (6.5 mL) | 100% | 2.4 mL (4 mL) (+68%) |
GTR 66% STR 33% PR 0% |
||
| Grade 4 glioma (6/20, 30%) | 47 mL (38 mL) | 10 mL (8 mL) | 100% | 2.3 mL (7 mL) (+77%) |
GTR 66% STR 17% PR 17% |
||
| Control Group 3 |
46 patients: 29 M, 17 F Avg. age 47 years (SD 16 years) Frontal 21, Temporal 8, Parietal 5, Insular 8, Occipital 2, Cerebellar 2 |
Grade 2 glioma (22/46, 48%) | 61 mL (48 mL) | 16 mL (20 mL) | GTR 41% STR 0% PR 59% |
||
| Grade 3 glioma (10/46, 22%) | 38 mL (23 mL) | 6.6 mL (10 mL) | GTR 30% STR 30% PR 40% |
||||
| Grade 4 glioma (14/46, 30%) | 48.6 mL (29 mL) | 2.7 mL (4.5 mL) | GTR 36% STR 50% PR 14% |
SDs are in parentheses.
Further resection represents how much tumor could be removed after the ioMRI and is calculated with the formula: rV/ioV.
EOR, extent of resection; V, initial volume; SD, standard deviation; ioV, average residual tumor at the ioMRI; ioMRI, intraoperative magnetic resonance imaging; rV, average residual tumor visible at the postoperative MRI; GA, general anesthesia; M, male; F, female; GTR, gross total resection (>98% resection); STR, subtotal resection (>90% resection); PR, partial resection (<90%).
Table 2.
Volumetric Details and Outcomes of Patients with Incomplete Resections
| Grade | Group | Number of Patients | IR (Patients) (<98%) | Residual Volume | Recurrences | Deaths |
|---|---|---|---|---|---|---|
| >15 mL: | ||||||
| Grade 2 | 1. ioMRI, GA | 11 | 54% (6/11) | 18% (2/11) | IR: 2/6 (33%) GTR: 0/5 (0%) |
IR: 0/4 (0%) GTR: 0/7 (0%) |
| 2. ioMRI, awake | 11 | 45% (5/11) | 9% (1/11) | IR: 3/5 (60%) GTR: 1/6 (17%) |
IR: 0/5 (0%) GTR: 0/6 (0%) |
|
| 3. Control | 22 | 59% (13/22) | 18% (4/22) | IR: 7/13 (53.8%) GTR: 0/9 (0%) |
IR: 2/13 (15%) GTR: 0/9 (0%) |
|
| >5 mL | ||||||
| Grade 3 | 1. ioMRI, GA | 7 | 43% (3/7) | 0% (0/7) | IR: 0/3 (0%) GTR: 1/4 (25%) |
IR: 0/3 (0%) GTR 0/4 (0%) |
| 2. ioMRI, awake | 3 | 33% (1/3) | 33% (1/3) | IR: 0/1 (0%) GTR: 1/2 (50%) |
IR: 0/1 GTR: 1/2 (50%) |
|
| 3. Control | 10 | 73% (7/10) | 50% (5/10) | IR: 3/7 (43%) GTR: 0/5 (0%) |
IR: 3/7 (43%) GTR: 0/5 (0%) |
|
| >5 mL | ||||||
| Grade 4 | 1. ioMRI, GA | 8 | 37% (3/8) | 12% (1/8) | IR: 3/3 (100%) GTR: 4/5 (80%) |
IR: 3/3 (100%) GTR: 4/5 (80%) |
| 2. ioMRI, awake | 6 | 34% (2/6) | 17% (1/6) | IR: 2/2 (100%) GTR: 3/4 (75%) |
IR: 2/2 (100%) GTR: 0/4 (0%) |
|
| 3. Control | 14 | 64% (9/14) | 14% (2/14) | IR: 9/9 (100%) GTR: 3/5 (60%) |
IR: 9/9 (100%) GTR: 4/5 (80%) |
P < 0.05 are indicated in bold.
IR, incomplete resection; ioMRI, intraoperative magnetic resonance imaging; GA, general anesthesia; GTR, gross total resection.
Grade 2
Patients with grade 2 tumors had better GTR when surgery was performed awake with ioMRI (Group 2) as compared with Group 1 and control (P < 0.05). In patients with incomplete resections, residuals were significantly smaller in Group 2 (awake surgery with ioMRI) when compared with both Group 1 and control (P < 0.05).
The use of ioMRI in combination with awake (Group 2) resulted in greater GTR rates (55% vs. 46% in Group 1 and 41% in Group 3). Residuals were smaller than 15 mL in 91% (compared with 82% of the other groups) and the average postoperative volume was the lowest of the 3 groups: 5.3 mL, or 9% of the original mass.
ioMRI enabled further resections: +34.3%/5.1 mL (Group 1) and +44.8%/4.3 mL (Group 2). Smaller ioMRI residuals were detectable in Group 2 when compared with Group 1, probably because surgery proceeded more confidently through the margins thanks to the real-time monitoring, until the pathologic tissue was hardly distinguishable or getting too near to areas causing intraoperative symptoms.
The major part of progressions was represented by an increase in volume at the surveillance scans, except in 2 patients in Group 2 (at 7 and 24, months respectively) and 3 in Group 3 (6, 24, and 24 months, respectively) were tumors substantially changed features.
In Group 1, both the patients with progressions underwent an incomplete resection with more than 15 mL of residual tumor. All of them are alive at an average follow-up of 28 months.
Interestingly, in Group 2, the only patient with recurrence (and transformation to glioblastoma multiforme [GBM] after 7 months) had a grade 2 noncontrast-enhancing left frontal astrocytoma (IDH1+, methylated O6-methylguanine-DNA methyltransferase, albeit preservation of ATRX) and residual tumor was 1 mL (EOR 98%).
Although there was no mortality in Groups 1 and 2 at latest follow-up (average 28 months), 2 patients in Group 3 died from progression (average follow-up: 25 months): one had a partially removed right frontal–temporal–insular IDH1– wild type, unmethylated O6-methylguanine-DNA methyltransferase with preservation of ATRX astrocytoma (preoperative volume 95 mL, postoperative volume 36 mL EOR 63%); the other was a partially resected extensive oligodendroglioma (preoperative volume 147 mL, postoperative volume 29 mL EOR 80%) with a deep component already about to involve the basal ganglia at the time of the first operation.
Grade 3
The greatest rate of GTR (66%) was possible in Group 2; in the remnant 33% (subtotal resection + PR) the average residual (3 mL) was smaller than in the Group 3 (9.6 mL) and bigger than in Group 1 (0.8 mL). The statistical significance of these 2 findings cannot be established with enough power, due to the very small size of this subgroup. Overall, ioMRI helped in improving our resection of: +73.3%/2.2 mL in Group 1 and +68%/5.1 mL in Group 2.
In Group 1, 1 patient who had a total resection of a recurrent anaplastic oligodendroglioma (first operation in 2009; first PFS = 7 years) recurred 9 months later. In Group 2, it is worth noticing the case of a recurrent anaplastic oligodendroglioma (first operation in 2004; first PFS = 9 years) which recurred after 6 months and caused patient's death 18 months later.
In the control group, 3 of the 7 patients with incomplete resections recurred and died: all of them had residuals larger than 5 mL and partial resections (66%–87%); interestingly, among the remaining 4 who didn't experience recurrence at 21/36-month follow-up, 2 had residuals larger than 5 mL as well, with similar EOR (61%–66%).
Grade 4
Significantly smaller ioMRI residual tumors were detected in Group 1 (1.7 mL) as compared with Group 2 (10 mL) (P < 0.01): this could be possibly explained by a more conservative approach on margin resection for tumors lying in the vicinity of eloquent structures. A smaller but persistent difference remained at the postoperative MRI, showing greater residuals in Group 2 (2.3 mL), compared with Group 1 (1.2 mL, P < 0.05): remnant tumor couldn't be removed due to the involvement of eloquent areas. Nevertheless, GTR rate was the greatest in Group 2 (66%), closely followed by Group 1 (63%), both statistically greater than Group 3 (36%) (P < 0.02).
Overall, ioMRI helped in resecting +29.5%/0.5 mL in Group 1 and +77%/7.7 mL in Group 2. Residuals were comparably small in the 3 groups, with <5 mL remnant in about 83%–88% of cases.
All Group 1 incomplete resections and 80% GTRs died within 26 months (despite small residual of less than 5 mL, representing EORs within 50%–93%). In Group 2, 1 patient with 1 mL of residual (EOR 90%) had a recurrence at 8 months and died at 12 months; the only other incomplete resection in Group 2 (20 mL, EOR 84%) was originally an IDH-mutant GBM, was operated on at 14 months from the first operation and died 16 months after the second operation. It's worth highlighting all of patients in Group 2 undergoing GTRs are still alive at an average of 25-month follow-up; the average PFS in this subgroup is 21 months.
Intraoperative Complications
During awake surgeries (Group 2), 1 patient (5%) had intraoperative seizures, which only caused a prolongation of the procedure with eventual recovery to baseline during the course of surgery; NORAS pins displaced in 1 case (5%) before the ioMRI, when sedation was lightened to wake the patient up for performing the brain mapping. Registration was restored via the intraoperative landmarks previously acquired. In the control group (Group 3), Mayfield pins displacement occurred in 1 patient (2%), but also in this case we used the intraoperative landmarks to restore the registration.
In-Theater Permanence
Group 3 mean overall in-theater stay was 6 hours and 45 minutes (Table 3). Group 1 procedures were about 105 minutes longer, with an average theater occupancy of 8 hours and 30 minutes (about 2 sessions). Group 2 operations took 2 additional hours, with an overall in-theater stay of 10 hours and 30 minutes (3 sessions). In Group 2, the longer time spent for patient preparation and mapping was partially compensated by a shorter stay in the operative room after the operation, as it generally happens for patients operated on with the awake techniques.
Table 3.
Details of Perioperative and Operative Times
| Group 1 (SD) | Group 2 (SD) | Group 3 (SD) | |
|---|---|---|---|
| OT | 8 hours, 30 minutes (2 hours) (–2 hours compared with the beginning of the series in 2014) | 10 hours, 30 minutes (1 hour, 28 minutes) (–2.5 hours compared with the beginning of the series in 2014) | 6 hours, 45 minutes (2 hours, 29 minutes) |
| Prep | 1 hours, 15 minutes (21 minutes) | 2 hours (15 minutes) | 1 hour (20 minutes) |
| Skin to skin | 6 hours, 15 minutes (2 hours 30 minutes) | 7 hours, 50 minutes (1 hour 22 minutes) | 4 hours, 45 minutes (2 hours, 17 minutes) |
| ioMRI | 1 hour, 10 minutes (18 minutes) | 1 hour, 20 minutes (25 minutes) | |
| Net op | 5 hours, 5 minutes | 6 hours, 30 minutes | 4 hours, 45 minutes |
| Post | 1 hour (12 minutes) | 40 minutes (13 minutes) | 1 hour (12 minutes) |
All values represent averages; SDs are in parentheses.
SD, standard deviation; OT, overall time of in-theater stay; Prep, preoperative time (preparation, anesthetic time, positioning and prep and drape; Skin to skin, from the skin incision to the dressing of the wound; ioMRI (intraoperative magnetic resonance imaging) time, from the temporary closure to when operation was restarted; net op, skin to skin – ioMRI time; Post, from the undraping to when patient left the operating room.
Overall operative time was statistically longer for Group 2 compared with Group 1 (P < 0.01) and 3 (P < 0.0001) and for Group 1 compared with 3 (P < 0.0005) and skin-to-skin times was also longer in Group 2 compared with Group 1 (P < 0.05) and 3 (P < 0.0001) and for Group 1 compared with 3 (P < 0.005).
Net operative time is defined as the time spent operating (skin-to-skin time minus the time needed for the ioMRI procedure): net operation times in Group 2 patients are significantly longer than Group 1 (6 hours and 30 minutes vs. 5 hours and 5 minutes, P < 0.0001), whereas Group 1 and 3 do not statistically differ.
Clinical Outcomes and Morbidity
Transient and permanent post-operative morbidity is summarized in Table 4. In patients operated on under general anesthesia and receiving ioMRI (Group 1), 1 patient (4%) had a postoperative infection requiring wound wash out, removal of bone flap, and antibiotics. Transient left-sided weakness in a right-handed patient (4%) with a right insular anaplastic astrocytoma: he was independent (WHO-PS 1) at 3 months' follow-up. Transient memory and cognition disturbances in a patient with right middle frontal anaplastic astrocytoma (4%). Although formal preoperative neuropsychological assessment was not done, persistent memory and cognition disturbances (WHO-PS 2) were detected in a patient (4%) with a WHO grade 2 central neurocytoma.
Table 4.
Transient (at Discharge) and Persistent (at 3-Month Follow-Up) Morbidity
| Demographics | Morbidity at Discharge | Persistent Morbidity | |
|---|---|---|---|
| ioMRI in GA Group 1 |
26 patients: 15 M, 11 F Avg. age 44 years (SD 16 years) Frontal 10, temporal 3, parietal 5, Insular 4, occipital 2, cerebellar 2 |
Memory and cognition disturbances 8% (2 patients) Hemiparesis 4% (1 patient) Infections 4% (1 patient) |
Memory and cognition disturbances 4% (1 patient) |
| ioMRI awake surgery Group 2 |
20 patients: 14 M, 6 F Avg. age 46 years (SD 14 years) Frontal 11, Temporal 5, Insular 4, (language areas 10, motor cortex 4, both systems 6) |
Memory and cognition disturbances 10% (2 patients) Hemiparesis 10% (2 patients) Dysphasia 20% (4 patients) Parietal syndrome 5% (1 patient) |
Memory and cognition disturbances 5% (1 patient) Parietal syndrome 5% (1 patient) |
| Control Group 3 |
46 patients: 29 M, 1 7F Avg. age 47 years (SD 16 years) Frontal 21, temporal 8, parietal 5, insular 8, occipital 2, cerebellar 2 |
Memory and cognition disturbances 13% (6 patients) Hemiparesis 13% (6 patients) Dysphasia 15.2% (7 patients) Parietal syndrome 5% (1 patient) |
Memory and cognition disturbances 10.8% (5 patients) Hemiparesis 4.3% (2 patients) Dysphasia 4.3% (2 patients) |
ioMRI, intraoperative magnetic resonance imaging; GA, general anesthesia; M, male; F, female; SD, standard deviation.
In patients operated on using awake surgery and ioMRI (Group 2), 2 patients (10%) harboring lesions near the motor strip had a transient weakness that resolved within 2 weeks; 4 patients (20%) experienced a transient dysphasia, which resolved within 12 weeks (one left frontal middle gyrus GBM; one temporo-insular GBM; one left fronto-temporo-insular diffuse astrocytoma; and one left fusiform fibrillar astrocytoma); a transient parietal syndrome occurred in 1 patient (5%) with a large left parietal GBM and resolved in 1 month; transient memory and cognition disturbances were detected in 2 patients (10%): one with a left fronto-polar low-grade oligodendroglioma (WHO-PS 1) and one with a left frontal anaplastic astrocytoma. Although formal preoperative neuropsychological assessment was not done, a permanent degree of memory and cognition impairment was reported by 1 patient (5%) with a temporo-insular grade 3 astrocytoma (WHO-PS 2 from a preoperative WHO-PS 1). A parietal syndrome permanently affected 1 patient (5%) with a large left parietal GBM (unsteadiness and troubles orientating in the surrounding environment, WHO-PS 1).
In the control group (Group 3), transient weakness complicated 13% of cases (6 patients), and transient dysphasia was present in 15.2% of patients (7 patients); early postoperative memory and cognition disturbances were reported in 13% of cases (6 patients). Persistent weakness affected 4.3% of patients (2 patients), whereas a persistent dysphasia of variable severity was detected in 4.3% of cases (2 patients). Persistent memory and cognition problems were affecting daily activities (WHO-PS 1 or 2) of 10.8% of patients (5 patients).
Progression-Free Survival/Overall Survival
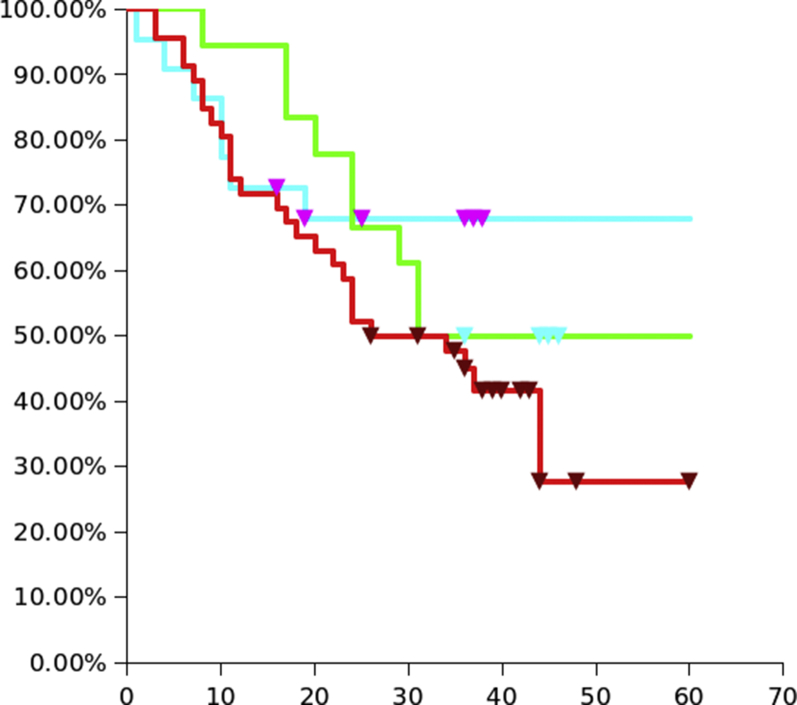
PFS curves did not differ statistically between the groups (Figures 1 and 2). Although there is an initial better trend in Group 2, the difference becomes less obvious at 30 months, whereas Group 1 maintains shows a wider gap starting at 20 months. Median PFS for Group 2 was 31 months, for Group 3 was 25 months, and it is not reached for Group 1 (68% free from progression at 5-year follow-up).
Figure 1.
Progression-free survival KM curve for the three groups: Groups 1 (ioMRI / GA) is light blue, Group 2 (ioMRI / awake) is green, Group 3 (control) is red.
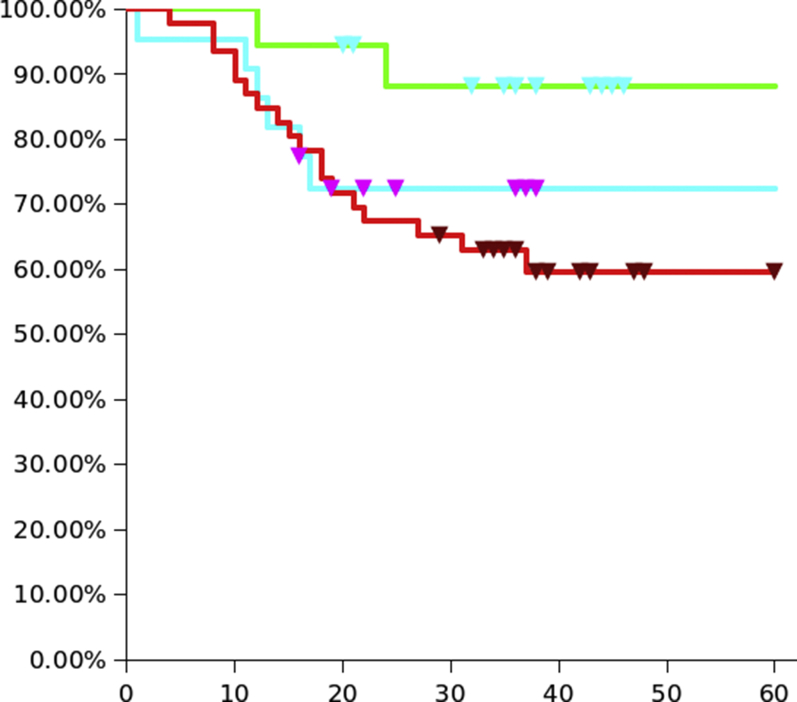
Figure 2.
Overall survival KM curve for the three groups: Groups 1 (ioMRI / GA) is light blue, Group 2 (ioMRI / awake) is green, Group 3 (control) is red.
Patients in Group 2 have a statistically significant longer OS when compared with Group 3 (P < 0.05). There seems to be a nonstatistically significant better trend in the second two thirds of the Group 1 curve compared with Group 3.
Discussion
Although still far from being perfect, various intraoperative aids and tools have made it possible to increase the GTR of gliomas from a disappointing 40% in the 1990s to an encouraging 80% of the contemporary series.1, 3, 22, 25
ioMRI helps in the recognition of the residual tumor with an unparalleled spatial and contrast resolution and also can detect early ischemic changes in the neighboring regions and the status of the surrounding white matter main tracts.5 It significantly minimizes the main limitation of the standard image-guided surgeries: the non-real time nature with brain shift and distortions that will eventually make the scans unusable toward the end of the resections, when they would actually be most needed.26 The issue can be partly limited by rationalizing the resection technique with an early circumferential dissection at the margins. This is often not possible because of the location, shape, size, and behavior of most of the gliomas: an initial internal debulking often is needed. The updated ioMRI scan can be navigated when back to theater to spot the residual areas with precision: a further brain shift is unlikely as a major debulking has already happened and residuals are often a few milliliters big and often lying on the cavity walls.
It is, of course, an expensive tool only available in a few hospitals. Its high costs are not only related to the initial installation (building the facilities, buying the machine, and the related accessories to be used in the operative room) but also to its maintenance and its use (radiographers and radiologists). Costs can be rationalized opting for the 2-room solution, as in our case: the operative theater and the MRI room are separated by a sliding door, allowing the same machine to be used also for regular scans. A recent metanalysis by the Scottish Neuroscience Group states the incremental cost of the ioMRI is below the threshold for cost-effectiveness of HGG therapy, denoting it is cost-effective on its own.5
Neurophysiology is a powerful tool for continuous monitoring of gross motor pathways, both cortical and subcortical. Subcortical stimulation is especially useful in localizing with millimetric precision the fibers of the pyramidal tract coming from the motor strip, making the dissection of the tumor from the white matter much safer.27 Unfortunately, supplementary, premotor, and extra-pyramidal fibers cannot be tested with the same accuracy, leading to their potential damage, which can indeed affect patient's daily life, especially those who must maintain the ability to execute fine motor patterns for their jobs and related quality of life. Monitoring other senses (vision, touch, and hearing) is also currently unacceptably inaccurate and therefore not routinely used.
Awake surgery allows accurate and extensive cortical and subcortical mapping of (virtually) all eloquent brain functions and their near-continuous monitoring.28, 29 It allows surgeons to choose safe corridors and strategies of resection based on the individual functional anatomy (Figure 3).30 When surgeons get too near to critical areas, red-flag events and alarms are often able to be spotted before permanent damage actually occurs (e.g., slurred speech, disturbed comprehension/linguistic production, slower movements, sparkles/shadows in the visual fields, auditory hallucinations, numbness). Continuous communication with the patient through a dedicated professional (speech and language therapist, neuropsychologist) is vital for the diagnosis and interpretation of these early signs. One of the main limitations of the technique is the patient's comfort and compliance, and the additional time needed for the anesthetic techniques (asleep–awake–asleep, MAC, or awake–awake–awake), and the mapping/monitoring.
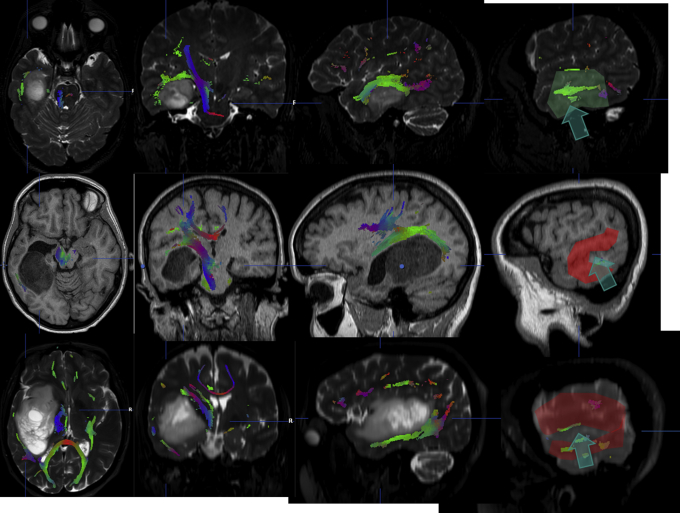
Figure 3.
Example of how the language mapping can change the sites of corticotomy for otherwise similarly located tumors. These images were taken from the BrainLab iPlan workstation, hence the left side corresponds to the left hemisphere. The integration of MRI and DTI gives us a pre-operative idea on how to plan the route of access, but the brain mapping (red and green areas on the cortical surface) enables us to avoid eloquent areas (language in these cases): note the large individual variability. The arrows show the access used in each case.
We looked with interest at some pioneering papers about the feasibility of combining 2 among the most powerful aids available: the ioMRI (for morphology) and the awake surgery (for function).18, 20, 31, 32, 33, 34, 35 We were particularly impressed by the recent series of Ghinda et al.35 of 106 patients with gliomas in eloquent areas, operated on with awake intraoperative mapping and high-field (3T) ioMRI: they reported GTR rates of 89% and 96% for LGG and HGG, respectively. The authors concluded that “combined awake craniotomy and ioMRI is a safe and efficient technique allowing maximal safe resection of eloquent area gliomas with possible subsequent OS and PFS benefits.”35
Both ioMRI and awake surgery have been criticized for being time-demanding, distressing for the patient and the anesthetist/theater/radiology staff and, in general, for requiring greater organizational and financial resources. From our experience, performing an ioMRI took about 1 hour and 45 minutes longer in terms of theater occupancy when compared with a control series where only standard neuronavigation and neurophysiology were used. Awake surgery added another 2 hours: about 45 minutes for preparation (before the skin incision), 30 minutes for the intraoperative mapping and pauses for monitoring, and the remnant additional time spent in resection, probably longer due to the critical location, size, and/or morphology of the tumors when compared with the control group but also to the Group 1, where tumors are far from languages areas and motor pathways can always be monitored with neurophysiology.
The near totality of Group 2 patients didn't report psychological distress nor during surgery or at the follow-up outpatient clinic. The asleep–awake–asleep or MAC anesthetic techniques also allow painless opening and closing phases and reduce the chances of distress and exhaustion, increasing patient compliance. There is a low risk (2.2%) of displacement from the NORAS pins if the patient is waking up agitated or not remembering being on a surgical table with the head in pins. The awake mapping was reported as an engaging and fascinating dreamy-like experience, whereas the ioMRI didn't raise any concerns at all. We think the preoperative neuropsychological filtering has a pivotal role in reducing the chances of dissatisfaction. Our experience is in line with Kiel's group36: they analyzed this aspect through a questionnaire and concluded 90% of patients would repeat the same procedure again if needed.
Criteria leading to use of ioMRI +/– awake surgery (irregular features and location) create a selection bias, making an accurate comparison between the 3 groups difficult: the most complex tumors underwent ioMRI +/– awake.
We didn't find relevant differences in terms of morbidity in the 3 groups if we excluded the transient symptoms, which will obviously be greater in patients with tumor in eloquent locations, more represented in Group 1 and 2. Group 3 is burdened from the occurrence of persistent weakness in 2 patients and persistent dysphasia in 2 patients: we can only argue whether the use of ioMRI or awake surgery in those cases would have led to an advantage in terms of intraoperative judgment of margins and infiltrated functional brain. The trend toward a lower long-term morbidity for the Groups 1 and 2 could be at least in part justified by the ability in distinguishing between tumor margins/edema, once an updated scan (ioMRI) is navigated: it is possible that surgeons relying on neurophysiology alone might resect edematous suspicious-looking tissue, leading to a deficit. Motor neurophysiology unipolar subcortical monitoring doesn't indeed give 3-dimensional information on the position of the tract which can be found in a spherical area whose diameter corresponds (in millimeters) to the mA of the given stimulus.37, 38 Furthermore, the stimulation is operator dependent: surgeons can apply variable pressure to the probe, which can slightly dive in the exposed subcortical interface; different technicians may interpret the neurophysiological data differently.
Preoperative tumor volumes are equally distributed throughout the groups, with minimal, nonstatistical differences, although the mean volume in Groups 1 and 2 is dragged down by a few relatively small tumors potentially difficult to localize because deep or only visible on FLAIR. Group 1 comprises tumors safely far from language areas whereas Group 2 consists of more challenging lesion characterized by an irregular shape, a nonhomogenous/scattered/absent contrast enhancement and the vicinity/involvement of eloquent (motor and/or language) areas. The use of ioMRI in combination with awake surgery made these cases as approachable as the less challenging ones in Group 3 as results in terms of morbidity, EOR, and PFS/OS show.
Although awake surgery is the only option when the integrity of language pathways is a major concern,13, 19, 35 valid alternatives to the use of ioMRI for the morphologic assessment of the tumor are represented by the modern intraoperative ultrasound devices (either conventional sector or linear array)39 and the fluorescent dyes, especially 5-aminolevulinic acid (5-ALA).40, 41 Ultrasound is a relatively cheap, direct, and repeatable tool for detecting both high- and low-grade lesions and large surrounding vessels. It requires a long learning curve due to the difficulty in interpreting the echoic signal and the probes are still bulky and wired, making its frequent (real-time indeed) use impractical.5, 42, 43, 44 In addition, its main limitations are the inability to scan sonically hidden corners and the low contrast and spatial resolution: 8% of surgeons have labelled as poor its image quality.5 Quality of image falls further in recurrent cases and in patients with previous radiation therapy.45 Ultrasound sensitivity and specificity vary from the beginning to the end of surgery when they fall to 26% and 58%, respectively, compared with 55% and 74% of the ioMRI.39, 46 GTRs across studies range between 72% and 75.4% GTR in HGG and 78% and 85% GTR in 3 LGG.5, 39, 45 A similar analysis of ioMRI series shows GTR rates between 42% and 99% for HGG and 89% for LGG.5, 35, 46 However, an accurate comparison between US and ioMRI series is challenging and may not be possible at the moment, as data are often heterogeneous, unmatched, and incomplete, with a quality of evidence ranging from low to very low.46
Eljamel and Coburger directly compared the use of US and ioMRI in their series: no differences were found in terms of EOR and PFS/OS, but they conclude combining the use of these tools may be the best solution for overcoming specific differences in accuracy.39 Moreover, although overall benefits in PFS were reported for both US and ioMRI,5 class I evidence of better PFS is available only for ioMRI studies.39 5-ALA is becoming the standard in HGGs, especially after the recognition of its cost-effectiveness.5, 35, 40, 7 5-ALA's main limitation remains the ineffectiveness in noncontrast-enhancing tumors.17, 40 A relatively long learning curve is needed to interpret the pathologic significance of a weak fluorescence.5, 47 Coburger et al.41 states that the ability of 5-ALA to detect tumor in the infiltration zone (specificity) is greater than for ioMRI (80% vs. 60%). In the same study, 5-ALA sensitivity is reported 91% (vs. 66% of the ioMRI).41 The authors conclude that “use of 5-ALA in addition to ioMRI may be beneficial to maximize EOR in HGGs.” They speculate that the 5-ALA greater specificity can be in part be explained by its ability to be detected in the infiltrated tissue beyond the pathologic contrast enhancement area, which is what the surgeon aims to remove when using navigation tool (both standard and with ioMRI).
More studies will be needed to establish the risk/benefit ratio of removing areas far beyond the contrast enhancement, especially since there is class 1 evidence that 5-ALA increases the EOR, but there are a lack of good data demonstrating 5-ALA increases PFS/OS.41 Although the lower ioMRI specificity could represent an issue in tumors surrounded by eloquent tissue, the awake surgery technique can be used to avoid functional deficits in these cases. Despite the more powerful sensitivity and specificity of 5-ALA compared with ioMRI, a systematic review by Barone et al.46 shows greater rates of resection with ioMRI (92%) than 5-ALA (67%): data quality is low due to heterogeneity and small sizes of populations and possible selection biases. Since this metanalysis, an over the years improvement in the learning curve led Stummer and Eljamer to publish their updated series reporting GTR rates ranging from 73% to 89%: it is not clear, however, if the residuals were calculated volumetrically and no details of the initial and final absolute volumes are given.5, 40
Our results align with the other ioMRI series. The rates of PR in LGGs is significantly less than in the control group (59% in Group 3, compared with 36% of Group 1 [P < 0.01] and 45% of Group 2 [P < 0.05]). The GTR rates in HGGs doubled thanks to the use of ioMRI and only 1 patient had a partial resection of an HGG in Group 1 and 2 combined. None of the HGGs in Group 1 (non-language areas) has been resected partially (<90%), with average postoperative residuals of 0.8 mL for grade 3 tumors and 1.2 mL for grade 4 tumors. About the same percentage of patients in Group 2 will have a resection less than total (34%) with larger residuals 3.2 mL and 3.7 mL for grade 3 and 4, respectively: this volumes fall within the thresholds set by Chaichana et al.2 affecting survival and recurrence (<5 mL). The significantly better OS in Group 2 patients seems to confirm the solidity of this threshold. In general, residual volumes in ioMRI are always smaller than in control group. It would be interesting to understand, in a future study, whether the geometry of the residual (one/multiple nodules, linear contrast enhancement area, discontinued scattered contrast enhanced areas, etc.) is also a predictive factor for the time to recurrence.
When we specifically compare Group 1 and 2, we cannot find statistically significant differences in terms of EOR, recurrences, and deaths. We think the addition of awake surgery adds a layer of safety to the whole procedure, enabling to get the same better results of the tumors far from speech areas.
Overall, the use of the ioMRI allowed greater resections in all grades, regardless of the greater complexity of tumors in groups 1 and 2. It's worth noticing that none of the high-grade tumors in Group 2 were entirely resected when checked intraoperatively. This reflects the surgeons' concerns when dealing with fast-growing tumors with irregular shapes that sit within/nearby eloquent tissue. Whereas a degree of intraoperative deficits is tolerated in LGG surgery because of the still useful brain reserve and plasticity that may lead to a recovery,4 we tend to avoid causing intraoperative deficits altogether when operating on HGGs, as there is a lower chance of improvement and the time required to achieve it may potentially supersede the PFS or even the OS.
Limitations
The aforementioned unavoidable bias in the selection of patients addressed to the ioMRI with or without awake surgery make the 3 groups hardly comparable. A prospective multicentric study comparing outcomes between departments with different settings and populations with equally challenging tumors is needed.
The analysis of PFS/OS suffers lack of statistical power due to the small numbers of patients together with a heterogeneous molecular signature and the presence of patients with recurrences or transformed tumors from lower grades. Also, initial management of patients with low-grade tumors was variable: some patients were only observed over the time, and others underwent surgery straight after the initial diagnosis, according to several factors including multidisciplinary team discussion and patients' will.
We omitted a stratification of tumors by genetic profiles because the samples would have become so small to hinder the interpretation of the results.
A reliable comparison with other published series is very difficult: previous attempt at formal comparisons concluded that data are not homogenous enough to draw high-quality conclusions.45, 46 A prospective multicentric study among centers using either the ioMRI or ultrasound or 5-ALA would enable us to bypass bias selection and collect data homogeneously for a fair comparison among these powerful aids.
Conclusions
The use of the ioMRI is effective in dealing with “complex” gliomas (location, eloquence, shape, contrast enhancement features, cloudy T1/T2 appearance) and making them more approachable, enabling a safe greater EOR than the control group. It prolongs the overall theater occupancy by about half a session. We found the addition of the awake technique useful for reaching greater resections in low-grade tumors: it can be always be safely used as far as inclusion/exclusion criteria are respected and prolongs the overall in-theater stay by only another half a session.
Footnotes
Conflict of interest statement: The authors declare that the article content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Lacroix M., Abi-Said D., Fourney D.R. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190–198. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 2.Chaichana K.L., Jusue-Torres I., Navarro-Ramirez R. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro Oncol. 2014;16:113–122. doi: 10.1093/neuonc/not137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acerbi F., Broggi M., Eoli M. Is fluorescein-guided technique able to help in resection of high-grade gliomas? Neurosurg Focus. 2014;36:E5. doi: 10.3171/2013.11.FOCUS13487. [DOI] [PubMed] [Google Scholar]
- 4.Duffau H. Long-term outcomes after supratotal resection of diffuse low-grade gliomas: a consecutive series with 11-year follow-up. Acta Neurochir (Wien) 2016;158:51–58. doi: 10.1007/s00701-015-2621-3. [DOI] [PubMed] [Google Scholar]
- 5.Eljamel M.S., Mahboob S.O. The effectiveness and cost-effectiveness of intraoperative imaging in high-grade glioma resection; a comparative review of intraoperative ALA, fluorescein, ultrasound and MRI. Photodiagnosis Photodyn Ther. 2016;16:35–43. doi: 10.1016/j.pdpdt.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 6.McGirt M.J., Chaichana K.L., Attenello F.J. Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery. 2008;63:700–707. doi: 10.1227/01.NEU.0000325729.41085.73. [author reply: 707] [DOI] [PubMed] [Google Scholar]
- 7.Keles G.E., Anderson B., Berger M.S. The effect of extent of resection on time to tumor progression and survival in patients with glioblastoma multiforme of the cerebral hemisphere. Surg Neurol. 1999;52:371–379. doi: 10.1016/s0090-3019(99)00103-2. [DOI] [PubMed] [Google Scholar]
- 8.Majchrzak K., Kaspera W., Bobek-Billewicz B. The assessment of prognostic factors in surgical treatment of low-grade gliomas: a prospective study. Clin Neurol Neurosurg. 2012;114:1135–1144. doi: 10.1016/j.clineuro.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 9.Stummer W., Reulen H.-J., Meinel T. Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery. 2008;62:564–576. doi: 10.1227/01.neu.0000317304.31579.17. [discussion: 564] [DOI] [PubMed] [Google Scholar]
- 10.Smith J.S., Chang E.F., Lamborn K.R. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26:1338–1345. doi: 10.1200/JCO.2007.13.9337. [DOI] [PubMed] [Google Scholar]
- 11.Sporns O. The human connectome: a complex network. Ann N Y Acad Sci. 2011;1224:109–125. doi: 10.1111/j.1749-6632.2010.05888.x. [DOI] [PubMed] [Google Scholar]
- 12.van den Heuvel M.P., Sporns O. Rich-club organization of the human connectome. J Neurosci. 2011;31:15775–15786. doi: 10.1523/JNEUROSCI.3539-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duffau H. The huge plastic potential of adult brain and the role of connectomics: new insights provided by serial mappings in glioma surgery. Cortex. 2014;58:325–337. doi: 10.1016/j.cortex.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Byrne R.W., editor. Functional Mapping of the Cerebral Cortex. Springer International Publishing; Cham: 2016. [Google Scholar]
- 15.Duffau H. Lessons from brain mapping in surgery for low-grade glioma: insights into associations between tumour and brain plasticity. Lancet Neurol. 2005;4:476–486. doi: 10.1016/S1474-4422(05)70140-X. [DOI] [PubMed] [Google Scholar]
- 16.Sherman J.H., Hoes K., Marcus J., Komotar R.J., Brennan C.W., Gutin P.H. Neurosurgery for brain tumors: update on recent technical advances. Curr Neurol Neurosci Rep. 2011;11:313–319. doi: 10.1007/s11910-011-0188-9. [DOI] [PubMed] [Google Scholar]
- 17.Coburger J., Merkel A., Scherer M. Low-grade glioma surgery in intraoperative magnetic resonance imaging: results of a multicenter retrospective assessment of the German study group for intraoperative magnetic resonance imaging. Neurosurgery. 2016;78:775–786. doi: 10.1227/NEU.0000000000001081. [DOI] [PubMed] [Google Scholar]
- 18.Peruzzi P., Puente E., Bergese S., Chiocca E.A. Intraoperative MRI (ioMRI) in the setting of awake craniotomies for supratentorial glioma resection. Acta Neurochir Suppl. 2011;109:43–48. doi: 10.1007/978-3-211-99651-5_7. [DOI] [PubMed] [Google Scholar]
- 19.Mehdorn H.M., Schwartz F., Becker J. Awake craniotomy for tumor resection: further optimizing therapy of brain tumors. Acta Neurochir Suppl. 2017;124:309–313. doi: 10.1007/978-3-319-39546-3_45. [DOI] [PubMed] [Google Scholar]
- 20.Maldaun M.V.C., Khawja S.N., Levine N.B. Awake craniotomy for gliomas in a high-field intraoperative magnetic resonance imaging suite: analysis of 42 cases. J Neurosurg. 2014;121:810–817. doi: 10.3171/2014.6.JNS132285. [DOI] [PubMed] [Google Scholar]
- 21.Duffau H., Moritz-Gasser S., Mandonnet E. A re-examination of neural basis of language processing: proposal of a dynamic hodotopical model from data provided by brain stimulation mapping during picture naming. Brain Lang. 2014;131:1–10. doi: 10.1016/j.bandl.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 22.McGirt M.J., Chaichana K.L., Gathinji M. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009;110:156–162. doi: 10.3171/2008.4.17536. [DOI] [PubMed] [Google Scholar]
- 23.Roelz R., Strohmaier D., Jabbarli R. Residual tumor volume as best outcome predictor in low grade glioma—a nine-years near-randomized survey of surgery vs. biopsy. Sci Rep. 2016;6:32286. doi: 10.1038/srep32286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louis D.N., Perry A., Reifenberger G. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 25.Bogaards A., Varma A., Collens S.P. Increased brain tumor resection using fluorescence image guidance in a preclinical model. Lasers Surg Med. 2004;35:181–190. doi: 10.1002/lsm.20088. [DOI] [PubMed] [Google Scholar]
- 26.Enchev Y. Neuronavigation: geneology, reality, and prospects. Neurosurg Focus. 2009;27:E11. doi: 10.3171/2009.6.FOCUS09109. [DOI] [PubMed] [Google Scholar]
- 27.Duffau H. Contribution of cortical and subcortical electrostimulation in brain glioma surgery: methodological and functional considerations. Neurophysiol Clin. 2007;37:373–382. doi: 10.1016/j.neucli.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Duffau H. Awake surgery for nonlanguage mapping. Neurosurgery. 2010;66:523–528. doi: 10.1227/01.NEU.0000364996.97762.73. [discussion: 528] [DOI] [PubMed] [Google Scholar]
- 29.Duffau H. The anatomo-functional connectivity of language revisited. New insights provided by electrostimulation and tractography. Neuropsychologia. 2008;46:927–934. doi: 10.1016/j.neuropsychologia.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 30.Picht T., Krieg S.M., Sollmann N. A comparison of language mapping by preoperative navigated transcranial magnetic stimulation and direct cortical stimulation during awake surgery. Neurosurgery. 2013;72:808–819. doi: 10.1227/NEU.0b013e3182889e01. [DOI] [PubMed] [Google Scholar]
- 31.Leuthardt E.C., Lim C.C.H., Shah M.N. Use of movable high-field-strength intraoperative magnetic resonance imaging with awake craniotomies for resection of gliomas: preliminary experience. Neurosurgery. 2011;69:194–205. doi: 10.1227/NEU.0b013e31821d0e4c. [discussion: 205] [DOI] [PubMed] [Google Scholar]
- 32.Parney I.F., Goerss S.J., McGee K., Huston J., Perkins W.J., Meyer F.B. Awake craniotomy, electrophysiologic mapping, and tumor resection with high-field intraoperative MRI. World Neurosurg. 2010;73:547–551. doi: 10.1016/j.wneu.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 33.Lu J., Wu J., Yao C. Awake language mapping and 3-Tesla intraoperative MRI-guided volumetric resection for gliomas in language areas. J Clin Neurosci. 2013;20:1280–1287. doi: 10.1016/j.jocn.2012.10.042. [DOI] [PubMed] [Google Scholar]
- 34.Nabavi A., Goebel S., Doerner L., Warneke N., Ulmer S., Mehdorn M. Awake craniotomy and intraoperative magnetic resonance imaging: patient selection, preparation, and technique. Top Magn Reson Imaging. 2009;19:191–196. doi: 10.1097/RMR.0b013e3181963b46. [DOI] [PubMed] [Google Scholar]
- 35.Ghinda D., Zhang N., Lu J., Yao C.-J., Yuan S., Wu J.-S. Contribution of combined intraoperative electrophysiological investigation with 3-T intraoperative MRI for awake cerebral glioma surgery: comprehensive review of the clinical implications and radiological outcomes. Neurosurg Focus. 2016;40:E14. doi: 10.3171/2015.12.FOCUS15572. [DOI] [PubMed] [Google Scholar]
- 36.Goebel S., Nabavi A., Schubert S., Mehdorn H.M. Patient perception of combined awake brain tumor surgery and intraoperative 1.5-T magnetic resonance imaging: the Kiel experience. Neurosurgery. 2010;67:594–600. doi: 10.1227/01.NEU.0000374870.46963.BB. [discussion: 600] [DOI] [PubMed] [Google Scholar]
- 37.Kombos T., Suess O., Kern B.C. Comparison between monopolar and bipolar electrical stimulation of the motor cortex. Acta Neurochir (Wien) 1999;141:1295–1301. doi: 10.1007/s007010050433. [DOI] [PubMed] [Google Scholar]
- 38.Kombos T., Süss O. Neurophysiological basis of direct cortical stimulation and applied neuroanatomy of the motor cortex: a review. Neurosurg Focus. 2009;27:E3. doi: 10.3171/2009.8.FOCUS09141. [DOI] [PubMed] [Google Scholar]
- 39.Coburger J., Scheuerle A., Kapapa T. Sensitivity and specificity of linear array intraoperative ultrasound in glioblastoma surgery: a comparative study with high field intraoperative MRI and conventional sector array ultrasound. Neurosurg Rev. 2015;38:499–509. doi: 10.1007/s10143-015-0627-1. [discussion: 509] [DOI] [PubMed] [Google Scholar]
- 40.Hadjipanayis C.G., Widhalm G., Stummer W. What is the surgical benefit of utilizing 5-aminolevulinic acid for fluorescence-guided surgery of malignant gliomas? Neurosurgery. 2015;77:663–673. doi: 10.1227/NEU.0000000000000929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coburger J., Engelke J., Scheuerle A. Tumor detection with 5-aminolevulinic acid fluorescence and Gd-DTPA-enhanced intraoperative MRI at the border of contrast-enhancing lesions: a prospective study based on histopathological assessment. Neurosurg Focus. 2014;36:E3. doi: 10.3171/2013.11.FOCUS13463. [DOI] [PubMed] [Google Scholar]
- 42.Moiyadi A.V. Intraoperative ultrasound technology in neuro-oncology practice-current role and future applications. World Neurosurg. 2016;93:81–93. doi: 10.1016/j.wneu.2016.05.083. [DOI] [PubMed] [Google Scholar]
- 43.Dubey S., Janu A., Chaudhari S., Moiyadi A. Navigable 3D-ultrasound facilitates supra-radical resections beyond the contrast-enhancing boundaries in malignant gliomas. J Neurol Surg A Cent Eur Neurosurg. 2016;77:372–375. doi: 10.1055/s-0035-1570005. [DOI] [PubMed] [Google Scholar]
- 44.Moiyadi A., Shetty P. Early experience with combining awake craniotomy and intraoperative navigable ultrasound for resection of eloquent region gliomas. J Neurol Surg A Cent Eur Neurosurg. 2017;78:105–112. doi: 10.1055/s-0036-1584512. [DOI] [PubMed] [Google Scholar]
- 45.Mahboob S., McPhillips R., Qiu Z. Intraoperative ultrasound-guided resection of gliomas: a meta-analysis and review of the literature. World Neurosurg. 2016;92:255–263. doi: 10.1016/j.wneu.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 46.Barone D.G., Lawrie T.A., Hart M.G. Image guided surgery for the resection of brain tumours. Cochrane Database Syst Rev. 2014;1:CD009685. doi: 10.1002/14651858.CD009685.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stummer W., Pichlmeier U., Meinel T. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]