Abstract
The central aim of this study was to investigate hormones as a predictor of individual vulnerability or resiliency on emotion processing tasks following one night of sleep restriction. The restriction group was instructed to sleep 3 a.m.–7 a.m. (13 men, 13 women in follicular phase, 10 women in luteal phase of menstrual cycle), and a control group slept 11 p.m.–7 a.m. (12 men, 12 follicular women, 12 luteal women). Sleep from home was verified with actigraphy. Saliva samples were collected on the evening prior to restriction, and in the morning and afternoon following restriction, to measure testosterone, estradiol, and progesterone. In the laboratory, event-related potentials (ERPs) were recorded during presentation of images and faces to index neural processing of emotional stimuli. Compared to controls, sleep-restricted participants had a larger amplitude Late Positive Potential (LPP) ERP to positive vs neutral images, reflecting greater motivated attention towards positive stimuli. Sleep-restricted participants were also less accurate categorizing sad faces and exhibited a larger N170 to sad faces, reflecting greater neural reactivity. Sleep-restricted luteal women were less accurate categorizing all images compared to control luteal women, and progesterone was related to several outcomes. Morning testosterone in men was lower in the sleep-restricted group compared to controls; lower testosterone was associated with lower accuracy to positive images, a greater difference between positive vs neutral LPP amplitude, and lower accuracy to sad and fearful faces. In summary, women higher in progesterone and men lower in testosterone were more vulnerable to the effects of sleep restriction on emotion processing tasks. This study highlights a role for sex and sex hormones in understanding individual differences in vulnerability to sleep loss.
Keywords: Sleep restriction, Emotion processing, Testosterone, Progesterone, Estradiol
Highlights
-
•
Sex and sex hormones play a role in individual differences in vulnerability to sleep loss.
-
•
Women higher in progesterone and men lower in testosterone were more vulnerable to the effects of sleep restriction on emotion processing tasks.
-
•
One night of sleep restriction was associated with lower testosterone in men.
-
•
Sleep-restricted participants showed greater motivated attention towards positive picture stimuli compared to rested controls.
-
•
Sleep-restricted participants were less accurate categorizing sad faces and exhibited a larger N170 ERP to sad faces, reflecting greater neural reactivity.
1. Introduction
Individual differences in response to sleep loss have long been an interest in the field of sleep (Kleitman, 1963). It has been proposed that vulnerability to sleep loss is a trait-like variable, which is supported by replicability across sleep deprivation events (Van Dongen et al., 2004, Van Dongen and Belenky, 2009). The major contributors to the trait component of variability have remained elusive (Van Dongen et al., 2004). The central focus of the current study was to investigate sex hormones as predictors of susceptibility to sleep loss on emotion processing tasks in men and women. Investigating the role of hormones in sleep loss vulnerability is important for understanding relationships between brain function and performance as well as for elucidating the functions of sleep.
Previous research has identified a role for testosterone in the processing of emotional stimuli in rested participants (Van Honk et al., 1999, Derntl et al., 2009, Goetz et al., 2014, Pereira and Moita, 2016). As well, sleep loss has been shown to impact emotion processing (for review, see Beattie et al., 2015), and to reduce testosterone (Carter et al., 2012, Cortés-Gallegos et al., 1983, González-Santos et al., 1989, Cote et al., 2013, Leproult and Van Cauter, 2011, Schmid et al., 2012, Wittert, 2014). Thus, testosterone in men may have a predictive role in emotion processing difficulties following sleep loss. The effect of sleep loss on sex hormones such as progesterone and estradiol in women has not been well studied, but there is a large body of research on the relationship between these hormones and emotion processing in women (Guapo et al., 2009, Derntl et al., 2008a, Derntl et al., 2008b, Van Wingen et al., 2008, Majewska et al., 1986, Van Wingen et al., 2011, Conway et al., 2007). Based on the findings that hormones play a role in emotion processing for well-rested participants, they were investigated as predictors for individual differences in the effects of sleep loss.
1.1. Effects of sleep loss on emotion processing
Sleep deprivation is associated with reduced recognition and production of, but increased reactivity to, facial emotional expressions (Beattie et al., 2015). A study by Van der Helm and colleagues (2010) found diminished performance in the recognition of subtle expressions of happy and angry faces in a sleep deprivation group compared to a control group. Similarly, a population of clinically diagnosed insomnia patients rated fearful and sad faces as less intense than good sleepers (Kyle et al., 2010). In another study, after sleep deprivation, participants displayed fewer facial expressions to emotional images than they had when well-rested (Minkel, 2011). Taken together, these studies indicate that overall emotion processing and expressiveness is blunted after sleep loss.
However, studies employing physiological measures of emotional reactivity have shown increased response to emotional stimuli after sleep loss. Using pupillary dilation as a measurement of emotional reactivity, Franzen and colleagues (2009) reported that sleep-deprived participants experienced greater pupil dilation for negative images than well-rested controls, illustrating enhanced autonomic arousal to emotional stimuli in the sleep-deprived. Brain imaging studies have found increased amygdala activity to negative stimuli after sleep deprivation, and reduced functional connectivity with regulatory regions (Yoo et al., 2007, Motomura et al., 2013). As well, total sleep deprivation was associated with increases in activation in mesolimbic reward areas for positive stimuli (Gujar et al., 2011).
Event-related potential (ERP) methods, which allow for temporal sensitivity in measuring information processing, have also been applied to investigate neural processing of emotional stimuli. For emotional faces, sleep deprived individuals generated a larger N170 ERP peak for threat (fear, angry) faces when the expressions were more subtle, in addition to a reduction in amplitude for sad faces; these data reflect greater encoding of the structural features of a threatening face perhaps due to salience of the emotion, yet diminished processing of sad faces (Cote et al., 2013). For emotional images, sleep deprived participants had a greater Late Positive Potential (LPP) ERP amplitude to emotional images than well-rested controls, but especially for negative images (Cote et al., 2014), reflecting enhanced motivated attention towards emotional stimuli after sleep loss. In contrast, a recent study (Alfarra et al., 2015) reported a failure to discriminate emotional from neutral stimuli that was driven by a larger LPP to neutral stimuli following a night of total sleep deprivation compared to a rested night. Although differences in design, and stimulus and response parameters of the tasks, may account for the different outcomes in these two studies, both converge to show deficits in attention allocated to processing emotional stimuli. In summary, the research converges to show that sleep loss results in reduced behavioural performance, but increased physiological reactivity (with fMRI and ERP studies) during emotion processing tasks particularly for salient emotions.
1.2. Effects of sleep loss on sex hormones
Several studies have reported effects of sleep loss on testosterone concentrations. Reports of decreased testosterone following one night of total sleep deprivation have varied from 18.5% (Carter at al., 2012) to 30.4% decreases in plasma testosterone (Cortés-Gallegos et al., 1983; González-Santos et al., 1989), and a 27% decrease in salivary testosterone that was associated with reductions in reactive aggression on a laboratory task (Cote et al., 2013). Sleep restriction has shown similar, yet smaller, effects of sleep loss on testosterone. When sleeping only the first half of the night for eight nights, men experienced 10–15% reductions in testosterone (Leproult and Van Cauter, 2011). Another study found that two nights of 4-h sleep with an early awakening reduced testosterone, but a late bedtime did not affect testosterone (Schmid et al., 2012). One report indicated that testosterone followed a typical rhythm if a minimum of three hours of slow wave sleep were obtained, and decreased across the duration of time spent awake (Wittert, 2014).
The effect of sleep loss on sex hormones in women is less studied. Women experience large fluctuations in estradiol and progesterone across the 28-day menstrual cycle. The follicular phase of the menstrual cycle is characterized by low concentrations of progesterone with higher levels of estradiol. During the luteal phase, women experience a decrease in estradiol with a significant increase in progesterone (Baker and Driver, 2007, Conway et al., 2007). In men, estradiol has been found to decrease over the first 24 h awake, and continue decreasing from 24 to 48 h of deprivation (González-Santos et al., 1989). Carter et al. (2012) reported that one night of sleep deprivation reduced progesterone but not estradiol in women. As well, in female nurses working nightshifts, 53% of the sample experienced alterations in their typical menstrual cycles (Labyak et al., 2002). Thus, it is possible that one night of sleep restriction would influence sex hormones in women.
1.3. The role of hormones in emotion processing
Testosterone in well-rested men has been found to have relationships to the processing of threat in a growing literature. For instance, higher concentrations of testosterone resulted in increased attention to angry faces in an Emotional Stroop task (Van Honk et al., 1999). Testosterone has been associated with reaction time to fearful faces, and amygdala activity to threatening images but not neutral stimuli in an emotion recognition task (Derntl et al., 2009). Also, testosterone administration increased hypothalamus, periaqueductal gray, and left corticomedial amygdala reactivity to angry versus neutral faces (Goetz et al., 2014), regions implicated in the fight, flight, and freeze responses to potential threats (Pereira and Moita, 2016).
In well-rested women, studies have found differences in performance on emotion processing tasks across the menstrual cycle. A study found follicular women to have greater accuracy for the categorization of angry and sad faces than luteal women and men; the authors argued a unique role for estradiol levels (Guapo et al., 2009). Similarly, for an emotional face categorization task, women in the follicular phase showed greater activation in the temporal lobe and hippocampus during viewing and also had greater behavioural performance on categorization (Derntl et al., 2008a). The authors suggested that there may be an evolutionary basis for women in the follicular phase to be able to accurately detect emotional faces for mate selection. A second study from this group found better emotional face categorization during the follicular phase and a negative correlation between progesterone and accuracy (Derntl et al., 2008b). Greater progesterone was also associated with a tendency to incorrectly categorize a face as angry.
Imaging studies also have revealed menstrual phase differences for emotion processing. Increases in amygdala activity and reductions in functional connectivity with the fusiform gyrus have been connected to progesterone administration due to progesterone increasing GABA activity through effects on GABA receptors (Van Wingen et al., 2008, Majewska et al., 1986). In a later review, van Wingen and colleagues (2011) summarized that the literature suggests higher amygdala activity during emotional tasks in the luteal phase, although, this finding may vary based on the nature of the task. Other studies have supported a possible role for progesterone in increased processing of threat. Conway and colleagues (2007) found that women with higher concentrations of progesterone tended to rate faces displaying fear and disgust as a greater intensity when the eyes were averted. As the luteal phase is a preparation for pregnancy, the authors suggested an increased response to threat and potential illness that may be damaging to a pregnancy, as an averted gaze suggests a health threat in the environment.
1.4. Aims of the current study
The current study investigated hormone concentrations measured pre- and post-sleep restriction as predictors of emotion processing deficits after short-term sleep restriction. Sleep times from a single night at home were verified with actigraphy. It was expected that there would be an effect of this degree of sleep loss on waking function and emotion processing. Specifically, it was predicted that the sleep-restricted group would perform worse than controls behaviourally, and show increased neural reactivity and attention, as measured by the N170 to faces and the LPP to images, particularly towards threatening or negative stimuli. Based on previous work showing effects of testosterone on emotion processing (Van Honk et al., 1999, Derntl et al., 2009, Goetz et al., 2014, Pereira and Moita, 2016), and effects of sleep loss on testosterone (Carter et al., 2012; Cortés-Gallegos, 1983; González-Santos, et al., 1989; Cote et al., 2013; Leproult and Van Cauter, 2011; Schmid et al., 2012; Wittert, 2014), we predicted that low concentrations of testosterone in men after sleep loss would be associated with worse emotion processing performance, particularly for negative and threatening stimuli. Based on the literature showing a relationship between progesterone and emotion processing (Derntl et al., 2008b, Van Wingen et al., 2008, Majewska et al., 1986), and superior performance in the follicular phase (Guapo et al., 2009, Derntl et al., 2008a, Derntl et al., 2008b), it was predicted that greater levels of progesterone in women would be associated with worse emotion processing performance. Given that the luteal phase is marked by higher concentrations of progesterone, it was predicted that women in the luteal phase may perform worse on emotion tasks after sleep loss than women in the follicular phase. While sex hormones have been shown to be related to emotion processing, and sleep loss leads to deficits in emotion processing, no research has investigated the role of hormones as predictors of vulnerability on emotion processing tasks under conditions of sleep loss. While we hypothesized that low testosterone in men and high progesterone in women would be associated with greater vulnerability to sleep loss, an alternative hypothesis is that the relationship between hormones and emotion would not be robust under conditions of sleep loss. Here we investigate the effects of a single night of sleep restricted to 4 h, because we propose that a moderating role of hormones will be revealed under these subtle sleep restriction conditions.
2. Methods
2.1. Participants
This study received ethics clearance from the university Biosciences Research Ethics Board. Of the 104 participants who expressed interest in the study, 18 participants were removed because of elevated mood scores on the Beck Depression Inventory, 3 were removed due to irregular sleep patterns, and 1 for head injury based on screening questionnaires. Another five participants were removed after screening: two for braids or weaves that prevented the use of an electrode cap, one for reporting a diagnosis of depression, one for daily marijuana usage, and one for severely shifted sleep during the baseline sleep diary period. Another three participants were removed after the full protocol due to not following study instructions for sleep times of their condition which was revealed from actigraphy data.
The final sample included 74 individuals, including 25 men, 25 follicular women, 22 luteal women, and 2 menstruating women, who were randomly assigned to a control group (in bed 11 to 7am) or a sleep restriction group (in bed 3 to 7 am). All participants slept the single experimental night at home and were instructed to awake at the same time to control for time awake prior to performance assessment. The sleep restriction group consisted of 37 individuals (13 men, Mage = 21.61, SD = 3.04, 13 follicular women, Mage = 20.23, SD = 2.42, and 10 luteal women (Mage = 19.60, SD = 1.58). The control group also consisted of 37 individuals (12 men, Mage = 22.08, SD = 3.29, 12 follicular women, Mage = 20.67, SD = 1.61, and 12 luteal women, Mage = 20.25, SD = 3.65). One woman in each group reported being actively menstruating on the study day and were removed from analyses when the effect of phase was being considered. Phase for women was determined through self-report of date of last onset of menses and length of cycle and verified through estradiol and progesterone concentrations. There were no significant differences between menstrual phases in estradiol concentrations, (p >.05), but as expected in the luteal group had higher progesterone concentrations than the follicular group at all measurement times (p <.05). Follicular women were between days 8–14 of their cycle, with day 1 being the onset of menses; luteal women were scheduled between days 15–26 and efforts were made not to run individuals on the cusp of phases. Because circadian phase preference may modulate hormone profiles, the Horne-Ostberg Morningness-Eveningness Questionnaire was administered to document chronotype category (Horne & Ostberg, 1976). There were no group differences on categorizations of morningness-eveningness (χ2(2) = 3.00, p=.223). Most participants reported being neither a morning nor an evening type, and none reported being extreme.
All participants reported being healthy good sleepers, non-smokers, right-handed, and were not taking any medications affecting sleep or hormones. They reported no history of psychiatric diagnoses, head injuries, or sleep disorders. Women reported having a regular menstrual cycle and were not taking hormone contraceptives.
2.2. Materials
2.2.1. Questionnaires
Participants were given several screening questionnaires to ensure study eligibility, including a sleep-wake history and habits questionnaire (developed in lab), a modified Beck Depression Inventory (Beck et al., 1996), the State-Trait Anxiety Questionnaire (Spielberger et al., 1983), the Fatigue Questionnaire (Yositake, 1978), and Epworth Sleepiness Scale (Johns, 1991). Women also completed a menstrual cycle questionnaire (Baker et al., 2001, Driver and Baker, 1998) to determine current menstrual phase. Participants were also given additional questionnaires to measure emotional style, and personality, the results of which are not reported here. Three times throughout the protocol, participants were surveyed on the Positive and Negative Affect Scale (PANAS) (Watson et al., 1988), the Stanford Sleepiness Scale (SSS)(Hoddes et al., 1973), and the NASA effort scale (Hitt et al., 1999).
2.2.2. Hormone assays
Cortisol (results not included here), testosterone in men, and estradiol, and progesterone in women were measured in saliva samples collected in 3 mL polystyrene tubes collected at five times: 10:30 pm (evening prior to sleep restriction), 7:00 am, 7:30 am, 4:00 pm, and 4:30 pm. The 7:00 am sample was used as a morning sample time for sex hormones to reflect the changes due to sleep restriction. The 4:00 pm sample was used as an afternoon sample for sex hormones that was closest in time to the performance of the emotion tasks. The 7:30 and 4:30 samples were collected for measures not included here. Saliva samples were centrifuged for 15 min to remove particles, and the supernatant was stored at −20 °C. For determination of hormone concentrations, the supernatant was then thawed and assayed, in duplicate, using commercial enzyme immunoassay kits (DRG International Inc). Optical densities were determined using a Biotek Synergy plate reader at 450 nm. The intra-assay coefficients of variation were 8.3%, 11.4%, and 19.9% for progesterone, testosterone and estradiol, respectively. The inter-assay coefficients of variation were 14.8%, 15.9%, and 20.6% for progesterone, testosterone, and estradiol.
2.2.3. Emotional images task
Images were selected from the International Affective Picture Set (IAPS) (Lang et al., 2005) and included 60 negative images (valence < 3.85, mean valence = 2.71, mean arousal = 5.71), 45 neutral images (valence = 4.5 to 5.5, mean valence = 4.98, arousal = 2.90), and 60 positive images (valence > 7, mean valence = 7.49, arousal = 4.93) (as used in Cote et al., 2014). Images were presented in colour on the full computer screen for 1500 ms with a variable 2–4 s inter-stimulus-interval. The task had two breaks and took approximately 20 min to complete. Participants were asked to rate the emotional valence of the images using keyboard responses (D, F, Space, J, K) on a gradient from very negative to very positive, with the central space bar being “neutral”; the keyboard order was counterbalanced across participants.
2.2.4. Emotional face task
Face stimuli were used from the NIMSTIM database containing cropped black and white emotional faces (Tottenham et al., 2009). Faces were morphed with neutral faces with Norrkross MorphX software to create an emotional gradient from 20–60% of the full emotion (as used in Cote et al. 2013). Because of near chance performance on 20% and 30% morph levels, the analysis focuses on the average across the 40–60% morph levels. Eight models (4 men, 4 women) displayed four different emotion expressions (happy, sad, fearful, angry) and neutral faces. Each face was presented for 400 ms with a 1–2 s inter-stimulus interval. There were eight breaks within the task, and the task took approximately 40 min to complete. Each stimulus was repeated four times overall, for a total of 640 presented stimuli (8 models × 5 morph levels × 4 emotions × 4 repeats). Participants were asked to indicate which emotion was being expressed with a keyboard button press (D, F, Space, J, K) with the central space bar being “neutral”; the keyboard order of emotions was counterbalanced across participants.
2.2.5. Electrophysiological recordings
Recordings were conducted using Neuroscan 64 channel Synamps II amplifiers and Scan 4.5.1 software (Neuroscan Inc, El Paso). Participants were fitted with a 64 electrode Ag/AgCl EEG quikcap and recordings were conducted at a sampling rate of 1000 Hz using DC-200 Hz hardware filters and a 60 Hz notch filter. Online recording references were between Cz and Cpz with AFz as a ground. A vertical and horizontal eye movement and a submental EMG were recorded to assist with artifact rejection prior to ERP analysis. Offline the recordings were re-referenced to an average of the left and right mastoids. A 30 Hz display filter was applied for display only in ERP grand average waveforms.
2.3. Procedure
Participants first underwent a screening process beginning with a phone interview that inquired about their sleep habits, general physical and mental health, menstrual cycle and demographic information. Eligible participants came to a laboratory orientation session where they signed consent, completed further screening questionnaires, and were given a short practice version of the performance assessment battery.
Between the orientation and main study day, a six to eight-day period, participants were asked to keep a regular sleep schedule sleeping from approximately 11 p.m. to 7 a.m. each night. They were also asked to wear an Actigraph monitor (Actigraph, Pensacola) and to complete online sleep diaries to verify study compliance. At 10:30 p.m. on the experimental night, participants provided a pre-sleep restriction 3 mL saliva sample while at home. They were also informed of their experimental group by email and asked to reply. The control group slept from 11:00 p.m. to 7:00 a.m. and the sleep restriction (SR) group slept from 3:00 a.m. to 7:00 a.m. Participants were instructed to awake at 7:00 a.m. at the latest, and to provide the first saliva sample immediately upon awakening and the second sample a half hour later. Participants were told to refrain from exercise, caffeine usage, and napping after the manipulation night. They were also told to eat a healthy breakfast and lunch at regular times. At 1:00 p.m., participants came to the laboratory and were fitted with an electrode cap. Participants performed the emotional face and emotional image tasks as a part of a larger test battery from 2:30 to 3:30 p.m. The final saliva samples were taken at 4:00 and 4:30. Participants were debriefed and free to leave the laboratory by 6:00 p.m.
2.4. Data analysis
Behavioural data were first compared on accuracy and reaction time for both emotion tasks. Outlier trials on reaction time were removed (greater than 6 seconds for faces and 10 seconds for the complex images; fewer than 1% of trials for faces and images). ERP analysis focused on the N170 amplitude at the PO8 site for emotional faces and the Late Positive Potential (LPP) as the average amplitude from 400 to 800 ms at CPz where these potentials were observed to be largest on the scalp from inspection of the topography of grand averages. To analyze ERPs, EEG data were epoched within each task from -100 to 900 ms around all categories of stimuli. A baseline correction was applied to the pre-stimulus interval (-100 to 0 ms). Eye movement artifacts were removed using regression technique in the Scan 4.3.1 software. Data were also inspected visually for artifact and trials removed. For the control condition, there was an average of 67.06 trials for happy, 52.63 for sad, 57.63 for fear, and 56.63 for angry for N170 amplitude. In the SR condition, there was an average of 63.81 trials for happy, 46.00 for sad, 53.13 for fear, and 49.00 for angry. There were no significant group differences on number of trials for face categories. For LPP amplitude, controls had an average of 39.00 trials for positive, 26.06 for neutral, and 41.06 for negative; SR had an average of 37.20 trials for positive, 26.78 for neutral, and 38.89 for negative. There were no significant group differences on number of trials for image categories.
All variables were examined for the presence of outliers and transformed if violating the assumption of normality. A Z score of greater than 3SD was used throughout the study. Participants were only removed from the analysis they were outliers on to be able to maximize the number of participants within the analyses. Hormone and accuracy data were transformed with a square root transformation. Figures are displayed with non-transformed data to show the relationship on the appropriate scale. For violations of variance or sphericity, the Greenhouse-Geisser was applied in all relevant models. Planned analyses included Group (C, SR) by Emotion (4 for faces or 2 for images) mixed-model ANOVAs for behavioural and electrophysiological variables. As well, planned analyses were done examining Group by Emotion by Sex (2) models, and Group by Emotion by Phase (2) models in women. Sex and phase effects are reported when they played a role, and when not a factor, the Group by Emotion model is reported. Testing the hypotheses of the role of hormones in performance included planned correlation and regression analyses. As a planned follow up to significant bivariate correlations, a moderation model testing the relationship between Group (C, SR) and emotional performance was done separately for accuracy, reaction time, and ERP amplitude variables. This model individually tested hormone concentrations at each time point as potential moderators. Planned follow-up used sex, and then phase as additional moderators.
3. Results
Participants in the sleep restriction group were significantly impaired compared to controls on subjective and behavioural measurements, including visual analogue mood scales, PANAS positive mood, subjective sleepiness, standard deviation of RT and 10% slowest RT (p’s < .05). Groups were only marginally different on mean RT, and did not differ on PANAS negative mood (see Table 1). These findings support expectations that one night restricted to 4 hours of sleep was sufficient to produce deficits on classic measures of subjective sleepiness, mood and behavioural performance. The SR and control groups did not differ significantly on total sleep time from diaries or actigraphy during the week prior to the manipulation night (p > .05); however, sleep restricted participants (M = 3 h and 59 min on diaries, range = 180–270 min; M = 3 h and 40 min on actigraphy, range = 169 to 266 min) did receive less sleep than controls on the experimental night, confirming the experimental manipulation (M = 7 h and 37 min on diaries, range = 360 to 480 min; M = 6 h and 32 min on actigraphy, range = 309 to 462 min) (diaries: t(64) = 36.59, p <.001; actigraphy (t(57) = 18.66, p <.001). Table 4 outlines the mean, SD, and range for sleep times in the baseline week and on the experimental night for control and SR groups within men, follicular women, and luteal women.
Table 1.
Effects of sleep loss on subjective mood, and reaction time.
| Controls |
Sleep Restriction |
|||||
|---|---|---|---|---|---|---|
| Scale | M | SD | M | SD | t | p |
| 2:00 PM | ||||||
| VAS:Calm-irritable | 10.62 | 13.02 | 16.67 | 12.73 | - 2.01 | 0.049 * |
| VAS: Happy-Sad | 13.38 | 12.39 | 24.42 | 18.33 | - 3.01 | 0.004* |
| VAS: Energetic-Sluggish | 27.05 | 20.74 | 57.81 | 22.24 | - 6.11 | <.001* |
| VAS:Relaxed-Tense | 12.27 | 12.52 | 22.33 | 15.84 | - 3.01 | 0.004* |
| Subjective Sleepiness | 1.89 | 0.70 | 3.42 | 1.18 | - 6.69 | <.001* |
| PANAS: Positive | 34.19 | 7.15 | 26.94 | 8.76 | 3.83 | <.001* |
| PANAS: Negative | 11.46 | 1.92 | 11.73 | 2.09 | - 0.579 | 0.565 |
| STAI: State | 27.08 | 6.45 | 31.41 | 6.33 | - 2.896 | 0.005* |
| Mean Reaction Time (RT) | 252.81 | 34.14 | 266.68 | 31.88 | -1.90 | 0.061 |
| 10% Slowest RT (ms) | 338.69 | 68.41 | 401.47 | 117.02 | 3.113 | 0.003* |
| Standard Deviation of RT | 41.54 | 18.42 | 64.32 | 50.01 | - 3.15 | 0.002* |
Table 4.
Mean, SD, and range of TST for experimental night and premanipulation period for actigraphy and subjective diary data by subgroup.
| Experimental Night |
Average TST from Premanipulation Period |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Actigraphy | Group | Mean (min) | SD (min) | Range (min) | Actigraphy | Group | Mean (min) | SD (min) | Range (min) |
| Control Men | 408.88 | 21.21 | 378–452 | Control Men | 396.93 | 35.11 | 341–456 | ||
| SR Men | 216.17 | 25.94 | 169–252 | SR Men | 391.73 | 64.71 | 285–505 | ||
| Control Follicular | 394.92 | 42.70 | 325–461 | Control Follicular | 427.85 | 22.09 | 389–470 | ||
| SR Follicular | 217.41 | 17.99 | 177–244 | SR Follicular | 400.86 | 29.74 | 327–438 | ||
| Control Luteal | 372.56 | 56.46 | 309–462 | Control Luteal | 410.88 | 43.15 | 340–507 | ||
| SR Luteal | 233.83 | 23.94 | 206–266 | SR Luteal | 443.06 | 54.22 | 358–505 | ||
| Diary | Diary | ||||||||
| Control Men | 461.09 | 21.17 | 420–482 | Control Men | 473.10 | 43.64 | 397–560 | ||
| SR Men | 242.50 | 8.92 | 230–260 | SR Men | 488.24 | 49.62 | 418–588 | ||
| Control Follicular | 454.09 | 37.90 | 360–480 | Control Follicular | 475.68 | 37.66 | 410–540 | ||
| SR Follicular | 241.17 | 10.13 | 229–270 | SR Follicular | 457.73 | 36.51 | 393–509 | ||
| Control Luteal | 458.18 | 33.64 | 390–490 | Control Luteal | 471.99 | 36.01 | 403–508 | ||
| SR Luteal | 233.78 | 24.23 | 180–270 | SR Luteal | 487.17 | 32.40 | 443–530 | ||
3.1. Effects of sleep restriction on emotion processing
Sleep restriction influenced performance on the emotion processing tasks. Two participants were excluded from emotional image analysis for very low behavioural accuracy (1 C, 1 SR). For behavioural accuracy, the Group (2) by Emotion (3) by Phase (2) ANOVA resulted in a main effect of Emotion (F(2,84) = 20.47, p < .001), and an interaction of Group by Phase (F(1,42) = 4.134, p = .048). Follow up t-tests showed that sleep restricted women in the luteal phase (M = 79.04, SD = 7.17) had lower accuracy than rested controls in the luteal phase (M = 87.10, SD = 4.51) when collapsed across emotion type, (t(19) = 3.161, p = .005). To follow up on the main effect, paired t-test on Emotion Type showed that overall, participants were more accurate for negative images than neutral (t(71) = - 4.01, p < .001) or positive (t(71) = - 3.19, p = .002) (see Table 2 for means). There were no effects for RT.
Table 2.
Group comparisons on emotional behavioural accuracy.
| Controls |
Sleep Restriction |
||||
|---|---|---|---|---|---|
| Behavioural Measure | M | SD | M | SD | p |
| Positive Accuracy | 87.18 | 9.49 | 83.86 | 14.48 | .272 |
| Neutral Accuracy | 83.28 | 12.66 | 81.63 | 17.09 | .880 |
| Negative Accuracy | 91.93 | 7.34 | 88.38 | 9.52 | .115 |
| Happy Face Accuracy | 91.73 | 7.41 | 89.90 | 9.95 | .450 |
| Sad Face Accuracy | 69.05 | 11.52 | 62.46 | 14.24 | .044 |
| Fear Face Accuracy | 78.92 | 12.85 | 74.24 | 14.84 | .168 |
| Angry Face Accuracy | 73.44 | 10.89 | 66.61 | 17.03 | .088 |
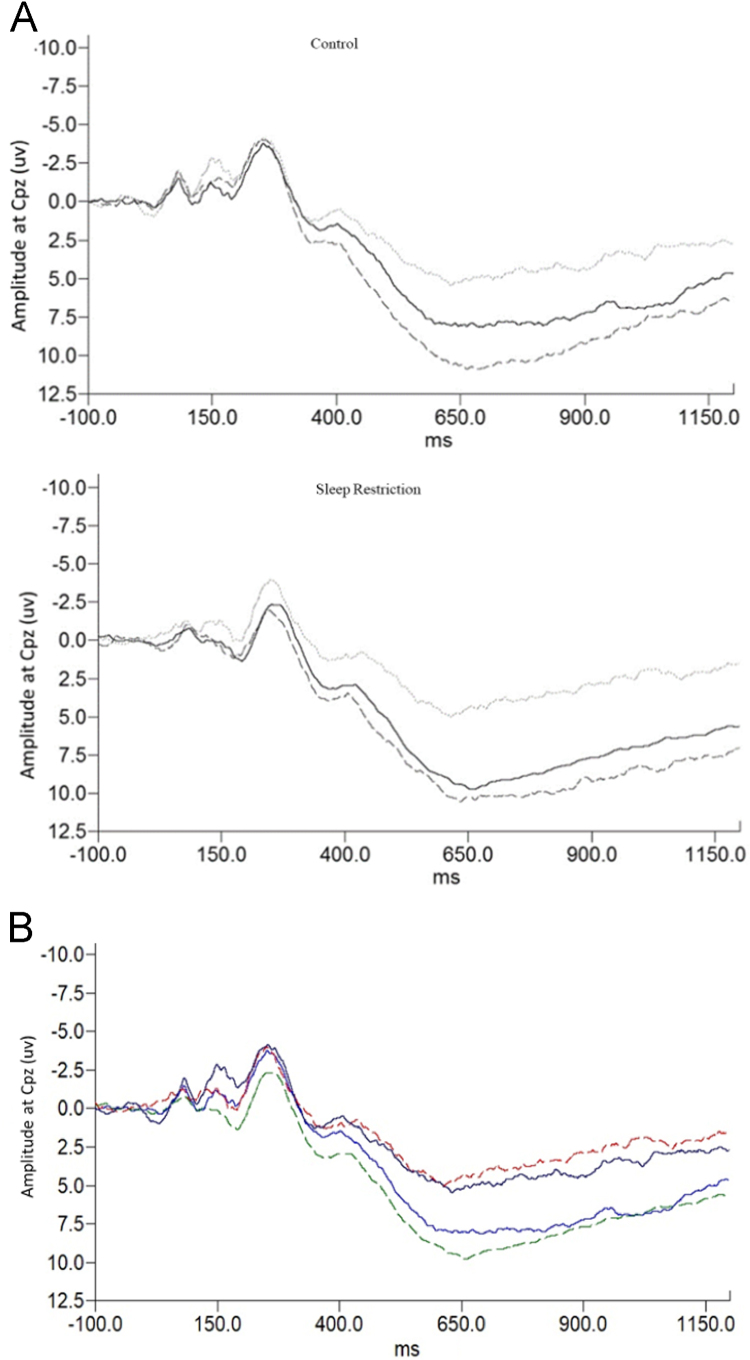
For LPP amplitude at CPz, a Group (2) by Emotion (3) ANOVA resulted in a significant Group by Emotion interaction (F(2,138) = 3.414, p = .036). This relationship was also significant at surrounding sites C1, C4, P2, and a trend at Cz, C2, CP1, and CP2. Inspection of the grand average ERP waveforms (see Fig. 1) illustrated that the nature of the difference was that the LPP was relatively larger to positive stimuli for the sleep restriction group. Thus, to illustrate the significant interaction, a difference score was calculated between negative minus neutral LPP amplitude, and positive minus neutral LPP amplitude. T-tests on this difference score showed that SR participants had a greater difference than controls between the amplitude to positive minus neutral images (t(69) = -2.391, p = .020), but not negative minus neutral images (see Fig. 1). Thus, sleep restricted participants exhibited greater sustained attention towards positive relative to neutral pictures than controls. A Group (2) by Emotion Type (3) by Sex (2) ANOVA revealed a main effect of sex, such that women had a higher LPP amplitude than men regardless of group (F(1,67)=7.836, p=.007), but there was no effect of phase.
Fig. 1.
A. Waveforms for LPP at CPz for control and sleep restricted participants. Solid line indicates positive images, broken grey line indicates negative images, and light grey line indicates neutral images. Note the different pattern of the LPP in sleep restricted participants, where the LPP is larger for positive (relative to neutral) and similar to the negative (relative to neutral). B. Waveforms for LPP at CPz for control and sleep restricted participants. Solid lines indicate controls, and broken lines indicate sleep restricted participants. The red and navy lines are amplitudes to neutral stimuli, and the blue and green lines are amplitudes to positive stimuli. Note the different pattern of the LPP in sleep restricted participants, where the LPP is larger for positive (relative to neutral).
For data from the face processing task, three statistical outliers were removed (1 SR, 2 C). A Group (2) by Face (4) ANOVA for accuracy identifying emotion in faces was carried out on the average of the 40–60% morph levels. There was a significant main effect of Face (F(3,207) = 119.09, p < .001), and a trend for a main effect of Group (F(1,69) = 3.942, p = .051), but no interaction. Given the a priori hypothesis about differences for specific emotions, outcomes of prior research, and group differences apparent in means plots, a more liberal analysis was conducted to explore data by emotion types. Independent t-tests revealed a significant difference between groups (see Table 2 for means) on accuracy for identifying sad faces (t(69) = 2.051, p = .044), and a trend for angry faces (t(69) = 1.732, p = .088). There were no group effects on reaction time, nor effects of menstrual phase or sex.
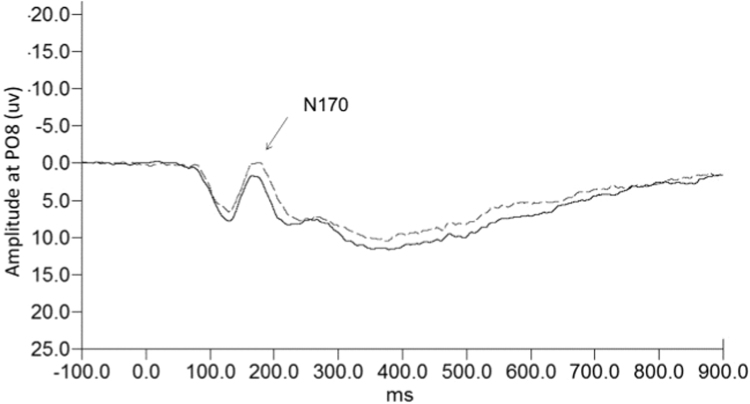
Ten more participants were excluded from the ERP analysis because of too few trials, unclear ERP peak data, or being statistical outliers (5 SR, 5C). Two more participants were excluded on one emotion only, sad and happy respectively, due to unclear peak data for those emotions. In order to have reliable data from ERPs, a sufficient number of trials is needed; thus, it is common to exclude participants who had fewer trials due to low accuracy or artifact. A Group (2) by Face (4) mixed model ANOVA for N170 amplitude at PO8 yielded a significant Group by Face interaction (F(3,180) = 3.461, p = .018). Follow-up planned t-tests to compare groups on the different face types revealed only trends for group differences for the N170 to happy faces (t(61) = 1.916, p = .061) and sad faces (t(61) = 1.951, p = .056). For both face types, sleep restricted participants had a greater amplitude than controls (see Fig. 2 for waveforms to sad faces). To follow up the significant interaction further, a repeated measures ANOVA in the sleep restriction group alone yielded a main effect of emotion (F(3,87) = 3.916, p = .011). There was no main effect of emotion in the control group. A paired samples t-test in the sleep restriction group showed happy was larger than fear (t(30) = -2.209, p = .035), sad was larger than both angry (t(30) – 2.493, p = .018) and fear (t(30) = -2.289, p = .007) for N170 amplitude. There was no effect of sex or phase. Altogether, while the effects were modest, they converge to show behavioural and ERP effects in the sleep restriction group across two tasks, with some evidence of emotion-specific outcomes.
Fig. 2.
Waveforms for N170 at PO8 to sad faces. Broken line is sleep restriction and solid line is controls.
3.2. Effects of sleep restriction on sex hormones
For men, salivary testosterone concentrations were affected by the sleep restriction manipulation. There was a significant interaction between Group and Time (evening, morning) in a mixed model ANOVA (F(1,21) = 5.643, p = .027). Follow-up t-tests revealed no group differences in the evening baseline at 10:30 p.m., and significant group differences at 7:00 a.m. in the morning, with sleep restricted men (M = 64.26 pg/mL, SD = 18.04) having lower testosterone concentrations compared to control men (M = 82.93 pg/mL, SD = 20.36), (t(21) = 3.33, p = .03). The mean change over the night in testosterone was 37.54 pg/mL (SD = 18.33) for control men and 19.81 pg/mL (SD = 17.46) for sleep restricted men. There was no effect of one night of sleep restriction on women’s sex hormone concentrations (see Table 3 for means and standard deviations).
Table 3.
Hormone concentrations (pg/mL) by sex, phase, and time.
| Sex/Phase | Group | Time | |||
|---|---|---|---|---|---|
| 10:30 PM | 7:00 AM | 4:00 PM | |||
| Testosterone | Men | C | 45.39 (17.58) | 82.93 (20.36) | 42.66 (22.95) |
| M(SD) | SR | 43.78 (15.69) | 64.26 (18.04) | 50.40 (29.90) | |
| p | .815 | .030 | .491 | ||
| Estradiol | Follicular | C | 1.93 (1.68) | 2.29 (2.68) | 2.49 (1.98) |
| M(SD) | SR | 2.40 (2.72) | 2.22 (2.31) | 2.55 (2.45) | |
| p | .608 | .949 | .894 | ||
| Total | 2.20 (2.29) | 2.25 (2.43) | 2.52 (2.20) | ||
| Luteal | C | 2.91 (3.35) | 2.93 (2.54) | 2.60 (1.91) | |
| SR | 2.97 (1.52) | 3.24 (2.57) | 3.80 (1.72) | ||
| p | .664 | .774 | .127 | ||
| Total | 2.93 (2.77) | 3.06 (2.49) | 3.08 (1.89) | ||
| Progesterone | Follicular | C | 40.58 (24.68) | 44.31 (18.27) | 34.63 (6.55) |
| M(SD) | SR | 29.57 (8.62) | 35.16 (10.77) | 30.04 (12.78) | |
| p | .130 | .128 | .191 | ||
| Total | 34.35 (17.91) | 39.35 (15.08) | 32.14 (10.45) | ||
| Luteal | C | 67.58 (24.25) | 67.27 (32.76) | 70.50 (32.83) | |
| SR | 87.12 (41.04) | 76.93 (37.59) | 71.68 (37.87) | ||
| p | .236 | .554 | .968 | ||
| Total | 75.40 (32.52) | 71.14 (34.14) | 70.97 (33.95) |
3.3. Relationships with testosterone, sleep loss, and emotion
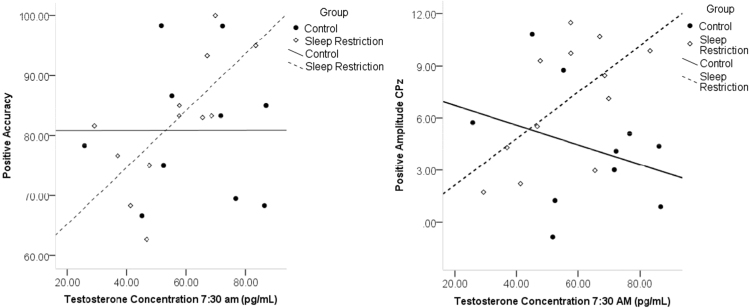
The primary goal of this study was to investigate the role of hormones in emotion processing following sleep restriction. For emotional images, morning testosterone (7:30 a.m.) was significantly correlated with accuracy to positive images (r = .697, p = .012), and the LPP amplitude to positive images (r =.610, p = .035; see Fig. 3) in sleep restricted men, such that lower testosterone was associated with lower performance and processing of positive images. As well, morning testosterone (7:30 a.m.) was positively correlated with accuracy to sad faces (r = .732, p = .007; see Fig. 4). Follow-up moderation analyses yielded a significant overall model between group and sad accuracy for testosterone measures at 7:30 a.m. (F(3,19)= 4.58, p = .041, R2 = .345). There was a significant interaction (b = .749, t = 2.53, p = .020), and simple effects revealed an effect of group at low levels of testosterone (t = - 2.55, p = .019), indicating that sleep restriction was associated with worse sad accuracy only in men with low levels of testosterone. As well, in the sleep restriction group, pre-sleep restriction testosterone (10:30 p.m.) showed positive correlations with fear accuracy (r = .647, p = .017), and this also resulted in a significant moderation model (F(3,20) = 3.58, p = .032, R2 = .350). There was a significant group by hormone interaction (b = .946, t = 2.93, p = .008). Simple effects again revealed a significant effect of group at low levels of testosterone (t = - 3.04, p = .006), meaning sleep restriction was associated with lower fear accuracy only in men with low levels of baseline testosterone.
Fig. 3.
Correlations of morning testosterone with accuracy and LPP amplitude for positive images.
Fig. 4.
Relationship of morning testosterone and sad accuracy. Less testosterone was associated with worse performance after sleep loss.
3.4. Relationships with female sex hormones, sleep loss and emotion
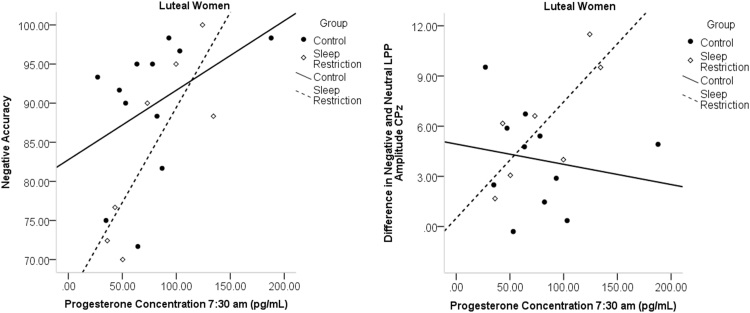
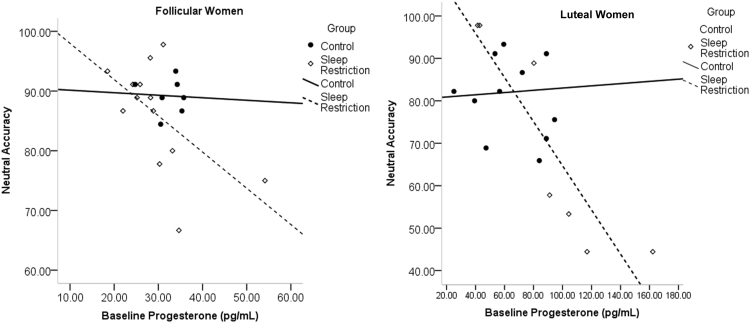
For analysis of estradiol and progesterone, one follicular woman (C), and two luteal women (SR) were excluded for having z-scores three standard deviations above the mean. For emotional images, morning progesterone (7:30 a.m.) was significantly correlated with accuracy to negative images in sleep-restricted luteal women (r = .826, p = .022), and the LPP amplitude difference between negative and neutral images (r = .778, p = .039; see Fig. 5) in sleep-restricted luteal women. Higher progesterone concentrations were associated with higher accuracy to negative images and a larger amplitude difference between the LPP to negative minus neutral images, reflecting greater neural reactivity to negative stimuli. In sleep-restricted luteal women, there was also a significant negative relationship between accuracy for neutral stimuli and evening progesterone (r = - .923, p = .003; see Fig. 6); this relationship was a trend in follicular women (r = - .547, p = .053; see Fig. 6). In the full sample of women, there was a significant moderation model (F(3,37)=- 5.01, p = .005, R2 = .289) for evening progesterone and accuracy for neutral stimuli. There was a significant interaction of evening progesterone and group (b = -.619, t = -2.70, p = .008, R2 = .150). Simple effects analysis showed an effect of group at high concentration of progesterone (b = -1.27, t = -2.12, p = .040). These analyses indicate higher progesterone was associated with lower accuracy to neutral images after sleep loss. Including phase in the model yielded a significant overall model, but lacked statistical power for significant interactions.
Fig. 5.
Correlations of morning progesterone and negative accuracy, and the difference of negative and neutral LPP amplitudes in luteal women.
Fig. 6.
Correlations of baseline progesterone in follicular (left) and luteal (right) women with accuracy to neutral images. More progesterone in women overall was associated with worse performance on neutral images.
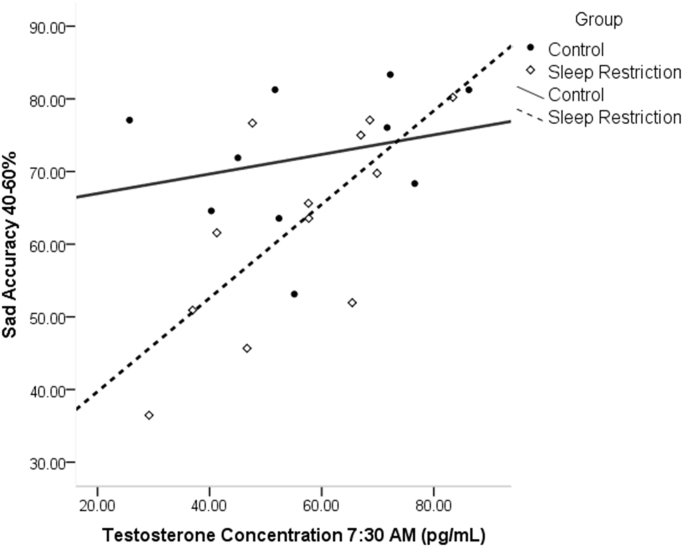
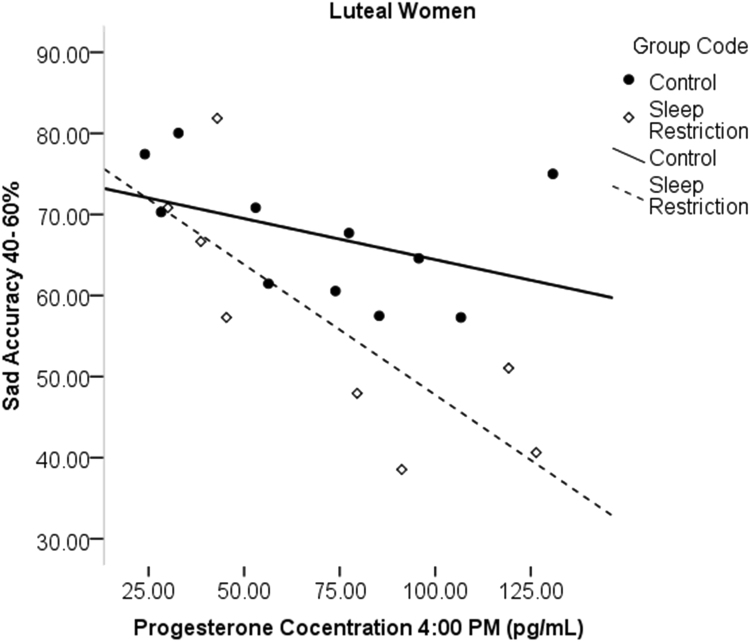
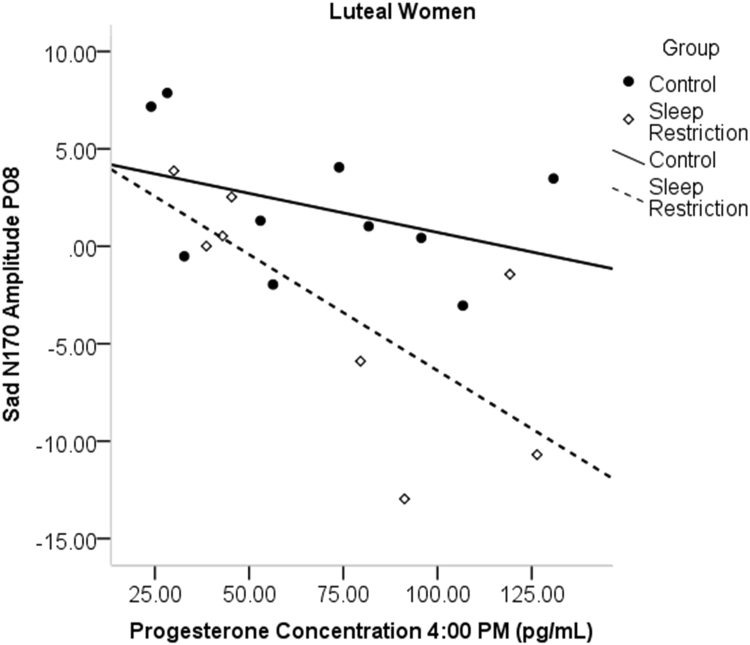
In luteal women, progesterone sampled at various times was correlated with sad accuracy, such that higher progesterone was associated with lower accuracy to sad face categorization after sleep restriction (7:00 a.m.: r = - .842, p = .009; 7:30 a.m.: r = - .867, p = .012, 4:00 p.m.: r = - .772, p = .025; see Fig. 7). Afternoon progesterone concentrations, the sample time point closest to the task, was also significantly correlated with the amplitude of the N170 in sleep restricted luteal women to sad (r = - .754, p = .031; see Fig. 8), happy (r = - .716, p = .046), and fearful faces (r = - .771, p = .025), with more progesterone related to greater N170 amplitude. Estradiol did not show significant associations with emotion processing outcomes in the sleep restriction group.
Fig. 7.
Correlations of progesterone and sad accuracy in luteal women at 4:00 p.m. More progesterone was associated with reduced accuracy after sleep loss.
Fig. 8.
Correlations of afternoon progesterone with N170 amplitude to sad faces in luteal women only. More progesterone was associated with a greater amplitude.
4. Discussion
The current study investigated hormones as predictors of emotion processing deficits experienced after short-term sleep restriction. One night of sleep restriction led to expected deficits in mood, subjective sleepiness, and variability in reaction time. Sleep restriction also affected emotion processing, such that the sleep restricted group had a greater LPP reactivity to positive relative to neutral images when compared to rested controls, and sleep restricted women in the luteal phase had lower accuracy overall on the emotion image categorization task. The sleep restricted group also had lower accuracy for sad face categorization compared to rested controls, and tended to display a larger N170 to sad faces. Sleep restricted men showed a blunted concentration of testosterone in the morning relative to rested control men; however, there were no effects of sleep restriction on hormone concentrations in women noted. Lower testosterone in sleep-restricted men was associated with poorer accuracy to positive images and smaller LPP amplitude to those images. There were also associations between testosterone and accuracy to sad and fearful faces, such that lower testosterone was associated with lower accuracy in sleep-restricted men. In sleep-restricted luteal women, higher progesterone was associated with higher accuracy to negative images, the LPP reactivity to negative relative to neutral images, and larger N170 amplitude to happy, sad and fearful faces; however, higher progesterone was associated with lower accuracy to neutral images and sad faces. Together, these findings indicate that testosterone in men and progesterone in women play a role in the effects of sleep loss on processing emotional information.
We previously investigated the effects of sleep loss on processing emotional facial expressions and images following one night of total sleep deprivation (Cote et al., 2013, Cote et al., 2014). In the current study, we aimed to investigate emotion processing following a more common level of sleep loss, a single night of sleep restricted to four hours by delaying bedtime (03:00 to 07:00) in comparison to a control group in bed eight hours (23:00 to 07:00). The timing of sleep restriction was chosen such that waking performance would be assessed at the same time of day and when all participants had been awake for an equivalent amount of time. It was expected that restriction for even a single night (without opportunity for homeostatic compensation through deeper sleep on a subsequent night of restricted sleep) would lead to the commonly reported effects of sleep loss. Indeed, we found one night of sleep restriction led to expected deficits in subjective mood and sleepiness, and neurobehavioral performance.
Moreover, we supported findings from previous studies showing enhanced neural reactivity to emotional images while sleepy (Yoo et al., 2007, Gujar et al., 2011, Cote et al., 2014). The LPP ERP amplitude of positive relative to neutral images was larger in sleep restricted participants compared to controls, reflecting neural reactivity or more attention allocation towards positive stimuli. Previous research has reported increases in reactivity in multiple mesolimbic regions, including the amygdala, to positive images in sleep deprived individuals when presented with positive and neutral stimuli only (Gujar et al., 2011). Cote et al. (2014) reported larger LPP ERPs to both positive and negative relative to neutral stimuli when all three stimulus types were presented together at random after one night of total sleep deprivation. The increased response to positive stimuli here and in Gujar et al. (2011) may reflect attention bias towards rewarding stimuli following sleep disruption (e.g., greater attention to images of hedonic value such as high-calorie foods). It is possible that changes to processing of the negative IAPS stimuli may only emerge after more substantial sleep disturbance.
Also in the current study, the sleep restricted group had lower accuracy for sad face categorization compared to rested controls, which is consistent with our prior total sleep deprivation study (Cote et al., 2013), and other work that reported a reduced ability to discriminate happy and sad faces and reduced intensity ratings (Killgore et al., 2011, Killgore, 2011). The sleep restricted participants in the current study displayed a larger N170 to sad faces, where as our previous study of total sleep deprivation observed smaller N170 to sad faces. It is possible that there are dose-response effects with the amount of sleep loss and effects of processing of sad faces. Given sad faces are less salient and not as important for survival, effort to process them may be abandoned under extreme level of sleep deprivation; the larger N170 observed here following sleep restriction may represent increased effort to maintain attentional control and accuracy when facing a subtle sleep challenge that is not strong enough to override motivation and drive to perform.
One goal of the current study was to examine the effects of one night of sleep restriction on sex hormone concentrations in men and women. Sleep restricted men had a blunted increase in testosterone over the night, and a lower concentration upon awakening, compared to rested control men. This finding compliments previous research findings of reductions in testosterone after sleep disruption (Carter et al., 2012; Cortés-Gallegos, 1983; González-Santos et al., 1989; Cote et al., 2013; Leproult and Van Cauter, 2011; Schmid et al., 2012; Wittert, 2014), but extends the findings to illustrate that a single night of sleep restriction is sufficient to disrupt testosterone in men. We found no effects of this level of sleep restriction on hormone concentrations in women. Given that Carter et al. (2012) reported reductions in progesterone but not estradiol in a sample of women awake for one night, there may be a dose-response relationship with the degree of sleep loss and alteration to hormone concentrations in women. There is therefore a need for further research into the effects of sleep loss on sex hormones in women.
The primary goal of this investigation was to examine the relationship between hormones and emotion processing following a night of sleep restriction. Based on previous research, it was predicted that lower testosterone in men would be associated with impaired performance on the emotion tasks after sleep loss, especially for negative and threatening stimuli (Van Honk et al., 1999, Derntl et al., 2009, Goetz et al., 2014, Pereira and Moita, 2016). In the emotional images task, morning testosterone concentrations were correlated with accuracy to the positive images and LPP amplitude to positive images in the sleep restriction group, such that lower testosterone was related to poorer performance and processing. The LPP is thought to be generated by a large network associated with emotion processing (Liu et al., 2012), and testosterone has been associated with increased amygdala response (Bos et al., 2013). Thus, the lower testosterone in men after sleep restriction may account for the reduced attention to positive images because of a reduced neural response.
The current study results further supported a relationship between testosterone and performance in face processing. Specifically, baseline testosterone taken in the evening was a predictor of fearful face processing; men with low testosterone at baseline showed deficits in accuracy to fearful faces after sleep loss. In addition, relationships were apparent with morning testosterone and accuracy to sad faces; the men who were lowest in testosterone in the morning, due to sleep loss, also showed lower accuracy identifying sad faces. So, while overall the sleep restriction group showed lowered accuracy for sad face processing, the moderation analysis with hormones allows to more specifically report that men with lower testosterone were uniquely affected following sleep restriction. Overall, the results with testosterone and emotional processing of both faces and images indicate that men with lower levels of testosterone were more vulnerable to the effects of sleep restriction.
For women, it was predicted that progesterone would be negatively associated with emotional processing performance, based on previous research reporting lower accuracy with higher progesterone (Guapo et al., 2009, Derntl et al., 2008a, Derntl et al., 2008b). This prediction was supported with neutral accuracy, and accuracy for sad faces. More progesterone was associated with an increased tendency to rate a neutral image as emotional; specifically neutral images were rated as more positive. This relationship was similar to findings from previous research with a negative relationship of progesterone and neutral accuracy, and general relationships of progesterone with diminished accuracy (Derntl et al., 2008b). For sad faces, more progesterone was again associated with worse performance after sleep restriction in luteal women. As the luteal phase is biologically associated with pregnancy preparation, the processing of sad faces may be selectively reduced as sad faces would be less salient for protection (Jones et al., 2005, Conway et al., 2007). Similarly, other research has found a greater accuracy on sad face categorization for women in the follicular phase than for women in the luteal phase (Guapo, 2009).
There were, however, also positive associations with progesterone and accuracy to negative images in sleep restricted women in the luteal phase. These findings suggest a negativity bias in women in the luteal phase; it is possible that identification of threatening stimuli is of greater salience during the luteal phase due to harm for a potential pregnancy. This bias could be heightened by the effects of sleep loss on processing of negative stimuli (Conway et al., 2007, Jones et al., 2005, Franzen et al., 2009, Cote et al., 2013, Cote et al., 2014). This interpretation is consistent with the findings of reduced accuracy for neutral images, which indicates a tendency to rate images as more emotional. Some research has indicated an increased response to threat in luteal women; for instance, Masataka and Shibasaki (2012) showed faster response times in luteal women for detection if a snake was present in a visual search array, as well as higher intensity ratings for averted disgust and fearful faces (Conway et al., 2007).
Evidence for increased emotional reactivity associated with progesterone is also supported by the electrophysiology data in the current study. There was a relationship with the LPP amplitude difference between negative relative to neutral images, with more progesterone being related to greater relative amplitude to the negative image in luteal women. The LPP data reflect greater allocation of attention towards negative images in sleep restricted luteal women, further supporting an increased salience of these images compared to neutral images. Progesterone in sleep restricted luteal women was also associated with greater N170 amplitude to sad, happy, and fearful faces. Research has found greater amygdala reactivity during the luteal phase and after progesterone administration (Van Wingen et al., 2008, Van Wingen et al., 2011).
Overall, the relationships with progesterone and emotion processing support a difference in salience detection between the phases. In the follicular phase, the accurate categorization of emotional faces may be critical as a factor in mate selection (Derntl et al., 2008a). However, in the luteal phase, it may be that the detection of threat is more salient, because of the need to protect a potential pregnancy (Conway et al., 2007). After sleep loss, it may be that women in the luteal phase become highly sensitive to threat at the expense of processing of other emotions. Overall, results indicate that women with higher concentrations of progesterone, and therefore women in the luteal phase, were more vulnerable to the effects of sleep restriction on emotion processing tasks. In light of research showing inhibitory GABA activity is enhanced by progesterone administration (Van Wingen et al., 2008, Majewska et al., 1986), increasing sleep pressure (via sleep restriction or deprivation) at a time when progesterone is high (luteal phase) may lead to additive effects impacting sleepiness and performance.
There were some design choices and limitations in the current study that may be addressed in future research. We opted for home monitoring of sleep by actigraphy because it is not difficult for young adults to comply with instruction to partially restrict sleep on a single night by staying up late. Sleep was verified by both actigraphy and diary, and groups differed on sleep time on the experimental night, verifying compliance and successful manipulation of sleep. Moreover, the significant and robust group differences on sleepiness, mood, and performance on a variety of measures validate that the experimental manipulation was successful. The addition of baseline measures of these metrics would have allowed for within subject comparisons to further validate the experimental manipulation. While there are benefits to sleeping in a naturalistic home setting, future studies should consider monitoring sleep under stricter laboratory controls particularly since longer and dose-response studies are warranted. As well, studies of continuous sleep restriction in the laboratory would permit investigation of homeostatic changes in polysomnography over multiple nights and the relation to hormones and performance. The pre-study period was also examined at home with diary reports and Actigraphy, and overall the sleep restriction and control groups did not differ on sleep time during this baseline period. However, when subgroups were examined separately in men, follicular and luteal women (presented in Table 4), the Actigraphy data indicated there was a significant mean difference between groups for sleep time within follicular women (p < .05). There were no significant difference between the control and the SR groups when examined for men or for luteal women subgroups. The difference between groups in follicular women was only 27 min, likely reaching significance due to the small variability (note also that other groups that differed by more than 27 min were not significantly different). Importantly, the outcomes of the current study showed the luteal women were more vulnerable to sleep restriction on various measures, and thus the small difference in home sleep time during the pre-experimental baseline week that was found for follicular women does not account for the results reported in the study. Another design consideration is that a between groups design was implemented in the current study because it is not ideal to repeat emotional stimuli within participants, especially the highly salient stimuli like the IAPS task. It would be advantageous to develop new emotion processing tasks that are more appropriate for repeated measures designs, which would permit comparison of participants to their own previous performance for better understanding of individual differences, or comparison between women within their own menstrual cycle.
Great care was taken in this study to enroll naturally-cycling women who reported regular menstrual cycles. However, due to the younger age of our university sample, and the potential for a younger gynecological age, we recognize that some of these women may have had anovulatory cycles since all cycles may not be ovulatory until 8–12 years post menarche (Vihko and Apter, 1984). The mean concentration of progesterone in our sample was lower than the expected values reported by the kit supplier for ages 21 to 50 years (99.1–332.6 pg/mL; DRG International Inc), and highly variable, for luteal women, but normative for follicular women. Our data is similar to data from Finn and colleagues (1988) who in a sample of women from the age of 20 to 39 had a post-ovulation mean salivary progesterone concentration of 74.2 pg/mL or 236 pmol/L. In future research with young adult samples, ovulation could be tested directly with the measurement of luteinizing hormone to address this issue. Another limitation and area for future research is to consider the timing and frequency of hormone collection. For instance, it may have been preferable to measure hormones immediately prior to, during, and following the emotion tasks in the current study. In the current study, emotion tasks were administered between 2 and 3 p.m. and a saliva sample was taken at 4 p.m. The testosterone sample at 4 p.m. appears to higher in SR than controls at the 4 p.m. time point, although the difference was not significant. It is therefore possible that any effects from sleep restriction on testosterone were short-lived and had recovered by the time of testing emotion tasks. However, research has supported a delay of the effect of hormone concentrations on emotion processing performance. For instance, a study by van Honk et al. (1999) found that salivary testosterone concentrations 6 h before the task showed the greatest relationship with emotion processing performance, and relationships were not as strong 4 h before or immediately preceding the task. As well, while we did not find an effect of sleep loss on women’s sex hormones, there were strong effects in the relationship between progesterone and emotion processing in the sleep restricted group. Another issue related to hormone sampling was that hormones were measured in a single sample at each time point. It is recognized that there may be some error introduced due to the pulsatile nature of hormones; however, such measurement error is likely minimized in the current study because it takes several minutes to produce a saliva sample and the within time-point variability is likely much smaller than the between time-point variability over the day. Finally, hormone administration studies during varying degrees of sleep loss would allow for a more systematic investigation of hormones as a mechanism for emotion processing deficits. While the current study showed convergence of effects using two different types of emotion processing tasks (images and faces), there is a need to further investigate the role of sex hormones on vulnerability to sleep loss using tasks from a wide range of cognitive domains.
The current study contributes to the literature by investigating the role of sex hormones in emotion processing following sleep loss. This relationship was investigated in a short-term sleep restriction paradigm that has considerable ecological validity and less of an impact on motivational systems than total sleep deprivation commonly employed. The findings of this study implicate a role for sex hormones in the effects of sleep loss on emotion processing, and their use as a predictor of individual vulnerability to sleep loss. Specifically, low testosterone in men was found to predict poor performance on emotion processing tasks. A highly novel finding of this study was the role of female sex hormones and phase of the menstrual cycle in emotion processing after sleep loss. Progesterone emerged as a strong predictor of performance vulnerability in women, highlighting the luteal phase of the menstrual cycle as a vulnerability period for emotional processing deficits following sleep restriction.
Acknowledgements
This research was funded by the Natural Science and Engineering Research Council (NSERC) (2014-05146) of Canada and publication was funded by the Brock Library Open Access Publishing Fund.
Acknowledgments
Conflicts of interest
None of the authors has any conflicts of interest.
References
- Alfarra R., Fins A.I., Chayo I., Tartar J.L. Changes in attention to an emotional task after sleep deprivation: neurophysiological and behavioral findings. Biol. Psychol. 2015;104:1–7. doi: 10.1016/j.biopsycho.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Baker F., Driver H. Circadian rhythms, sleep, and the menstrual cycle. Sleep. Med. 2007;8(6):613–622. doi: 10.1016/j.sleep.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Baker H., Waner J., Vieira E., Taylor S., Driver H., Mitchell D. Sleep and 24-hour body temperatures: a comparison in young men, naturally-cycling women, and women taking hormonal contraceptives. J. Physiol. 2001;530:565–574. doi: 10.1111/j.1469-7793.2001.0565k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie L., Kyle S.D., Espie C.A., Biello S.M. Social interactions, emotion and sleep: a systematic review and research agenda. Sleep. Med. Rev. 2015;24:83–100. doi: 10.1016/j.smrv.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Beck A., Steer R., Brown G. Psychological Corporation; San Antonio, TX: 1996. Manual for the Beck Depression Inventory-II. [Google Scholar]
- Bos P., van Honk J., Ramsey N., Stein D., Hermans E. Testosterone administration in women increases amygdala responses to fearful and happy faces. Psychoneuroendocrinology. 2013;38:808–817. doi: 10.1016/j.psyneuen.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Carter J.R., Durocher J.J., Larson R.A., DellaValla J.P., Yang H. Sympathetic neural responses to 24-hour sleep deprivation in humans: sex differences. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H1991–H1997. doi: 10.1152/ajpheart.01132.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway C., Jones B., DeBruine L. Salience of emotional displays of danger and contagion in faces is enhances when progesterone levels are raised. Horm. Behav. 2007;51:202–206. doi: 10.1016/j.yhbeh.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Cote K., Jancsar C., Hunt B. Event-related neural response to emotional picture stimuli following sleep deprivation. Psychol. Neurosci. 2014;8:102–113. [Google Scholar]
- Cote K., McCormick C., Geniole S., Renn R., MacAulay S. Sleep deprivation lowers reactive aggression and testosterone in men. Biol. Psychol. 2013;92:249–256. doi: 10.1016/j.biopsycho.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Cote K., Mondloch C., Sergeeva T., Semplonius T. Impact of total sleep deprivation on behavioural neural processing of emotionally expressive faces. Exp. Brain Res. 2013;232(5):1429–1442. doi: 10.1007/s00221-013-3780-1. [DOI] [PubMed] [Google Scholar]
- Cortés-Gallegos V., Castañeda G., Alonso R., Sojo I., Carranco A., Cervantes C., Parra A. Sleep deprivation reduces circulating androgens in healthy men. Arch. Androl. 1983;10(1):33–37. doi: 10.3109/01485018308990167. [DOI] [PubMed] [Google Scholar]
- Derntl B., Kryspin-Exner I., Fernbach E., Moser E., Habel U. Emotion recognition accuracy in healthy young females is associated with cycle phase. Horm. Behav. 2008;53:90–95. doi: 10.1016/j.yhbeh.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Derntl B., Windischberger C., Robinson S. Facial emotion recognition and amygdala activation are associated with menstrual cycle phase. Psychoneuroendocrinology. 2008;33(8):1031–1040. doi: 10.1016/j.psyneuen.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derntl B., Windischberger C., Robinson S., Kryspin-Exner I., Gur R.C., Moser E., Habel U. Amygdala activity to fear and anger in healthy young males is associated with testosterone. Psychoneuroendocrinology. 2009;34:687–693. doi: 10.1016/j.psyneuen.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Driver H., Baker F. Menstrual factors in sleep. Sleep. Med. Rev. 1998;2:213–229. doi: 10.1016/s1087-0792(98)90009-0. [DOI] [PubMed] [Google Scholar]
- Finn M.A., Madden A.T.S., Gosling J.P., Meehan F.P., Tallon D.F., Fottrell P.F. Normal salivary progesterone levels throughout the ovarian cycle as determined by direct enzyme immunoassay. Fertil. Steril. 1988;50(6):882–887. doi: 10.1016/s0015-0282(16)60366-7. [DOI] [PubMed] [Google Scholar]
- Franzen P., Buysse D., Dahl R., Thompson W., Siegle G. Sleep deprivation alters pupillary reactivity to emotional stimuli in healthy young adults. Biol. Psychol. 2009;80(3):300–305. doi: 10.1016/j.biopsycho.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz S., Tang L., Thomason M., Diamond M., Hariri A., Carre J. Testosterone rapidly increases neural reactivity to threat in healthy men: a novel two-step pharmacological challenge paradigm. Biol. Psychiatry. 2014;76(4):324–331. doi: 10.1016/j.biopsych.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Santos M., Gajá-Rodíguez O., Alonso-Uriarte R., Sojo-Aranda I., Cortés-Gallegos V. Sleep deprivation and adaptive hormonal responses of healthy men. Syst. Biol. Reprod. Med. 1989;22(3):203–207. doi: 10.3109/01485018908986773. [DOI] [PubMed] [Google Scholar]
- Guapo V., Graeff F., Zani A., Labate C., dos Reis R., Del-Ben C. Effects of sex hormonal levels and phases of the menstrual cycle in the processing of emotional faces. Psychoneuroendocrinology. 2009;34(7):1087–1094. doi: 10.1016/j.psyneuen.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Gujar N., Yoo S., Hu P., Walker M. Sleep deprivation amplifies reactivity of brain reward networks, biasing the appraisal of positive emotional experiences. J. Neurosci. 2011;31(12):4466–4474. doi: 10.1523/JNEUROSCI.3220-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitt, I., Kring, J., Daskarolis, E., Morris, C., & Mouloua M., 1999. Assessing mental workload with subjective measures: An analytical review of the NASA-TLX index since its reception.
- Hoddes E., Zarcone V., Smythe H., Phillips R., Dement W.C. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10:431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Johns M. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991:50–55. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Jones B., Little A., Boothroyd L. Commitment to relationships and preferences for femininity and apparent health in faces are strongest on days of the menstrual cycle when progesterone level is high. Horm. Behav. 2005;48:283–290. doi: 10.1016/j.yhbeh.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Killgore, D., Balkin, T., & Killgore, W., 2011. Sleep deprivation blunts the perception of emotional intensity in faces. In: Proceedings of the 25th Annual Meeting of the Associated Professional Sleep Societies; June; Minneapolis, MN.
- Killgore, W., 2011. Sleep deprivation impairs recognition of specific emotions. In: Proceedings of the 25th Annual Meeting of the Associated Professional Sleep Societies; June; Minneapolis, MN.
- Kleitman N. Sleep and Wakefulness. University of Chicago Press; Chicago, IL: 1963. Deprivation of sleep. [Google Scholar]
- Kyle S., Beattie L., Spiegelhalder K., Rogers Z., Espie C. Altered emotion perception in insomnia disorder. Sleep. 2014;37(4):775–783. doi: 10.5665/sleep.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labyak S., Lava S., Turek F., Zee P. Effects of shiftwork on sleep and menstrual function in nurses. Health Care Women Int. 2002;23:703–714. doi: 10.1080/07399330290107449. [DOI] [PubMed] [Google Scholar]
- Lang P., Bradley M., Cuthbert B. University of Florida; Gainesville, FL: 2005. International Affective Picture System (IAPS): Instruction Manual and Affective Ratings (Technical Report A-6) pp. 1–9. [Google Scholar]
- Leproult R., Van Cauter E. Effect of 1 week of sleep restriction on testosterone levels in young healthy men. JAMA. 2011;305(21):2173–2174. doi: 10.1001/jama.2011.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Huang H., McGinnis-Deweese M., Keil A., Ding M. Neural substrate of the late positive potential in emotional processing. J. Neurosci. 2012;32(42):14563–14572. doi: 10.1523/JNEUROSCI.3109-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska M., Harrison N., Schwartz R., Barker J., Paul S. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Masataka N., Shibasaki M. Premenstrual enhancement of snake detection in visual search in healthy women. Sci. Rep. 2012;2(307):1–4. doi: 10.1038/srep00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkel J., Htaik O., Banks S., Dinges D. Emotional expressiveness in sleep-deprived healthy adults. Behav. Sleep. Med. 2011;9(1):5–14. doi: 10.1080/15402002.2011.533987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motomura Y., Kitamura S., Oba K. Sleep debt elicits negative emotional reaction through diminished amygdala-anterior cingulate functional connectivity. PLoS One. 2013;8(2):1–10. doi: 10.1371/journal.pone.0056578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira A., Moita M. Is there anybody out there? Neural circuits of threat detection in vertebrates. Curr. Opin. Neurobiol. 2016;41:179–187. doi: 10.1016/j.conb.2016.09.011. [DOI] [PubMed] [Google Scholar]
- Schmid S., Hallschmid M., Jauch-Chara K., Lehnert H., Schultes B. Sleep timing may modulate the effect of sleep loss on testosterone. Clin. Endocrinol. 2012;77(5):749–754. doi: 10.1111/j.1365-2265.2012.04419.x. [DOI] [PubMed] [Google Scholar]
- Spielberger C., Gorsuch R., Lushene R., Vagg P., Jacobs G. Consulting Psychologists Press; Palo Alto, CA: 1983. Manual for the State-Trait Anxiety Inventory. [Google Scholar]
- Tottenham N., Tanaka J., Leon A., McCarry T., Nurse M., Hare T., Nelson C. The NIMSTIM set of facial expressions: judgements from untrained research participants. Psychiatry Res. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dongen H., Baynard M., Maislin G., Dinges D. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;17(4):423–432. [PubMed] [Google Scholar]
- Van Dongen H.P.A., Belenky G. Individual differences in vulnerability to sleep loss in the work environment. Ind. Health. 2009;47:518–526. doi: 10.2486/indhealth.47.518. [DOI] [PubMed] [Google Scholar]
- Van Honk J., Tuiten A., Verbaten R., Van den Hout M., Koppeschaar H., Thijssen J., de Haan E. Correlations among salivary testosterone, mood, and selective attention to threat in humans. Horm. Behav. 1999;36:17–24. doi: 10.1006/hbeh.1999.1521. [DOI] [PubMed] [Google Scholar]
- Van Wingen G., Ossewaarde L., Bäckstrӧm T., Hermans E., Fernández G. Gonadal hormone regulation of the emotion circuitry in humans. Neuroscience. 2011;191:38–45. doi: 10.1016/j.neuroscience.2011.04.042. [DOI] [PubMed] [Google Scholar]
- Van Wingen G.A., van Broekhoven F., Verkes R.J., Petersson M., Bäckstrӧm T., Buitelaar J.K., Fernández G. Progesterone selectively increases amygdala reactivity in women. Mol. Psychiatry. 2008;13:325–333. doi: 10.1038/sj.mp.4002030. [DOI] [PubMed] [Google Scholar]
- Van der Helm E., Gujar N., Walker M. Sleep deprivation impairs the accurate recognition of human emotions. Sleep. 2010;33(3):335–342. doi: 10.1093/sleep/33.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vihko R., Apter D. Endocrine characteristics of adolescent menstrual cycles: impact of early menarche. J. Ster. Biochem. 1984;20(1):231–236. doi: 10.1016/0022-4731(84)90209-7. [DOI] [PubMed] [Google Scholar]
- Watson D., Clark D., Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wittert G. The relationship between sleep disorders and testosterone in men. Asian J. Androl. 2014;16(2):262–265. doi: 10.4103/1008-682X.122586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yositake H. Three characteristic patterns of subjective fatigue symptoms. Ergonomics. 1978;21:231–233. doi: 10.1080/00140137808931718. [DOI] [PubMed] [Google Scholar]
- Yoo S., Gujar N., Hu P., Jolesz F., Walker M. The human emotional brain without sleep – a prefrontal amygdala disconnect. Curr. Biol. 2007;17(20):R877–R878. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]