Abstract
Circadian entrainment to the solar light:dark schedule is thought to be maintained by a simple photon counting method. According to this hypothesis, the pacemaker adjusts the phase of the body’s endogenous rhythms in accordance to the intensity and duration with which it encounters a perceived twilight signal. While previous data have generally supported the hypothesis, more recent analysis has codified other factors besides irradiance that influence the magnitude of resetting responses to light delivered within the same phase of the circadian cycle. In particular, the frequency with which light is alternated with darkness, or whether it’s packaged in millisecond flashes versus continuous blocks, can significantly alter the dose-response relationship. Here, we used a drosophilid model to test whether circadian photon-counting trends can be broken with light administration protocols spanning just 15 minutes. In the early part of the delay zone, a 15-min continuous light pulse was fragmented until it could no longer produce a full-magnitude shift of the flies’ locomotor activity rhythms. The remaining exposure was then reorganized along various fractionation schemes that employed pulses with different widths and interstimulus intervals. Our results suggest that the pacemaker integrates the phase-shifting effects of equiluminous light differently depending on the stimulus pattern with which light is made available. For example, despite having fewer photons, certain ratios of light and darkness could be optimized on a timescale of seconds and minutes so as to achieve pacemaker resetting close to par with steady luminance. These data provide further evidence that the circadian pacemaker’s responses to light entail more than photon counting and motivate continued discussion on how phototherapy can be best optimized in clinical practice to improve conditions linked to circadian impairment.
Keywords: Light, Circadian, Phase shift, Entrainment, Phototherapy
Highlights
-
•
Intermittent delivery of light over seconds and minutes can reset the circadian pacemaker almost as well as continuous exposure.
-
•
Drosophila have the capacity to integrate photic information presented across a series of millisecond xenon flashes (just as rodents and humans).
-
•
Drosophila ananassae offer a potential platform by which to derive generalizable findings concerning intermittent light’s effects on the metazoan circadian system.
1. Introduction
The reciprocity hypothesis summarizes much of the dogma surrounding the study of light’s effects on the circadian pacemaker. It postulates that any timekeeping shift made to light at a given phase of the subjective evening is based solely on the number of photons registered by the pacemaker: the brighter or longer the pulse, the greater the resulting phase jump one should see up to some pseudosaturation level (Takahashi et al., 1984). Given the complexities of photoentrainment, not the least of which are the various signal-to-noise problems encountered within the dynamic light environment of the twilight zones, the reciprocity hypothesis might appear at first glance to be ill-suited to explain the process by which light information gets translated into phase-shifting drive. However, to a first approximation, reciprocity trends appear to hold when conventional artificial lighting is shone on animals for periods exceeding 5 min up to about an hour. The pacemaker integrates photic input the same way over this span such that different trains and durations of non-saturating pulses from ~5 to 60 min will elicit the same final phase shift as long as the overall photon flux is conserved (Best et al., 1999, Dkhissi-Benyahya et al., 2000, Nelson and Takahashi, 1991, Nelson and Takahashi, 1999, Takahashi et al., 1984). In a long-running series of experiments, Czeisler and colleagues have shown that the human pacemaker also tracks reciprocity trends; volunteers exposed to higher illuminance or longer pulses in the early and late biological night exhibit greater delay and advance resetting, respectively (Boivin et al., 1996, Chang et al., 2012, Gronfier et al., 2004, Rimmer et al., 2000, Zeitzer et al., 2000, Zeitzer et al., 2005).
A novel feature of photic resetting that fell out of the Czeisler experimental series was the added observation that, after a particular threshold of exposure, further introduction of light produced diminishing returns on phase movement (i.e., the more the light was shown, the less the photic information got translated into the magnitude of the phase shift; Boivin et al., 1996; Chang et al., 2012; Rahman et al., 2017). This nonlinear duration-efficacy relationship prompted Kronauer to develop a mathematical model for how pacemaker photosensitivity changes throughout continued light exposure (Kronauer et al., 1999). Broadly construed, the Kronauer model proposes that light stimulation always prompts an initial response by the pacemaker that persists in decaying fashion for a period of time after the stimulation has stopped (like the initial pedals of a bicycle wheel). In order to sustain phase-shifting drive, the onsets of the pulse must be long enough to reach full phase-shifting strength and then be balanced with periods of darkness so that steady activation of the pacemaker can occur without triggering competing processes that curb photosensitivity. Maximal phase-shifts are achieved when the rate constants for drive build-up and decay are optimized against the rate constant by which the pacemaker loses photosensitivity (Jewett et al., 1999, Kronauer et al., 1999). Upon testing these assumptions, the Czeisler group found that light delivered intermittently could, indeed, elicit circadian responses almost as effective as those seen after constant light despite the sizable difference in overall exposure (Gronfier et al., 2004, Rimmer et al., 2000).
The pacemaker’s reaction to intermittent light has been explored mainly in the context of protocols where stimulation is delivered in recurring millisecond bursts over an hour (Najjar and Zeitzer, 2016, Van Den Pol et al., 1998, Vidal and Morin, 2007) or within wider segments (5–45 min) that alternate with an hour or half-hour of darkness throughout a large portion of the subjective night (Gronfier et al., 2004, Rimmer et al., 2000). In the current paper, we have asked whether reciprocity trends are “broken” with simple 15-min protocols that do not take advantage of flash perturbation strategies or longer stimulation windows more conducive to building phase-shifting momentum. From circadian time 13 (CT13) to CT13.25, a continuous 15-min light pulse was whittled down until it could no longer produce a full-magnitude delay shift. The remaining exposure was then rearranged into a sequence of patterns involving second and minute-long episodes of light and darkness to see if certain combinations of stimulation and rest could overcome the exposure deficit to reinstate full pacemaker resetting. Our results suggest that the pacemaker is impacted by the pace at which light is introduced on the order of seconds and minutes. When reconciled with the observations that have been made with intermittent light at other time scales, these results hint at a Matryoshka or “nesting doll” operational logic for the metazoan circadian system. They raise the possibility that the pacemaker calculates phase-shifting drive based on Kronauer-like principles functioning at several decreasing temporal resolutions (placed one inside another), where drive is a running computation of pacemaker sensitization and desensitization moving from milliseconds and seconds, seconds to minutes, and minutes to an hour.
2. Materials and methods
To obtain the clearest picture possible on light-induced phase resetting, we tracked the locomotor activity rhythms of Drosophila ananassae, a particular cosmopolitan species of fruit fly that co-evolved with human society. Ananassae show a unimodal pattern of locomotor activity during the day—and consolidated sleep at night—that mimics the diurnal sleep/wake patterns of people and offer a realistic model of human circadian behavior (Prabhakaran and Sheeba, 2012, Prabhakaran and Sheeba, 2013, Prabhakaran and Sheeba, 2014). Ananassae were purchased and regularly replenished from an isofemale line maintained at the Drosophila Species Stock Center (DSSC) at the University of California, San Diego (since relocated to Cornell University; stock # 14024-0371.16; NSF Award #1351502). The animals were reared at 25 °C in DigiTherm® incubators (Tritech Research, Inc., Los Angeles, CA), entrained to a 12:12 LD cycle (600 lx, compact white fluorescent lighting, lights-on at 0700 h, MST), and transferred daily to generate a steady supply of offspring. For phase-shifting experiments, female flies were selected as late-stage, “pharate-adult” pupae, moved onto fresh food, and housed in groups of 5 to 6. A few days post-eclosion, individual animals were placed into Pyrex glass chambers (5 mm outside diameter, 65 mm long) containing a plug of corn flour-nutritional yeast-agar medium on one end (0.8% agar, 3.5% sucrose, 1.7% glucose, 6% fine-grained masa, 1% yeast) and a cotton fitting on the other, and loaded into Trikinetics DAM2 Drosophila Activity Monitors (TriKinetics, Inc., Waltham, MA). Motion was detected and counted by cross-sectioned infrared beams, which transmitted movement information over modem/USB to a computer acquisition software (DAMSystem-308) every 30 s. DAM2 units were situated in climate-controlled vivariums identical to the ones used in colony management and under the same ambient conditions. Two independent environmental trackers (TriKinetics DEnM Drosophila Environment Monitor and the Tritech DeviceCom3 log) continuously measured the temperature and relative humidity of each enclosure’s surrounding air, and archived the intensity of visible-band illumination, providing a quality control record for all the experiments.
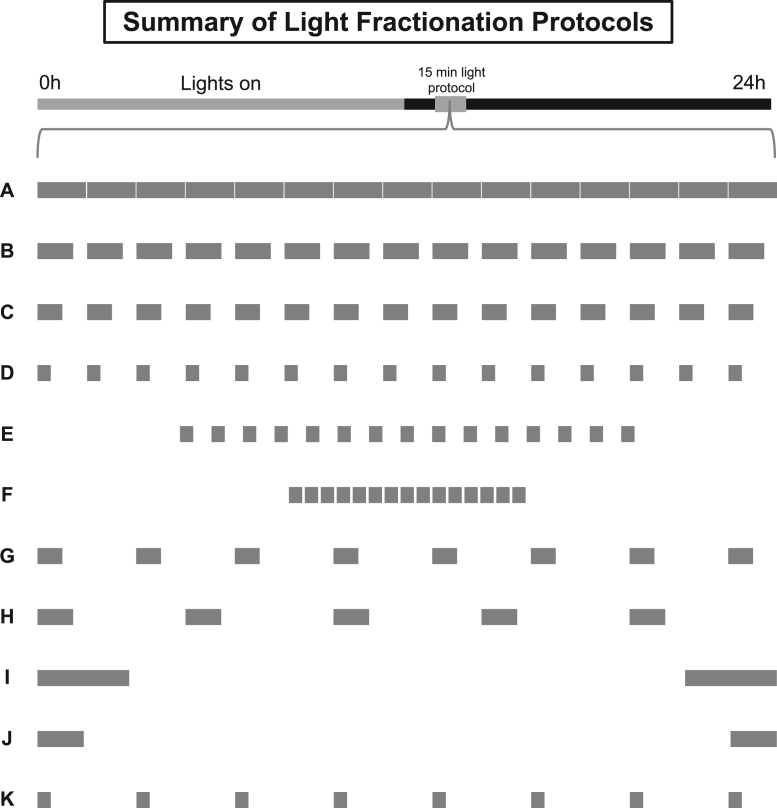
An Aschoff Type II paradigm was used to generate an ananassae PRC to broad spectrum fluorescent light, to establish a proof of concept that flies have the capacity to integrate photic information presented across a series of millisecond xenon flashes (just as rodents and humans), and to quantify the effects of pulse fractionation on phase resetting of locomotor activity rhythms (Aschoff, 1965). This procedure offers an accurate assessment of the natural field shape of the PRC vis-à-vis photoentrainment by avoiding the amplitude inflation that develops after long-term housing in DD (Johnson, 1999, Mrosovsky, 1996) and might be especially relevant for applying data across animal and human models (Mistlberger et al., 1996). For the PRC experiment, flies continued entrainment to the 12:12 LD schedule under which they were reared for 3 days. After lights-off on the last day of the schedule, independent groups received a single 15-min pulse at one of the hourly intervals of the subjective night (i.e., CT13, CT14, etc) or within half-hour increments near the previous LD schedule’s transitions (CT12.5, 13.5, 22.5, or 23.5). This was accomplished by software-controlled activation of the house lamp (600 lx, white fluorescent light; Tritech Research, DeviceCom3™). Post-pulse, animals were left to free-run in DD for 5 days. The millisecond flash and second/minute long fractionation experiments were carried out using the same general steps described above, except that all stimuli were delivered at CT13. For the flash experiment, animals were temporarily removed from their vivarium, placed onto a titanium dioxide paint-coated platform, and exposed to 4ms pulses of xenon light (205 lx) delivered at 1 Hz for 15 min with a ColorDome Ganzfeld lamp (Diagnosys LLC, Lowell, MA). For the fractionation experiment, separate cohorts of flies were administered 1 of the following 11 light regimens (A-K) with the house lamp in the 15 min between CT13 and CT13.25:
-
A.
A uniform, uninterrupted light pulse delivered over 15 min.
-
B.
Intermittent delivery of light for 45 s each minute on the minute (referred to as a 45 s duty cycle).
-
C.
Intermittent delivery of light for 30 s each minute on the minute (referred to as a 30 s duty cycle).
-
D.
Intermittent delivery of light for 15 s each minute on the minute (referred to as a 15 s duty cycle).
-
E.
A series of fifteen 15 s light pulses separated by an interstimulus interval of 30 s (centered in the middle of the CT13-CT13.25 timeframe).
-
F.
A series of fifteen 15 s light pulses separated by an interstimulus interval of 15 s (centered in the middle of the CT13-CT13.25 timeframe).
-
G.
Intermittent delivery of light for 30 s every 2 min.
-
H.
Intermittent delivery of light for 45 s every 3 min.
-
I.
A 225 s light stimulus delivered within two symmetrical 112.5 s blocks distributed at the tail-ends of CT13 and CT13.25. The first bookend pulse began precisely at CT13, while the second pulse ended precisely at CT13.25.
-
J.
A ~113 s light stimulus delivered within two symmetrical 56.5 s blocks distributed at the tail-ends of CT13 and CT13.25. The first bookend pulse began precisely at CT13, while the second pulse ended precisely at CT13.25.
-
K.
Intermittent delivery of light for 15 s every 2 min.
Actogram plots reflecting the daily activity profile for each fly in a given treatment group were created by binning raw 30-sec time series data of individual ananassae. Phase shifts of behavior were calculated by determining the horizontal distance between regression lines fitted through software-called activity onsets and offsets 2 days prior and 2–4 days after light administration (ClockLab Analysis Version 6, Actimetrics, Wilmette, IL). Two days prior to the pulse, the activity onsets of ananassae were always phase-locked to the timing of lights-on in the LD schedule. Post-pulse, transients were observed for a day, but the flies’ behavioral rhythms stably reset by the second DD cycle (hence the start of the regression here). To correct for phase movements that might simply accompany transitions from LD to DD, a control group was transferred into DD without light treatment. Net calculations of onset/offset shifts in the PRC experiment and net calculations of onset shifts in the millisecond flash and fractionation experiments were normalized for the effects of LD schedule removal. Statistical comparisons between onset and offset shifts in the ananassae PRC were made via two-way analysis of variance (ANOVA) followed by Bonferroni-Šídák multiple comparisons testing. Changes in phase-shift magnitude resulting from the various light fractionation schemes were evaluated by one-way ANOVA with Tukey’s post hoc correction. In total, 787 animals were independently evaluated in the PRC experiment (623 with light treatment, 164 DD controls), while 22 were sampled in the millisecond flash experiment. Another 1052 individual flies were queried to examine the effects of second and minute-long bouts of intermittent light.
3. Results
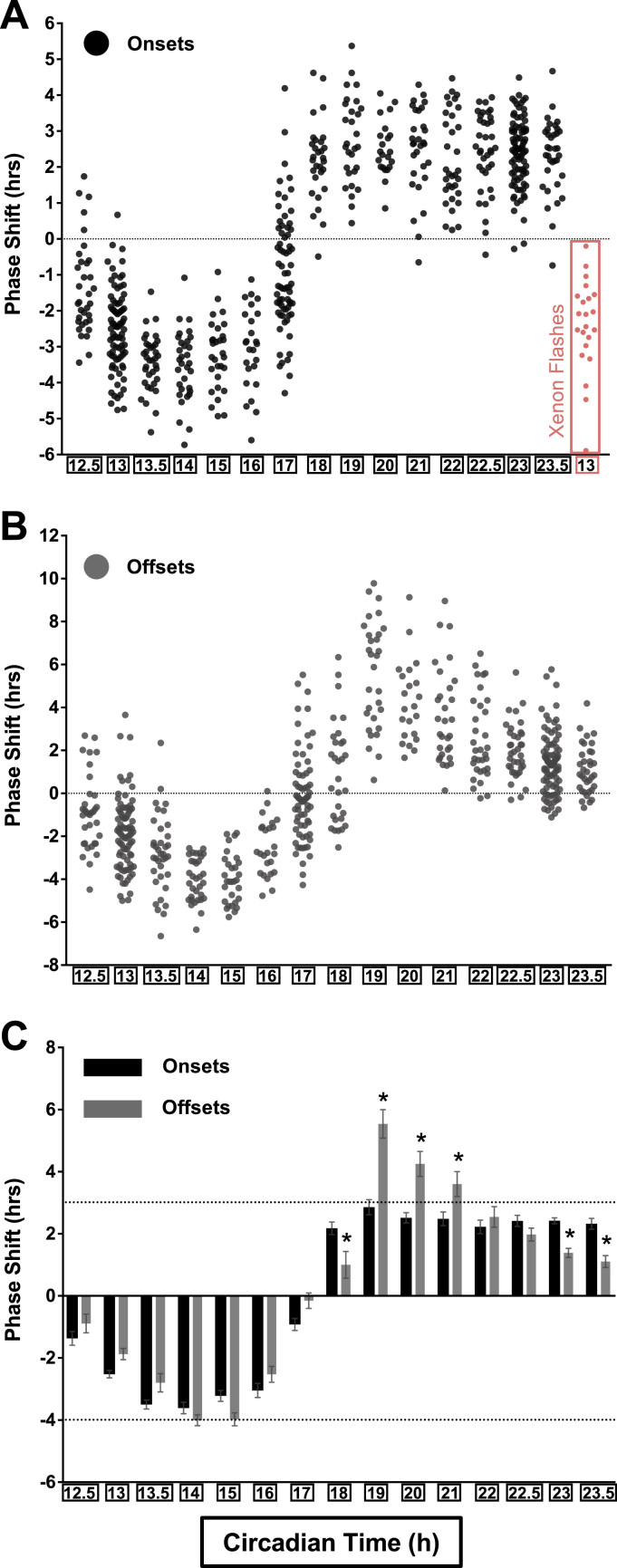
The results of the PRC experiment are reported in two phase marker scatterplots visualizing changes in activity onsets (Fig. 1A) and offsets (Fig. 1B) for individual animals post-pulse. Light administration from CT12.5 to CT17 delayed fly wakeup times, with stimulation at CT14 triggering max delays of almost 4 h. By contrast, pulses given in the latter-half-of-the-night prompted earlier awakenings (main effect of circadian time, F14,1216 = 252.4, p < 0.001). Light presentation at CT19 reset locomotor rhythms by +3–5 h (+2.86 for activity onsets, +5.54 for activity offsets). While targeting of the rest of the advance zone produced steadier shifts that waned only slightly in magnitude from +3 to +2 h for activity onsets (Fig. 1A), the magnitude of the resetting response for activity offsets was significantly more phase-dependent, with graded adjustments occurring between CT18 and CT23.5 (main effect of phase marker, F1,1216 = 8.44, p = 0.0037; phase marker X circadian time interaction, F14,1216 = 10.08, p < 0.001; Sidak’s multiple comparisons test, onset vs. offset, ps < 0.05 at CT18, 19, 20, 21, 23, and 23.5; Fig. 1C). Even so, the variability of the data for the ananassae offset PRC was significantly greater than that observed in the onset PRC at most timepoints tested (F test to compare variances, ps < 0.05 at CT13, 13.5, 17, 18, 19, 20, 21, 22, and 23). The contours of the delay zone and most of the advance zone are conserved between ananassae, wild-type strains of melanogaster, rodents, and humans (Hall and Rosbash, 1987, Khalsa et al., 2003, Schwartz and Zimmerman, 1990). For Drosophila, these similarities do not seem to be influenced by the Aschoff paradigm (Konopka et al., 1991, Saunders et al., 1994, Rutila et al., 1998) or the sex of the animals used to map light resetting (Hall and Rosbash, 1987, Levine et al., 1994, Suri et al., 1998, Saunders et al., 1994).
Fig. 1.
A phase response curve (PRC) to light inDrosophila Ananassae. (A-C) After lights-off on the last day of a 12:12 LD schedule, independent groups of flies were given a single pulse of white fluorescent light (600 lx, 15 min) at one of the 11 hours associated with the subjective evening, or within half-hour increments near the previous LD schedule’s transitions (CT12.5, 13.5, 22.5, and 23.5). They were then kept in DD. The phase shift observed in each fly’s activity rhythm is shown in scatter plot (1 circle = 1 animal) for both behavioral onsets (A; black circles) and offsets (B; gray circles). The average resetting response (± SEM) for the onset and offset markers are graphed side-by-side for each circadian timepoint tested in panel C. Asterisks indicate onset and offset responses that were statistically different (p < 0.05). Delays are plotted in hours with negative numbers, while advances are plotted with positive numbers. A separate group of flies was treated with flashes of xenon light (4ms, 1 Hz, 205 lx, 15 min) at CT13 (insert, salmon color circles). For comparison’s sake, the broken dotted lines at +3 h and -4 h define the amplitude of phase shifts usually observed after light administration in the delay and advance zones of melanogaster (Hall and Rosbash, 1987, Suri et al., 1998). Data were collected from the following number of flies: 34 (CT12.5), 85 (CT13), 37 (CT13.5), 33 (CT14), 30 (CT15), 25 (CT16), 70 (CT17), 30 (CT18), 30 (CT19), 22 (CT20), 30 (CT21), 33 (CT22), 37 (CT22.5), 92 (CT23), 35 (CT23.5), and 22 (Xenon CT13).
Having established an ananassae PRC atlas, we proceeded to examine the effects of intermittent light on locomotor rhythms using 15-min continuous exposure at CT13 as a baseline and activity onset as a phase marker. At the millisecond timescale, 1-Hz flashes of 4ms xenon light (205 lx) delayed locomotor rhythms to the same extent as 15 min of continuous illumination despite a 250× difference in exposure duration (flashes are represented by salmon-colored data points within the insert at the end of Fig. 1A; Mean ± SD of the delay shift for full 15-min exposure, −2.5 ± 1.1 h; for 1-Hz 4ms flashes delivered over 15 min, -2.4 ± 1.3 h). These observations match those that have been made in hamsters, mice, as well as humans (Najjar and Zeitzer, 2016, Van Den Pol et al., 1998, Vidal and Morin, 2007).
The results for the second and minute-long fractionation experiment are summarized in Table 1 alongside a graphical illustration of the different light administration protocols tested (Fig. 2). The average onset shift achieved with 15 s of white fluorescent light (600 lx) delivered on-the-minute for 15 min at CT13 was significantly smaller than that achieved with an unbroken 15-min pulse or with a 45 s or 30 s duty cycle (F10,1126 = 22.87, p < 0.0001; Tukey’s post-hoc test, ps < 0.004; Table 1, protocols A-D). This disparity gave us an opportunity to test how we could take the photon flux implied in the 15 s duty cycle—225 s exposure to 600-lux fluorescent light—and rearrange it so that it created phase shifts rivaling those created with constant illumination between CT13 and CT13.25. In our first attempt at repackaging this stimulus, we evaluated circadian responses to protocols where the fifteen 15 s pulses were separated by shorter interstimulus intervals (ISIs; Table 1, protocols D-F). Instead of the 45 s ISIs used in the 15 s duty cycle regimen, we used ISIs of 30 s and 15 s. Shortening the timeframe in between the 15 s pulses from 45-to-30s did not influence the size of the phase shift to 225 s light exposure (45 s vs. 30 s ISI, p > 0.99). However, shortening the ISI to 15 s engineered significantly larger delays in activity onset that were ~60% of the delays seen with 15 min of uninterrupted light (45 s vs. 15 s ISI, p < 0.02).
Table 1.
|
CT13 Stimulation Protocol |
Light Exposure | Behavior Onset | |
|---|---|---|---|
| ID | Description | Time (sec) | Δ Phase Shift, h (n) |
| A | Continuous illumination (15 min) | 900 | −2.52 ± 0.12 (85)* |
| B | Intermittent pulse 45 out of every 60 s | 675 | −2.36 ± 0.12 (113)* |
| C | Intermittent pulse 30 out of every 60 s | 450 | −1.52 ± 0.09 (120)* |
| D | Intermittent pulse 15 out of every 60 s | 225 | −0.91 ± 0.10 (129) |
| E | Fifteen 15 s light pulses spaced 30 s apart | 225 | −0.94 ± 0.11 (91) |
| F | Fifteen 15 s light pulses spaced 15 s apart | 225 | −1.50 ± 0.13 (91)* |
| G | Eight 30 s light pulses spaced 90 s apart | 240 | −1.87 ± 0.14 (84)* |
| H | Five 45 s light pulses spaced 135 s apart | 225 | −1.65 ± 0.12 (119)* |
| I | Two 112.5 s light pulses spaced 11 min, 15 s apart | 225 | −2.10 ± 0.15 (97)* |
| J | Two 56.5 s light pulses spaced 13 min, 7 s apart | 113 | −1.18 ± 0.10 (176) |
| K | Intermittent pulse 15 out of every 120 s | 120 | −0.54 ± 0.21 (32) |
Fig. 2.
Illustration of light fractionation protocols. After lights-off on the last day of a 12:12 LD schedule at CT13-CT13.25 (grey box), separate groups of flies received either a 15-min pulse of uninterrupted, constant light (A), or intermittent delivery of light according to the following logic: (B) stimulation for 45 s on the minute, (C) stimulation for 30 s on the minute, (D) stimulation for 15 s on the minute, (E) stimulation with fifteen 15 s pulses positioned 30 s apart, (F) stimulation with fifteen 15 s pulses positioned 15 s apart, (G) stimulation for 30 s every 2 min, (H) stimulation for 45 s every 3 min, (I) stimulation for 225 s within two symmetrical 112.5 s blocks distributed at the tail-ends of CT13-CT13.25, (J) stimulation for ~113 s within two symmetrical 56.5 s blocks distributed at the tail-ends of CT13-CT13.25, and (K) stimulation for 15 s every 2 min.
Next, we determined how phase-shifting might be better optimized by condensing the fifteen 15 s pulses into longer duration stimuli separated by incrementally larger relaxation intervals. Instead of delivering 15 s pulses every min between CT13 and CT13.25, we tested regimens where animals were administered 30 s of light every 2 min or 45 s of light every 3 min (Table 1, protocols D, G, and H). Here, the total amount of exposure was generally, but not precisely, conserved: the 45s-every-3min group was stimulated with fluorescent light for a total of 225 s, but for the purpose of preserving the 30 s pulse width, the 30s-every-2min group was stimulated for a touch longer—240 s. That caveat aside, animals treated with both of these condensed protocols showed onset shifts that significantly exceeded the shift achieved with a 15 s duty cycle (ps < <0.0001); moreover, the magnitude of this response reached 66–75% of the shift produced by 15 min of continuous stimulation (Table 1, protocols A, G, and H).
We explored a third and final strategy for improving the information drive of ~225 s of 600-lux fluorescent light by redistributing the 225 s of exposure into 2 large blocks that bookended the window between CT13 and CT13.25 (Table 1, protocols D and I). Notably, although the phase movements induced by 225 s of bookend stimulation fall a little short of the responses seen with uniform light (protocol A), the fractionation scheme still produces onset shifts not statistically different from the 15-min baseline (Tukey’s post-hoc test, p = 0.428). Truncating the bookend stimulation by half, using two 56.5 s blocks (protocol J) instead of two 112.5 s blocks, does not outperform the 15 s duty cycle (protocol D; p = 0.691). Surprisingly, however, the pacemaker drive set in motion by the smaller bookend regimen is comparable to the drive produced by the 15 s duty cycle and is descriptively larger than that produced from another longer exposure regimen with little appreciable circadian effect (i.e., protocol K, 15 s of light presented every 2 min).
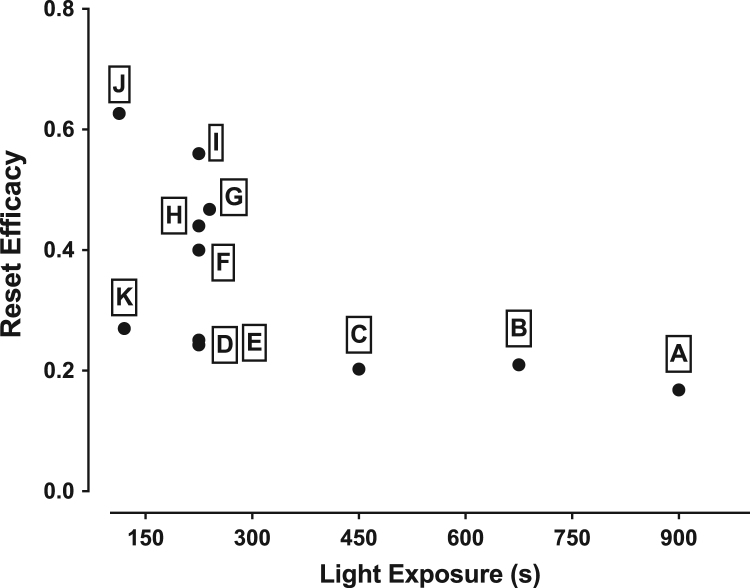
Together, these data suggest that condensing light pulses into macro-second signals—with commensurately larger rest or “decay” intervals—and introducing blocks of stimulation that span the poles of the time window targeted by light treatment can create inordinate circadian responses to an otherwise fixed amount of light at the second-to-minute timescale. In order to further visualize the impact that these light administration strategies had on the pacemaker, we plotted the size of the phase shift mobilized for every 600-lux fractionation protocol tested in the current study as a function of total light exposure. The efficacy plot is shown in Fig. 3. Consistent with predictions made by the Kronauer model, the reset efficacy of our protocols decayed as the exposure period lengthened, from a ratio of 0.25 (min of phase shift produced per second of light invested) for the 120 s, 15s-every-2-min protocol (K) down to a ratio of about 0.17 for the 900 s constant illumination protocol (A). However, intermittent photic stimulation was able to counter the trend near both the 120 s (protocol J) and 225 s (protocols F-I) exposure marks and achieve a reset efficacy per quanta higher-than-expected for the light that was delivered. The larger takeaway from these data in ananassae, and for similarly reported data in humans (Gronfier et al., 2004, Najjar and Zeitzer, 2016, Rimmer et al., 2000), is that light interspersed with periods of darkness is a powerful strategy that can be used to communicate more efficiently with the pacemaker.
Fig. 3.
Pacemaker responses to intermittent versus continuous light. The efficiency with which continuous or intermittent light phase-shifts the ananassae locomotor rhythm is shown for protocols A-K. Reset efficacy is calculated by dividing the size of the shift (in minutes) by the duration of 600-lux fluorescent light used to produce it (in seconds). This ratio is then plotted as a function of the total light exposure.
4. Discussion
Since 1991, the pacemaker has been conceptualized as a photon counter in its computation of twilight (Brown, 2016). Independent of intensity or duration, light is purported to evoke shifts in pacemaker rhythm that increase monotonically with the number of photons received from the light source until the response becomes saturated. From there, further photic stimulation does little to augment phase resetting (Nelson and Takahashi, 1991, Nelson and Takahashi, 1999). One of the assumptions of this model, often dubbed the “reciprocity” hypothesis, is that the pacemaker is agnostic to dynamic fluctuations in the intensity, pulse duration, and repetition with which light is delivered. This agnosticism is supposed to endure despite the staggered differences in processing speed—and differences in temporal lag—that would be expected to occur as a light signal is transduced along several steps in the chain of microprocessors stretching from photoreceptors to the pacemaker to the molecular oscillators residing in and between the pacemaker cells. Owing to limitations in control systems that have historically managed incandescent or fluorescent lamps, little-to-no-study has been done on the circadian effects of dynamic light regimens that change stimulus parameters mid-train or from one syllable to the next, parameters such as pulse shape (e.g., patterns of intensity modulation—boxcar, root raised cosine, sinc, ramp, sawtooth), width (e.g., nanoseconds to minutes), illuminance (e.g., 0–100 lx), or ISI. Nor has any consideration been given to the development of light-streaming methods that apply principles of signal processing or telecommunications optimization (e.g., faster-than-Nyquist signaling, Anderson et al., 2013). In general, the idea that dynamic protocols can enhance phase-shifting when they are tailored for multistep neurophysiology transfer between photoreceptors and the pacemaker has not been met with much empirical study (exploratory work is only starting; e.g., Dobb et al., 2017). However, history would suggest that we haven’t seen the upper limits possible with Type 1 pacemaker resetting yet.
The very existence of a photosensitive circadian system was revealed not through better scientific equipment, but rather due to the advent of a consumer product: electric lighting. To our knowledge, no prior attempts at pacemaker resetting were done before the 1950s (Johnson, 1999), and certainly not before the 20th century, not with wax candles or gas lamps for example. Control of electric lighting made investigation of the pacemaker’s relationship with the photoperiod and twilight possible. It is equally possible that our understanding of those relationships has been colored by the technical limitations of working with incandescent, fluorescent, or xenon light—where temporal control of emission onsets/offsets, wavelength, spot-intensity, spatial coherence, and broad facets of pulse modulation are lacking relative to light-emitting diodes (LEDs) or lasers. Newer light technologies might unlock further principles of light-induced phase resetting. Some of these principles might speak to how the pacemaker reads twilight progressions. Others might be far more synthetic, exploiting photoreceptor and retinal cell behavior to cause massive “time jumps” or to accelerate or decelerate pacemaker oscillations over the course of the photoperiod.
A more basic assumption of the reciprocity hypothesis—and one that is currently more amenable to testing—is that the pacemaker will respond the same way to intermittent trains of light with different pulse widths and ISIs so long as the photon count is identical. This assumption was codified around studies employing a circumscribed set of uniform stimulus trains (Nelson and Takahashi, 1999). In one seminal experiment, for instance, the generalizability of the phase-shifting response mobilized by 300 s of equiluminous light was demonstrated when the exposure was subdivided into: 10 30 s pulses separated by 30 s of darkness; 100 3 s pulses separated by 3 s of darkness; or 1,000 300ms pulses separated by 300ms of darkness (Nelson and Takahashi, 1999). A drawback of this experiment was that no effort was made to vary the ISI in multiples of the pulse duration, possibly leading to a homogenization of the results. In one case, the reset efficacy of the 30 s regimen might have been undercut because the pacemaker required more time (a larger ISI) to translate the drive accumulated from each pulse’s light exposure (Kronauer et al., 1999). In another, the reset efficacy of the shorter regimens might have unduly benefited from quicker ISIs (e.g., facilitating temporal summation; Najjar and Zeitzer, 2016). Our results in ananassae are congruent with these suggestions. Beyond the present work, at least one other investigation has found that varying the pulse width and ISI of an intermittent light regimen can lead to significantly different outcomes for the non-image forming system, of which the pacemaker is a part. Applying pupil constriction as a readout, Vartanian et al. (2015) showed that reciprocity trends could be shattered when human volunteers were administered 63 combinations of light varying in intensity, pulse width, and ISI (i.e., 3 total photon counts × 3 pulse widths × 7 flicker frequencies). Intermittent regimens with lower photon counts could be optimized such that they produced constriction responses exceeding those generated with high-photon yielding regimens and could handily outperform constant light treatments possessing up to 8.5-fold more energy (Vartanian et al., 2015). Counter to the reciprocity hypothesis, the amplitude of responses made by the non-image forming system could not be predicted—in any capacity—by the amount of light a subject was shown (ibid). Our data add to a rapidly growing literature suggesting that the metazoan pacemaker factors in more than just photons when calculating phase shifts that will realign its internal timekeeping to twilight (Gronfier et al., 2004, Najjar and Zeitzer, 2016, Van Den Pol et al., 1998, Vidal and Morin, 2007, Zeitzer et al., 2011, Zeitzer et al., 2014).
Ananassae exhibit circadian responses to millisecond bursts of light that are little different from those manufactured with continuous illumination. This finding—the first in Drosophila—suggests remarkable similarities among animals with regards to how the pacemaker processes different patterns of photic stimulation and suggests that ananassae offer a potential platform by which to derive generalizable findings concerning intermittent light presented at other timescales. After documenting the ananassae response to xenon flashes, we went on to ask whether a continuous 15-min light pulse delivered in the early part of the PRC delay zone could also be broken apart on the order of seconds and minutes so as to produce phase shifts on par with steady light. We hypothesized that these smaller “bits” could be optimized to maximize pacemaker drive with the leftover photons. Two second and minute-long fractionation schemes were able to achieve some semblance of the reset efficacy observed with constant light. One scheme divided the remaining exposure into two 112.5 s pulses that bookended the 15-min timeframe, whereas the others divided the remainder into 30 s and 45 s pulses with 90 s and 135 s ISIs, respectively (i.e., periods of darkness 3× the pulse duration). Speculation is merited on what the significance might be of these various protocols for the substrate pacemaker’s reading interval.
The Kronauer model writ large suggests that light resetting will be maximized when exposure occurs for the duration necessary to mobilize full pacemaker drive without incurring the penalties that come with building photosensitivity (1999). Therefore, the duration with which light is shown must reach, but not exceed, the threshold above which further exposure will be processed inefficiently. Additional light stimulation should be applied in darkness once the resetting drive set in motion by the previous pulse has significantly waned. The Kronauer model makes specific assumptions about the rate constants that mediate drive accumulation and decay for a physiologically relevant range of illuminance. A critical appraisal of these assumptions—and how they were mathematically derived—is outside the scope of this discussion. However, we would like to put forward the suggestion that the balance of light and darkness that will optimize phase resetting depends on the timescale in which a pulse is delivered.
Consistent with previous Kronauer projections (1999), work by Czeisler and colleagues suggests that the optimal LD ratio at the minute-to-hour level is about 1:8 to 1:3 (Gronfier et al., 2004, Rimmer et al., 2000). Here, based on our bookend and 30 and 45 s fractionation schemes, we estimate that circadian drive moving from seconds-to-minutes is optimized at a similar “golden” ratio somewhere between 1:6 to 1:3. No models exist yet on what the circadian effects of nanosecond, microsecond, or millisecond introductions of light should be or how they should be tuned for pacemaker resetting. Nevertheless, our work here and empirical work by several investigators in rodents (Van Den Pol et al., 1998, Vidal and Morin, 2007)—and humans (Zeitzer et al., 2011, Zeitzer et al., 2014)—suggest that the optimal LD ratio to elicit phase-shifting plummets at the millisecond-to-second scale to 1:250–1:500. This means that intermittent flashes of light mount a powerful drive on the pacemaker that accumulates through periods of darkness free of any noticeable feedback penalty. Because the pacemaker is most impressionable to the information available at the beginning of a light pulse, the flashes might generate drive that is highly disproportionate to exposures that are sustained longer (even if only for a second or two), providing an explanation for why xenon flashes in the current study were able to produce larger phase-shifting responses in ananassae than even the best second and minute-long light fractionation strategies tested. Irrespective of the efficacy gap, the data suggest that different optimal patterns of photic stimulation co-exist at multiple timescales over which the pacemaker is likely to sample light. Future experiments will be necessary to determine if Matryoshka-like duty cycles tuned for light delivery over milliseconds, seconds, as well as minutes might be feasibly integrated into a single protocol to maximize pacemaker communication within hourly segments of the subjective night.
Circadian timekeeping is fundamental to mental and physical health. With maturity and age, the temporal organization of the mind and body strays slowly from the Universal Time (UT) that is set with the Earth’s rotation. This disorganization has been linked to progression of several age-related and psychiatric diseases (e.g., Tranah et al., 2011; Walsh et al., 2014). Non-invasive phototherapy has the potential to improve disease outcomes, but the information that the pacemaker tracks in twilight (or any artificial light signal) to assure that a person stays entrained to the outside world is not understood (Kaladchibachi and Fernandez, 2018). By filling in this blank, and by institutionalizing more precise light administration protocols at the level of organizations such as the American Medical Association and American Psychiatric Association (Morgenthaler et al., 2007), circadian science has the opportunity to change the current standard of care for a wide variety of conditions that impair quality of life.
Acknowledgements
We are indebted to Science Foundation Arizona (SFAz), the BIO5 Institute at the University of Arizona, and the State of Arizona and Arizona Department of Health Services for their financial support.
Conflict of interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome. We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us. We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
References
- Anderson J.B., Rusek F., Öwall V. Faster-than-Nyquist signaling. Proc. IEEE. 2013;101:1817–1830. [Google Scholar]
- Aschoff, J., 1965. Response curves in circadian periodicity. Circadian Clocks, pp. 95–111.
- Best J.D., Maywood E.S., Smith K.L., Hastings M.H. Rapid resetting of the mammalian circadian clock. J. Neurosci. 1999;19:828–835. doi: 10.1523/JNEUROSCI.19-02-00828.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin D.B., Duffy J.F., Kronauer R.E., Czeisler C.A. Dose-response relationships for resetting of human circadian clock by light. Nature. 1996;379:540–542. doi: 10.1038/379540a0. [DOI] [PubMed] [Google Scholar]
- Brown T.M. Using light to tell the time of day: sensory coding in the mammalian circadian visual network. J. Exp. Biol. 2016;219:1779–1792. doi: 10.1242/jeb.132167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A.M., Santhi N., St, Hilaire M. Human responses to bright light of different durations. J. Physiol. 2012;590:3103–3112. doi: 10.1113/jphysiol.2011.226555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dkhissi-Benyahya O., Sicard B., Cooper H.M. Effects of irradiance and stimulus duration on early gene expression (Fos) in the suprachiasmatic nucleus: temporal summation and reciprocity. J. Neurosci. 2000;20:7790–7797. doi: 10.1523/JNEUROSCI.20-20-07790.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobb R., Martial F., Elijah D., Storchi R., Brown T.M., Lucas R.J. The impact of temporal modulations in irradiance under light adapted conditions on the mouse suprachiasmatic nuclei (SCN) Sci. Rep. 2017;7:10582. doi: 10.1038/s41598-017-11184-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronfier C., Wright K.P., Kronauer R.E., Jewett M.E., Czeisler C.A. Efficacy of a single sequence of intermittent bright light pulses for delaying circadian phase in humans. Am. J. Physiol. Endocrinol. Metab. 2004;287:E174–E181. doi: 10.1152/ajpendo.00385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J.C., Rosbash M. Genes and biological rhythms. Trends Genet. 1987;3:185–191. [Google Scholar]
- Jewett M.E., Forger D.B., Kronauer R.E. Revised limit cycle oscillator model of human circadian pacemaker. J. Biol. Rhythm. 1999;14:493–500. doi: 10.1177/074873049901400608. [DOI] [PubMed] [Google Scholar]
- Johnson C.H. Forty years of PRCs-What have we learned? Chronobiol. Int. 1999;16:711–743. doi: 10.3109/07420529909016940. [DOI] [PubMed] [Google Scholar]
- Kaladchibachi S., Fernandez F. Precision light for the treatment of psychiatric disorders. Neural Plast. 2018:5868570. doi: 10.1155/2018/5868570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa S.B.S., Jewett M.E., Cajochen C., Czeisler C.A. A phase response curve to single bright light pulses in human subjects. J. Physiol. 2003;549:945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka R.J., Smith R.F., Orr D. Characterization of Andante, a new Drosophila clock mutant, and its interactions with other clock mutants. J. Neurogenet. 1991;7:103–114. doi: 10.3109/01677069109066214. [DOI] [PubMed] [Google Scholar]
- Kronauer R.E., Forger D.B., Jewett M.E. Quantifying human circadian pacemaker response to brief, extended, and repeated light stimuli over the phototopic range. J. Biol. Rhythm. 1999;14:501–516. doi: 10.1177/074873099129001073. [DOI] [PubMed] [Google Scholar]
- Levine J.D., Casey C.I., Kalderon D.D., Jackson F.R. Altered circadian pacemaker functions and cyclic AMP rhythms in the Drosophila learning mutant dunce. Neuron. 1994;13:967–974. doi: 10.1016/0896-6273(94)90262-3. [DOI] [PubMed] [Google Scholar]
- Mistlberger R.E., Marchant E.G., Sinclair S.V. Nonphotic phase-shifting and the motivation to run: cold exposure reexamined. J. Biol. Rhythm. 1996;11:208–215. doi: 10.1177/074873049601100303. [DOI] [PubMed] [Google Scholar]
- Morgenthaler T.I., Lee-Chiong T., Alessi C. Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. Sleep. 2007;30:1445–1459. doi: 10.1093/sleep/30.11.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrosovsky N. Methods of measuring phase shifts: why I continue to use an Aschoff type II procedure despite the skepticism of referees. Chronobiol. Int. 1996;13:387–392. doi: 10.3109/07420529609012662. [DOI] [PubMed] [Google Scholar]
- Najjar R.P., Zeitzer J.M. Temporal integration of light flashes by the human circadian system. J. Clin. Investig. 2016;126:938–947. doi: 10.1172/JCI82306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D.E., Takahashi J.S. Sensitivity and integration in a visual pathway for circadian entrainment in the hamster (Mesocricetus auratus) J. Physiol. 1991;439:115–145. doi: 10.1113/jphysiol.1991.sp018660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D.E., Takahashi J.S. Integration and saturation within the circadian photic entrainment pathway of hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1999;277:R1351–R1361. doi: 10.1152/ajpregu.1999.277.5.R1351. [DOI] [PubMed] [Google Scholar]
- Prabhakaran P.M., Sheeba V. Sympatric Drosophilid species melanogaster and ananassae differ in temporal patterns of activity. J. Biol. Rhythm. 2012;27:365–376. doi: 10.1177/0748730412458661. [DOI] [PubMed] [Google Scholar]
- Prabhakaran P.M., Sheeba V. Insights into differential activity patterns of drosophilids under semi-natural conditions. J. Exp. Biol. 2013;216:4691–4702. doi: 10.1242/jeb.092270. [DOI] [PubMed] [Google Scholar]
- Prabhakaran P.M., Sheeba V. Simulating natural light and temperature cycles in the laboratory reveals differential effects on activity/rest rhythm of four Drosophilids. J. Comp. Physiol. A. 2014;200:849–862. doi: 10.1007/s00359-014-0927-x. [DOI] [PubMed] [Google Scholar]
- Rahman S.A., Hilaire M.A.S., Chang A.M. Circadian phase resetting by a single short-duration light exposure. JCI Insight. 2017;2:e89494. doi: 10.1172/jci.insight.89494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmer D.W., Boivin D.B., Shanahan T.L., Kronauer R.E., Duffy J.F., Czeisler C.A. Dynamic resetting of the human circadian pacemaker by intermittent bright light. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R1574–R1579. doi: 10.1152/ajpregu.2000.279.5.R1574. [DOI] [PubMed] [Google Scholar]
- Rutila J.E., Maltseva O., Rosbash M. The tim SL mutant affects a restricted portion of the Drosophila melanogaster circadian cycle. J. Biol. Rhythm. 1998;13:380–392. doi: 10.1177/074873098129000200. [DOI] [PubMed] [Google Scholar]
- Saunders D.S., Gillanders S.W., Lewis R.D. Light-pulse phase response curves for the locomotor activity rhythm in period mutants of Drosophila melanogaster. J. Insect Physiol. 1994;40:957–968. [Google Scholar]
- Schwartz W.J., Zimmerman P. Circadian timekeeping in BALB/c and C57BL/6 inbred mouse strains. J. Neurosci. 1990;10:3685–3694. doi: 10.1523/JNEUROSCI.10-11-03685.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri V., Qian Z., Hall J.C., Rosbash M. Evidence that the TIM light response is relevant to light-induced phase shifts in Drosophila melanogaster. Neuron. 1998;21:225–234. doi: 10.1016/s0896-6273(00)80529-2. [DOI] [PubMed] [Google Scholar]
- Takahashi J.S., DeCoursey P.J., Bauman L., Menaker M. Spectral sensitivity of a novel photoreceptive system mediating entrainment of mammalian circadian rhythms. Nature. 1984;308:186–188. doi: 10.1038/308186a0. [DOI] [PubMed] [Google Scholar]
- Tranah G.J., Blackwell T., Stone K.L. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann. Neurol. 2011;70:722–732. doi: 10.1002/ana.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Pol A.N., Cao V., Heller H.C. Circadian system of mice integrates brief light stimuli. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1998;275:R654–R657. doi: 10.1152/ajpregu.1998.275.2.R654. [DOI] [PubMed] [Google Scholar]
- Vartanian G.V., Zhao X., Wong K.Y. Using flickering light to enhance nonimage-forming visual stimulation in humans. Investig. Ophthalmol. Vis. Sci. 2015;56:4680–4688. doi: 10.1167/iovs.15-16468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal L., Morin L.P. Absence of normal photic integration in the circadian visual system: response to millisecond light flashes. J. Neurosci. 2007;27:3375–3382. doi: 10.1523/JNEUROSCI.5496-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C.M., Blackwell T., Tranah G.J. Weaker circadian activity rhythms are associated with poorer executive function in older women. Sleep. 2014;37:2009–2016. doi: 10.5665/sleep.4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitzer J.M., Dijk D.J., Kronauer R.E., Brown E.N., Czeisler C.A. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J. Physiol. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitzer J.M., Fisicaro R.A., Ruby N.F., Heller H.C. Millisecond flashes of light phase delay the human circadian clock during sleep. J. Biol. Rhythm. 2014;29:370–376. doi: 10.1177/0748730414546532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitzer J.M., Khalsa S.B.S., Boivin D.B., Duffy J.F., Shanahan T.L., Kronauer R.E., Czeisler C.A. Temporal dynamics of late-night photic stimulation of the human circadian timing system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;289:R839–R844. doi: 10.1152/ajpregu.00232.2005. [DOI] [PubMed] [Google Scholar]
- Zeitzer J.M., Ruby N.F., Fisicaro R.A., Heller H.C. Response of the human circadian system to millisecond flashes of light. PLoS One. 2011;6:e22078. doi: 10.1371/journal.pone.0022078. [DOI] [PMC free article] [PubMed] [Google Scholar]