Abstract
Giant cell tumor of the bone (GCTB) is a locally aggressive tumor with a certain distant metastatic rate. For sacral GCT (SGCT) and pelvic GCT (PGCT), surgery has its limitations, especially for unresectable or recurrent tumors. Selective arterial embolization (SAE) is reported to be an option for treatment in several cases, but there are few systematic reviews on the effects of SAE on SGCT and/or PGCT. Medline and Embase databases were searched for eligible English articles. Inclusion and exclusion criteria were conducted before searching. All the clinical factors were measured by SPSS software, with P‐values ≤0.05 considered statistically significant. A total of 9 articles were retrieved, including 44 patients receiving SAE ranging from 1 to 10 times. During the mean follow‐up period of 85.8 months, the radiographic response rate was 81.8%, with a local control and overall survival rate of 75% and 81.8%, respectively. No bowel, bladder, or sexual dysfunction was observed. Three patients developed distant metastases and finally died. Patients with primary tumors tended to have better prognosis than those with recurrence (P = 0.039). The favorable outcomes of SAE suggest that it may be an alternative treatment for SGCT and PGCT patients for whom surgery is not appropriate.
Keywords: Local control, Overall survival, Pelvis, Sacral giant cell tumor, Selective arterial embolization
Introduction
Giant cell tumor of bone (GCTB) is defined as a potential locally aggressive tumor with a distant metastatic rate of approximately 6.6%1, 2, 3, 4, 5, 6. It occurs most in the second to fourth decades of young adults, but also can be found in patients at any other period. Morbidities between men and women are almost equal. Epiphysis and metaphysis of long bones are the most prevalent sites among the GCT occurrence sites, especially at the distal femur and proximal tibia. Sacral giant cell tumor (SGCT) is the third most common GCTB. SGCT accounts for 2%–8% of all GCTB cases, while pelvic giant cell tumors (PGCT) are less frequent7, 8, 9, 10, 11. Pain is the most common complaint due to the osteolytic destruction. Other clinical manifestations include pathological fracture, losing control of bladder and bowel, as well as sexual dysfunction. Pathological fracture is generally considered as a potential predictor of higher local recurrence and distant metastases rate. GCTB are usually a solid brown mass. Histologically, they contain substantial multinucleated osteoclast‐like giant cells and mononuclear stromal cells. According to their radiological appearance, GCTB are classified by Campanacci et al. 5 into three grades: Stage I, latent; Stage II, active; and Stage III, aggressive.
Although surgery remains the treatment of choice for local SGCT, it is limited by a high recurrence rate and heavy surgical bleeding due to the complex anatomic structures, regardless of wide excision or intralesional curretage8, 12. In addition, neurological dysfunction at the expense of incontinence and sexual dysfunction could lead to psychological issues after surgery13. Pelvic instability is also a problem for surgery, especially for en‐bloc resection, and gives rise to difficulties in reconstruction14, 15. Moreover, surgical intervention plays an extremely limited role in those refractory and unresectable GCTB. Adjuvant therapies such as radiotherapy (RT), cryosurgery, phenol, cementation and bisphosphonate, as well as denosumab, were used alone or in conjunction with other treatments. RT was reported to be an alternative modality for SGCT or PGCT, but RT‐induced malignant transformation cannot be overlooked16.
Selective arterial embolization (SAE), as a form of non‐operative management, has been proved as a viable option for the treatment of GCTB because it can reduce the tumor size and/or induce ossification. On that condition, patients could acquire pain relief and regain further surgical opportunities. However, most published reports focus on the adjuvant preoperative treatment followed by surgery, or on only a few patients that received SAE as a major treatment; on top of this, few systematic reviews have been reported so far17, 18, 19. In this study, we systemically searched the relevant published case reports (series) and did a comprehensive evaluation of the value of SAE, seeking credible references for evidence‐based decision‐making.
Materials and Methods
Medline and Embase databases are the main sources of our research for eligible English articles from 1 January 1978 to 31 December 2015, using the search term “(arterial embolization OR arterial occlusion) AND giant cell tumor.” The inclusion criteria included: (i) histologically confirmed SGCT or PGCT by biopsies; (ii) SGCT or PGCT not suitable for surgical treatment (e.g. poor general condition or rejection of surgeries); (iii) SGCT or PGCT patients receiving SAE; and (iv) clinical features, prognosis and follow‐up time were precisely recorded. The exclusion criteria were as follows: (i) SGCT or PGCT patients receiving adjuvant preoperative SAE followed by surgery; (ii) non‐specific follow‐up time; and (iii) vague results of prognostic factors. The databases were initially reviewed by title and abstract content, and then corresponding articles were read and analyzed completely according to the inclusion and exclusion criteria.
Information on the included patients was collected, including basic information (ID, age, and sex), status of tumor (site, primary or recurrent, previous treatments, main symptoms, and the initial and latest volume of tumor), embolization materials and frequency, various prognostic factors, and follow‐up time. The prognostic factors were as follows: response to SAE was defined as pain relief, tumor size decreasing, as well as radiographic response. Radiographic response was defined as ossification of the tumor margin. Local control (LC) was defined as confirmed lack of disease progression irrespective of distant metastasis. The LC rate was discreetly recorded as 2‐, 5‐, 10‐year, and overall LC rate. The follow‐up time was from the beginning of the first SAE to the patient's death or loss to follow‐up. Two researchers searched the data independently, and differences were discussed for resolution.
As for statistical analysis, we used the mean, median, range, and confidence interval to describe quantitative data, counts, and percentage for qualitative data. Kaplan–Meier survival analysis was adopted to estimate the LC and overall survival (OS) rate; different factors were compared using the log‐rank test. Patients who died of unrelated disease were included as censored data during survival analysis. The χ2‐test or the Fisher exact test was performed to identify possible factors that could predict SAE outcomes. Statistical significance was identified with P‐values <0.05 by using SPSS statistics version 21.
Result
Article Selection Results
Our strategy of article selection is described concretely in the flow chart (Fig. 1). A total of 104 articles from Medline and Embase as well as the relevant references were used for searching. Based on the previous inclusion and exclusion criteria, 44 patients from 9 articles were involved in our systematic review; 1 study showing similar data to another was excluded. Among 9 articles, the publication time varied from 1978 to 2013, comprising 34 females and 10 males, with a mean age of 34.4 ± 13.3 years (median, 31.5 years; range, 15–68 years)20.
Figure 1.
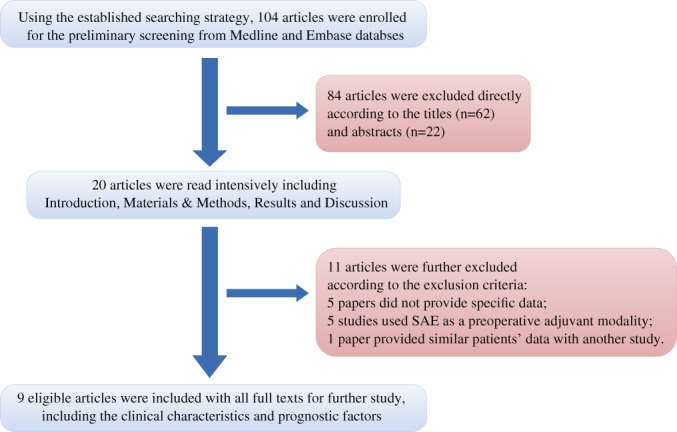
A flow chart of article selection strategy.
Description of Patients' Clinical Features
The clinical data of all patients are shown comprehensively (please see the Appendix)9, 21, 22, 23, 24, 25, 26, 27. For the location of lesions, 41 (93.2%) patients' lesions were detected in the sacrum, while only 4 patients' lesions (1 had GCTB of sacrum and pelvis) were found in the ilium. For clinical manifestation, lower back pain was the most common symptom, followed by leg pain, lower extremity numbness, and neurological deficit. Nine patients presented with recurrent diseases who had failed to respond to the previous treatments; the additional 2 patients with primary tumors received RT or curettage before SAE. For embolization materials, 40 (90.9%) cases were reported to use Gelfoam or morselized polyvinyl alcohol particles for peripheral occlusion and stainless steel coils for central occlusion, while superabsorbent polymer microsphere (SAP‐MS) was applied in 4 patients instead (reported by Nakanishi and his collaborators)28. No bowel, bladder, or sexual dysfunction was observed, except that 6 patients had varying levels of neurological complications, including permanent left‐peroneal nerve palsy (Case 11), foot drop (Case 40), and mild numbness and muscle weakness (4 cases).
Statistical Description of Prognostic Indicators
During a mean follow‐up period of 85.8 months (median, 74.6 months; range, 2–277 months), the SAE ranged from 1 to 10 times (mean, 4.1 times; median, 4.0 times). All patients were responsive to SAE, and the objective radiographic response rate was 81.8% (36/44). Notably, 12 (27.3%) patients were followed up for more than 120 months. In our study, according to the Kaplan–Meier curve, the 2‐, 5‐, 10‐year LC rate was 93.2% (41/44), 90.9% (40/44), and 81.8% (36/44), respectively. A total of 33 (33/44, 75.0%) patients had LC at the final follow‐up. A total of 8 (8/44, 18.2%) patients died of disease, among whom 1 had lung metastasis before SAE, while 2 after series SAE, with a 2‐, 5‐, 10‐year, and OS rate of 90.9% (40/44), 88.6% (39/44), 86.3% (38/44), and 81.8% (36/44), respectively. The mean survival time of these 8 deceased patients was 65.2 months (range, 8.0–172.0 months). Of these, 1 died of renal cell carcinoma during the 205‐month follow‐up period, and 6 experienced neurological complications, including permanent left‐peroneal nerve palsy, foot drop, unilateral ankle dorsiflexion weakness, transient toe numbness, and minimum hamstring muscle weakness, but none had complaints of incontinence or sexual dysfunction. The overall LC and OS rate are illustrated by the Kaplan–Meier function curve exhibited in Figs 2 and 3.
Figure 2.
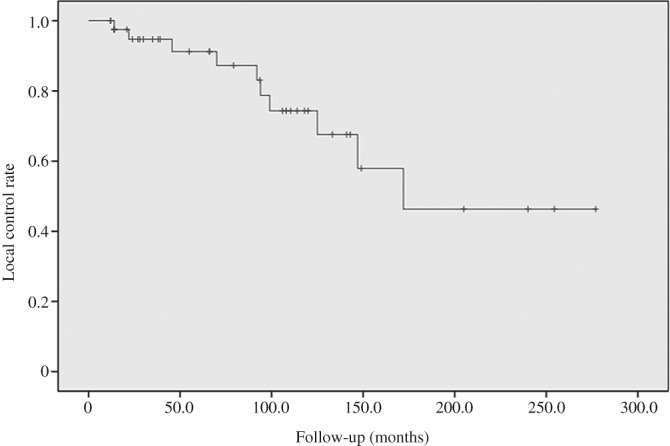
Kaplan–Meier curve of local control concerning selective arterial embolization (SAE) for sacral or pelvic giant cell tumor (GCT).
Figure 3.
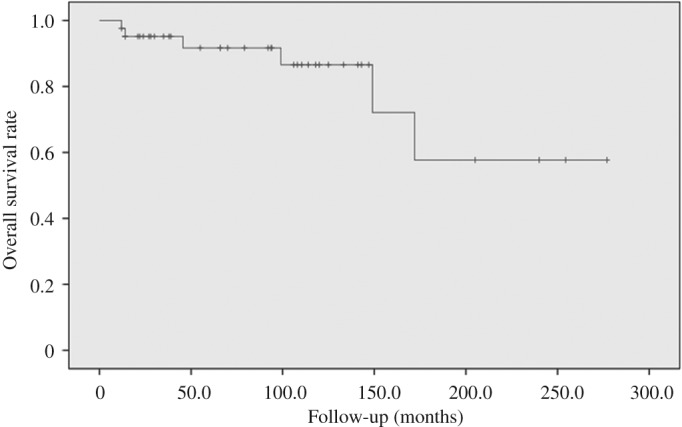
Kaplan–Meier curve of overall survival concerning selective arterial embolization (SAE) for sacral or pelvic giant cell tumor (GCT).
Statistical Analysis of Prognosis Indicators
We examined the correlations between tumor status, previous treatments, and overall LC, OS rate. Statistical analysis showed patients with primary tumors tended to have better prognosis than recurrent ones (P = 0.039). However, no significance was found in terms of previous treatments and overall LC or OS rate. In our research, the most prevalent number of SAE times was 4 or 5, followed by 6, according to the evidence of angiography, including absence of new vascularization, persistent stabilization, or improvements in patients' clinical signs. Log‐rank analysis failed to show significant association between embolization times and LC or OS, displayed in Table 1. Women appeared to have better prognosis for 1‐year and 2‐year survival (P 1 = 0.008, P 2 = 0.008); however this finding may not have clinical significance because of the limited sample size. In addition, patients' age and embolization materials were found to be insignificant for the prognosis.
Table 1.
Log‐rank analysis of embolization times in local control and overall survival (37 cases*)
| Threshold | Number | Percent (%) | P‐values† | P‐values‡ |
|---|---|---|---|---|
| <3/≥3 | 8/29 | 21.6/78.4 | 0.403 | 0.788 |
| <4/≥4 | 16/21 | 43.2/56.8 | 0.944 | 0.815 |
| <5/≥5 | 23/14 | 62.2/37.8 | 0.819 | 0.385 |
| <6/≥6 | 29/8 | 78.4/21.6 | 0.765 | 0.730 |
Specific embolization times were provided in 37 of the 44 patients;
Local control;
Overall survival.
Discussion
Giant cell tumor of bone (GCTB), accounting for 4%–5% of all primary bone tumors, has been recognized as a locally aggressive tumor with or without multi‐centric lesions29, 30. Distant metastasis occurs at a low rate1, 2, 3, 4, 5, 6. GCTB is observed predominantly at the epiphysis and metaphysic of long bones, especially at the distal femur and proximal tibia. The sacrum is the third most common site, accounting for 2%–8% of GCTB, while pelvic involvement of GCTB is quite rare; only 4 patients were eligible for inclusion in our study7, 8. Both the sacrum and pelvis have complex anatomic structures, often resulting in large size at initial presentation.
Disadvantages of Surgery
Even though surgery remains the treatment of choice, other conservative and alternative therapies, such as SAE and therapeutic RT, are also widely applied in clinical practice. The local recurrence rate can be as high as 40%–50% after intralesional curettage or marginal resection with or without bone cement packing31, 32, 33, 34. Goldenberg et al. report that five SGCT with curettage alone had recurrence in the end35. Yet wide en‐bloc resection was considered as overtreatment due to excessive blood loss or postoperative pelvic instability. Moreover, the neurological functional sacrifice was serious, including loss of bowel, bladder control, or sexual dysfunction15, 36, 37. Thus, SAE had been presented as an alternative treatment for unresectable or recurrent GCT after surgery. However, early on in clinical practice, SAE was adopted as an adjuvant preoperative management so as to create the opportunity for subsequent surgery17, 18, 19. Indeed, case series on SAE as a primary therapy have reported favorable effects, with high LC and OS rates, as well as minimal long‐term complications, but few systematic and comprehensive reviews have been published on SAE. Therefore, we conducted this systematic review to analyze the prognosis of SAE for SGCT and PGCT.
Assessments of Embolization
The mean age (34.4 ± 13.3 years) of patients was similar to that of most published articles. All patients responded in our study, which demonstrated an instant improvement of patients with SAE in clinical symptoms and signs. The reossification rate reached as high as 81.8% (36/44) on CT and MRI images, indicating that SAE might have capacity to stimulate the functioning of osteoblasts indirectly. Because the LC status was considered of great significance in vertebral tumors, our study showed favorable results in the 1‐year, 2‐year, 5‐year, 10‐year, and overall LC rate after SAE, which is in parallel with the relative survival rate16. Of 11 patients failing to have LC, 8 were within 120 months, indicating that 10‐year survival might be a prognostic threshold to forecast the overall LC rate. Of all included patients, 6 were classified as Campanacci Grade II and 3 as Grade III, but no prognostic significance was observed between the two subgroups in our study.
Although no significant difference was shown among embolization materials in our research, as previous demonstrated, Gelfoam, polyvinyl alcohol (PVA), and stainless steel coils for most SAE ran the risk of a short occlusive time of tumor‐feeding arteries as well as non‐target embolization, which could result in the subsequent failure of LC or even the OS38, 40. Therefore, superabsorbent polymer microsphere (SAP‐MS) is recommended for SAE due to its precise and everlasting occlusion with minimum harm to normal tissue. SAP‐MS has gained popularity in various treatment modalities, including ovarian cancer, hepatocellular carcinoma, and colorectal cancer with liver metastasis41, 42, 43, 44. Embolization frequency was also found to be insignificant in increasing the survival rate. However, we believed it could, to some extent, contribute to continuous pain relief as well as tumor volume‐control and devascularization, thus improving the quality of patients' life. In addition, in our study, SAE was not continued until no new vessel formation was observed by angiography or patients gained persistent stabilization and improvements in clinical features. The time interval of SAE mentioned in related articles was not extremely consistent, ranging from 3 to 8 weeks, which indicates that determining the standard guidelines for series SAE require clinical trials.
The tumor status appeared to impact the prognosis. Previous studies also reported that recurrence status was a potential negative prognostic factor for SAE. Lin et al. revealed that 4 of 8 patients with recurrent disease died, compared to 1 of 10 with primary status who died23. Of the 44 patients, 13 cases were managed to measure and provide the tumor size precisely by the original authors in the articles. The mean decrease in ratios was 25.2% (median, 24.5%; range, −82.4% to 38.71%); however, in 1 patient, due to mere occlusion at the very proximal level, there was collateral circulation to the residual tumors. This objective index showed a good response of tumors towards SAE.
The post‐treatment neurological complication rate was low (6/44, 13.6%), which was confirmed to be related to the occlusive arteries. Among 44 patients, although each had an angiography before SAE to detect the tumor‐feeding arteries, SAE was unable to occlude the tumor‐supplying arteries at a precise and proper level, which may damage the adjacent normal tissue. None had compromised bowel, bladder, or sexual function control due to SAE; conversely, 1 patient who had bowel and bladder dysfunction before SAE gained favorable results to have a functional return of micturition and defecation (case 41). Compared with surgery or RT, the neurological deficit rate was acceptable and could be lower in the highly‐selective arterial embolization. Two patients who developed lung metastases after SAE died in the end, one who had lung metastases before SAE; this was comparable to the overall metastatic rate of 1.5%–7% in GCTB45. This might indicate that SAE had no impact on the distant metastases of GCTB.
Other Adjuvant Treatments
Denosumab, as a fully humanized monoclonal antibody directly inhabiting the receptor activator of nuclear factor κB ligand (RANKL), has played a very significant role in recent clinical practice in treating unsalvageable GCTB. It was reported to have favorable efficacy and safety, but was limited due to its lack of long‐term evaluation and adverse effects, such as the adverse impact on bone density, hypocalcemia, and osteonecrosis of the jaw (ONJ)46. With respect to RT, a serious and fatal problem to be faced is the post‐RT sarcomatous transformation noticed in many studies, which can be as high as 11% despite its effectiveness in increasing the overall LC rate47. More high‐quality clinical research needs to be undertaken to define the optimal strategies for various unresectable SGCT and PGCT.
Limitation
Although this is a novel and large systematic review of SGCT and PGCT treated by SAE, there are some limitations. First, the sample size of this retrospective study is relatively small, which may decrease the power of the statistics. Second, some information on the included patients was not presented in previous articles (please see the Appendix, marked with “—”), resulting in incomplete statistical analysis of some prognostic factors.
Conclusion
Favorable results for LC and survival rates with SAE were suggested for unresectable SGTC and PGCT in our study. The recurrent tumor status appeared to be a negative factor for prognosis in SAE. SAP‐MS was recommended for use with embolization materials. More well‐designed randomized control trials are required to validate the value of SAE in SGCT and PGCT that are not suitable for surgery.
Acknowledgements
The authors thank Professor Jianru Xiao for great supports to this study and conscientious guidance. The authors also thank all the colleagues for their kind help.
Data of the patients included in the study
| References | ID | Age/sex | Site | SoT | Pre. Tx | No. SAE. | Pain relief | Local control | Ossi. | NC | Distal metas. | Last status | FU (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hosalkar et al. 21 | 1 | 21/F | S1‐S4 | P | N | 7 | Y | Y | Y | N | N | Alive | 254.4 |
| 2 | 42/F | S2‐S5 | P | N | 4 | Y | Y | Y | N | Alive | 120 | ||
| 3 | 19/M | S2‐S4 | P | N | 4 | Y | Y | Y | N | Alive | 110.4 | ||
| 4 | 34/F | S1‐S5 | P | N | 4 | Y | Y | Y | N | Alive | 133.2 | ||
| 5 | 27/F | S2‐S5 | P | N | 6 | Y | Y | Y | N | Alive | 66 | ||
| 6 | 20/F | S2‐S4 | P | N | 5 | Y | Y | Y | N | Alive | 66 | ||
| 7 | 56/F | S1‐S4 | P | N | 6 | Y | Y | Y | N | Alive | 93.6 | ||
| 8 | 24/F | S2‐S5 | P | N | 3 | N | N | Y | Y | DOD | 45.6 | ||
| 9 | 36/F | S1‐S5 | P | N | 5 | Y | Y | Y | N | Alive | 79.2 | ||
| Martin & McCarthy 9 | 10 | 35/F | Sacrum | — given | N | — | N | Y | Y | — | N | Alive | 14 |
| Nakanishi et al. 28 | 11 | 30/M | Sacrum | P | Rx. | 3 | Y | Y | Y | Y | N | Alive | 118 |
| 12 | 68/F | Sacrum | P | N | 3 | Y | Y | Y | N | N | Alive | 141 | |
| 13 | 40/F | Sacrum | P | N | 4 | Y | Y | Y | N | N | Alive | 39 | |
| 14 | 32/M | Sacrum | R | Cur. | 5 | N | N | N | N | N | DOD | 14 | |
| Onishi et al. 22 | 15 | 56/F | SacrumSacrum | P | N | 5 | Y | Y | Y | N | N | Alive | 28 |
| Lin et al. 23 | 16 | 59/M | S1‐S5: 3 cases | P | N | 2 | Y | N | 14Y | 3Y | N | Alive | 147 |
| 17 | 19/F | P | N | 5 | N | N | Alive | 92 | |||||
| 18 | 39/M | P | N | 2 | N | N | Alive | 94 | |||||
| 19 | 38/M | P | N | 3 | Y | N | Alive | 27 | |||||
| 20 | 32/F | P | N | 3 | Y | N | Alive | 114 | |||||
| 21 | 59/M | P | N | 3 | Y | N | Alive | 24 | |||||
| 22 | 34/F | S3‐S4: 6 cases | P | N | 5 | Y | N | Alive | 35 | ||||
| 23 | 19/F | R | Cur&Rx | 10 | N | N | DOD | 99 | |||||
| 24 | 15/F | R | Cur | 7 | Y | N | Alive | 240 | |||||
| 25 | 28/F | R | Cur | 3 | Y | 4N | 15N | N | Alive | 277 | |||
| 26 | 46/F | R | Cur&Rx | 1 | Y | N | DU | 205 | |||||
| 27 | 26/M | R | Cur&Rx | 4 | N | N | Alive | 70 | |||||
| 28 | 52/F | S1‐S2: 9 cases | R | Cur&Rx | 4 | Y | N | DOD | 149 | ||||
| 29 | 27/F | R | Cur&Rx | 6 | Y | N | Alive | 106 | |||||
| 30 | 23/F | R | Cur | 8 | N | Y | DOD | 172 | |||||
| 31 | 33/F | P | N | 6 | N | N | Alive | 125 | |||||
| 32 | 17/M | P | N | 3 | Y | N | DOD | 12 | |||||
| 33 | 27/F | P | N | 5 | Y | N | Alive | 143 | |||||
| Wallace et al. 24 | 34 | 27/F | R ilium | P | N | 1 | Y | Y | Y | — | N | Alive | 12 |
| 35 | 46/F | Sacrum | P | 3Sur&Rx | 1 | Y | Y | Y | N | Alive | 30 | ||
| Chuang et al. 25 | 36 | 46/F | Sacrum | — | — | — | Y | Y | Y | N | N | Alive | 55 |
| 37 | 31/F | R ilium | — | — | Y | Y | Y | N | N | Alive | 38 | ||
| 38 | 31/F | S1‐S2, L5 | — | — | Y | Y | Y | N | N | Alive | 21 | ||
| 39 | 29/F | Sacrum | — | — | Y | Y | Y | N | N | Alive | 14 | ||
| 40 | 43/M | S ilium | — | 2 | Y | N | N | Y | Y* | DOD | 8 | ||
| 41 | 25/F | L ilium | — | — | N | N | N | N | N | DOD | 22 | ||
| 42 | 57/F | Sacrum | — | — | Y | Y | N | N | N | Alive | 2 | ||
| Eftekhari et al. 26 | 43 | 15/F | Sacrum | R | Sur&Che Chemo | 2 | Y | Y | Y | Y | N | Alive | 14 |
| Yu et al. 27 | 44 | 31/F | Sacrum LSIJ | P | N | 2 | Y | Y | Y | N | N | Alive | 108 |
Had lung metastasis before SAE.
Che, chemotherapy; Cur, curretage; Distal metas., distal metastasis; DOD, died of disease; DU, died of unrelated diseases; FU, follow‐up; LSIJ, left sacroiliac joint; NC, neurological complication; Ossi, ossification; P, primary; Pre.Tx, previous treatment; R, recurrent; Rx, radiation; SAE, selective arterial embolization; SoT, Status of tumor; 3Sur, Surgery*3.
Disclosure: The authors disclose no conflicts.
References
- 1. Muheremu A, Niu X. Pulmonary metastasis of giant cell tumor of bones. World J Surg Oncol, 2014, 12: 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chan CM, Adler Z, Reith JD, Gibbs CP Jr. Risk factors for pulmonary metastases from giant cell tumor of bone. J Bone Joint Surg Am, 2015, 97: 420–428. [DOI] [PubMed] [Google Scholar]
- 3. Quattrini I, Pollino S, Pazzaglia L, et al. Prognostic role of nuclear factor/IB and bone remodeling proteins in metastatic giant cell tumor of bone: a retrospective study. J Orthop Res, 2015, 33: 1205–1211. [DOI] [PubMed] [Google Scholar]
- 4. Balke M, Schremper L, Gebert C, et al. Giant cell tumor of bone: treatment and outcome of 214 cases. J Cancer Res Clin Oncol, 2008, 134: 969–978. [DOI] [PubMed] [Google Scholar]
- 5. Campanacci M, Baldini N, Boriani S, Sudanese A. Giant‐cell tumor of bone. J Bone Joint Surg Am, 1987, 69: 106–114. [PubMed] [Google Scholar]
- 6. Kay RM, Eckardt JJ, Seeger LL, Mirra JM, Hak DJ. Pulmonary metastasis of benign giant cell tumor of bone. Six histologically confirmed cases, including one of spontaneous regression. Clin Orthop Relat Res, 1994, 302: 219–230. [PubMed] [Google Scholar]
- 7. Thangaraj R, Grimer RJ, Carter SR, Stirling AJ, Spilsbury J, Spooner D. Giant cell tumour of the sacrum: a suggested algorithm for treatment. Eur Spine J, 2010, 19: 1189–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van der Heijden L, van de Sande MA, van der Geest IC, et al. Giant cell tumors of the sacrum‐‐a nationwide study on midterm results in 26 patients after intralesional excision. Eur Spine J, 2014, 23: 1949–1962. [DOI] [PubMed] [Google Scholar]
- 9. Martin C, McCarthy EF. Giant cell tumor of the sacrum and spine: series of 23 cases and a review of the literature. Iowa Orthop J, 2010, 30: 69–75. [PMC free article] [PubMed] [Google Scholar]
- 10. Zheng K, Wang Z, Wu SJ, et al. Giant cell tumor of the pelvis: a systematic review. Orthop Surg, 2015, 7: 102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sanjay BK, Frassica FJ, Frassica DA, Unni KK, McLeod RA, Sim FH. Treatment of giant‐cell tumor of the pelvis. J Bone Joint Surg Am, 1993, 75: 1466–1475. [DOI] [PubMed] [Google Scholar]
- 12. Shen CC, Li H, Shi ZL, Tao HM, Yang ZM. Current treatment of sacral giant cell tumour of bone: a review. J Int Med Res, 2012, 40: 415–425. [DOI] [PubMed] [Google Scholar]
- 13. Nakai S, Yoshizawa H, Kobayashi S, Maeda K, Okumura Y. Anorectal and bladder function after sacrifice of the sacral nerves. Spine (Phila Pa 1976), 2000, 25: 2234–2239. [DOI] [PubMed] [Google Scholar]
- 14. Luther N, Bilsky MH, Hartl R. Giant cell tumor of the spine. Neurosurg Clin N Am, 2008, 19: 49–55. [DOI] [PubMed] [Google Scholar]
- 15. Beadel GP, McLaughlin CE, Aljassir F, et al. Iliosacral resection for primary bone tumors: is pelvic reconstruction necessary?. Clin Orthop Relat Res, 2005, 438: 22–29. [DOI] [PubMed] [Google Scholar]
- 16. Ma Y, Xu W, Yin H, et al. Therapeutic radiotherapy for giant cell tumor of the spine: a systemic review. Eur Spine J, 2015, 24: 1754–1760. [DOI] [PubMed] [Google Scholar]
- 17. Rangel‐Castilla L, Shah AH, Klucznik RP, Diaz OM. Preoperative Onyx embolization of hypervascular head, neck, and spinal tumors: experience with 100 consecutive cases from a single tertiary center. J Neurointerv Surg, 2014, 6: 51–56. [DOI] [PubMed] [Google Scholar]
- 18. Emori M, Kaya M, Sasaki M, Wada T, Yamaguchi T, Yamashita T. Pre‐operative selective arterial embolization as a neoadjuvant therapy for proximal humerus giant cell tumor of bone: radiological and histological evaluation. Jpn J Clin Oncol, 2012, 42: 851–855. [DOI] [PubMed] [Google Scholar]
- 19. Broaddus WC, Grady MS, Delashaw JB Jr, Ferguson RD, Jane JA. Preoperative superselective arteriolar embolization: a new approach to enhance resectability of spinal tumors. Neurosurgery, 1990, 27: 755–759. [PubMed] [Google Scholar]
- 20. Lackman RD, Khoury LD, Esmail A, Donthineni‐Rao R. The treatment of sacral giant‐cell tumours by serial arterial embolisation. J Bone Joint Surg Br, 2002, 84: 873–877. [DOI] [PubMed] [Google Scholar]
- 21. Hosalkar HS, Jones KJ, King JJ, Lackman RD. Serial arterial embolization for large sacral giant‐cell tumors: mid‐ to long‐term results. Spine (Phila Pa 1976), 2007, 32: 1107–1115. [DOI] [PubMed] [Google Scholar]
- 22. Onishi H, Kaya M, Wada T, Nagoya S, Sasaki M, Yamashita T. Giant cell tumor of the sacrum treated with selective arterial embolization. Int J Clin Oncol, 2010, 15: 416–419. [DOI] [PubMed] [Google Scholar]
- 23. Lin PP, Guzel VB, Moura MF, et al. Long‐term follow‐up of patients with giant cell tumor of the sacrum treated with selective arterial embolization. Cancer, 2002, 95: 1317–1325. [DOI] [PubMed] [Google Scholar]
- 24. Wallace S, Granmayeh M, deSantos LA, et al. Arterial occlusion of pelvic bone tumors. Cancer, 1979, 43: 322–328. [DOI] [PubMed] [Google Scholar]
- 25. Chuang VP, Soo CS, Wallace S, Benjamin RS. Arterial occlusion: management of giant cell tumor and aneurysmal bone cyst. AJR Am J Roentgenol, 1981, 136: 1127–1130. [DOI] [PubMed] [Google Scholar]
- 26. Eftekhari F, Wallace S, Chuang VP, et al. Intraarterial management of giant‐cell tumors of the spine in children. Pediatr Radiol, 1982, 12: 289–293. [DOI] [PubMed] [Google Scholar]
- 27. Yu X, Xu M, Xu S, Fu Z. Long‐term outcome of giant cell tumor of bone involving sacroiliac joint treated with selective arterial embolization and curettage: a case report and literature review. World J Surg Oncol, 2013, 11: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nakanishi K, Osuga K, Hori S, et al. Transarterial embolization (TAE) of sacral giant cell tumor (GCT) using spherical parmanent embolic material superabsorbant polymer microsphere (SAP‐MS). Springerplus, 2013, 2: 666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tandra VS, Kotha KM, Satyanarayana MG, Vadlamani KV, Yerravalli V. Synchronous multicentric giant cell tumour of distal radius and sacrum with pulmonary metastases. Case Rep Oncol Med, 2015, 2015: 354158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Naam NH, Jones SL, Floyd J, Memisoglu EI. Multicentric giant cell tumor of the fourth and fifth metacarpals with lung metastases. Hand (N Y), 2014, 9: 389–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hart RA, Boriani S, Biagini R, Currier B, Weinstein JN. A system for surgical staging and management of spine tumors. A clinical outcome study of giant cell tumors of the spine. Spine (Phila Pa 1976), 1997, 22: 1773–1782. [DOI] [PubMed] [Google Scholar]
- 32. Turcotte RE, Sim FH, Unni KK. Giant cell tumor of the sacrum. Clin Orthop Relat Res, 1993, 291: 215–221. [PubMed] [Google Scholar]
- 33. Sanjay BK, Sim FH, Unni KK, McLeod RA, Klassen RA. Giant‐cell tumours of the spine. J Bone Joint Surg Br, 1993, 75: 148–154. [DOI] [PubMed] [Google Scholar]
- 34. Becker WT, Dohle J, Bernd L, et al. Local recurrence of giant cell tumor of bone after intralesional treatment with and without adjuvant therapy. J Bone Joint Surg Am, 2008, 90: 1060–1067. [DOI] [PubMed] [Google Scholar]
- 35. Goldenberg RR, Campbell CJ, Bonfiglio M. Giant‐cell tumor of bone. An analysis of two hundred and eighteen cases. J Bone Joint Surg Am, 1970, 52: 619–664. [PubMed] [Google Scholar]
- 36. Guo W, Ji T, Tang X, Yang Y. Outcome of conservative surgery for giant cell tumor of the sacrum. Spine (Phila Pa 1976), 2009, 34: 1025–1031. [DOI] [PubMed] [Google Scholar]
- 37. Marcove RC, Sheth DS, Brien EW, Huvos AG, Healey JH. Conservative surgery for giant cell tumors of the sacrum. The role of cryosurgery as a supplement to curettage and partial excision. Cancer, 1994, 74: 1253–1260. [DOI] [PubMed] [Google Scholar]
- 38. Maeda N, Osuga K, Higashihara H, et al. Transarterial chemoembolization with cisplatin as second‐line treatment for hepatocellular carcinoma unresponsive to chemoembolization with epirubicin‐Lipiodol emulsion. Cardiovasc Intervent Radiol, 2012, 35: 82–89. [DOI] [PubMed] [Google Scholar]
- 39. Osuga K, Hori S, Hiraishi K, et al. Bland embolization of hepatocellular carcinoma using superabsorbent polymer microspheres. Cardiovasc Intervent Radiol, 2008, 31: 1108–1116. [DOI] [PubMed] [Google Scholar]
- 40. Basile A, Rand T, Lomoschitz F, et al. Trisacryl gelatin microspheres versus polyvinyl alcohol particles in the preoperative embolization of bone neoplasms. Cardiovasc Intervent Radiol, 2004, 27: 495–502. [DOI] [PubMed] [Google Scholar]
- 41. Seki A, Hori S. Switching the loaded agent from epirubicin to cisplatin: salvage transcatheter arterial chemoembolization with drug‐eluting microspheres for unresectable hepatocellular carcinoma. Cardiovasc Intervent Radiol, 2012, 35: 555–562. [DOI] [PubMed] [Google Scholar]
- 42. Seki A, Hori S, Kobayashi K, Narumiya S. Transcatheter arterial chemoembolization with epirubicin‐loaded superabsorbent polymer microspheres for 135 hepatocellular carcinoma patients: single‐center experience. Cardiovasc Intervent Radiol, 2011, 34: 557–565. [DOI] [PubMed] [Google Scholar]
- 43. Huppert P, Wenzel T, Wietholtz H. Transcatheter arterial chemoembolization (TACE) of colorectal cancer liver metastases by irinotecan‐eluting microspheres in a salvage patient population. Cardiovasc Intervent Radiol, 2014, 37: 154–164. [DOI] [PubMed] [Google Scholar]
- 44. Seki A, Hori S, Sueyoshi S, Hori A. Local control and prognostic significance of transarterial treatment for limited recurrence of ovarian cancer as third‐line and beyond therapy. Int J Clin Oncol, 2014, 19: 1065–1073. [DOI] [PubMed] [Google Scholar]
- 45. Muheremu A, Huang Z, Niu X. Treatment for giant cell tumor of the spine metastasizing to the lung: a report of two cases and a literature review. Oncol Lett, 2015, 9: 1321–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Singh AS, Chawla NS, Chawla SP. Giant‐cell tumor of bone: treatment options and role of denosumab. Biologics, 2015, 9: 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Leggon RE, Zlotecki R, Reith J, Scarborough MT. Giant cell tumor of the pelvis and sacrum: 17 cases and analysis of the literature. Clin Orthop Relat Res, 2004, 423: 196–207. [DOI] [PubMed] [Google Scholar]


