Key Points
Question
How is light sensitivity of the posterior pole compromised in Stargardt disease?
Findings
In this multicenter prospective cohort study, microperimetric mean sensitivity was lower in the fovea than in the peripheral macula. Overall mean sensitivity was lower in older patients and those stating longer disease duration. There are cases with obvious discrepancies of low mean sensitivity measurements with good visual acuity; these cases showed foveal sparing on fundus autofluorescence testing.
Meaning
These findings suggest microperimetry allows a more comprehensive assessment of the function of the central retina, and it may serve as an outcome measure in future clinical trials for Stargardt disease and other macular diseases.
Abstract
Importance
New outcome measures for treatment trials for Stargardt disease type 1 (STGD1) and other macular diseases are needed. Microperimetry allows mapping of light sensitivity of the macula and provides topographic information on visual function beyond visual acuity.
Objective
To measure and analyze retinal light sensitivity of the macula in STGD1 using fundus-controlled perimetry (microperimetry).
Design, Setting, and Participants
This was a multicenter prospective cohort study. A total of 199 patients and 326 eyes with molecularly confirmed (ABCA4) STGD1 underwent testing with the Nidek MP-1 microperimeter as part of the multicenter, prospective Natural History of the Progression of Atrophy Secondary to Stargardt Disease (ProgStar) study. Sensitivity of 68 retinal loci was tested, and the mean sensitivity (MS) was determined; each point was categorized as “normal,” “relative,” or “deep” scotoma.
Main Outcomes and Measures
Mean sensitivity and the number of points with normal sensitivity, relative, or deep scotomas.
Results
Mean (SD) patient age was 34.2 (14.7) years, mean (SD) best-corrected visual acuity of all eyes was 47.8 (16.9) Early Treatment Diabetic Retinopathy Study letter score (approximately 20/100 Snellen equivalent), and mean MS of all eyes of all 68 points was 11.0 (5.0) dB. The median number of normal points per eye was 49 (mean [SD], 41.3 [20.8]; range, 0-68); abnormal sensitivity and deep scotomas were more prevalent in the central macula. Mean sensitivity was lower in the fovea (mean [SD], 2.7 [4.4] dB) than in the inner (mean [SD], 6.8 [5.8] dB) and outer ring (mean [SD], 12.7 [5.3] dB). Overall MS per eye was 0.086 dB lower per year of additional age (95% CI, −0.13 to −0.041; P < .001) and 0.21 dB lower per additional year of duration of STGD1 (95% CI, −0.28 to −0.14; P < .001). Longer duration of STGD1 was associated with worse MS (β = −0.18; P < .001), with a lower number of normal test points (β = −0.71; P < .001), and with a higher number of deep scotoma points (β = −0.70; P < .001). We found 11 eyes with low MS (<6 dB) but very good best-corrected visual acuity of at least 72 Early Treatment Diabetic Retinopathy Study letter score (20/40 Snellen equivalent).
Conclusions and Relevance
We provide an extensive analysis of macular sensitivity parameters in STGD1 and demonstrate their association with demographic characteristics and vision. These data suggest microperimetry testing provides a more comprehensive assessment of retinal function and will be an important outcome measure in future clinical trials.
This study measures and analyzes retinal light sensitivity of the macula in Stargardt disease tye 1 using fundus-controlled perimetry (microperimetry).
Introduction
Stargardt disease type 1 (STGD1; OMIM: 248200) is the most common form of juvenile-onset macular dystrophy.1 Pathogenic mutations in the ABCA4 gene lead to atrophic-appearing macular lesions and variable loss of best-corrected visual acuity (BCVA).2 Best-corrected visual acuity can range from 20/20 to 20/400 or worse.3 Measuring the BCVA is a straightforward approach to quantify visual performance and has been widely studied.1,3,4,5 However, the Progression of Atrophy Secondary to Stargardt disease (ProgStar) study group showed that BCVA is unlikely to be a sensitive outcome measure for treatment trials in STGD1 except in certain subgroups owing to the slow rate of BCVA loss.3,6 Visual field testing is another psychophysical approach, but it misses small scotomas and is often complicated by unstable fixation in macular diseases. Fundus-controlled perimetry (microperimetry) allows for precise sensitivity analysis of the macula by displaying stimuli in preplanned retinal areas.7,8 An eye tracker ensures an exact correlation of macular pathology with functional defects in the Nidek MP-1 microperimeter (MP-1; Nidek Technologies).7 The MP-1 has been shown to be a reliable technique for testing of macular function in longitudinal treatment studies for STGD1.9
In this study, we examined the cross-sectional associations of microperimetric sensitivity data with demographic characteristics and the BCVA in our study cohort with molecularly confirmed STGD1, using data from the baseline examination of the prospective ProgStar study.
Methods
The ProgStar study received approval from the Western Institutional Review Board and all institutional review boards of all involved institutions and is in compliance with Health Insurance Portability and Accountability Act of 1996 and the Declaration of Helsinki. Details on all regulatory requirements, the design, organization, inclusion and exclusion criteria, the data collection, and management processes are described in ProgStar Report No. 1.10 This report also details the certification processes, the coordinating, and data management centers, and the strategies to ensure quality and completeness of the examinations and the gradings.
Microperimetry
The Nidek MP-1 microperimeter (NAVIS Software 1.7.7 or higher; Nidek Technologies SRL) was used to perform testing. Sensitivity was tested in 68 macular test locations in a pattern comparable with the Humphrey 10-2 pattern (eFigure 1 in the Supplement) using white size 3 stimuli (0.43 arc degrees; comparable with Goldmann III) with a duration of 200 milliseconds on a white monochromatic background and a 4-2 strategy. In the ProgStar study, optical coherence tomography data were used to center the test pattern manually onto the anatomical fovea as accurately as possible following a standard operating procedure.10,11 In cases with foveal atrophy, the graders looked for the point of maximal inner retinal layer convergence and used the adjoining B-scans immediately superior and inferior to the approximate foveal center to determine this center as precisely as possible. Accurate pattern placement is necessary to ensure proper structure-function correlation, ie, to ensure that the central 4 foveal points actually test the function of the foveal region, although this approach leads to exclusion of a significant amount of eyes from the analysis. All tests were performed under almost dark (mesopic) light conditions. The sensitivity at each retinal location was determined by iteratively adjusting the light intensity until the dimmest visible stimulus was found. The sensitivity for each test location was determined on a scale of 0 dB to 20 dB. Test locations with 0 dB (ie, retinal locations where only the brightest stimulus was detected or no stimulus at all was detected) were defined as “deep scotoma,” and test locations with more than 0 dB but less than 12 dB were defined as “relative scotoma.” The ProgStar protocol did not include the testing of normal individuals but based on the literature12,13 and experience to date, loci with 12 dB or higher sensitivity were considered to be normal, near-normal, or at least not substantially abnormal. For simplicity, these test locations with 12 dB to 20 dB are therefore referred to as “normal.” All examinations were performed monocularly, with the contralateral eye patched. The central 4 points were 1.7° away from the anatomical fovea and were grouped as “foveal”; the 12 perifoveal points were at a distance of 3.5° to 4.7° from the center of the anatomical fovea and were grouped as the “inner ring”; and the remainder of the test locations between 5.6° to 10.1° from the fovea were grouped as the “outer ring” (eFigure 1 in the Supplement).
The central reading center categorized the pattern placement on the fovea as “adequate,” “poor,” or “cannot grade.” When the center of the grid was within less than 1° from the anatomical fovea, this was graded as “adequate.” Distances from 1° to less than 2° were “fair.” “Poor” pattern placement means that the grid was improperly placed at a distance of at least 2° from the fovea (eyes with such “poor” placement were excluded from the main analysis).
Statistical Analyses
Linear models with generalized estimating equations were used to estimate the associations between the outcome parameters while accounting for between-eye correlations. Aggregated residuals were used for model assessment. If the assumption of a linear relationship between an independent variable and the outcome variable was not met, piecewise linear models with generalized estimating equations were used where the cutoff value for the final model was determined through iterative model fitting and was the value that yielded the minimum information criteria in the model fit. Multivariable regression models were used including explanatory variables that were significant in univariate analyses, with the appropriate P values ≤ .10.
Results
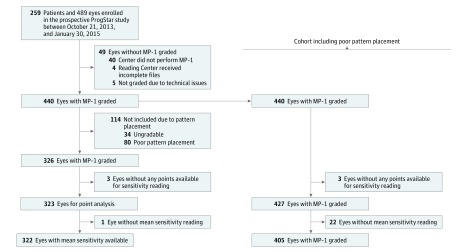
Between October 21, 2013, and January 30, 2015, a total of 259 patients and 489 eyes were enrolled at 9 centers in the prospective ProgStar study.10 The MP-1 testing was available and graded for 440 eyes. To increase the quality of our data set, 80 eyes with poor pattern placement and 34 eyes with ungradable pattern placement were excluded from the final analysis; a final cohort of 326 eyes (199 participants; 114 women and 85 men) was included. eTable 1 in the Supplement shows the demographic features for all patients and the subgroups. No differences in most characteristics between those with adequate vs poor or ungradable pattern placement were found (with the only exception that in the latter group, African American and Asian ethnicities appeared to be more prevalent). For the qualifying cohort (at least 1 eye with adequate or fair pattern placement), the mean (SD) patients age was 34.2 (14.7) years, and the median BCVA was 43 Early Treatment Diabetic Retinopathy Study (ETDRS) letter score (approximately 20/125; mean [SD], 47.8 [16.9]; range, 20-88). Best-corrected visual acuity was 4.0 ETDRS letter score lower (95% CI, −6.13 to −1.80; P < .001) among patients with poor or ungradable pattern placement.
Figure 1 presents the detailed enrollment count. We present herein the results from the 326 eyes following exclusion of eyes with poor or ungradable pattern placement; data for all 440 eyes are provided in eTables 3-5 in the Supplement.
Figure 1. Enrollment and Outcomes.
Among 489 eyes enrolled in the prospective ProgStar Study, a total of 326 were included in the sensitivity analysis of the macula and 322 eyes were included in the analysis of mean sensitivity.
Measures of Macular Sensitivity in STGD1
The median of the mean sensitivity per eye (MS) throughout all 68 points assessed with the MP-1 was 12.4 dB (mean [SD], 11 [5] dB; range, 0- 19.9 dB; n = 322 eyes). The number of normal points and relatively and deeply scotomatous points is shown in Table 1. The median MS was worse when measured in the foveal 4 points (0 dB) and best in the outer ring (14.5 dB). This trend was also reflected by the distribution of deeply scotomatous points, the mean percentage of which among all tested locations was highest at the fovea (73%), intermediate in the inner ring (46%), and smallest in the outer ring (15%) (eTable 2 in the Supplement). Overall, MS measurements were highly correlated between the right and left eyes (overall MS ρ = 0.95; P < .001; n = 124). The correlation of MS between the right and left eye was weaker in the foveal 4 points (foveal MS ρ = 0.75; P < .001; n = 125) than in the inner ring (inner ring MS ρ = 0.91; P < .001; n = 125) or outer ring (outer ring MS ρ = 0.95; P < .001; n = 125). The Pearson correlation coefficient of the BCVA between the right and left eye (BCVA ρ = 0.78; P < .001; n = 127) was similar to that of the foveal MS.
Table 1. Macular Sensitivity in Stargardt Disease and Its Association With Demographic Features.
| Outcome, Independent Variable | Eyes With Good and Fair Pattern Placement | |||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| β Estimate (95% CI) | P Value | β Estimate (95% CI) | P Value | |
| Mean sensitivity, dBa | ||||
| Current age per y | −0.086 (−0.13 to −0.041) | <.001 | −0.04 (−0.09 to 0.02) | .20 |
| Current age ≥50 y | −3.13 (−4.78 to −1.47) | <.001 | NA | NA |
| Age at onset >18 y | 0.01 (−1.44 to 1.47) | .98 | NA | NA |
| Duration of Stargardt disease, y | −0.21 (−0.28 to −0.14) | <.001 | −0.18 (−0.27 to −0.09) | <.001 |
| Male sex | 0.55 (−0.86 to 1.96) | .44 | NA | NA |
| Nonwhite race/ethnicity | 0.26 (−1.75 to 2.27) | .80 | NA | NA |
| Count of normal points | ||||
| Current age per y | −0.34 (−0.54 to −0.15) | <.001 | −0.14 (−0.39 to 0.10) | .25 |
| Current age ≥50 y | −13.48 (−20.71 to −6.26) | <.001 | NA | NA |
| Age of onset >18 y | 0.77 (−5.29 to 6.82) | .80 | NA | NA |
| Duration of Stargardt disease, y | −0.83 (−1.13 to −0.52) | <.001 | −0.71 (−1.10 to −0.32) | <.001 |
| Male sex | 0.65 (−5.23 to 6.54) | .83 | NA | NA |
| Nonwhite race/ethnicity | 2.05 (−6.11 to 10.21) | .63 | NA | NA |
| Count of deep scotoma points | ||||
| Current age per y | 0.29 (0.13 to 0.45) | <.001 | 0.09 (−0.09 to 0.27) | .32 |
| Current age ≥50 y | 9.25 (2.98 to 15.52) | .004 | NA | NA |
| Age of onset >18 y | −0.13 (−5.08 to 4.81) | .96 | NA | NA |
| Duration of Stargardt disease, y | 0.78 (0.50 to 1.05) | <.001 | 0.70 (0.37 to 1.04) | <.001 |
| Male sex | −1.37 (−6.13 to 3.40) | .57 | NA | NA |
| Nonwhite race/ethnicity | 1.1 (−5.67 to 7.86) | .75 | NA | NA |
Abbreviation: NA, not applicable.
n = 323 eyes.
The total number of normal, relatively scotomatous, and deeply scotomatous points among the 68 test locations for each eye showed that the median number of locations with normal sensitivity was higher (median, 49 points; range, 0-68 points) than for points categorized as a relative scotoma (median, 8 points; range, 0-43 points) or deep scotoma (median, 10 points; range, 0-68 points). eTable 3 in the Supplement shows the detailed results from sensitivity testing in the entire cohort including eyes with poor pattern placement.
Measures of Macular Sensitivity and Their Association With Demographic Features
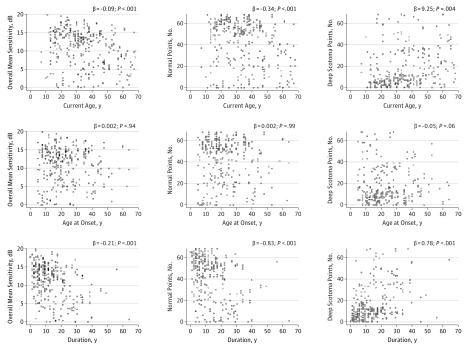
Table 1 summarizes the association of sensitivity testing with demographic features. Overall MS was 0.086 dB lower per year of additional age (95% CI, −0.13 to −0.041; P < .001) in the univariate model. Better MS of all 68 test locations was significantly associated with younger current patient age (β = −0.09; 95% CI, −0.13 to −0.04; P < .001, Figure 2) and with shorter duration of STGD1 symptoms (β = −0.71; 95% CI, −1.10 to −0.32; P < .001, Figure 2) but not with age at diagnosis of STGD1 older than 18 years (β = 0.014; 95% CI, −1.44 to 1.47; P = .98), race/ethnicity (β = 0.26; 95% CI, −1.75 to 2.27; P = .80), or sex (β = 0.55; 95% CI, −0.86 to 1.96; P = .44). The number of normal test locations (test locations ≥12 dB) was significantly associated with age younger than 50 years, with those patients’ eyes having a mean of 13.4 more normal points (P < .001). A larger number of normal test locations was also associated with shorter duration of STGD1 (P < .001). Similarly, a higher number of deeply scotomatous locations was associated with longer duration of STGD1 symptoms (P < .001) and with an age older than 50 years (P = .004) with their eyes having on average 9.3 additional deep scotomas.
Figure 2. Microperimetric Sensitivity and Demographics.
Scatterplots showing the correlation between microperimetric sensitivity with the duration of Stargardt disease (STGD1), the age at onset of STGD1, and the duration of STGD1 symptoms. Univariate linear regression analyses.
On multivariate analysis accounting for the different demographic measures, duration of STGD1 was the demographic feature associated with most measures of sensitivity in the macular region (Table 1). There were significant associations with the overall MS (β = −0.18; 95% CI, −0.27 to −0.09; P < .001), with the number of normal test locations (β = −0.71; 95% CI, −1.10 to −0.32; P < .001), and the number of deep scotoma points (β = −0.70; 95% CI, 0.37-1.04; P < .001). After accounting for duration of STGD1, there were no longer associations with current age for any of the 3 measures of sensitivity in the macula. eTable 4 in the Supplement presents the results from the entire cohort (including poor pattern placement) which were essentially equivalent.
Measures of Macular Sensitivity and Their Association With Visual Acuity
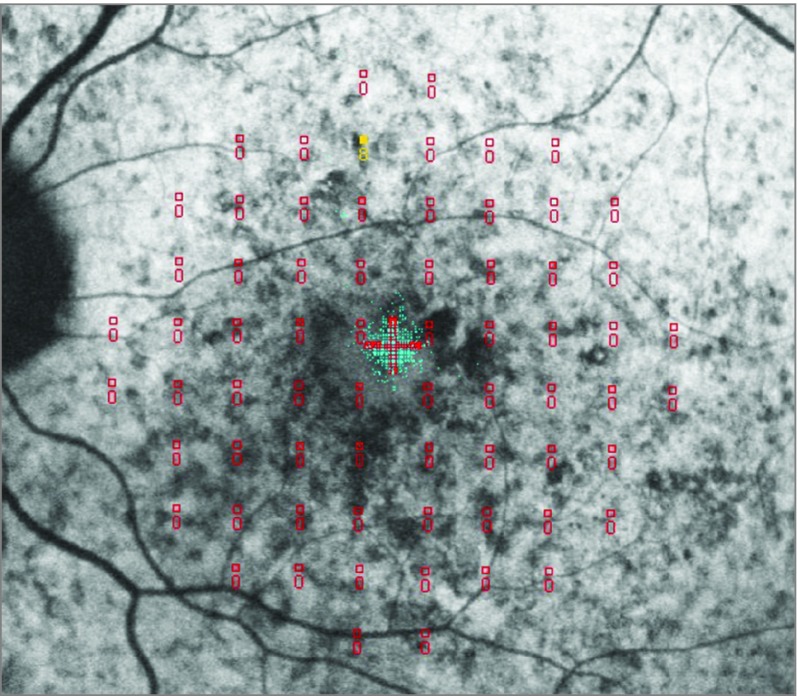
The association of macular sensitivity and BCVA are presented in Table 2. Best-corrected visual acuity was 1.2 ETDRS letter score lower per 1 dB worse MS (95% CI, 0.75 to 1.64; P < .001). On multivariate analysis simultaneously, including the mean sensitivities in the fovea, in the inner, and in the outer ring, BCVA was 0.62 ETDRS letter score worse per 1 dB less MS among the foveal 4 points (95% CI, 0.1 to 1.14; P = .02); the association was similar for the inner ring where 1 dB decreased MS was associated with 0.59 ETDRS letter score worse BCVA (95% CI, 0.05 to 1.14; P = .03); there was no association of the MS in the outer ring with the BCVA (P = .22). In both the univariate and multivariate analyses, a higher number of deep scotoma points was associated with worse BCVA (β = −0.30; P < .001). eFigure 2 in the Supplement shows these associations in the univariate analysis. Closer analysis of the scatterplot (eFigure 2 in the Supplement) revealed that there are some cases with low MS (<6 dB) but very good BCVA (≥72 ETDRS letter score; ≥20/40). We observed 11 such eyes. Figure 3 shows an illustrative example: all 11 eyes had a small preserved foveal island of nonatrophic retina used for fixation accounting for the good BCVA. However, the preserved island was too small to be covered by any of the central 4 test locations. eTable 5 in the Supplement shows these correlations for the entire cohort.
Table 2. Macular Sensitivity in Stargardt Disease and Its Association With Visual Acuity.
| Independent Variable | BCVA in Eyes With Good and Fair Pattern Placement (n = 323) |
|||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| β Estimate (95% CI) | P Value | β Estimate (95% CI) | P Value | |
| MS all points | 1.19 (0.75-1.64) | <.001 | NA | NA |
| MS fovea | 1.04 (0.57-1.51) | <.001 | 0.62 (0.1-1.14) | .02 |
| MS inner ring | 1.08 (0.69-1.47) | <.001 | 0.59 (0.05- 1.14) | .03 |
| MS outer ring | 0.95 (0.54-1.36) | <.001 | 0.34 (−0.2 to 0.87) | .22 |
| Count | ||||
| Normal points | 0.27 (0.16-0.38) | <.001 | NA | NA |
| Deep scotoma | −0.30 (−0.43 to −0.18) | <.001 | −0.29 (0.42 to −0.17) | <.001 |
Abbreviations: BCVA, best-corrected visual acuity; MS, mean sensitivity; NA, not applicable.
Figure 3. Case of Discrepancy Between Microperimetric Sensitivity and Visual Acuity.
The right eye of a 35-year-old man with mean sensitivity of 0.12 dB and best-corrected visual acuity of 77 Early Treatment Diabetic Retinopathy Study letter score (about 20/32 Snellen equivalent). Fixation was within an island of nonatrophic retina on a coregistered fundus autofluorescence image.
Discussion
This study investigated light sensitivity parameters of the macula obtained with fundus-controlled (micro)perimetry in patients with STGD1 and found an overall MS (SD) of 11.0 (5.0) dB. Both the MS and the number of scotomatous points show that the ability to perceive light stimuli of the central retina is reduced in STGD1. Longer duration of symptoms was associated with worse sensitivity of the macula, and the sensitivity parameters correlated with BCVA, although not tightly. Using the MP-1, Testa et al14 reported comparable mean MS results of around 10 dB in a cross-sectional analysis of molecularly confirmed patients with STGD1 with an age at onset of first symptoms younger than 30 years.14 In our cohort, the mean of the MS in all tested locations was 11.0 dB, which is consistent with the report by Testa et al14 given the later age at first symptoms of the disease in our cohort. Later onset of symptoms has been shown to be associated with a milder course of the disease.3 Because macular sensitivity in any tested location should be close to 20 dB in age-matched healthy control individuals,15 our results suggest that the functional deficit in STGD1 is most pronounced in the foveal center, while it is relatively preserved in the peripheral macula.
Our results show that better ability to perceive light stimuli in the macula is significantly associated with younger patient age (P < .001) (univariate analysis) and shorter duration of STGD1 (multivariate analysis). Both a higher overall MS as well as a higher number of test locations categorized as “normal” and a lower number of “deeply scotomatous” points were associated with shorter duration of symptoms and younger patient age. Using the Nidek MP-1, Midena et al15 showed that healthy individuals in their 20s have an MS that is about 1 dB higher than those of healthy people aged 70 to 75 years. The reasons for the age-related decrease of light sensitivity have been attributed to preretinal factors, such as ocular media opacities or smaller pupil size,16 or to neural losses.17 However, this natural decline in sensitivity of the macula is negligible when compared with the loss in sensitivity we found in our STGD1 cohort. According to our cross-sectional results, 1 year of additional reported duration of STGD1 was associated with 0.21 dB lower MS. Therefore, if compared with the Midena et al study,15 a decline in MS throughout 50 years of normal aging is comparable with what we would expect after 5 years in these patients with STGD1. Our results are in accordance with prior reports on better BCVA outcomes in late-onset cases of STGD118 and shorter duration of STGD1.14 Further investigation of longitudinal macular sensitivity data will be conducted in the ProgStar study, differentiating between early- and late-onset cases because patients with an early onset of the disease are expected to have a more rapid decline in visual function.19
We found that better MS is associated with better BCVA. However, there were sporadic cases with eyes with an MS better than 16 dB and a BCVA of less than 40 ETDRS letter score. On the other hand, we encountered 11 eyes with a discrepancy between the BCVA and MS readings, with BCVA of at least 72 ETDRS letter score (approximately 20/40) and MS less than 6 dB. The one example outlined in Figure 3 shows that focally preserved retinal structure as seen on fundus autofluorescence imaging accounts for such cases. A fovea-sparing phenotype of STGD1 has been described, and macular pigment may play an important role in such cases.9,11,20,21 Microperimetry underestimates the true visual acuity potential for this phenotype, whereas BCVA alone does not capture the extensive loss of sensitivity in the rest of the macula.8 Therefore, for a comprehensive assessment of retinal function in STGD1 and possibly in a larger group of macular diseases including age-related macular degeneration, both BCVA and microperimetric sensitivity testing should be considered, although both show strong associations.
Because the visual resolution is highest in the foveal center, where it is limited only by cone spacing,22 we had expected a strong relationship between the MS in the fovea with BCVA. Surprisingly, we found an almost identical association for the inner ring. This suggests that a relatively large area extending to an eccentricity of about 4° from the foveal center is critical for BCVA testing and high-resolution visual perception, possibly with eccentric fixation within 4° from the foveal center.23 It is also possible that our inclusion criteria may account for this finding because they allowed a freedom of 2° grid displacement. Therefore, it is possible that some of the inner ring points were actually in the fovea. On multivariate analysis, the sensitivity in the outer ring did not correlate with BCVA (β = 0.34; P = .22) suggesting that the visual resolution in the outer ring is relatively poor and functional loss of the outer ring area is not associated with additional loss of BCVA.
Our results also show that eyes with less than 20 locations of relative scotoma or less than 20 deep scotoma points were associated with a wide range of BCVA outcomes, from 20 to more than 80 ETDRS letter score. A higher number of deep scotoma points was associated with worse BCVA (β = −0.30; P < .001).
Limitations
Limitations of the presented research include the cross-sectional nature of the data. The primary outcome parameter of the ProgStar studies is the size of atrophic lesions on fundus autofluorescence testing that resulted in a minimum fundus autofluorescence lesion size as an inclusion criterion. Hence, very early stages of disease without any obvious anatomical changes were not included, and we did not analyze the function of the macula in such cases.
Conclusions
We show that microperimetry is as an important outcome measure for describing the functional status of the macula in STGD1. Our forthcoming longitudinal data may show whether it is useful for also detecting functional change. Microperimetry adds to the information provided by BCVA testing and allows for a more comprehensive assessment of macular function. The microperimetric sensitivity data may also be correlated with the location of the center of fixation. Investigating the correlation of fixation data with sensitivity data of the central macula would permit the identification of a lower threshold of retinal sensitivity beneath which fixation is more liable to become eccentric. In addition, further correlation of the presented data with optical coherence tomographic 24,25 and fundus autofluorescence–derived26,27 measures to show structure-function correlations will be calculated with the longitudinal 12- and 24-months follow-up data from the ProgStar study.10
eFigure 1: Modified Humphrey 10-2 Pattern (68 Points) for Sensitivity Testing of the Macula.
eFigure 2: Correlation of Visual Acuity with Microperimetric Sensitivity.
eTable 1: Demographic Characteristics by Inclusion in the Analyses.
eTable 2: Macular Sensitivity in Stargardt Disease.
eTable 3: Macular Sensitivity in Stargardt Disease including Patients with Poor Pattern Placement.
eTable 4: Macular Sensitivity in Stargardt Disease and Its Association with Demographic Features including Patients with Poor Pattern Placement.
eTable 5: Macular Sensitivity in Stargardt Disease and Its Association with Visual Acuity including Patients with Poor Pattern Placement. ProgStar Study Team as of 10 Feb 2017
References
- 1.Walia S, Fishman GA. Natural history of phenotypic changes in Stargardt macular dystrophy. Ophthalmic Genet. 2009;30(2):63-68. [DOI] [PubMed] [Google Scholar]
- 2.Tanna P, Strauss RW, Fujinami K, Michaelides M. Stargardt disease: clinical features, molecular genetics, animal models and therapeutic options. Br J Ophthalmol. 2017;101(1):25-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kong X, Strauss RW, Michaelides M, et al. ; ProgStar Study Group . Visual Acuity Loss and Associated Risk Factors in the Retrospective Progression of Stargardt Disease Study (ProgStar Report No. 2). Ophthalmology. 2016;123(9):1887-1897. [DOI] [PubMed] [Google Scholar]
- 4.Rotenstreich Y, Fishman GA, Anderson RJ. Visual acuity loss and clinical observations in a large series of patients with Stargardt disease. Ophthalmology. 2003;110(6):1151-1158. [DOI] [PubMed] [Google Scholar]
- 5.Fishman GA, Farber M, Patel BS, Derlacki DJ. Visual acuity loss in patients with Stargardt’s macular dystrophy. Ophthalmology. 1987;94(7):809-814. [DOI] [PubMed] [Google Scholar]
- 6.Scholl HP, Strauss RW, Singh MS, et al. Emerging therapies for inherited retinal degeneration. Sci Transl Med. 2016;8(368):368rv6. [DOI] [PubMed] [Google Scholar]
- 7.Charbel Issa P, Helb HM, Rohrschneider K, Holz FG, Scholl HP. Microperimetric assessment of patients with type 2 idiopathic macular telangiectasia. Invest Ophthalmol Vis Sci. 2007;48(8):3788-3795. [DOI] [PubMed] [Google Scholar]
- 8.Schönbach EM, Scholl HP. Fundus autofluorescence in a subclinical case of best disease. Retin Cases Brief Rep. 2017;11(suppl 1):S159-S162. [DOI] [PubMed] [Google Scholar]
- 9.Cideciyan AV, Swider M, Aleman TS, et al. Macular function in macular degenerations: repeatability of microperimetry as a potential outcome measure for ABCA4-associated retinopathy trials. Invest Ophthalmol Vis Sci. 2012;53(2):841-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strauss RW, Ho A, Muñoz B, et al. ; Progression of Stargardt Disease Study Group . The natural history of the progression of atrophy secondary to stargardt disease (progstar) studies: design and baseline characteristics: progstar report no. 1. Ophthalmology. 2016;123(4):817-828. [DOI] [PubMed] [Google Scholar]
- 11.Feucht N, Schönbach EM, Lanzl I, Kotliar K, Lohmann CP, Maier M. Changes in the foveal microstructure after intravitreal bevacizumab application in patients with retinal vascular disease. Clin Ophthalmol. 2013;7:173-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finger RP, Charbel Issa P, Fimmers R, Holz FG, Rubin GS, Scholl HP. Reading performance is reduced by parafoveal scotomas in patients with macular telangiectasia type 2. Invest Ophthalmol Vis Sci. 2009;50(3):1366-1370. [DOI] [PubMed] [Google Scholar]
- 13.Chen FK, Patel PJ, Xing W, et al. Test-retest variability of microperimetry using the Nidek MP1 in patients with macular disease. Invest Ophthalmol Vis Sci. 2009;50(7):3464-3472. [DOI] [PubMed] [Google Scholar]
- 14.Testa F, Melillo P, Di Iorio V, et al. Macular function and morphologic features in juvenile stargardt disease: longitudinal study. Ophthalmology. 2014;121(12):2399-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Midena E, Vujosevic S, Cavarzeran F; Microperimetry Study Group . Normal values for fundus perimetry with the microperimeter MP1. Ophthalmology. 2010;117(8):1571-1576, 1576.e1. [DOI] [PubMed] [Google Scholar]
- 16.Lachenmayr BJ, Kojetinsky S, Ostermaier N, Angstwurm K, Vivell PM, Schaumberger M. The different effects of aging on normal sensitivity in flicker and light-sense perimetry. Invest Ophthalmol Vis Sci. 1994;35(6):2741-2748. [PubMed] [Google Scholar]
- 17.Johnson CA, Adams AJ, Lewis RA. Evidence for a neural basis of age-related visual field loss in normal observers. Invest Ophthalmol Vis Sci. 1989;30(9):2056-2064. [PubMed] [Google Scholar]
- 18.Lewis RA, Shroyer NF, Singh N, et al. Genotype/phenotype analysis of a photoreceptor-specific ATP-binding cassette transporter gene, ABCR, in Stargardt disease. Am J Hum Genet. 1999;64(2):422-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westeneng-van Haaften SC, Boon CJ, Cremers FP, Hoefsloot LH, den Hollander AI, Hoyng CB. Clinical and genetic characteristics of late-onset Stargardt’s disease. Ophthalmology. 2012;119(6):1199-1210. [DOI] [PubMed] [Google Scholar]
- 20.Wolfson Y, Fletcher E, Strauss RW, Scholl HP. Evidence of macular pigment in the central macula in albinism. Exp Eye Res. 2016;145:468-471. [DOI] [PubMed] [Google Scholar]
- 21.Aleman TS, Cideciyan AV, Windsor EA, et al. Macular pigment and lutein supplementation in ABCA4-associated retinal degenerations. Invest Ophthalmol Vis Sci. 2007;48(3):1319-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossi EA, Roorda A. The relationship between visual resolution and cone spacing in the human fovea. Nat Neurosci. 2010;13(2):156-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schönbach EM, Ibrahim MA, Strauss RW, et al. Fixation location and stability using the mp1 microperimeter in stargardt disease. Ophthalmology Retina. 2017. doi: 10.1016/j.oret.2016.08.009 [DOI] [PubMed] [Google Scholar]
- 24.Strauss RW, Munoz B, Wolfson Y, et al. Assessment of estimated retinal atrophy progression in Stargardt macular dystrophy using spectral-domain optical coherence tomography. Br J Ophthalmol. 2016;100(7):956-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mashayekhi A, Schönbach E, Shields CL, Shields JA. Early subclinical macular edema in eyes with uveal melanoma: association with future cystoid macular edema. Ophthalmology. 2015;122(5):1023-1029. [DOI] [PubMed] [Google Scholar]
- 26.Kuehlewein L, Hariri AH, Ho A, et al. Comparison of manual and semiautomated fundus autofluorescence analysis of macular atrophy in stargardt disease phenotype. Retina. 2016;36(6):1216-1221. [DOI] [PubMed] [Google Scholar]
- 27.Strauss RW, Muñoz B, Jha A, et al. Comparison of short-wavelength reduced-illuminance and conventional autofluorescence imaging in stargardt macular dystrophy. Am J Ophthalmol. 2016;168:269-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1: Modified Humphrey 10-2 Pattern (68 Points) for Sensitivity Testing of the Macula.
eFigure 2: Correlation of Visual Acuity with Microperimetric Sensitivity.
eTable 1: Demographic Characteristics by Inclusion in the Analyses.
eTable 2: Macular Sensitivity in Stargardt Disease.
eTable 3: Macular Sensitivity in Stargardt Disease including Patients with Poor Pattern Placement.
eTable 4: Macular Sensitivity in Stargardt Disease and Its Association with Demographic Features including Patients with Poor Pattern Placement.
eTable 5: Macular Sensitivity in Stargardt Disease and Its Association with Visual Acuity including Patients with Poor Pattern Placement. ProgStar Study Team as of 10 Feb 2017