Abstract
The desired target steady-state average colistin concentration (Css,avg) to balance between therapeutic effectiveness and nephrotoxicity is largely unclear. The objective of this study was to evaluate the effect of the desired target colistin Css,avg on the effectiveness and safety of IV colistin therapy in critically ill patients. Overall, 153 critically ill patients (71% males) receiving IV colistin were retrospectively analyzed. The desired target colistin Css,avg was estimated based on the daily colistin dose and creatinine clearance of each patient. No significant predictor for clinical cure was identified. However, microbiological outcome was significantly associated with pneumonia compared to bacteremia (odds ratio [OR] 0.092, 95% confidence interval [CI] [0.033–0.251], P < 0.001) and the use of IV colistin loading dose (OR 2.783, 95% CI [1.126–6.880], P = 0.027). Colistin-associated nephrotoxicity was significantly less likely to occur in patients who received inhaled colistin close to the time of IV colistin therapy (OR 0.331, CI [0.119–0.925], P = 0.035). The desired target Css,avg of colistin was not associated with treatment outcomes or the risk of nephrotoxicity. Loading dose and inhaled colistin use near the time of IV colistin therapy may be considered to maximize therapeutic effectiveness and minimize the risk of colistin-associated nephrotoxicity, respectively.
Subject terms: Bacterial infection, Outcomes research, Risk factors
Introduction
Treatment of infections caused by multidrug-resistant (MDR) gram-negative bacterial pathogens is challenging due to limited treatment options1. Due to altered physiological characteristics related to critical illness and the care provided in an intensive care unit (ICU) such as common use of broad-spectrum antibiotic agents and invasive procedures, critically ill patients are more susceptible to MDR bacterial infections associated with substantially increased morbidity and mortality2–6. Globally, the most clinically significant MDR bacterial pathogens include Acinetobacter baumannii and Pseudomonas aeruginosa2,7. Some strains of these MDR pathogens are resistant to nearly all antimicrobial agents including aminoglycosides, cephalosporins, fluoroquinolones, and carbapenems, leaving very few antibiotic options for the treatment of infections caused by these organisms2. Among the limited therapeutic options, colistin is one of the most commonly used antibacterial medication for the treatment of life-threatening invasive infections caused by MDR pathogens2,8.
Colistin is a polymyxin antimicrobial agent, specifically polymyxin E9,10. It is intermittently infused via an intravenous (IV) route as a prodrug called colistin methanesulfonate (CMS)9,10. It is rapidly bactericidal with a substantial postantibiotic effect against gram-negative organisms including Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella species9,11. The bactericidal activity of colistin appears concentration-dependent; the ratio of the area under the unbound plasma concentration-time curve over a dosing interval to minimum inhibitory concentration (fAUC:MIC) was suggested to be the pharmacokinetic-pharmacodynamic parameter most predictive of colistin activity9,12,13. According to a previous study using the animal model infected by Pseudomonas aeruginosa, a colistin fAUC:MIC of 12 to 48 was associated with near-optimal to optimal bacterial killing12. However, accurate estimation of fAUC requires serial collection of blood samples over a dosing interval, which is not feasible in routine clinical practice. Therefore, the fAUC is commonly expressed as the average steady-state plasma concentration, which is fAUC divided by 12 hours (typical one dosing interval for patients with normal renal function). To achieve the colistin target of fAUC:MIC of 12 to 48 for an organism with an MIC of 1 mg/L, the fAUC:MIC of 12 to 48 correspond to the target average steady-state total plasma colistin concentrations (Css,avg) of 1 to 4 mg/L. This target Css,avg was not considered in the current dosages approved by the Food and Drug Administration (FDA) in the United States: 2.5 to 5 mg/kg daily in 2 to 4 divided doses for patients with normal renal function with dosage adjusted based on the renal function of each patient14. More recently, the target Css,avg-driven colistin dosing algorithms were developed by modeling and simulation approaches15,16. Currently, many clinicians use these dosing algorithms to determine colistin dose for each individual patient for optimal systemic colistin exposure. However, the target colistin Css,avg range is relatively wide for dosing calculation, and it has not been prospectively validated in large-scale human studies. Moreover, although controversial, the potential association between the risk of nephrotoxicity and the colistin Css,avg of ≥2.5 mg/L further complicates the clinical decision to choose the appropriate colistin dosing17–19. Consequently, the target colistin Css,avg value for dosing calculation to optimally balance between clinical effectiveness and nephrotoxicity is largely unclear, resulting in highly variable colistin dosages used in clinical practice13,15,16,19,20.
Although colistin pharmacokinetics have been extensively studied in various patient populations, to our knowledge, the relationship between the desired target colistin Css,avg for colistin dosing and the treatment outcomes in critically ill patients with acute infections has not been elaborated. Therefore, the objective of this study was to evaluate the effect of the desired target colistin Css,avg for dosing on the effectiveness and safety of IV colistin therapy in critically ill patients.
Results
A total of 170 patient records were identified to meet the inclusion and exclusion criteria. Due to the limited number of patients with infectious diseases other than pneumonia and bacteremia (n = 4 with urinary tract infection, n = 9 with intra-abdominal infection, n = 2 with skin and soft tissue infection), only the patients with pneumonia (n = 114) and bacteremia (n = 41) were included in the analysis cohort. In terms of causative pathogens, Klebsiella pneumoniae caused pneumonia in two patients only, and thus, these patients were excluded from our final analysis cohort. Overall, our final analysis cohort included 153 patients (Table 1). Loading dose of IV colistin was administered to 80 patients (52%). The median (range) desired target colistin Css,avg, which was estimated using Eqs 1 and 2 constructed in a previous study, was 3.10 (2.24–7.24) mg/L from loading doses and 3.17 (0.37–15.56) mg/L from maintenance doses:
| 1 |
| 2 |
where body weight is the lower of ideal body weight (IBW) or total body weight (TBW) in kg, and CrCln is the estimated creatinine clearance (CrCl) normalized to calculated body surface area at baseline in mL/min/1.73 m2 15,21,22. Treatment outcome data were missing in 30 patients each for clinical response and microbiological eradication. In the patients with treatment outcome data, most of the causative organisms had a colistin MIC of ≤0.5 mg/L (n = 97; 97/123 = 79%), which was determined by the Vitek 2 AST N212 card for nonfermenters in the Vitek 2 automated system (bioMerieux, Durham, NC, USA) (Fig. 1). Table 2 summarizes antimicrobial susceptibilities of the cultured isolates of Acinetobacter baumannii and Pseudomonas aeruginosa. All of the clinical cultured isolates were susceptible to colistin with the exception of three Acinetobacter baumannii isolates; they were resistant to colistin. Bacterial susceptibility data were not available in 15 patients. Overall, a total of 123, 108, and 153 patient records were included in the analysis of clinical cure, microbiological eradication, and colistin-associated nephrotoxicity, respectively.
Table 1.
Patient characteristics (n = 153).
| Characteristics | Mean ± standard deviation or median (range) unless otherwise stated |
|---|---|
| Age (years) | 66 (21–91) |
| Male sex (No.) | 109 (71%) |
| Height (cm) | 165 (125–186) |
| Weight (kg) | 57 (37–99) |
| Body mass index (kg/m2) | 21.5 ± 4.1 |
| Creatinine clearance at the beginning of therapy (mL/min) | 55 ± 19 |
| Disease severity score | |
| Charlson comorbidity index | 2 (0–12) |
| APACHE IIa | 24 (2–42) |
| Infectious diseases | |
| Pneumonia (No.) | 112 (73%) |
| Bacteremia (No.) | 41 (27%) |
| Causative organism | |
| Acinetobacter baumannii (No.) | 121 (79%) |
| Pseudomonas aeruginosa (No.) | 32 (21%) |
| No. of concurrently used antibiotics other than intravenous colistin | 3 (0–9)b |
| Use of inhaled colistin therapy immediately prior to the initiation or after the end of intravenous colistin treatment | 19 (12%) |
| Concomitant antibacterials (No.) | |
| Piperacillin-Tazobactam | 123 (80%) |
| Third generation cephalosporins | 130 (85%) |
| Fourth generation cephalosporins | 128 (84%) |
| Aminoglycosides | 16 (10%) |
| Glycopeptides | 139 (91%) |
| Rifampin | 26 (17%) |
| Fluoroquinolones | 46 (30%) |
| Sulfonamides | 55 (36%) |
| No. of concurrently used nephrotoxins other than intravenous colistin | 3 (1–5) |
| Concurrent nephrotoxins other than antibacterials | |
| Vasopressors | 139 (91%) |
| Diuretics | 141 (92%) |
| Intravenous contrast media | 23 (15%) |
| Polyene | 57 (37%) |
| Loading dose of intravenous colistinc (mg) | 300 (225–990) |
| Maintenance dose of intravenous colistin (mg) | 300 (75–1,080) |
| Average daily dose of intravenous colistin (mg) | 312 (84–1,004) |
| Per ideal body weight (mg/kg) | 5.4 (1.5–16.0) |
| Per total body weight (mg/kg) | 5.7 (1.2–21.0) |
| Cumulative intravenous colistin dose (mg) | 4,500 (700–94,220) |
| Duration of intravenous colistin therapy (days) | 14 (3–156) |
aAcute Physiology and Chronic Health Evaluation II (APACHE II) score ranging from 0 to 71 with higher scores corresponding to more severe disease and increased risk of death; data available in 82 patients only.
bOnly 2 patients were treated with intravenous colistin monotherapy.
cOnly 80 patients (52%) received loading dose.
Figure 1.
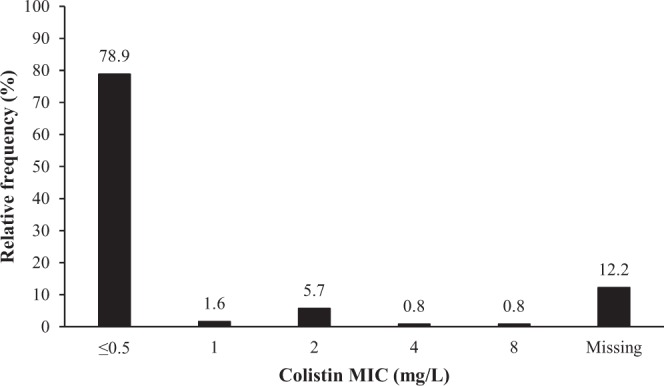
Distribution of the minimum inhibitory concentrations (MICs, mg/L) for colistin (n = 108).
Table 2.
Antimicrobial susceptibilities of the cultured clinical isolates (n = 108a).
| Acinetobacter baumannii (n = 85) | Pseudomonas aeruginosa (n = 23) | ||
|---|---|---|---|
| Antimicrobial agents | No. of susceptible isolates (%) | Antimicrobial agents | No. of susceptible isolates (%) |
| Ampicillin | 18 (21) | Amikacin | 16 (70) |
| Ampicillin/sulbactam | 7 (8) | Aztreonam | 3 (13) |
| Ceftazidime | 0 (0) | Ceftazidime | 3 (13) |
| Ciprofloxacin | 0 (0) | Ciprofloxacin | 2 (9) |
| Colistin | 82 (96) | Colistin | 23 (100) |
| Cefepime | 1 (1) | Cefepime | 4 (17) |
| Cefotaxime | 0 (0) | Gentamicin | 8 (35) |
| Gentamicin | 8 (9) | Imipenem | 2 (9) |
| Imipenem | 1 (1) | Levofloxacin | 1 (4) |
| Levofloxacin | 0 (0) | Meropenem | 2 (9) |
| Meropenem | 1 (1) | Piperacillin | 2 (9) |
| Minocycline | 66 (78) | Piperacillin/tazobactam | 3 (13) |
| Piperacillin | 0 (0) | ||
| Piperacillin/tazobactam | 0 (0) | ||
| Sulfamethoxazole/trimethoprim | 11 (13) | ||
| Tigecycline | 71 (84) | ||
aData to evaluate microbiological response and bacterial susceptibilities were missing in 30 and 18 patients, respectively, and data for both were missing in three patients; therefore, antimicrobial susceptibility data were collected from 108 patient records only.
In the univariate analysis, clinical cure was more likely to occur in patients with bacteremia, those receiving loading dose, those receiving lower cumulative dose, and those treated for a shorter period of time (Table 3). For the microbiological outcome, microbiological eradication was more likely to be achieved in patients with higher baseline CrCl, those with bacteremia, those infected with Acinetobacter baumannii, those receiving an aminoglycoside concurrently, those who received IV colistin loading dose, and those treated for a shorter period of time (Table 4). In terms of safety, colistin-induced nephrotoxicity was more likely to occur in patients with lower baseline CrCl, those not receiving inhaled colistin therapy immediately prior to the initiation or after the end of systemic colistin treatment, those receiving smaller cumulative IV colistin dose, and those treated for a shorter period of time (Table 5). Dose-related factors of colistin such as colistin maintenance dose and the desired target Css,avg were not significantly associated with colistin treatment outcomes. Based on the multiple logistic regression analysis, none of the tested factors were significantly associated with clinical cure (Table 3). However, microbiological eradication was significantly associated with the infectious indication (odds ratio [OR] with 95% confidence interval [CI]: 0.092 [0.033–0.251] for pneumonia compared to bacteremia, P < 0.001) and the use of IV colistin loading dose (OR with 95% CI: 2.783 [1.126–6.880], P = 0.027) (Table 4). Colistin-induced nephrotoxicity, assessed using the Acute Kidney Injury Network (AKIN) criteria, was significantly less likely to occur in patients who received inhaled colistin treatment immediately prior to the initiation or after the end of systemic colistin therapy (OR with 95% CI: 0.331 [0.119–0.925], P = 0.035) (Table 5)23.
Table 3.
Factors evaluated for the association with clinical cure (n = 123a).
| Characteristics | Univariate analysisb,c | Multivariable analysis | |||
|---|---|---|---|---|---|
| Clinical cure (n = 43) | Clinical failure (n = 80) | P value | Odds ratio (95% CI) | P-value | |
| Age (years) | 64 (23–90) | 66 (21–91) | 0.208 | N/E | N/E |
| Male sex (No.) | 27 (63%) | 57 (71%) | 0.336 | N/E | N/E |
| Weight (kg) | 55.2 (40.6–80.5) | 56.9 (37.5–99.2) | 0.561 | N/E | N/E |
| Body mass index (kg/m2) | 21.0 ± 4.1 | 21.7 ± 3.9 | 0.352 | N/E | N/E |
| Creatinine clearance (CrCl) at the beginning of therapyd (mL/min) | 54 ± 17 | 57 ± 19 | 0.352 | N/E | N/E |
| Charlson comorbidity index | 2 (0–10) | 2.5 (0–12) | 0.529 | N/E | N/E |
| Infectious diseases | 0.082 | 0.099 | |||
| Pneumonia (No.) | 27 (63%) | 62 (78%) | |||
| Bacteremia (No.) | 16 (37%) | 18 (22%) | |||
| Causative organism | 0.614 | N/E | |||
| Acinetobacter baumannii (No.) | 36 (84%) | 64 (80%) | N/E | ||
| Pseudomonas aeruginosa (No.) | 7 (16%) | 16 (20%) | N/E | ||
| No. of concurrent antibiotics used other than intravenous colistin | 3 (1–9) | 4 (1–9) | 0.803 | N/E | N/E |
| Use of inhaled colistin immediately prior to the initiation or after the end of systemic colistin treatment (No.) | 7 (16%) | 11 (14%) | 0.705 | N/E | N/E |
| Concurrent antimicrobials (No.) | |||||
| Piperacillin-tazobactam | 11 (26%) | 16 (20%) | 0.476 | N/E | N/E |
| Third generation cephalosporins | 7 (16%) | 15 (19%) | 0.733 | N/E | N/E |
| Fourth generation cephalosporins | 7 (16%) | 17 (21%) | 0.507 | N/E | N/E |
| Aminoglycosides | 6 (14%) | 8 (10%) | 0.510 | N/E | N/E |
| Rifampin | 5 (12%) | 19 (24%) | 0.106 | N/E | N/E |
| Fluoroquinolones | 13 (30%) | 30 (38%) | 0.420 | N/E | N/E |
| Sulfonamides | 19 (44%) | 27 (34%) | 0.254 | N/E | N/E |
| Use of intravenous colistin loading dose (No.) | 26 (60%) | 34 (42%) | 0.057 | 0.068 | |
| Maintenance dose of intravenous colistin (mg) | 337.5 (75–1080) | 300 (100–900) | 0.426 | N/E | N/E |
| Desired target colistin Css,avg estimated from the maintenance dosee (mg/L) | 2.95 (0.37–15.56) | 3.175 (0.78–6.29) | 0.701 | N/E | N/E |
| Desired target colistin Css,avge ≥ 2 mg/L (No.) | 33 (77%) | 62 (78%) | 0.924 | N/E | N/E |
| Average daily dose of intravenous colistin (mg) | 321 (84–1004) | 300 (92–588) | 0.414 | N/E | N/E |
| Per ideal body weight (mg/kg) | 5.4 (1.5–16.0) | 4.9 (1.5–13.8) | 0.570 | N/E | N/E |
| Per total body weight (mg/kg) | 6.2 (2.1–21.0) | 5.4 (1.2–13.7) | 0.319 | N/E | N/E |
| Cumulative intravenous colistin dose (mg) | 4225 (1350–94220) | 5802 (925–43800) | 0.085 | N/E | N/E |
| Duration of intravenous colistin therapy (days) | 14 (4–156) | 17.5 (4–146) | 0.047 | N/E | N/E |
Abbreviation: Css,avg, average steady-state plasma concentration; CI, confidence interval; N/E, not estimated.
aData to determine clinical cure were not available in 30 patients, so 123 patient records were included in the analysis.
bMean ± standard deviation or median (range) unless otherwise noted.
cBolded indicate factors significantly associated with clinical cure.
dEstimated by the Cockcroft-Gault equation using the actual measured serum creatinine concentration (SCr) if SCr was ≥1 mg/dL and the lower of ideal body weight (IBW) or total body weight (TBW); if the patient’s SCr was <1 mg/dL, SCr was rounded up to 1 mg/dL for CrCl estimation.
eEstimated by where CrCln is CrCl normalized to body surface area estimated by the Mosteller method at baseline in mL/min/1.73 m2.
Table 4.
Factors evaluated for the association with microbiological eradication (n = 108a).
| Characteristics | Univariate analysisb,c | Multivariable analysisc | |||
|---|---|---|---|---|---|
| Microbiological eradication (n = 48) | Microbiological failure (n = 60) | P value | Odds ratio (95% CI) | P value | |
| Age (years) | 64 (31–83) | 66 (21–90) | 0.581 | N/E | N/E |
| Male sex (No.) | 34 (71%) | 41 (68%) | 0.779 | N/E | N/E |
| Weight (kg) | 55.6 (42.0–99.2) | 56.6 (37.5–76.5) | 0.358 | N/E | N/E |
| Body mass index (kg/m2) | 21.3 ± 4.1 | 21.5 ± 3.9 | 0.736 | N/E | N/E |
| Creatinine clearance (CrCl) at the beginning of therapyd (mL/min) | 60 ± 19 | 55 ± 16 | 0.094 | 0.373 | |
| Charlson comorbidity index | 2 (0–12) | 2.5 (0–11) | 0.146 | N/E | N/E |
| Susceptibility | 0.406 | N/E | |||
| MDR strains susceptible to colistin only (No.) | 8 (17%) | 5 (8%) | N/E | ||
| MDR strains susceptible to ≥2 antibacterial agents including colistin (No.) | 39 (81%) | 54 (90%) | N/E | ||
| MDR strains resistant to colistin (No.) | 1 (2%) | 1 (2%) | N/E | ||
| Infectious diseases | <0.001 | <0.001 | |||
| Pneumonia (No.) | 20 (42%) | 53 (88%) | 0.092 (0.033–0.251) | ||
| Bacteremia (No.) | 28 (58%) | 7 (12%) | 1 (reference) | ||
| Causative organism | 0.014 | 0.360 | |||
| Acinetobacter baumannii (No.) | 43 (90%) | 42 (70%) | |||
| Pseudomonas aeruginosa (No.) | 5 (10%) | 18 (30%) | |||
| No. of concurrent antibiotics used other than intravenous colistin | 4 (1–9) | 4 (1–8) | 0.451 | N/E | N/E |
| Use of inhaled colistin immediately prior to the initiation or after the end of systemic colistin treatment (No.) | 7 (15%) | 10 (17%) | 0.768 | N/E | N/E |
| Concurrent antimicrobials (No.) | |||||
| Piperacillin-tazobactam | 15 (31%) | 12 (20%) | 0.180 | N/E | N/E |
| Third generation cephalosporins | 9 (19%) | 11 (18%) | 0.956 | N/E | N/E |
| Fourth generation cephalosporins | 10 (21%) | 12 (20%) | 0.915 | N/E | N/E |
| Aminoglycosides | 9 (19%) | 4 (7%) | 0.055 | 0.115 | |
| Rifampin | 11 (23%) | 9 (15%) | 0.293 | N/E | N/E |
| Fluoroquinolones | 20 (42%) | 19 (32%) | 0.282 | N/E | N/E |
| Sulfonamides | 21 (44%) | 20 (33%) | 0.268 | N/E | N/E |
| Use of intravenous colistin loading dose (No.) | 29 (60%) | 22 (37%) | 0.014 | 2.783 (1.126–6.880) | 0.027 |
| Maintenance dose of intravenous colistin (mg) | 360 (120–900) | 75 (300–1080) | 0.432 | N/E | N/E |
| Desired target colistin Css,avg estimated from the maintenance dosee (mg/L) | 3.18 (1.03–8.29) | 2.90 (0.37–15.56) | 0.826 | N/E | N/E |
| Desired target colistin Css,avge ≥ 2 mg/L (No.) | 38 (79%) | 46 (77%) | 0.756 | N/E | N/E |
| Average daily dose of intravenous colistin (mg) | 341 (92–733) | 300 (97–1004) | 0.824 | N/E | N/E |
| Per ideal body weight (mg/kg) | 5.4 (1.5–11.6) | 5.2 (1.7–16.0) | 0.931 | N/E | N/E |
| Per total body weight (mg/kg) | 6.1 (1.2–13.7) | 5.7 (2.0–21.0) | 0.574 | N/E | N/E |
| Cumulative intravenous colistin dose (mg) | 4655 (1040–33684) | 5888 (925–94220) | 0.119 | N/E | N/E |
| Duration of intravenous colistin therapy (days) | 14 (4–134) | 18 (4–156) | 0.023 | 0.313 | |
Abbreviations: Css,avg, average steady-state plasma concentration; CI, confidence interval; MDR, multi-drug resistant; N/E, not estimated.
aData to evaluate microbiological response and bacterial susceptibility were missing in 30 and 18 patients, respectively, and data for both were missing in three patients; therefore, only 108 patient records were included in the analysis of microbiological eradication.
bMean ± standard deviation or median (range) unless otherwise noted.
cBolded indicate factors significantly associated with microbiological eradication.
dEstimated by the Cockcroft-Gault equation using the actual measured serum creatinine concentration (SCr) if SCr was ≥1 mg/dL and the lower of ideal body weight (IBW) or total body weight (TBW); if the patient’s SCr was <1 mg/dL, SCr was rounded up to 1 mg/dL for CrCl estimation.
eEstimated by where CrCln is CrCl normalized to body surface area estimated by the Mosteller method at baseline in mL/min/1.73 m2.
Table 5.
Factors evaluated for the association with nephrotoxicity (n = 153).
| Characteristics | Univariate analysisa,b | Multivariable analysisb | |||
|---|---|---|---|---|---|
| Nephrotoxicity (n = 84) | No nephrotoxicity (n = 69) | P value | Odds ratio (95% CI) | P value | |
| Age (years) | 66 (23–90) | 66 (21–91) | 0.504 | N/E | N/E |
| Male sex (No.) | 58 (69%) | 51 (74%) | 0.508 | N/E | N/E |
| Weight (kg) | 56.6 (37.0–86.1) | 56.7 (37.5–99.2) | 0.765 | N/E | N/E |
| Body mass index (kg/m2) | 21.7 ± 4.1 | 21.4 ± 4.1 | 0.653 | N/E | N/E |
| Creatinine clearance (CrCl) at the beginning of therapyc (mL/min) | 53 ± 16 | 58 ± 21 | 0.058 | 0.233 | |
| Charlson comorbidity index | 2 (0–12) | 2 (0–12) | 0.411 | N/E | N/E |
| Use of inhaled colistin immediately prior to the initiation or after the end of systemic colistin treatment (No.) | 6 (7%) | 13 (19%) | 0.029 | 0.331 (0.119–0.925) | 0.035 |
| No. of concurrent nephrotoxins used other than intravenous colistin | 4 (1–5) | 3 (1–5) | 0.234 | N/E | N/E |
| Concurrent nephrotoxins (No.) | |||||
| Vasopressor | 76 (90%) | 63 (91%) | 0.860 | N/E | N/E |
| Aminoglycoside | 11 (13%) | 5 (7%) | 0.239 | N/E | N/E |
| Polyene | 35 (42%) | 22 (32%) | 0.213 | N/E | N/E |
| Glycopeptide | 75 (89%) | 64 (93%) | 0.459 | N/E | N/E |
| Diuretic | 79 (94%) | 62 (90%) | 0.337 | N/E | N/E |
| Intravenous contrast | 13 (15%) | 10 (14%) | 0.866 | N/E | N/E |
| Use of intravenous colistin loading dose (No.) | 46 (55%) | 34 (49%) | 0.499 | N/E | N/E |
| Maintenance dose of intravenous colistin (mg) | 300 (100–1080) | 300 (75–900) | 0.999 | N/E | N/E |
| Desired target colistin Css,avg estimated from the maintenance dosed (mg/L) | 3.17 (0.98–8.29) | 3.18 (0.37–15.56) | 0.448 | N/E | N/E |
| Desired target colistin Css,avgd ≥ 2.5 mg/L (No.) | 58 (89%) | 45 (65%) | 0.615 | N/E | N/E |
| Average daily dose of intravenous colistin (mg) | 320 (84–1004) | 309 (97–900) | 0.768 | N/E | N/E |
| Per ideal body weight (mg/kg) | 5.7 (1.5–16.0) | 5.0 (1.7–15.4) | 0.685 | N/E | N/E |
| Per total body weight (mg/kg) | 5.8 (1.2–21.0) | 5.7 (2.2–18.0) | 0.946 | N/E | N/E |
| Cumulative intravenous colistin dose (mg) | 4025 (720–33684) | 5325 (700–94220) | 0.010 | N/E | N/E |
| Duration of intravenous colistin therapy (days) | 13 (4–134) | 18 (3–156) | 0.007 | N/E | N/E |
Abbreviation: Css,avg, average steady-state plasma concentration; CI, confidence interval; N/E, not estimated.
aMean ± standard deviation or median (range) unless otherwise noted.
bBolded indicate factors significantly associated with nephrotoxicity.
cEstimated by the Cockcroft-Gault equation using the actual measured serum creatinine concentration (SCr) if SCr was ≥1 mg/dL and the lower of ideal body weight (IBW) or total body weight (TBW); if the patient’s SCr was <1 mg/dL, SCr was rounded up to 1 mg/dL for CrCl estimation.
dEstimated by where CrCln is CrCl normalized to body surface area estimated by the Mosteller method at baseline in mL/min/1.73 m2.
Discussion
With the development of the colistin target Css,avg-based dosing algorithm, colistin doses are mostly determined based on the desired target Css,avg upon the discretion of the clinician treating the patient15,16. Considering the narrow therapeutic window of colistin, it is pertinent for clinicians to use the most appropriate desired target Css,avg for optimal treatment outcomes13. Previous studies suggested conflicting evidence regarding the relationship of colistin dose or concentration with treatment outcomes24–33. Similar to our current study, several previous studies showed the lack of significant association between treatment outcomes and colistin dose or systemic exposure (Tables 3 and 4)24–28. In contrast, other previous studies suggested significantly improved therapeutic effectiveness of colistin at higher doses or systemic exposures29–33. Compared to our current study, the patient population included in these previous studies was relatively homogeneous; patients with a specific indication such as burn, bacteremia, and pneumonia were exclusively included in these studies29–33. In addition, colistin was administered to patients as a fixed or weight-based dosing (e.g., 2.5–5 mg/kg/day divided into 2–4 times per day) without a desired target Css,avg29–33. Similar to treatment outcomes, the relationship between the risk of colistin-associated nephrotoxicity and colistin dose or systemic exposure is controversial24–33. Several previous studies reported no significant association between the colistin-associated nephrotoxicity risk and colistin dose or systemic exposure, which is consistent with our current study findings (Table 5)24,29–31,33. In contrast, other previous studies suggested significantly increased risk of colistin-associated nephrotoxicity at higher colistin dose or systemic exposures25,27,32. While our current study assessed nephrotoxicity using the AKIN criteria, these previous studies evaluated nephrotoxicity based on the RIFLE criteria categorizing nephrotoxicity into risk, injury, failure, loss, and end-stage renal disease25,27,32. Overall, these differences in the study population, outcome definition, and colistin treatment strategy between our current study and previous studies might account for the discrepancy in the study finding for the association of colistin dose or systemic exposures with treatment outcomes and the risk of nephrotoxicity.
Although our current study identified no significant predictors for clinical response of colistin therapy (Table 3), several previous studies reported various factors significantly associated with clinical response, including use of colistin loading dose, Charlson comorbidity index, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, presence of severe sepsis, absence of septic shock, sex, absence of nephrotoxicity at the end of colistin therapy, and higher colistin dosages24,26,29–31,34. Considering substantial heterogeneity in demographic and clinical characteristics of our study patients with a limited sample size, it might be more challenging in our current study to identify significant predictors for clinical response of colistin therapy. For microbiological outcome, our current study showed significantly higher likelihood of bacterial eradication in patients with bacteremia compared to those with pneumonia and in those receiving IV colistin loading dose (Table 4). Consistently, a previous study using mouse infection models reported colistin to be substantially less effective for the treatment of pneumonia compared to skin and soft tissue infection12. This may be because of the possible inadequate antimicrobial exposures in the lungs since pneumonia is often considered a deep tissue infection12. The significant association between the use of IV colistin loading dose and microbiological eradication may highlight the importance of adequate initial antimicrobial therapy for optimal treatment outcome. In a previous study by Martinez and colleagues, antimicrobial exposure during the first dose was suggested as the critical factor for the outcome of an infectious disease35. This was further supported in a previous in vitro study, where the dose-dependent bactericidal activity of colistin was primarily observed during the early phase of therapy36. For nephrotoxicity, patients who received inhaled colistin immediately prior to the initiation or after the end of systemic colistin treatment were at a lower risk of developing colistin-associated nephrotoxicity (Table 5), which is consistent with previous study findings37. The protective effect of inhaled colistin use may be related to using lower doses of IV colistin; in our study, the median (range) colistin maintenance dose, average daily dose, and the desired target Css,avg in patients who received inhaled colistin vs. in those who did not were 360 mg (220–600 mg) vs. 400 mg (120–900 mg), 340 mg (174–525 mg) vs. 355 mg (128–773 mg), and 3.37 mg/L (1.59–5.08 mg/L) vs. 3.42 mg/L (1.43–9.63 mg/L) (P > 0.05; data not shown). Therefore, IV loading dose and inhaled use of colistin may be considered to maximize bacterial eradication and to minimize the risk of nephrotoxicity, respectively.
Our study supports the importance of using IV colistin loading dose in critically ill patients with infections caused by MDR organisms, as suggested by several previous studies15,18,20,34,38–45. Although the use of IV colistin loading dose was not significantly associated with clinical response or colistin-induced nephrotoxicity (Tables 3 and 5), bacterial eradication was significantly more likely to occur in patients receiving IV colistin loading dose (Table 4). Similarly, Katip and colleagues suggested higher likelihood of microbiological clearance in patients receiving IV colistin loading dose for the treatment of various infections caused by MDR Acinetobacter baumannii compared to those receiving the maintenance dose only (87.9% vs. 70.4%, P = 0.0006)39. As aforementioned, this may highlight the importance of appropriate antimicrobial exposure during the first dose to optimize the treatment outcome of an infectious disease35. According to a previous study performed by Kumar and colleagues to evaluate survival after septic shock caused by Escherichia coli in a murine model, mortality was significantly increased with hourly delays of adequate antimicrobial therapy46. Consistently, Mohamed and colleagues suggested higher likelihood of adequate bacterial killing, defined as ≥3 log killing from the baseline, with colistin loading dose administration, and the extent of bacterial killing was dependent on the amount of colistin loading dose based on the pharmacokinetic-pharmacodynamic modeling and simulation41,43,45. The improved bacteriological response with the colistin loading dose may be associated with rapid achievement of the target therapeutic colistin concentrations in plasma15,20,34,42. Due to the slow conversion rate of the prodrug (i.e., CMS) to the active drug (i.e., colistin) and the long half-life of colistin (approximately 14.4 h), it can take 2 to 3 days to attain adequate colistin concentrations without a loading dose; administration of a loading dose may reduce the time to reach the target therapeutic colistin concentration within the first few hours20,34,43. In contrast to our present study, other previous studies suggested no association between the use of IV colistin loading dose and microbiological response47,48. This discrepancy in the association between bacteriological response and the use of IV colistin loading dose may be due to the inclusion of a smaller number of patients in the previous studies compared to our current study and unbalanced sample size between the case (i.e., loading dose used) and the control (i.e., no loading dose) groups47,48. The previous population pharmacokinetic study conducted by Grégoire and colleagues in critically ill patients further complicated the issue of IV colistin loading dose49. In this study, the estimated half-life of colistin was relatively short (3.1 hours) compared to the half-lives calculated in other previous studies (9.0 to 14.4 hours)15,20,42,49, suggesting no need to use IV colistin loading dose49. However, caution should be exercised when interpreting the half-life estimates of colistin because of the uncertainty in the fraction converted from CMS to colistin in most of the clinical studies49. A recent survey study in physicians, pharmacists, and microbiologists (n = 420) reported only half (52.5%) of the respondents utilized a loading dose always or very often, possibly due to the conflicting evidence available regarding the effect of IV colistin loading dose on treatment outcomes50. Large-scale, prospective, clinical studies need to be performed in the near future to determine the effect of IV colistin loading dose on treatment outcomes robustly.
There are some study limitations to be addressed. First, this study may not be adequately powered to identify all of the factors associated with treatment outcomes and nephrotoxicity. Considering the relatively small sample size, caution needs to be exercised when interpreting and applying our study findings to clinical practice. Second, the desired target colistin Css,avg was retrospectively estimated based on a previously published equation using the actual administered doses15. Because plasma colistin concentrations were not measured in our current study, it could not be assessed whether or not our study patients achieved the desired target Css,avg at the given colistin dose. Third, due to the retrospective nature of this study based on the electronic medical records (EMRs), causality between the tested factors and treatment outcomes or nephrotoxicity risk could not be evaluated. Important information such as APACHE II score and dehydration status was missing for many, if not all, patients in our study. Moreover, clinical microbiology culture and susceptibility data were missing in several patients. Furthermore, colistin susceptibilities in our current study were not determined by the standard broth dilution method proposed by the Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST), which is considered as the current gold standard for antimicrobial susceptibility testing51,52. Colistin susceptibility testing was performed in compliance with the standard method at the time of care for patients included in our study (i.e., 2013 to 2014), which was the disk diffusion method to confirm the colistin susceptibility measured by the automated antimicrobial susceptibility testing based on the broth microdilution method (e.g., Vitek 2). Due to the unavailability of the cultured clinical strains currently, colistin susceptibility testing could not be repeated with the standard broth microdilution method proposed by the CLSI and the EUCAST. Although we acknowledge the failure to use the current gold standard antimicrobial susceptibility testing method as a study limitation, our colistin MICs and susceptibility results might be sufficiently reliable because Vitek 2 systems, used to measure colistin MICs in our study patients, are based on the broth microdilution method53. Lastly, confounding factors related to human behavior such as prescribing pattern might lead to spurious results for the relationship of tested factors with treatment outcomes or the risk of nephrotoxicity. In the univariate analysis of our current study, larger cumulative doses and longer treatment duration of IV colistin were associated with lower likelihood of clinical cure, bacterial eradication, and nephrotoxicity (Tables 3–5). According to previous studies, these relationships were not considered clinically plausible24–33. Our institutional observation revealed this as a unique prescribing pattern. Colistin has been used at the same or higher doses over a prolonged period for patients with inadequate treatment response. In addition, IV colistin was discontinued immediately after the development of clinical signs and/or symptoms of nephrotoxicity even in patients with critical infection. Future clinical studies, preferably prospective studies, are warranted to clarify the effect of colistin doses or systemic exposures on treatment outcomes and safety and to develop and validate an appropriate model to predict colistin exposure in various patient populations for optimal colistin therapy and monitoring.
In conclusion, the desired target Css,avg of colistin is not associated with treatment outcomes or the risk of colistin-associated nephrotoxicity. However, bacterial eradication is more likely to occur in critically ill patients with bacteremia compared to pneumonia caused by MDR Acinetobacter baumannii or Pseudomonas aeruginosa and in those receiving a loading dose of IV colistin. Use of inhaled colistin immediately prior to the initiation or after the end of systemic colistin therapy lowers the likelihood of developing colistin-associated nephrotoxicity. Use of an IV colistin loading dose and inhaled colistin near the time of systemic colistin treatment may be considered to maximize therapeutic effectiveness and minimize the risk of colistin-associated nephrotoxicity, respectively.
Methods
Study design and patients
This is a retrospective study by reviewing EMRs for patients hospitalized in an ICU at a 2,000-bed tertiary university hospital from January 2013 to December 2014. Adult patients (≥18 years of age) were included if they had a documented acute infection caused by multidrug-resistant bacterial pathogens and received IV colistin for 72 hours or longer. If colistin treatment course was interrupted for more than a week, only the treatment course before interruption was included. Exclusion criteria included pregnancy, breastfeeding, and severe kidney function impairment defined as a baseline serum creatinine concentration (SCr) of ≥4 mg/dL or undergoing renal replacement therapy of any type. Patients who simultaneously received inhaled colistin with IV colistin were excluded from the analysis; however, those who received inhaled colistin prior to the initiation or after the cessation of IV colistin were included. This study was approved by the institutional review board at the study site (Severance Hospital of Yonsei University Medical Center, Seoul, Republic of Korea). All methods were carried out in accordance with relevant guidelines and regulations. The need for informed consent was waived due to the retrospective nature of the study.
Data collection
The following data were collected from EMRs for patients who satisfied our inclusion and exclusion criteria: demographics including age, sex, weight, and height; disease severity including APACHE II and Charlson comorbidity index; infection-related information including indication, causative organisms identified using the calorimetric Vitek 2 GN ID card (bioMerieux, Durham, NC, USA) in the Vitek 2 automated system, and antibiotic susceptibility of the causative organisms; clinical laboratory test results such as white blood cell count (WBC) and SCr; colistin dosing regimen such as use of a loading dose, the amount of loading dose, maintenance dose and frequency, and duration of therapy; additional antimicrobial agents used besides IV colistin; use of inhaled colistin immediately prior to the initiation or after the cessation of IV colistin; and concurrent use of nephrotoxins other than colistin such as aminoglycosides, glycopeptides, IV contrast, diuretics, polyene, and vasopressors. The antimicrobial MICs of the cultured isolates were determined for clinical practice by the Vitek 2 AST N212 card for nonfermenters in the Vitek 2 automated system (bioMerieux, Durham, NC, USA). The measured MICs were interpreted based on the guidelines published by the CLSI54,55. Colistin susceptibility results obtained by the Vitek 2 system were confirmed using the disk diffusion method in accordance with the CLSI standards at the time of patient care54,55. The Cockcroft-Gault method was used to estimate CrCl by using the actual measured SCr if SCr was ≥1 mg/dL and the lower of IBW or TBW; if the patient’s SCr was <1 mg/dL, SCr was rounded up to 1 mg/dL21.
The desired target colistin Css,avg was retrospectively estimated using the following equations (Eqs 3 and 4) modified from those suggested in a previous study:
| 3 |
| 4 |
where body weight is the lower of IBW or TBW in kg, and CrCln is CrCl normalized to body surface area at baseline in mL/min/1.73 m2 15. Body surface area was estimated by the Mosteller method22.
Treatment effectiveness was evaluated based on clinical cure and microbiological eradication. Clinical cure was defined as the resolution of infection signified by clinical improvement such as temperature <37 °C for ≥72 hours, WBC < 12,000 cells/mm3, no radiologic evidence of active infection, no other clinical infectious signs and symptoms, and the lack of re-occurrence of the same infection during the hospitalization period. Microbiological eradication/cure was defined as the documented elimination of the original causative bacterial pathogen from the site of isolation through follow-up culture studies during or at the end of colistin therapy. Otherwise, the cases were classified as either clinical or microbiological failure. Nephrotoxicity was defined as an increase in SCr by 0.3 mg/dL or 1.5- to 2-fold increase in SCr from baseline according to the Acute Kidney Injury Network (AKIN) criteria23. Nephrotoxicity that occurred at ≥48 hours after the initiation of IV colistin therapy was deemed to be associated with colistin.
Statistical analysis
All statistical analyses were performed using SPSS Statistics 23.0 (IBM SPSS Statistics for Windows, Version 23.0, Armonk, NY: IBM Corp.). Categorical variables were analyzed using the chi-square or Fisher’s exact test. Continuous variables were compared between the treatment success and failure groups using the two independent sample Student’s t test for normally distributed data or the Mann-Whitney U test for non-normally distributed data based on the Kolmogorov-Smirnov normality test results. Factors evaluated for the association with clinical response in the univariate analysis included all of the demographic and clinical characteristics of study patients. For microbiological eradication, bacterial susceptibility in addition to all of the factors tested for the association with clinical response were evaluated. For nephrotoxicity, the followings were tested in addition to the factors assessed for clinical response: the number of concurrently used nephrotoxins; and use of a concurrent vasopressor, nephrotoxic antimicrobial agents, diuretics, and an IV contrast media.
Univariate analyses were performed to compare patients with and without clinical cure, microbiological eradication, and nephrotoxicity. Based on the univariate analysis results, multiple logistic regression was performed using a stepwise forward method by evaluating the factors identified as significantly associated with clinical cure, microbiological eradication, and nephrotoxicity, respectively. In order to ensure the inclusion of all potentially pertinent factors, variables with P < 0.10 from the univariate analysis and clinical plausibility were assessed in the multiple logistic regression. Statistical significance in the multivariable logistic regression was defined as P < 0.05.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Education (2017R1D1A1B03033389). E. K. Chung received research grant support from the NRF funded by the Korea government (Ministry of Science and ICT; MSIT) (2017R1C1B5015865). The funder had no involvement in the study design; data collection, analysis, and interpretation; manuscript writing; and manuscript submission for publication.
Author Contributions
S. Jung contributed to study conceptualization; data collection, analysis, and interpretation; and manuscript review. E.K. Chung contributed to study methodology, data analysis and interpretation, and manuscript writing and review. M.S. Jun and E.S. Son were involved in the research administration and manuscript review. S.J. Rhie contributed to study conceptualization, data interpretation, overall study supervision, funding, and manuscript review.
Data Availability
The datasets generated and/or analysed during the current study are not publicly available due to the inclusion of private medical information at our institution, but may be available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sunmi Jung and Eun Kyoung Chung contributed equally.
References
- 1.World Health Organization. Antimicrobial resistance. Geneva, Switzerland: WHO, http://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (2018).
- 2.Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Atlanta, Georgia, USA: CDC, https://www.cdc.gov/drugresistance/threat-report-2013/index.html (2014).
- 3.Aloush V, Navon-Venezia S, Seigman-Igra Y, Cabili S, Carmeli Y. Multidrug-resistant Pseudomonas aeruginosa: risk factors and clinical impact. Antimicrob Agents Chemother. 2006;50:43–48. doi: 10.1128/AAC.50.1.43-48.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maragakis LL. Recognition and prevention of multidrug-resistant Gram-negative bacteria in the intensive care unit. Crit Care Med. 2010;38((8 Suppl)):S345–351. doi: 10.1097/CCM.0b013e3181e6cbc5. [DOI] [PubMed] [Google Scholar]
- 5.Kalil AC, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mermel LA, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryu S. The new Korean action plan for containment of antimicrobial resistance. J Glob Antimicrob Resist. 2017;8:70–73. doi: 10.1016/j.jgar.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Garnacho-Montero J, et al. European Society of Intensive Care Medicine. Task force on management and prevention of Acinetobacter baumannii infections in the ICU. Intensive Care Med. 2015;41:2057–2075. doi: 10.1007/s00134-015-4079-4. [DOI] [PubMed] [Google Scholar]
- 9.Lim LM, et al. Resurgence of colistin: a review of resistance, toxicity, pharmacodynamics, and dosing. Pharmacotherapy. 2010;30:1279–1291. doi: 10.1592/phco.30.12.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergen PJ, et al. Polymyxin combinations: pharmacokinetics and pharmacodynamics for rationale use. Pharmacotherapy. 2015;35:34–42. doi: 10.1002/phar.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, et al. In vitro pharmacodynamic properties of colistin and colistin methanesulfonate against Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Antimicrob Agents Chemother. 2001;45:781–785. doi: 10.1128/AAC.45.3.781-785.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheah SE, et al. New pharmacokinetic/pharmacodynamic studies of systemically administered colistin against Pseudomonas aeruginosa and Acinetobacter baumannii in mouse thigh and lung infection models: smaller response in lung infection. J. Antimicrob Chemother. 2015;70:3291–3297. doi: 10.1093/jac/dkv267. [DOI] [PubMed] [Google Scholar]
- 13.Nation RL, et al. Updated US and European Dose Recommendations for Intravenous Colistin: How Do They Perform? Clin Infect Dis. 2016;62:552–558. doi: 10.1093/cid/civ964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coly-Mycin, M. Parenteral [prescribing information]. Par Pharmaceutical. Available at, http://www.parsterileproducts.com/products/assets/pdf/PI/2018/Coly-MycinM-3000818K.pdf, Accessed September 10, 2018.
- 15.Garonzik SM, et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother. 2011;55:3284–3294. doi: 10.1128/AAC.01733-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nation RL, et al. Dosing guidance for intravenous colistin in critically-ill patients. Clin Infect Dis. 2017;64:565–571. doi: 10.1093/cid/ciw839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorlí L, et al. Trough colistin plasma level is an independent risk factor for nephrotoxicity: a prospective observational cohort study. BMC Infect Dis. 2013;13:380. doi: 10.1186/1471-2334-13-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landersdorfer CB, Nation RL. Colistin: how should it be dosed for the critically ill? Semin Respir Crit Care Med. 2015;36:126–135. doi: 10.1055/s-0034-1398390. [DOI] [PubMed] [Google Scholar]
- 19.Nation RL, et al. Framework for optimisation of the clinical use of colistin and polymyxin B: the Prato polymyxin consensus. Lancet Infect Dis. 2015;15:225–234. doi: 10.1016/S1473-3099(14)70850-3. [DOI] [PubMed] [Google Scholar]
- 20.Plachouras D, et al. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrob Agents Chemother. 2009;53:3430–3436. doi: 10.1128/AAC.01361-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 22.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 23.Mehta RL, et al. Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dalfino L, et al. High-dose, extended-interval colistin administration in critically ill patients: is this the right dosing strategy? A preliminary study. Clin Infect Dis. 2012;54:1720–1726. doi: 10.1093/cid/cis286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vicari G, Bauer SR, Neuner EA, Lam SW. Association between colistin dose and microbiologic outcomes in patients with multidrug-resistant gram-negative bacteremia. Clin Infect Dis. 2013;56:398–404. doi: 10.1093/cid/cis909. [DOI] [PubMed] [Google Scholar]
- 26.Sorlí L, et al. Impact of colistin plasma levels on the clinical outcome of patients with infections caused by extremely drug-resistant Pseudomonas aeruginosa. BMC Infect Dis. 2017;17:11. doi: 10.1186/s12879-016-2117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benattar YD, et al. The effectiveness and safety of high-dose colistin: prospective cohort study. Clin Infect Dis. 2016;63:1605–1612. doi: 10.1093/cid/ciw684. [DOI] [PubMed] [Google Scholar]
- 28.Choi IS, et al. Predictors of mortality in patients with extensively drug-resistant Acinetobacter baumannii pneumonia receiving colistin therapy. Int J Antimicrob Agents. 2016;48:175–180. doi: 10.1016/j.ijantimicag.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Wilkinson RE, Hill DM, Hickerson WL. Outcome analysis of colistin-treated burn center patients. Burns. 2017;43:1244–1249. doi: 10.1016/j.burns.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Trifi A, et al. Efficacy and toxicity of high-dose colistin in multidrug-resistant Gram-negative bacilli infections: a comparative study of a matched series. Chemotherapy. 2016;61:190–196. doi: 10.1159/000442786. [DOI] [PubMed] [Google Scholar]
- 31.Gibson GA, et al. Influence of colistin dose on global cure in patients with bacteremia due to carbapenem-resistant Gram-negative bacilli. Antimicrob Agents Chemother. 2015;60:431–436. doi: 10.1128/AAC.01414-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwon KH, et al. Colistin treatment in carbapenem-resistant Acinetobacter baumannii pneumonia patients: Incidence of nephrotoxicity and outcomes. Int J Antimicrob Agents. 2015;45:605–609. doi: 10.1016/j.ijantimicag.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Falagas ME, et al. Colistin therapy for microbiologically documented multidrug-resistant Gram-negative bacterial infections: a retrospective cohort study of 258 patients. Int J Antimicrob Agents. 2010;35:194–199. doi: 10.1016/j.ijantimicag.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 34.Nazer LH, Anabtawi N. Optimizing colistin dosing: Is a loading dose necessary? Am J Health Syst Pharm. 2017;74:e9–e16. doi: 10.2146/ajhp150876. [DOI] [PubMed] [Google Scholar]
- 35.Martinez MN, Papich MG, Drusano GL. Dosing regimen matters: the importance of early intervention and rapid attainment of the pharmacokinetic/pharmacodynamic target. Antimicrob Agents Chemother. 2012;56:2795–2805. doi: 10.1128/AAC.05360-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bergen PJ, et al. Pharmacokinetic/pharmacodynamic investigation of colistin against Pseudomonas aeruginosa using an in vitro model. Antimicrob Agents Chemother. 2010;54:3783–3789. doi: 10.1128/AAC.00903-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdellatif S, et al. Efficacy and toxicity of aerosolised colistin in ventilator-associated pneumonia: a prospective, randomised trial. Ann Intensive Care. 2016;6:26. doi: 10.1186/s13613-016-0127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garnacho-Montero J, Timsit JF. Managing Acinetobacter baumannii infections. Curr Opin Infect Dis. 2019;32:69–76. doi: 10.1097/QCO.0000000000000518. [DOI] [PubMed] [Google Scholar]
- 39.Katip, W., Meechoui, M., Thawornwittayakom, P., Chinwong, D. & Oberdorfer, P. Efficacy and safety of high loading dose of colistin in multidrug-resistant Acinetobacter baumannii: a prospective cohort study. J. Intensive Care Med. 885066617725694, 10.1177/0885066617725694 (2017). [DOI] [PubMed]
- 40.Grégoire N, Aranzana-Climent V, Magréault S, Marchand S, Couet W. Clinical pharmacokinetics and pharmacodynamics of colistin. Clin Pharmacokinet. 2017;56:1441–1460. doi: 10.1007/s40262-017-0561-1. [DOI] [PubMed] [Google Scholar]
- 41.Mohamed AF, et al. Dynamic interaction of colistin and meropenem on a WT and a resistant strain of Pseudomonas aeruginosa as quantified in a PK/PD model. J. Antimicrob Chemother. 2016;71:1279–1290. doi: 10.1093/jac/dkv488. [DOI] [PubMed] [Google Scholar]
- 42.Karaiskos I, et al. Colistin population pharmacokinetics after application of a loading dose of 9 MU colistin methanesulfonate in critically ill patients. Antimicrob Agents Chemother. 2015;59:7240–7248. doi: 10.1128/AAC.00554-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohamed AF, et al. Application of a loading dose of colistin methanesulfonate in critically ill patients: population pharmacokinetics, protein binding, and prediction of bacterial kill. Antimicrob Agents Chemother. 2012;56:4241–4249. doi: 10.1128/AAC.06426-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garnacho-Montero J, Corcia-Palomo Y, Amaya-Villar R, Martin-Villen L. How to treat VAP due to MDR pathogens in ICU patients. BMC Infect Dis. 2014;28(14):135. doi: 10.1186/1471-2334-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohamed AF, Cars O, Friberg LE. A pharmacokinetic/pharmacodynamic model developed for the effect of colistin on Pseudomonas aeruginosa in vitro with evaluation of population pharmacokinetic variability on simulated bacterial killing. J. Antimicrob Chemother. 2014;69:1350–1361. doi: 10.1093/jac/dkt520. [DOI] [PubMed] [Google Scholar]
- 46.Kumar A, et al. The duration of hypotension before the initiation of antibiotic treatment is a critical determinant of survival in a murine model of Escherichia coli septic shock: association with serum lactate and inflammatory cytokine levels. J. Infect Dis. 2006;193:251–258. doi: 10.1086/498909. [DOI] [PubMed] [Google Scholar]
- 47.Alp E, et al. Efficacy of loading dose of colistin in Acinetobacter baumannii ventilator-associated pneumonia. Infez Med. 2017;25:311–319. [PubMed] [Google Scholar]
- 48.Katip W, Uitrakul S, Oberdorfer P. Clinical outcomes and nephrotoxicity of colistin loading dose for treatment of extensively drug-resistant Acinetobacter baumannii in cancer patients. Infect Drug Resist. 2017;10:293–298. doi: 10.2147/IDR.S144314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grégoire N, et al. New colistin population pharmacokinetic data in critically ill patients suggesting an alternative loading dose rationale. Antimicrob Agents Chemother. 2014;58:7324–7330. doi: 10.1128/AAC.03508-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wenzler E, Bunnell KL, Danziger LH. Clinical use of the polymyxins: the tale of the fox and the cat. Int J Antimicrob Agents. 2018;51:700–706. doi: 10.1016/j.ijantimicag.2017.12.023. [DOI] [PubMed] [Google Scholar]
- 51.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; twenty-third informational supplement. Document M100-S29. Wayne, P. A.: CLSI; 2019.
- 52.CLSI-EUCAST Polymyxin Breakpoints Working Group. Recommendations for MIC determination of colistin (polymyxin E). EUCAST http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/General_documents/Recommendations_for_MIC_determination_of_colistin_March_2016.pdf (2016).
- 53.Burgos, R. M., Erdman, S. M. & Rodvold, K. A. Infectious Diseases in Basic Skills in Interpreting Laboratory Data (ed. Lee, M.) 421–492 (American Society of Health-System Pharmacists, 2017).
- 54.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; twenty-third informational supplement. Document M100-S23. Wayne, P. A.: CLSI; 2013.
- 55.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; twenty-fourth informational supplement. Document M100-S24. Wayne, P. A.: CLSI; 2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due to the inclusion of private medical information at our institution, but may be available from the corresponding author on reasonable request.


