LETTER TO THE EDITOR
Paraganglioma-related clinical sequela results from catecholamine secretion that can cause hypertension, tachyarrhythmia, multi-organ failure, and death.1 Radiolabeled 177Lu-DOTATATE (Lutathera®) is a systemic therapy given at 200 mCi as a 30–60 minute intravenous infusion every 8 weeks for 4 cycles. After binding as a somatostatin analogue to overexpressed somatostatin receptors on a tumor, Lutathera® undergoes radioactive decay releasing beta particles that subsequently cause cellular disruption, which causes profound catecholamine release.2 A pharmacologic catecholamine blockade is achieved with α/β-adrenoceptor blockers or calcium-channel blockers, with or without catecholamine synthesis inhibitor α-methyl-para-tyrosine (Demser®).1 Until now, no other medications have been evaluated in catecholamine-induced sinus tachycardia.
A fifty-four-year-old male with inoperable metastatic paragangliomas required radio- or chemotherapy. His hypertension and tachycardia were well controlled on phenoxybenzamine, metoprolol, and metyrosine, and his cardiac and functional status (NYHA I, EF 60%) were excellent. Prior to therapy, his plasma norepinephrine and epinephrine were 20,456 pg/mL (84–794 pg/mL) and <20 pg/mL (0–57 pg/mL), respectively. After one treatment of Lutathera®, he experienced hypertension and tachycardia, requiring occasional 5 mg phentolamine intravenous pushes, esmolol 200 mcg/kg/min, labetalol 2 mg/min, and diltiazem 15 mg/hr to establish circulatory control. Despite adequate medical management, his tachycardia and hypertension were becoming treatment resistant. As predicted, norepinephrine increased, reaching 172,339 pg/ml. Subsequently, the patient developed systolic heart failure (EF 30%) and acute kidney injury. Persistent tachycardia despite maximum doses of β- and calcium-channel blockers, as well as Demser®, warranted immediate consideration of additional rate control medications. With an excellent safety profile and benefit in systolic heart failure3, 5 mg 2x/day of oral Ivabradine was given. Heart rate control was achieved with a decrease in maximal pulse from 143 to 92 bpm on the day prior to Ivabradine and the third day of Ivabradine use, respectively. When Ivabradine was tapered to 2.5 mg 2x/day, the patient developed tachycardia and was therefore sent home on 5 mg 2x/day. Cardiac function likewise improved, achieving an EF of 45%. Owing to the patient’s clinical course, subsequent Lutathera® cycles were deferred.
Catecholamines increase heart rate via β-adrenoceptor stimulation (Fig. 1). β- and calcium-channel blockers modulate this effect by inhibiting the signal cascade. Although β-adrenoceptor desensitization occurs with prolonged catecholamine stimulation4, tachycardia persists despite a maximal β-adrenoceptor blockade.
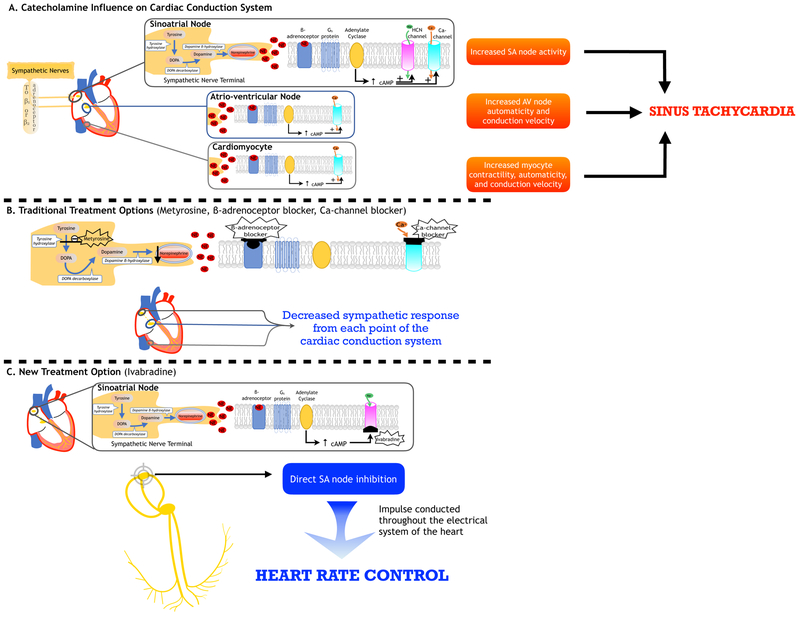
Figure 1. Sites of action for heart rate control in catecholamine-induced tachycardia.
Heart rate is controlled by the autonomic nervous system, with the sinus node acting as the pacemaker. Sinus tachycardia from sympathetic overstimulation is the most prevalent cardiovascular manifestation of paragangliomas. (A) Norepinephrine and/or epinephrine are released from sympathetic nerve terminals (or catecholamine-secreting tumors) and bind to β1- or β2- adrenoceptors in the heart (sinoatrial (SA) node, atrioventricular (AV) node, cardiomyocyte). A subsequent cascade results, wherein a β-receptor coupled with Gs-protein activates adenylate cyclase, which increases cAMP levels and promotes ion-channel permeability thereby increasing node activity, automaticity, and conduction velocity leading to sinus tachycardia. (B) Traditional treatment options for sinus tachycardia include β- and calcium-channel blockers, which provide negative chronotropic effects on the SA node, depression of the AV node, and decreased conduction velocity and contractility in cardiomyocytes. In addition, α-methyl-para-tyrosine (Demser®), an inhibitor of the rate-limiting enzyme tyrosine hydroxylase, works by decreasing catecholamine synthesis, thereby decreasing catecholamine-induced sympathetic stimulation of the heart. (C) A new treatment option using a novel drug, ivabradine, works by targeting the If current in the SA node through intracellular blockade of HCN channel leading to reduced diastolic depolarization of the pacemaker action potential hence decreasing heart rate. In this manner, despite β-adrenoceptor activation and cAMP production, electrical impulse would not be generated due to If current blockade. With our present knowledge, it is important to note that ivabradine acts solely on the SA node; therefore, catecholamine effects on the AV node and cardiomyocytes will not be mitigated by this drug. Nevertheless, the regulation of the intrinsic pacemaker in the SA node is paramount in heart rate control. Abbreviations: DOPA: 3,4-dihydroxyphenylalanine; HCN: hyperpolarization-activated cyclic nucleotide-gated channel; cAMP: cyclic adenosine monophosphate.
Ivabradine is the only commercially available If current inhibitor, acting directly in the sinus node without adverse cardiovascular events.5 The If current acts in the hyperpolarization-activated cyclic nucleotide-gated (HCN) channel of the sinoatrial node, which increases with sympathetic stimulation resulting in the generation and modulation of cardiac rhythmicity.5 Thus, regulated impulse generation with Ivabradine is one possible approach in managing sinus tachycardia in patients with confounding elevated catecholamine levels and β−adrenoceptor desensitization, and therefore diminished responses to β-adrenoceptor blockers.
Footnotes
Disclosure: Nothing to disclose
References
- 1.Pacak K Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab 2007;92:4069–79. [DOI] [PubMed] [Google Scholar]
- 2.Makis W, McCann K, McEwan AJ. The Challenges of Treating Paraganglioma Patients with (177)Lu-DOTATATE PRRT: Catecholamine Crises, Tumor Lysis Syndrome and the Need for Modification of Treatment Protocols. Nucl Med Mol Imaging 2015;49:223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koruth JS, Lala A, Pinney S, Reddy VY, Dukkipati SR. The Clinical Use of Ivabradine. J Am Coll Cardiol 2017;70:1777–84. [DOI] [PubMed] [Google Scholar]
- 4.Tsujimoto G, Manger WM, Hoffman BB. Desensitization of beta-adrenergic receptors by pheochromocytoma. Endocrinology 1984;114:1272–8. [DOI] [PubMed] [Google Scholar]
- 5.DiFrancesco D The role of the funny current in pacemaker activity. Circ Res 2010;106:434–46. [DOI] [PubMed] [Google Scholar]