Abstract
Objective:
Previous studies have suggested that suicide rates are elevated among cancer patients relative to the general population. In this analysis, we comprehensively evaluated characteristics associated with higher suicide rates among patients with cancers of the digestive system.
Methods:
Using the United States (U.S.) Surveillance, Epidemiology, and End Results (SEER) database, we identified all patients diagnosed with digestive system cancers during 2000–2014. Patients were classified as having died from suicide if their cause of death in SEER was listed as “suicide and self-inflicted injury.” Suicide rates were compared to age-, sex-, and race-adjusted rates in the general population.
Results:
A total of 881 suicides were identified among 856,293 patients diagnosed with digestive system cancers. The suicide rate in this population was 32.8 per 100,000 person-years, and was nearly twice that in the general population (standardized mortality ratio [SMR]=1.91; 95% CI: 1.79, 2.04). Suicide rates were significantly elevated for all cancer sites, but were highest for esophageal (SMR=5.03), pancreatic (SMR=5.28), stomach (SMR=2.84), and liver (SMR=2.14) cancers. SMRs for suicide were highest within the first five years of diagnosis, and increased with age at diagnosis for all sites except colon and stomach.
Conclusions:
Patients with cancers of the digestive system have a higher incidence of suicide than the general population. Suicide rates among esophageal and pancreatic cancer patients are more than five times general population rates. The involvement of psychiatrists and other mental health professionals may be a critical component of cancer care for these high-risk patient subgroups.
Keywords: cancer, oncology, esophageal cancer, liver cancer, pancreatic cancer, suicide
Introduction
Suicide is the 10th leading cause of death in the United States (U.S.), accounting for over 40,000 deaths in 2014.1 After a consistent decline in the late 1980s through the 1990s, U.S. suicide rates increased steadily from 1999 through 2014, with increases observed among adolescents and both young and middle-aged adults.2 Characteristics typically associated with higher suicide rates in the general population include older age, white race, and male sex. Chronic or debilitating physical illnesses, such as cancer, have also been included among the major risk factors for suicide.3
Previous U.S-based studies have demonstrated that suicide rates in cancer patients are significantly higher than in the general population. In a study of U.S. cancer patients diagnosed from 1973 to 2002 using data from the Surveillance, Epidemiology, and End Results (SEER) program, the age-, sex-, and race-adjusted suicide rate among cancer patients (31.4/100,000 person-years) was nearly twice that in the general U.S. population (16.7/100,000 person-years).4 More recently, Kam et al. conducted an in-depth analysis of suicide among patients diagnosed with head and neck cancer between 1973 and 2011, finding higher rates among those with hypopharyngeal and laryngeal cancers, sites linked to loss of function in speaking and swallowing ability.5 To our knowledge, such a comprehensive analysis has not been conducted among cancers of the digestive system, many of which also have poor prognoses and physical symptoms associated with reduced quality of life. Identifying cancer patient subgroups with higher suicide rates may help in directing mental health services and other forms of psychological support to those most in need.
The aim of this study was to investigate suicide rates among patients with cancers of the digestive system compared to the general population. We also examined whether suicide rates were higher in subgroups defined by anatomic cancer site, stage, time since diagnosis, and patient characteristics.
Materials and methods
Data Source
We identified patients with cancers of the digestive system using data from the SEER program of the National Cancer Institute. The SEER program is a system of population-based cancer registries which are located strategically throughout the country and cover approximately 28% of the total U.S. population.6 In addition to cancer incidence data, SEER also conducts active follow-up for patient vital status and reports survival and mortality data, including cause of death as ascertained from death certificates. For this study, we used the Public Use version of data collected from the SEER 18 registries from 2000–2014, based on the November 2016 submission.7 The year 2000 is the earliest year for which data is available for all cases in the SEER 18 registries.8 Variables available from the SEER database include patient age at diagnosis, cancer site, stage, year of diagnosis, surgery, demographic characteristics (sex, race, and marital status), and survival time calculated from the date of diagnosis. This study was considered exempt by the Institutional Review Board of the University of North Carolina (IRB number 17–3233).
Study population
We included all patients with digestive system tumors, as defined by the SEER site recode ICD-O-3/WHO 2008, who were diagnosed during 2000–2014. Cancer sites of the digestive system include the esophagus, stomach, small intestine, colon, rectum and rectosigmoid junction, anus, anal canal and anorectum, liver and intrahepatic bile duct, gallbladder, other biliary, pancreas, retroperitoneum, peritoneum, omentum and mesentery, and other digestive organs.9 Our study cohort was restricted to those with a first primary tumor with malignant behavior and known patient age. Cases identified through death certificate or autopsy only were excluded. We classified patient deaths as suicides if the cause of death, as defined by the SEER cause of death recode,10 was listed as ‘suicide and self-inflicted injury.’
Statistical analysis
Suicide rates among patients with cancers of the digestive system were calculated as the number of observed suicides per 100,000 person-years of follow-up. To compare suicide rates in our cohort with those of the general population, we used U.S. population suicide rates from the National Center for Health Statistics, available through the SEER program. Standardized mortality ratios (SMRs) were calculated as the ratio of observed suicides among digestive system cancer patients to the number of expected suicides in the general population. The number of expected suicides was calculated by multiplying the general population suicide rate by the person-time in our cohort, summed across strata of age, race, and sex. For SMR calculations, individuals of unknown race were excluded, since general population rates are not available for unknown race. Confidence intervals for the SMRs were calculated using exact methods. We estimated SMRs according to anatomic site, sex, age at diagnosis (<50, 50–59, 60–69, 70–79, 80+), race (White, Black, Other), marital status (married, unmarried), stage (localized, regional, distant), and surgery (yes, no). In cancer site-specific analyses, we also estimated SMRs according time since diagnosis (<2 months, 2 months-<1 year, 1 years-<5 years, 5+ years), selecting the initial 2 month cutoff as our best estimate of a reasonable window between diagnosis and the start of cancer treatment, the time period which we hypothesized would be associated with the highest suicide rates. We also calculated absolute excess risks (AERs) for suicide as the observed minus expected counts, divided by the total person-time at risk. AERs are reported as the number of excess suicides per 10,000 person-years. As a secondary analysis, we estimated rate ratios for suicide among digestive system cancer patients using multivariable-adjusted Poisson regression models. For these analyses, patients with 0 completed months of follow-up were assigned a value of 0.5 months.11 Analyses were performed using SEER*Stat version 8.3.4.12 and SAS version 9.4 (SAS Institute, Cary, NC).
Results
A total of 856,292 patients with cancers of the digestive system were identified (Table 1). The most common cancer sites among patients in this cohort were colon (37%), rectum and rectosigmoid junction (16%), and pancreas (14%). The majority of patients were white (78%), male (54%), married (53%), and diagnosed at ages 60 and older (69%) with localized or regional stage cancers (64%). Overall, a total of 881 suicides were observed over 2,688,837 person-years of follow-up (mean=3.14 years) (Table 1). This corresponded to an overall suicide rate of 32.77 per 100,000 person-years, and an SMR of 1.91 (95% CI: 1.79, 2.04) relative to the general U.S. population, with adjustment for age, sex, and race.
Table 1.
Suicide rates among patients with cancers of the digestive system according to patient characteristics
| Persons (%) | Person-years (%) | Observed | Expected | SMR (95% CI) | Absolute excess riska |
Suicides per 100,000 person-years |
|
|---|---|---|---|---|---|---|---|
| Overall | 856,292 (100.0) | 2,688,837 (100.0) | 881 | 461.38 | 1.91 (1.79, 2.04) | 1.56 | 32.77 |
| Site | |||||||
| Esophagus | 43912 (5.1) | 75,514 (2.8) | 88 | 17.50 | 5.03 (4.03, 6.19) | 9.34 | 116.54 |
| Colon | 318861 (37.2) | 1,364,509 (50.7) | 311 | 228.21 | 1.36 (1.22, 1.52) | 0.61 | 22.79 |
| Rectum and rectosigmoid junction | 134913 (15.8) | 619,867 (23.1) | 185 | 112.27 | 1.65 (1.42, 1.90) | 1.17 | 29.85 |
| Liver and intrahepatic bile duct | 81684 (9.5) | 118,615 (4.4) | 47 | 21.93 | 2.14 (1.58, 2.85) | 2.11 | 39.62 |
| Pancreas | 119194 (13.9) | 108,093 (4.0) | 93 | 17.62 | 5.28 (4.26, 6.47) | 6.97 | 86.04 |
| Stomach | 76313 (8.9) | 165,987 (6.2) | 81 | 28.52 | 2.84 (2.26, 3.53) | 3.16 | 48.80 |
| Otherb | 81415 (9.5) | 236,253 (8.8) | 76 | 35.34 | 2.15 (1.69, 2.69) | 1.72 | 32.17 |
| Sex | |||||||
| Men | 465,628 (54.4) | 1,412,882 (52.5) | 775 | 393.19 | 1.97 (1.83, 2.11) | 2.70 | 54.85 |
| Women | 390,664 (45.6) | 1,275,955 (47.5) | 106 | 68.19 | 1.55 (1.27, 1.88) | 0.30 | 8.31 |
| Age at diagnosis | |||||||
| <50 | 94,086 (11.0) | 380,553 (14.2) | 102 | 66.43 | 1.54 (1.25, 1.86) | 0.93 | 26.80 |
| 50–59 | 174,665 (20.4) | 635,007 (23.6) | 158 | 110.95 | 1.42 (1.21, 1.66) | 0.74 | 24.88 |
| 60–69 | 212,613 (24.8) | 712,769 (26.5) | 261 | 110.71 | 2.36 (2.08, 2.66) | 2.11 | 36.62 |
| 70–79 | 206,133 (24.1) | 626,220 (23.3) | 231 | 110.86 | 2.08 (1.82, 2.37) | 1.92 | 36.89 |
| 80+ | 168,795 (19.7) | 334,287 (12.4) | 129 | 62.43 | 2.07 (1.73, 2.46) | 1.99 | 38.59 |
| Race | |||||||
| White | 670,545 (78.3) | 2,143,777 (79.7) | 783 | 423.02 | 1.85 (1.72, 1.99) | 1.68 | 36.52 |
| Black | 104,437 (12.2) | 296,543 (11.0) | 34 | 15.94 | 2.13 (1.48, 2.98) | 0.61 | 11.47 |
| Other | 81,310 (9.5) | 248,517 (9.2) | 64 | 22.43 | 2.85 (2.20, 3.64) | 1.67 | 25.75 |
| Marital status | |||||||
| Unmarriedc | 361,662 (42.2) | 967,374 (36.0) | 381 | 130.56 | 2.92 (2.63, 3.23) | 2.59 | 39.38 |
| Married | 452,271 (52.8) | 1,588,600 (59.1) | 454 | 308.62 | 1.47 (1.34, 1.61) | 0.92 | 28.58 |
| Unknown | 42,359 (4.9) | 132,863 (4.9) | 46 | ||||
| Stage | |||||||
| Localized | 280,517 (32.8) | 1,327,814 (49.4) | 326 | 230.69 | 1.41 (1.26, 1.58) | 0.72 | 24.55 |
| Regional | 269,625 (31.5) | 976,891 (36.3) | 293 | 166.85 | 1.76 (1.56, 1.97) | 1.29 | 29.99 |
| Distant | 240,693 (28.1) | 273,669 (10.2) | 209 | 45.48 | 4.60 (3.99, 5.26) | 5.97 | 76.37 |
| Unknown | 65,457 (7.6) | 110,463 (4.1) | 53 | ||||
| Surgery | |||||||
| Yes | 515,121 (60.2) | 2,317,761 (86.2) | 551 | 397.01 | 1.39 (1.27, 1.51) | 0.66 | 23.77 |
| No | 330,539 (38.6) | 350,903 (13.1) | 316 | 60.75 | 5.20 (4.64, 5.81) | 7.27 | 90.05 |
| Unknown | 10,632 (1.2) | 20,173 (0.8) | 14 | ||||
Excess per 10,000 person-years
Other includes small intestine, anus, anal canal and anorectum, gallbladder, other biliary, retroperitoneum, peritoneum, omentum, and mesentery, and other digestive organs
Includes single, widowed, divorced, and unmarried or domestic partner
All cancer sites had significantly higher suicide rates than the general population. The highest SMRs were observed for esophageal cancer (SMR=5.03; 95% CI: 4.03, 6.19), and pancreatic cancer (SMR=5.28; 95% CI: 4.26, 6.47), with absolute rates of 117 and 86 suicides per 100,000 persons-years, respectively. Other cancer sites with more than double the suicide rate in the general population included stomach (SMR=2.84; 95% CI: 2.26, 3.53) and liver and intrahepatic bile duct (SMR=2.14; 95% CI: 1.58, 2.85). The SMRs were lowest for colon cancer and cancer of the rectum and rectosigmoid junction, but were slightly higher for rectum and rectosigmoid junction (SMR=1.65; 95% CI: 1.42, 1.90) than for colon (SMR=1.36; 95% CI: 1.22, 1.52).
For all sites combined, suicide rates were elevated for both men (SMR=1.97; 95% CI: 1.83, 2.11) and women (SMR=1.55; 95% CI: 1.27, 1.88) with digestive system tumors. The absolute suicide rate was highest for Whites, though SMRs were higher for Blacks (SMR=2.13; 95% CI: 1.48, 2.98) and individuals of other races (SMR=2.85; 95% CI: 2.20, 3.64). In analyses according to age at cancer diagnosis, we did not observe consistent trends with increasing age, though both absolute rates and SMRs were lowest among those aged 50–59 years (rate=24.88 per 100,000 person-years; SMR=1.42; 95% CI: 1.21, 1.66). The absolute rate was highest among those aged 80+ years (38.59 per 100,000 person-years), while the SMR was highest among those aged 60–69 years (SMR=2.36; 95% CI: 2.08, 2.66). In other subgroup analyses, those who were unmarried at diagnosis, had distant stage disease, or did not receive surgery had higher suicide rates and corresponding SMRs than those who were married, had localized or regional disease, or received surgery, respectively.
In analyses stratified by sex, the suicide rate among men was higher than that in the general population for all cancer sites, with the highest SMRs among those with pancreatic or esophageal cancer (Table 2). Suicide rates were substantially lower among female patients than among males. Although the highest suicide rate was observed among women with esophageal cancer, the SMRs were only significantly elevated for women with colon cancer (SMR=1.43; 95% CI: 1.06, 1.88) and other digestive cancers (SMR=2.32; 95% CI: 1.40, 3.62).
Table 2.
Cancer site-specific suicide rates according to sex
| Men |
Women |
|||||||
|---|---|---|---|---|---|---|---|---|
| Persons | Suicides | SMR (95% CI) | Suicides per 100,000 person- years |
Persons | Suicides | SMR (95% CI) | Suicides per 100,000 person- ears |
|
| Esophagus | 34,287 | 85 | 5.10 (4.08, 6.31) | 143.69 | 9,625 | 3 | 3.54 (0.73, 10.35) | 18.34 |
| Colon | 155,735 | 261 | 1.35 (1.19, 1.53) | 38.99 | 163,126 | 50 | 1.43 (1.06, 1.88) | 7.19 |
| Rectum and rectosigmoid junction | 76,732 | 166 | 1.72 (1.47, 2.00) | 47.41 | 58,181 | 19 | 1.21 (0.73, 1.89) | 7.04 |
| Liver and intrahepatic bile duct | 58,920 | 44 | 2.19 (1.59, 2.94) | 51.90 | 22,764 | 3 | 1.63 (0.34, 4.78) | 8.87 |
| Pancreas | 59,300 | 87 | 5.95 (4.76, 7.33) | 162.83 | 59,894 | 6 | 2.01 (0.74, 4.37) | 10.98 |
| Stomach | 46,143 | 75 | 3.01 (2.37, 3.77) | 77.84 | 30,170 | 6 | 1.68 (0.62, 3.65) | 8.62 |
| Othera | 34,511 | 57 | 2.10 (1.59, 2.72) | 57.17 | 46,904 | 19 | 2.32 (1.40, 3.62) | 13.91 |
Other includes small intestine, anus, anal canal and anorectum, gallbladder, other biliary, retroperitoneum, peritoneum, omentum, and mesentery, and other digestive organs
In site-specific analyses, SMRs increased with age at diagnosis, with the highest among those aged 70+ years, for all sites except colon cancer and stomach cancer (Figure 1). Among those with colon cancer, the SMR was highest among those aged <50 years, and lowest among those aged 50–69. The opposite pattern was observed for stomach cancer, with the SMR highest among those aged 50–69, and lowest among those aged <50.
Figure 1.
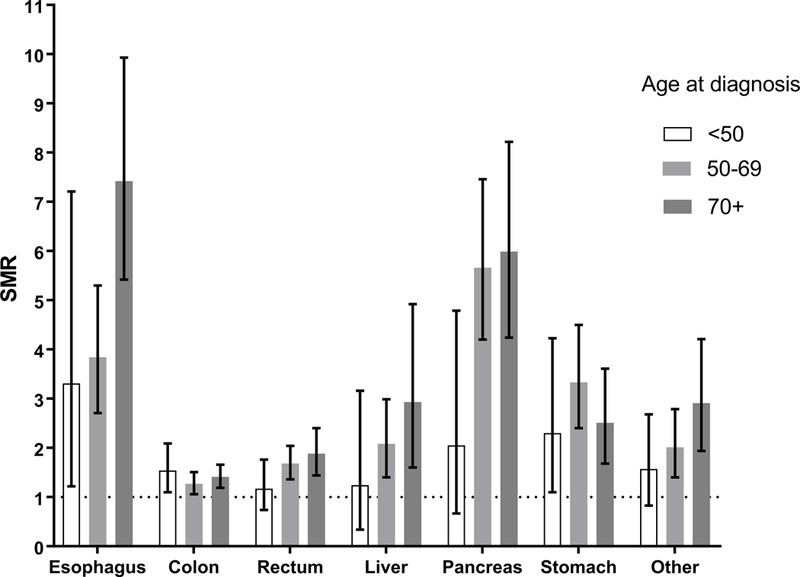
Standardized mortality ratios (SMRs) and 95% confidence intervals for suicide among digestive system cancer patients compared to the general population, according to age at diagnosis by cancer site.
Suicide rates were highest within the first two months after cancer diagnosis, with SMRs ranging from 2.36 (95% CI: 1.42, 3.69) among those with colon cancer, to 12.53 (95% CI: 7.54, 19.56) among those with esophageal cancer (Table 3). SMRs remained elevated for all cancer sites between two months and five years after diagnosis, but were not significantly different from the general population at five or more years post-diagnosis.
Table 3.
Cancer site-specific suicide rates according to time since diagnosis
| <2 months | 2 months - <1 year | 1 year - <5 years | 5+ years | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Suicides | SMR (95% CI) | Suicides per 100,000 person- years |
Suicides | SMR (95% CI) | Suicides per 100,000 person- years |
Suicides | SMR (95% CI) | Suicides per 100,000 person- years |
Suicides | SMR (95% CI) | Suicides per 100,000 person- years |
|
| Overall | 114 | 5.16 (4.26, 6.20) | 87.70 | 261 | 3.17 (2.80, 3.58) | 53.62 | 341 | 1.59 (1.43, 1.77) | 27.02 | 165 | 1.16 (0.99, 1.35) | 20.37 |
| Esophagus | 19 | 12.53 (7.54, 19.56) | 283.33 | 45 | 9.16 (6.68, 12.26) | 208.81 | 20 | 2.64 (1.61, 4.07) | 61.07 | 4 | 1.15 (0.31, 2.94) | 27.57 |
| Colon | 19 | 2.36 (1.42, 3.69) | 38.08 | 56 | 1.63 (1.23, 2.12) | 26.43 | 142 | 1.32 (1.11, 1.55) | 21.79 | 94 | 1.21 (0.97, 1.48) | 20.84 |
| Rectum and rectosigmoid junction | 12 | 3.14 (1.62, 5.48) | 55.29 | 44 | 2.61 (1.90, 3.51) | 46.13 | 83 | 1.57 (1.25, 1.94) | 28.05 | 46 | 1.19 (0.87, 1.59) | 22.23 |
| Liver and intrahepatic bile duct | 6 | 2.76 (1.01, 6.00) | 52.43 | 18 | 2.85 (1.69, 4.51) | 53.86 | 18 | 1.81 (1.07, 2.85) | 33.20 | 5 | 1.44 (0.47, 3.36) | 25.59 |
| Pancreas | 26 | 9.72 (6.35, 14.25) | 157.74 | 48 | 7.31 (5.39, 9.70) | 118.92 | 17 | 2.65 (1.54, 4.24) | 43.42 | 2 | 1.02 (0.12, 3.68) | 16.53 |
| Stomach | 17 | 8.37 (4.88, 13.40) | 148.76 | 31 | 4.61 (3.13, 6.55) | 81.00 | 28 | 2.18 (1.45, 3.15) | 37.01 | 5 | 0.72 (0.23, 1.69) | 12.31 |
| Othera | 15 | 8.32 (4.65, 13.71) | 121.54 | 19 | 2.84 (1.71, 4.44) | 41.42 | 33 | 1.98 (1.36, 2.78) | 29.29 | 9 | 0.88 (0.40, 1.67) | 13.77 |
Other includes small intestine, anus, anal canal and anorectum, gallbladder, other biliary, retroperitoneum, peritoneum, omentum, and mesentery, and other digestive organs
In multivariable-adjusted analyses within the cancer patient cohort, characteristics associated with higher suicide rates included a diagnosis of esophageal, pancreatic, or stomach cancer, as well as male sex, White race, and older age at diagnosis. Being unmarried, having regional or distant stage disease, and not having surgery were also associated with higher suicide rates (Supplementary Table 1).
Conclusions
In this population-based study, we examined rates of suicide in U.S. patients diagnosed with cancers of the digestive system, and considered variation in rates according to cancer site, patient characteristics, and time since diagnosis. Overall, the suicide rate among digestive system cancer patients was nearly twice that of the general U.S. population. Our results confirm those of earlier reports suggesting higher suicide rates in patients with pancreatic and stomach cancers,4, 13 and further identify esophageal and liver cancer patients as potential high-risk groups. For all cancer sites, suicides rates were highest within the first year of cancer diagnosis—particularly within the first two months—and remained elevated for up to five years, but were not significantly different from the general population after five years. Our findings reflect recent trends in suicides rates among both cancer patients and the general population.
Prior reviews of suicide in cancer patients reflected patterns from the 1970s to early 2000s and concluded that many of the risk factors for suicide in the general population can be extended to populations with cancer.3, 14 In addition to depression, characteristics such as male gender, older age, and white race are consistently associated with higher suicide rates, regardless of cancer history.1, 3, 4, 14, 15 However, other risk factors, such as cancer type and recency of cancer diagnosis, are specific to populations with cancer.3 Pancreatic cancer has been cited as among the sites with the highest suicide rates, as have prostate, lung, and head and neck cancers.14 The first few months following a cancer diagnosis also tend to be associated with higher suicide risk, with rates remaining elevated up to 5 years postdiagnosis and generally declining thereafter.4, 5, 16–18
Our finding of an approximate doubling of the rate of suicide in patients with cancers of the digestive system relative to the general population is comparable to that previously reported for all cancer types combined in earlier time periods. Using SEER data for all patients diagnosed with cancer from 1973 to 2002, Misono et al. reported an overall SMR of 1.88 (95% CI: 1.83, 1.93).4 In general, their site-specific analyses suggested that cancer types with poorer prognosis on average tended to have the highest suicide rates. However, results were only reported for the more common cancer sites, including colorectal cancer and stomach cancer, but not other cancers of the digestive system. In their study, the SMR for colon and rectal cancer combined was 1.90 (95% CI: 1.74, 2.07). We analyzed the two sites separately, finding slightly lower SMRs for both sites than in their results from earlier years, and slightly higher SMRs for rectal cancer than colon cancer among males, but not females. Comparison of our results with those of Misono et al. also suggests that the suicide rate in stomach cancer patients has decreased in recent years, from 72 to 49 per 100,000 person-years in their study and ours, respectively. This is reflected in the lower SMR in our study than that reported by Misono et al. (SMR=4.68; 95% CI: 3.81, 5.70), and may partially stem from modest increases in five-year relative survival among stomach cancer patients since the 1970s.19
To our knowledge, little research has examined suicide rates among U.S. patients with esophageal and liver cancer, though each of these cancer types has an estimated five-year survival of less than 20%, and may have quality of life concerns as a result of debilitating symptoms or treatment side effects.20–23 These cancers are also associated with alcohol and tobacco use, factors related to suicide risk in the general population.24, 25 In a prior study of suicides among cancer patients diagnosed in California during 1997–2006, age-adjusted rates of suicide were 76.8 and 21.8 per 100,000 person-years for esophageal and liver cancers, respectively.26 Notably, in the current study, the absolute suicide rate among esophageal cancer patients was nearly 120 per 100,000 person-years, and exceeded that of all of other digestive system cancers in our study, including pancreatic. Dysphagia and weight loss, along with continued pain and fatigue following esophageal surgery, have been associated with reduced quality of life22, 23 and may be contributors to suicide in patients with esophageal cancer. Oncologists and other healthcare providers should be aware of the excess risk in this population, to help ensure that patients most likely to be experiencing psychological distress receive the support they need.
Though still more than five times that of the general population, the absolute rate of suicide among pancreatic patients in our analyses is lower than that previously reported. In a study of patients diagnosed with pancreatic adenocarcinoma between 1995 and 2005 identified using the SEER database, the suicide rate was 135 per 100,000 person-years, with a corresponding SMR of 10.8 (95% CI: 9.2–12.7).13 The reasons for this apparent decrease are not clear, given only slight improvements in pancreatic cancer prognosis since the early 2000s.27 It is possible that developments in symptom management and palliative care, or increasing availability and use of these approaches,28, 29 may be at least partially attributable.
The issues surrounding suicide in terminally ill patients are complex, and have been the subject of considerable moral and ethical debate. For many cancers of the digestive system, including esophageal, liver, and pancreatic, five-year relative survival is less than 50%, even among those with localized disease.30–32 Such poor prognoses may be accompanied by feelings of depression and hopelessness, which may precipitate suicidality in cancer patients.14 Routine screening for depression and suicidal ideation in cancer patients has been proposed, and several tools are available to aide in the early identification of those experiencing these symptoms.14, 33 While there remains limited evidence of the effectiveness of screening initiatives to reduce suicide rates in oncology settings, the involvement of psychiatrists and other mental health professionals may be a critical component of cancer care for high-risk patient subgroups, particularly within the first couple months of diagnosis when suicide risk is highest.
Study limitations
Our study has several limitations, including those inherent to the use of registry data sources such as SEER. Information on depressive symptoms, comorbidities, physical functioning, and substance abuse is not available in SEER, but would help to further refine the identification of patients at high risk for suicide. Cause of death ascertained from death certificates may also be subject to misclassification, and therefore suicides rates in our analyses may be underestimated. However, relatively high sensitivity and specificity have been reported for the coding of suicide on death certificates with a physician review panel as the gold standard for cause of death determination.34 Furthermore, any underestimation of suicides rates is likely similar between cancer patients and the general population, making it less likely that our SMR estimates are substantially biased. We also could not account for the impact of cancer recurrences on suicide rates, as recurrence information is not captured by SEER.
Supplementary Material
Clinical implications.
Our findings suggest that rates of suicide are significantly elevated among patients with cancers of the digestive system compared to the general population, with particularly high rates for those recently diagnosed with esophageal and pancreatic cancers. Although suicide is a relatively rare occurrence even among patients with advanced cancer, healthcare providers should be aware of high-risk subgroups, and should utilize appropriate screening tools and referrals to mental health services when indicated.
Footnotes
Ethical approval statement: This study was considered exempt by the Institutional Review Board of the University of North Carolina (IRB number 17–3233).
Patient consent statement: For this type of study, participant written informed consent was not required.
Conflict of interest: None
References
- 1.Kochanek KD, Murphy SL, Xu JQ, Tejada-Vera B. Deaths: Final data for 2014 National vital statistics reports; vol 65 no 4. Hyattsville, MD: National Center for Health Statistics; 2016. [PubMed] [Google Scholar]
- 2.Curtin SC, Warner M, Hedegaard H. Increase in suicide in the United States, 1999–2014 NCHS data brief, no 241. Hyattsville, MD: National Center for Health Statistics; 2016. [PubMed] [Google Scholar]
- 3.Robson A, Scrutton F, Wilkinson L, et al. The risk of suicide in cancer patients: a review of the literature Psychooncology 2010;19(12):1250–8. [DOI] [PubMed] [Google Scholar]
- 4.Misono S, Weiss NS, Fann JR, et al. Incidence of suicide in persons with cancer J Clin Oncol 2008;26(29):4731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kam D, Salib A, Gorgy G, et al. Incidence of Suicide in Patients With Head and Neck Cancer JAMA Otolaryngol Head Neck Surg 2015;141(12):1075–81. [DOI] [PubMed] [Google Scholar]
- 6.Surveillance, Epidemiology, and End Results Program. Overview of the SEER Program. Available from: https://seer.cancer.gov/about/overview.html.
- 7.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs excluding AK Research Data, Nov 2016 Sub (2000–2014) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2015 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2017,based on the November 2016 submission. [Google Scholar]
- 8.Surveillance, Epidemiology, and End Results Program. SEER Registry Groupings for Analyses. Available from: https://seer.cancer.gov/registries/terms.html.
- 9.Surveillance, Epidemiology, and End Results Program. Site Recode ICD-O-3/WHO 2008. Definition Available from: https://seer.cancer.gov/siterecode/icdo3_dwhoheme/.
- 10.Surveillance, Epidemiology, and End Results Program. SEER Cause of Death Recode 1969+ (04/16/2012). Available from: https://seer.cancer.gov/codrecode/1969+_d04162012/index.html.
- 11.Koepsell TD, Weiss NS. Epidemiologic Methods: Studying the Occurrence of Illness.New York,NY: Oxford University Press; 2003. [Google Scholar]
- 12.Surveillance, Epidemiology, and End Results Program. SEER*Stat Software, version 8.3.4. [Google Scholar]
- 13.Turaga KK, Malafa MP, Jacobsen PB, et al. Suicide in patients with pancreatic cancer Cancer 2011;117(3):642–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anguiano L, Mayer DK, Piven ML, et al. A literature review of suicide in cancer patients Cancer Nurs 2012;35(4):E14–26. [DOI] [PubMed] [Google Scholar]
- 15.Siracuse BL, Gorgy G, Ruskin J, et al. What is the Incidence of Suicide in Patients with Bone and Soft Tissue Cancer? : Suicide and Sarcoma Clin Orthop Relat Res 2017;475(5):1439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang F, Fall K, Mittleman MA, et al. Suicide and cardiovascular death after a cancer diagnosis N Engl J Med 2012;366(14):1310–8. [DOI] [PubMed] [Google Scholar]
- 17.Allebeck P, Bolund C, Ringback G. Increased suicide rate in cancer patients. A cohort study based on the Swedish Cancer-Environment Register J Clin Epidemiol 1989;42(7):611–6. [DOI] [PubMed] [Google Scholar]
- 18.Hem E, Loge JH, Haldorsen T, et al. Suicide risk in cancer patients from 1960 to 1999 J Clin Oncol 2004;22(20):4209–16. [DOI] [PubMed] [Google Scholar]
- 19.Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Stomach Cancer. Available from: https://seer.cancer.gov/statfacts/html/stomach.html. [Google Scholar]
- 20.Gandhi S, Khubchandani S, Iyer R. Quality of life and hepatocellular carcinoma J Gastrointest Oncol 2014;5(4):296–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chie WC, Yu F, Li M, et al. Quality of life changes in patients undergoing treatment for hepatocellular carcinoma Qual Life Res 2015;24(10):2499–506. [DOI] [PubMed] [Google Scholar]
- 22.Darling GE. Quality of life in patients with esophageal cancer Thorac Surg Clin 2013;23(4):569–75. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs M, Macefield RC, Elbers RG, et al. Meta-analysis shows clinically relevant and long-lasting deterioration in health-related quality of life after esophageal cancer surgery Qual Life Res 2014;23(4):1097–115. [DOI] [PubMed] [Google Scholar]
- 24.Han B, Compton WM, Blanco C. Tobacco Use and 12-Month Suicidality Among Adults in the United States Nicotine Tob Res 2017;19(1):39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glasheen C, Pemberton MR, Lipari R, et al. Binge drinking and the risk of suicidal thoughts, plans, and attempts Addict Behav 2015;43:42–9. [DOI] [PubMed] [Google Scholar]
- 26.Nasseri K, Mills PK, Mirshahidi HR, et al. Suicide in cancer patients in California, 1997–2006 Arch Suicide Res 2012;16(4):324–33. [DOI] [PubMed] [Google Scholar]
- 27.Sirri E, Castro FA, Kieschke J, et al. Recent Trends in Survival of Patients With Pancreatic Cancer in Germany and the United States Pancreas 2016;45(6):908–14. [DOI] [PubMed] [Google Scholar]
- 28.Hammer SL, Clark K, Grant M, et al. Seventeen years of progress for supportive care services: A resurvey of National Cancer Institute-designated comprehensive cancer centers Palliat Support Care 2015;13(4):917–25. [DOI] [PubMed] [Google Scholar]
- 29.Kulaylat AS, Mirkin KA, Hollenbeak CS, et al. Utilization and trends in palliative therapy for stage IV pancreatic adenocarcinoma patients: a U.S. population-based study J Gastrointest Oncol 2017;8(4):710–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Esophageal Cancer. Available from: https://seer.cancer.gov/statfacts/html/esoph.html.
- 31.Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Liver and Intrahepatic Bile Duct Cancer. Available from: https://seer.cancer.gov/statfacts/html/livibd.html.
- 32.Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Pancreas Cancer. Available from: https://seer.cancer.gov/statfacts/html/pancreas.html.
- 33.Leung YW, Li M, Devins G, et al. Routine screening for suicidal intention in patients with cancer Psychooncology 2013;22(11):2537–45. [DOI] [PubMed] [Google Scholar]
- 34.Moyer LA, Boyle CA, Pollock DA. Validity of death certificates for injury-related causes of death Am J Epidemiol 1989;130(5):1024–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


